Prognostic Performance of Three Lymph-Node Staging Systems on Gastric Signet-Ring-Cell Carcinoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Lymph-Node Staging System
2.3. Statistical Analysis
3. Results
3.1. Clinical Characteristics
3.2. Characteristics of Different Lymph-Node staging Systems
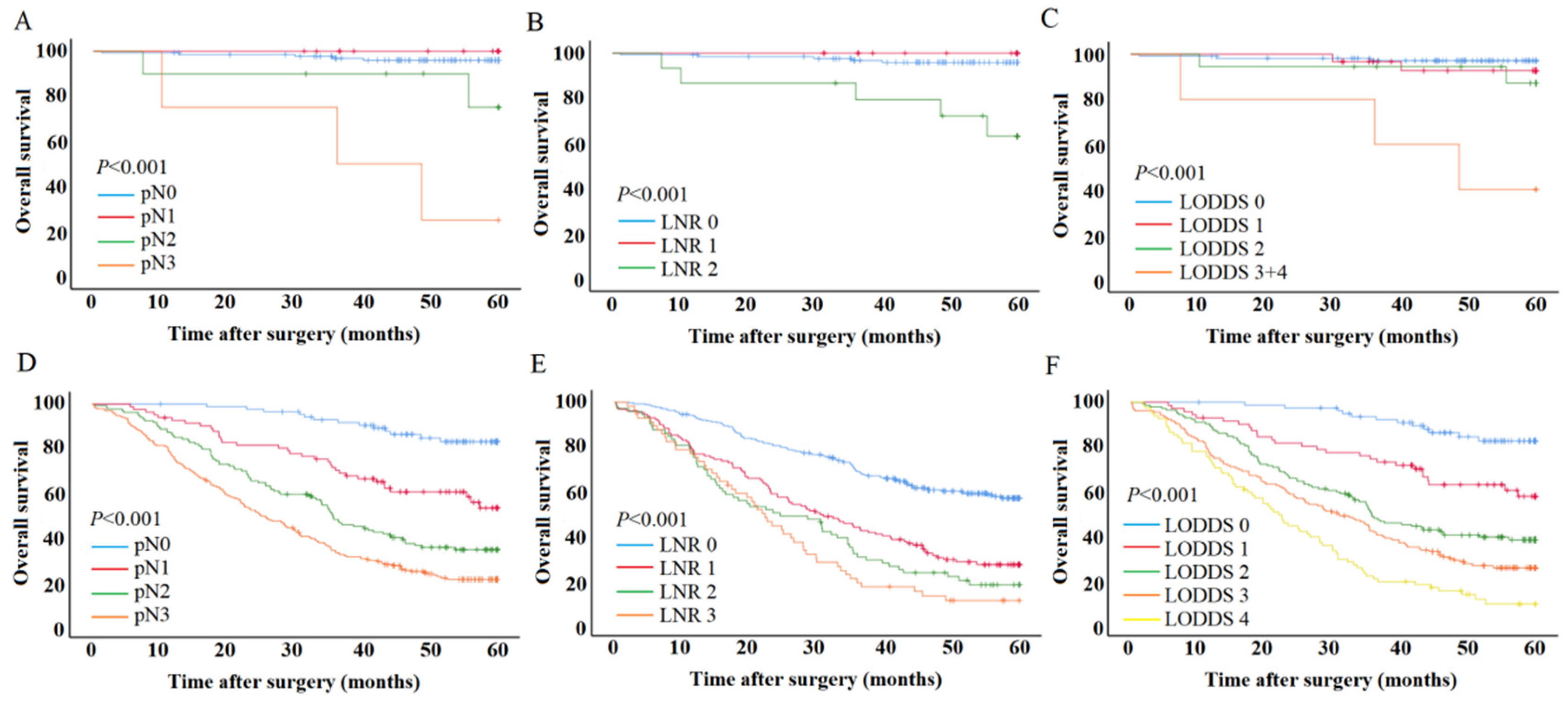
3.3. Prognostic Performance of Different Node Staging Systems
3.4. The Effect of LODDS on Patient Survival
3.5. Univariate and Multivariate Analysis for Patient Outcomes
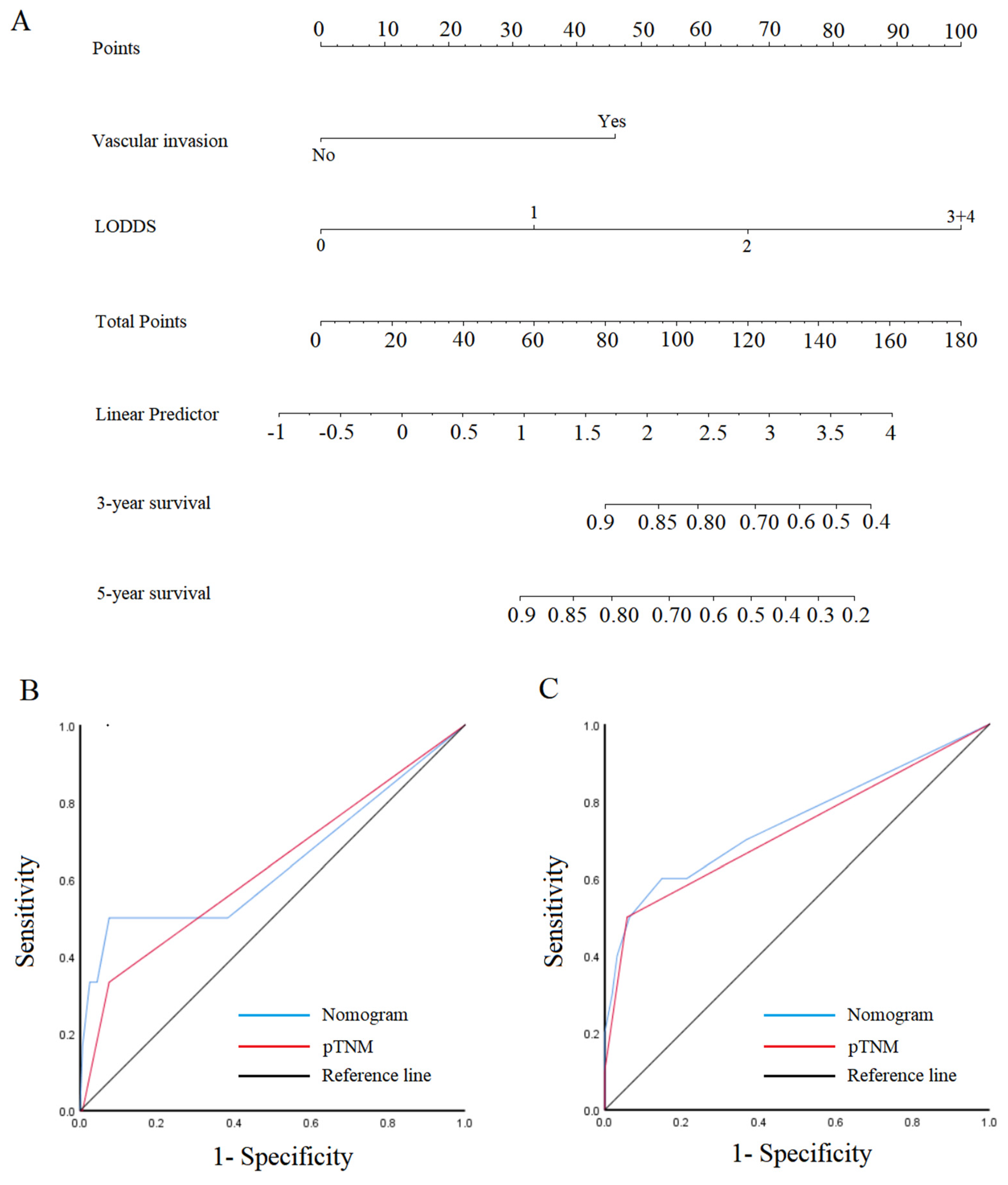
3.6. Nomogram in the Patient Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Henson, D.E.; Dittus, C.; Younes, M.; Nguyen, H.; Albores-Saavedra, J. Differential trends in the intestinal and diffuse types of gastric carcinoma in the United States, 1973–2000: Increase in the signet ring cell type. Arch. Pathol. Lab. Med. 2004, 128, 765–770. [Google Scholar] [CrossRef] [PubMed]
- Pernot, S.; Voron, T.; Perkins, G.; Lagorce-Pages, C.; Berger, A.; Taieb, J. Signet-ring cell carcinoma of the stomach: Impact on prognosis and specific therapeutic challenge. World J. Gastroenterol. 2015, 21, 11428–11438. [Google Scholar] [CrossRef]
- Dodge, O.G. Histological Typing of Gastric and Oesophageal Tumours (International Histological Classification of Tumours No. 18). Br. J. Cancer 1979, 39, 210. [Google Scholar] [CrossRef]
- Kao, Y.-C.; Fang, W.-L.; Wang, R.-F.; Li, A.F.-Y.; Yang, M.-H.; Wu, C.-W.; Shyr, Y.-M.; Huang, K.-H. Clinicopathological differences in signet ring cell adenocarcinoma between early and advanced gastric cancer. Gastric Cancer 2019, 22, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Lv, W.; Zhang, J.; Zhang, J.; Huang, B.; Lin, J. Different prognostic significance of signet ring cell histology for early and advanced gastric cancer patients: A systematic review and meta-analysis. Expert Rev. Gastroenterol. Hepatol. 2020, 14, 499–509. [Google Scholar] [CrossRef]
- Kwon, S.J.; Kim, G.S. Prognostic significance of lymph node metastasis in advanced carcinoma of the stomach. Br. J. Surg. 1996, 83, 1600–1603. [Google Scholar] [CrossRef]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef]
- Lee, H.K.; Yang, H.K.; Kim, W.H.; Lee, K.U.; Choe, K.J.; Kim, J.P. Influence of the number of lymph nodes examined on staging of gastric cancer. Br. J. Surg. 2001, 88, 1408–1412. [Google Scholar] [CrossRef]
- Jian-Hui, C.; Shi-Rong, C.; Hui, W.; Si-Le, C.; Jian-Bo, X.; Er-Tao, Z.; Chuang-Qi, C.; Yu-Long, H. Prognostic value of three different lymph node staging systems in the survival of patients with gastric cancer following D2 lymphadenectomy. Tumor Biol. 2016, 37, 11105–11113. [Google Scholar] [CrossRef]
- Wang, X.; Appleby, D.H.; Zhang, X.; Gan, L.; Wang, J.J.; Wan, F. Comparison of three lymph node staging schemes for predicting outcome in patients with gastric cancer. Br. J. Surg. 2013, 100, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Spolverato, G.; Ejaz, A.; Kim, Y.; Squires, M.H.; Poultsides, G.; Fields, R.C.; Bloomston, M.; Weber, S.M.; Votanopoulos, K.; Acher, A.; et al. Prognostic Performance of Different Lymph Node Staging Systems After Curative Intent Resection for Gastric Adenocarcinoma. Ann. Surg. 2015, 262, 991–998. [Google Scholar] [CrossRef] [PubMed]
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer 2021, 24, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Jeon, C.H.; Kim, S.J.; Seo, H.S.; Song, K.Y.; Lee, H.H. A Novel Approach for Gastric Cancer Staging in Elderly Patients Based on the Lymph Node Ratio. J. Gastric Cancer 2021, 21, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Kim, J.-H.; Kim, H.; Kim, H.; Lee, Y.C.; Kil Lee, S.; Shin, S.K.; Park, J.C.; Chung, H.S.; Park, J.J.; et al. Is the recent WHO histological classification for gastric cancer helpful for application to endoscopic resection? Gastric Cancer 2016, 19, 869–875. [Google Scholar] [CrossRef]
- Xu, Z.; Jing, J.; Ma, G. Development and validation of prognostic nomogram based on log odds of positive lymph nodes for patients with gastric signet ring cell carcinoma. Chin. J. Cancer Res. 2020, 32, 778–793. [Google Scholar] [CrossRef]
- Deng, J.; Zhang, R.; Wu, L.; Zhang, L.; Wang, X.; Liu, Y.; Hao, X.; Liangliang, W. Superiority of the ratio between negative and positive lymph nodes for predicting the prognosis for patients with gastric cancer. Ann. Surg. Oncol. 2015, 22, 1258–1266. [Google Scholar] [CrossRef]
- Lorenzon, L.; Mercantini, P.; Ferri, M.; La Torre, M.; Sparagna, A.; Cavallini, M.; Ziparo, V.; Balducci, G. Lymph-node ratio classification strongly correlates with cancer survivals of patients who underwent r0 resection for gastric cancer with more than 15 nodes harvested. Eur. Surg. Res. 2014, 53, 1–10. [Google Scholar] [CrossRef]
- Vinh-Hung, V.; Verschraegen, C.; I Promish, D.; Cserni, G.; Van De Steene, J.; Tai, P.; Vlastos, G.; Voordeckers, M.; Storme, G.; Royce, M. Ratios of involved nodes in early breast cancer. Breast Cancer Res. 2004, 6, R680–R688. [Google Scholar] [CrossRef] [PubMed]
- Feinstein, A.R.; Sosin, D.M.; Wells, C.K. The Will Rogers phenomenon. Stage migration and new diagnostic techniques as a source of misleading statistics for survival in cancer. N. Engl. J. Med. 1985, 312, 1604–1608. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Zhu, G.-L.; Lu, C.; Guo, P.-T.; Huang, B.-J.; Li, K.; Xu, Y.; Li, D.-M.; Wang, Z.-N.; Xu, H.-M. The impact of N-ratio in minimizing stage migration phenomenon in gastric cancer patients with insufficient number or level of lymph node retrieved: Results from a Chinese mono-institutional study in 2159 patients. Ann. Oncol. 2009, 20, 897–905. [Google Scholar] [CrossRef]
- Lee, J.H.; Choi, I.J.; Kook, M.C.; Nam, B.H.; Kim, Y.W.; Ryu, K.W. Risk factors for lymph node metastasis in patients with early gastric cancer and signet ring cell histology. Br. J. Surg. 2010, 97, 732–736. [Google Scholar] [CrossRef]
- Kunisaki, C.; Shimada, H.; Nomura, M.; Matsuda, G.; Otsuka, Y.; Akiyama, H. Therapeutic strategy for signet ring cell carcinoma of the stomach. Br. J. Surg. 2004, 91, 1319–1324. [Google Scholar] [CrossRef] [PubMed]
- Gu, P.; Deng, J.; Sun, Z.; Wang, Z.; Wang, W.; Liang, H.; Xu, H.; Zhou, Z. Superiority of log odds of positive lymph nodes (LODDS) for prognostic prediction after gastric cancer surgery: A multi-institutional analysis of 7620 patients in China. Surg. Today 2021, 51, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Ramos, M.F.K.P.; Pereira, M.A.; Dias, A.R.; Yagi, O.K.; Zaidan, E.P.; Junior, U.R.; Zilberstein, B.; Cecconello, I. Surgical outcomes of gastrectomy with D1 lymph node dissection performed for patients with unfavorable clinical conditions. Eur. J. Surg. Oncol. 2019, 45, 460–465. [Google Scholar] [CrossRef]
- Smith, D.D.; Schwarz, R.R.; Schwarz, R.E. Impact of total lymph node count on staging and survival after gastrectomy for gastric cancer: Data from a large US-population database. J. Clin. Oncol. 2005, 23, 7114–7124. [Google Scholar] [CrossRef]
- Li, S.; Desiderio, J.; Li, Z.; Tozzi, F.; Ji, J.; Parisi, A. The development and external validation of a nomogram predicting overall survival of gastric cancer patients with inadequate lymph nodes based on an international database. Int. J. Clin. Oncol. 2021, 26, 867–874. [Google Scholar] [CrossRef]




| Characteristics | pT1 (n = 165) | pT2–pT4 (n = 528) | p Value |
|---|---|---|---|
| Sex | 0.005 | ||
| Male | 90 (54.5) | 352 (66.7) | |
| Female | 75 (45.5) | 176 (33.3) | |
| Age, median, range | 55 (29–81) | 59 (23–82) | <0.001 |
| Tumor location | 0.001 | ||
| Upper | 8 (4.8) | 58 (11.0) | |
| Middle | 29 (17.6) | 80 (15.2) | |
| Lower | 128 (77.6) | 358 (67.8) | |
| Total | 0 (0.0) | 32 (6.1) | |
| Tumor size (mm) | <0.001 | ||
| ≤50 | 148 (89.7) | 240 (45.5) | |
| >50 | 17 (10.3) | 288 (54.5) | |
| pN | <0.001 | ||
| pN0 | 130 (78.8) | 86 (16.3) | |
| pN1 | 21 (12.7) | 81 (15.3) | |
| pN2 | 10 (6.1) | 132 (25.0) | |
| pN3 | 4 (2.4) | 229 (43.4) | |
| pTNM | <0.001 | ||
| Ⅰ | 151 (91.2) | 27 (5.1) | |
| Ⅱ | 13 (7.9) | 144 (27.3) | |
| Ⅲ | 1 (0.6) | 357 (67.6) | |
| Vascular invasion | <0.001 | ||
| No | 143 (86.7) | 226 (42.8) | |
| Yes | 22 (13.3) | 302 (57.2) | |
| Neural infiltration | <0.001 | ||
| No | 151 (91.5) | 100 (18.9) | |
| Yes | 14 (8.5) | 428 (81.1) | |
| LNR | <0.001 | ||
| 0–0.2 | 159 (96.4) | 272 (51.5) | |
| 0.2–0.4 | 5 (3.0) | 128 (24.2) | |
| 0.4–0.6 | 1 (0.6) | 72 (13.6) | |
| >0.6 | 0 (0.0) | 56 (10.6) | |
| LODDS | <0.001 | ||
| LODDS 0 | 111 (62.3) | 79 (15.0) | |
| LODDS 1 | 31 (18.8) | 71 (13.4) | |
| LODDS 2 | 18 (10.9) | 143 (27.1) | |
| LODDS 3 | 4 (2.4) | 154 (29.2) | |
| LODDS 4 | 1 (0.6) | 81 (15.3) |
| No. of LNs Retrieved | ||||
|---|---|---|---|---|
| Total | <16 | 16–30 | >30 | |
| C-Index | C-Index | C-Index | C-Index | |
| pT1 SRCC | ||||
| pN | 0.676 | 0.635 | 0.965 | 0.674 |
| LNR | 0.728 | 0.635 | 0.958 | 0.674 |
| LODDS | 0.723 | 0.656 | 0.976 | 0.731 |
| pT2-pT4 SRCC | ||||
| pN | 0.670 | 0.739 | 0.682 | 0.644 |
| LNR | 0.644 | 0.706 | 0.658 | 0.621 |
| LODDS | 0.684 | 0.779 | 0.699 | 0.657 |
| Characteristics | Multivariate Analysis for pN | Multivariate Analysis for LNR | Multivariate Analysis for LODDS | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Vascular invasion | 0.091 | 0.074 | 0.045 | |||
| No | 1 | 1 | 1 | |||
| Yes | 3.254 (0.828–12.792) | 3.423 (0.889–13.173) | 3.789 (1.031–13.919) | |||
| pN | 0.003 | |||||
| pN0 | 1 | |||||
| pN1 | 0.000 (0.000-inf) | 0.983 | ||||
| pN2 | 4.067 (0.745–22.187) | 0.105 | ||||
| pN3 | 18.219 (3.950–84.030) | <0.001 | ||||
| LNR | 0.017 | |||||
| 0 | 1 | |||||
| 0.1 | 0.000 | 0.984 | ||||
| >0.1 | 6.823 (1.831–25.427) | 0.004 | ||||
| LODDS | 0.002 | |||||
| LODDS 0 | 1 | |||||
| LODDS 1 | 2.207 (0.367–13.278) | 0.387 | ||||
| LODDS 2 | 3.595 (0.598–21.635) | 0.162 | ||||
| LODDS 3 + 4 | 21.966 (4.293–112.394) | <0.001 | ||||
| Characteristics | Multivariate Analysis for pN | Multivariate Analysis for LMR | Multivariate Analysis for LODDS | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Age | 1.012 (1.011–1.023) | 0.032 | 1.012 (1.001–1.023) | 0.036 | 1.010 (0.999–1.021) | 0.072 |
| Tumor location | <0.001 | 0.002 | 0.006 | |||
| Upper | 1 | 1 | 1 | |||
| Middle | 0.635 (0.405–0.996) | 0.048 | 0.712 (0.454–1.117) | 0.139 | 0.717 (0.457–1.125) | 0.148 |
| Lower | 0.843 (0.590–1.203) | 0.347 | 0.938 (0.655–1.343) | 0.726 | 0.914 (0.639–1.307) | 0.621 |
| Total | 1.785 (1.085–2.937) | 0.023 | 1.878 (1.125–3.133) | 0.016 | 1.734 (1.039–2.893) | 0.035 |
| Vascular invasion | 0.381 | 0.189 | 0.314 | |||
| No | 1 | 1 | 1 | |||
| Yes | 1.116 (0.873–1.427) | 1.184 (0.920–1.524) | 1.136 (0.886–1.456) | |||
| Neural infiltration | 0.026 | 0.023 | 0.023 | |||
| No | 1 | 1 | 1 | |||
| Yes | 1.471 (1.047–2.068) | 1.490 (1.056–2.102) | 1.489 (1.057–2.096) | |||
| pN | <0.001 | |||||
| pN1 | 1 | |||||
| pN2 | 2.917 (1.532–5.551) | 0.001 | ||||
| pN3 | 5.440 (3.012–9.825) | <0.001 | ||||
| LMR | <0.001 | |||||
| 0–0.2 | 1 | |||||
| 0.2–0.4 | 2.088 (1.565–2.785) | <0.001 | ||||
| 0.4–0.6 | 2.663 (1.892–3.748) | <0.001 | ||||
| >0.6 | 3.002 (2.056–4.385) | <0.001 | ||||
| LODDS | <0.001 | |||||
| LODDS 0 | 1 | |||||
| LODDS 1 | 2.733 (1.380–5.415) | 0.004 | ||||
| LODDS 2 | 5.055 (2.751–9.289) | <0.001 | ||||
| LODDS 3 | 6.786 (3.690–12.481) | <0.001 | ||||
| LODDS 4 | 9.565 (5.040–18.155) | <0.001 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Ma, Y.; Liu, B. Prognostic Performance of Three Lymph-Node Staging Systems on Gastric Signet-Ring-Cell Carcinoma. Cancers 2023, 15, 3170. https://doi.org/10.3390/cancers15123170
Zhang L, Ma Y, Liu B. Prognostic Performance of Three Lymph-Node Staging Systems on Gastric Signet-Ring-Cell Carcinoma. Cancers. 2023; 15(12):3170. https://doi.org/10.3390/cancers15123170
Chicago/Turabian StyleZhang, Limin, Yan Ma, and Bao Liu. 2023. "Prognostic Performance of Three Lymph-Node Staging Systems on Gastric Signet-Ring-Cell Carcinoma" Cancers 15, no. 12: 3170. https://doi.org/10.3390/cancers15123170
APA StyleZhang, L., Ma, Y., & Liu, B. (2023). Prognostic Performance of Three Lymph-Node Staging Systems on Gastric Signet-Ring-Cell Carcinoma. Cancers, 15(12), 3170. https://doi.org/10.3390/cancers15123170




