Inflammatory Bowel Disease Treatment in Cancer Patients—A Comprehensive Review
Abstract
Simple Summary
Abstract
1. Introduction
2. Inflammatory Bowel Disease and Malignancies
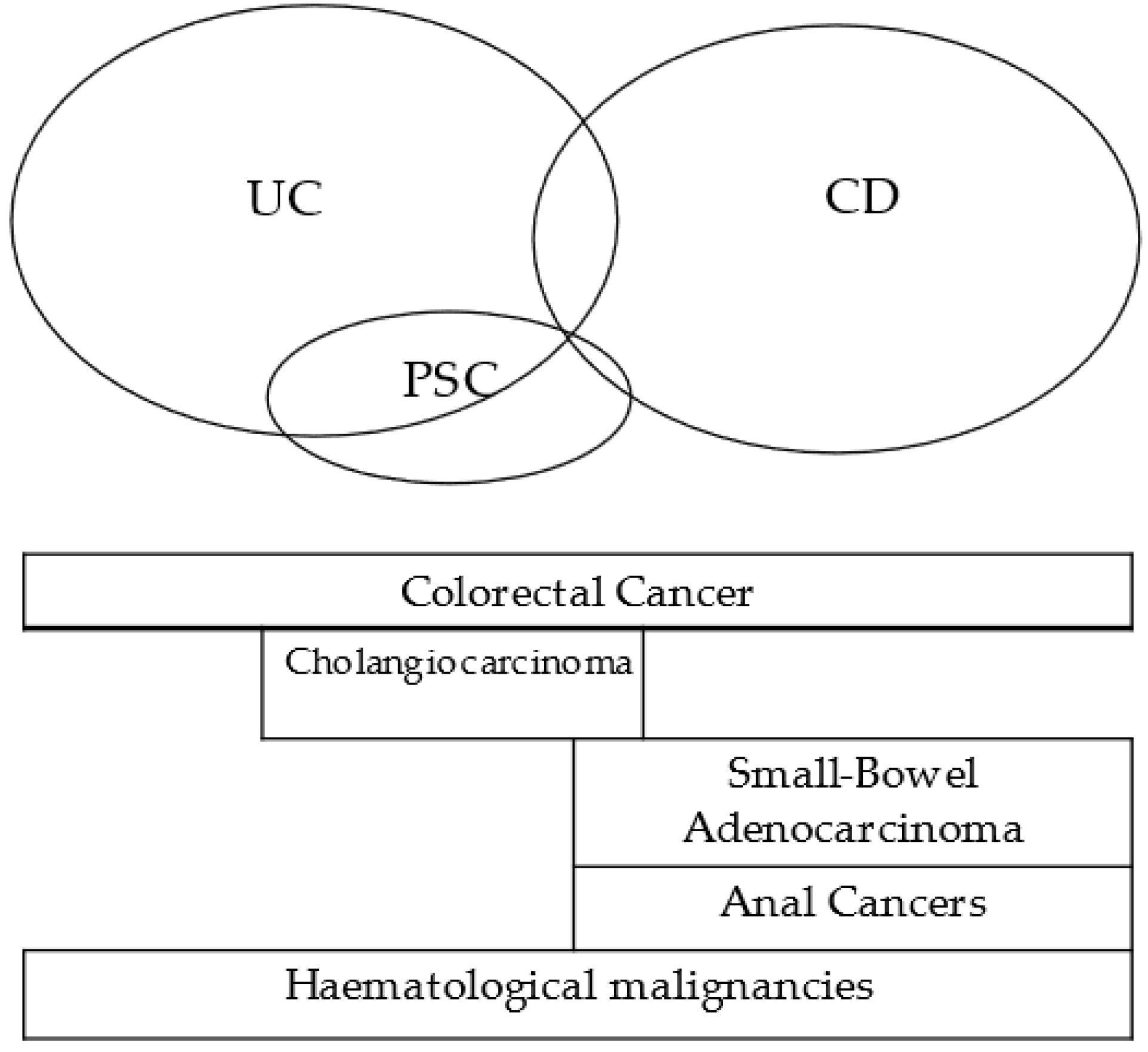
2.1. IBD Related Cancers
2.1.1. Colorectal Cancer
2.1.2. Small-Bowel Adenocarcinoma
2.1.3. Anal Cancers
2.1.4. Cholangiocarcinoma
2.1.5. Hematological Malignancies
2.2. IBD Drug-Related Malignancies
2.2.1. Thiopurines
2.2.2. Anti-TNFα
2.2.3. Anti-TNFα and Immunomodulator
2.2.4. Anti-Integrins
2.2.5. Anti IL-12/IL-23
2.2.6. JAK Inhibitors
2.2.7. Methotrexate
2.2.8. Novel IBD Drugs
3. Managing Inflammatory Bowel Disease during Oncological Treatment
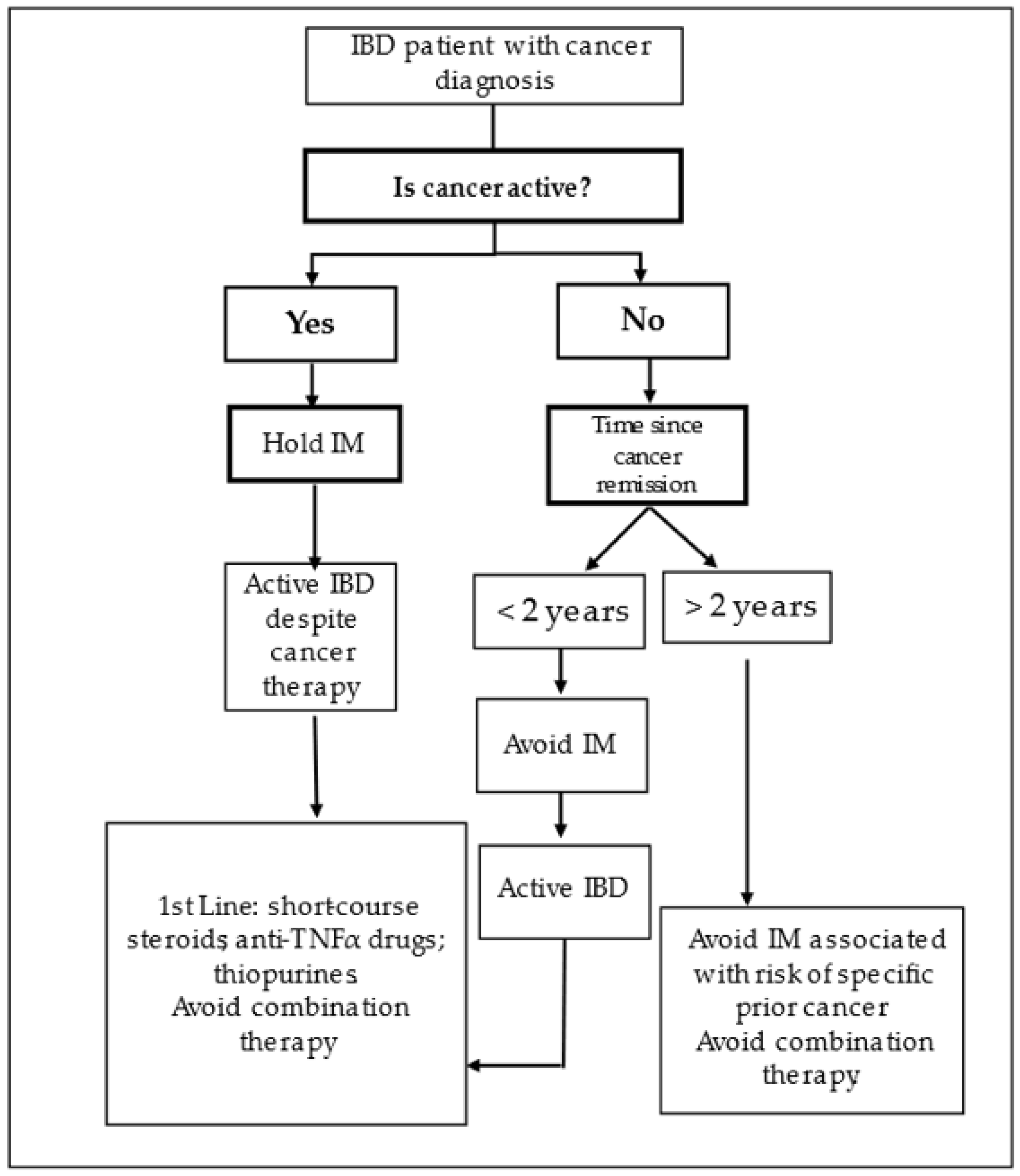
3.1. Risk of Maintaining IBD Immunomodulator Treatment
3.2. Recommendations for Management in Clinical Practice
3.3. Cancer Treatment Effect on IBD
4. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Beaugerie, L. Practical Considerations in Managing Patients with IBD at Risk of Future Cancer or with Current or Previous Cancer; Bristish Society of Gastroenterology: London, UK, 2019. [Google Scholar]
- Molodecky, N.A.; Soon, I.S.; Rabi, D.M.; Ghali, W.A.; Ferris, M.; Chernoff, G.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Barkema, H.W.; et al. Increasing Incidence and Prevalence of the Inflammatory Bowel Diseases with Time, Based on Systematic Review. Gastroenterology 2012, 142, 46–54.e42. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Li, Z.; Liu, S.; Zhang, D. Global, Regional and National Burden of Inflammatory Bowel Disease in 204 Countries and Territories from 1990 to 2019: A Systematic Analysis Based on the Global Burden of Disease Study 2019. BMJ Open 2023, 13, e065186. [Google Scholar] [CrossRef] [PubMed]
- Annese, V.; Beaugerie, L.; Egan, L.; Biancone, L.; Bolling, C.; Brandts, C.; Dierickx, D.; Dummer, R.; Fiorino, G.; Gornet, J.M.; et al. European Evidence-Based Consensus: Inflammatory Bowel Disease and Malignancies. J. Crohn’s Colitis 2015, 9, 945–965. [Google Scholar] [CrossRef] [PubMed]
- Poullenot, F.; Seksik, P.; Beaugerie, L.; Amiot, A.; Nachury, M.; Abitbol, V.; Stefanescu, C.; Reenaers, C.; Fumery, M.; Pelletier, A.L.; et al. Risk of Incident Cancer in Inflammatory Bowel Disease Patients Starting Anti-TNF Therapy While Having Recent Malignancy. Inflamm. Bowel Dis. 2016, 22, 1362–1369. [Google Scholar] [CrossRef]
- Beaugerie, L.; Itzkowitz, S.H. Cancers Complicating Inflammatory Bowel Disease. N. Engl. J. Med. 2015, 372, 1441–1452. [Google Scholar] [CrossRef]
- Jess, T.; Simonsen, J.; Jorgensen, K.T.; Pedersen, B.V.; Nielsen, N.M.; Frisch, M. Decreasing Risk of Colorectal Cancer in Patients with Inflammatory Bowel Disease over 30 Years. Gastroenterology 2012, 143, 375–381.e1. [Google Scholar] [CrossRef]
- Choi, C.H.R.; Rutter, M.D.; Askari, A.; Lee, G.H.; Warusavitarne, J.; Moorghen, M.; Thomas-Gibson, S.; Saunders, B.P.; Graham, T.A.; Hart, A.L. Forty-Year Analysis of Colonoscopic Surveillance Program for Neoplasia in Ulcerative Colitis: An Updated Overview. Am. J. Gastroenterol. 2015, 110, 1022–1034. [Google Scholar] [CrossRef]
- Kappelman, M.D.; Farkas, D.K.; Long, M.D.; Erichsen, R.; Sandler, R.S.; Sørensen, H.T.; Baron, J.A. Risk of Cancer in Patients with Inflammatory Bowel Diseases: A Nationwide Population-Based Cohort Study with 30 Years of Follow-up Evaluation. Clin. Gastroenterol. Hepatol. 2014, 12, 265–273.e1. [Google Scholar] [CrossRef]
- Lutgens, M.W.M.D.; van Oijen, M.G.H.; van der Heijden, G.J.M.G.; Vleggaar, F.P.; Siersema, P.D.; Oldenburg, B. Declining Risk of Colorectal Cancer in Inflammatory Bowel Disease: An Updated Meta-Analysis of Population-Based Cohort Studies. Inflamm. Bowel Dis. 2013, 19, 789–799. [Google Scholar] [CrossRef]
- Dixon, S.W.; Collard, T.J.; Mortensson, E.M.H.; Legge, D.N.; Chambers, A.C.; Greenhough, A.; Creed, T.J.; Williams, A.C. 5-Aminosalicylic Acid Inhibits Stem Cell Function in Human Adenoma-Derived Cells: Implications for Chemoprophylaxis in Colorectal Tumorigenesis. Br. J. Cancer 2021, 124, 1959–1969. [Google Scholar] [CrossRef]
- Qiu, X.; Ma, J.; Wang, K.; Zhang, H. Chemopreventive Effects of 5-Aminosalicylic Acid on Inflammatory Bowel Disease-Associated Colorectal Cancer and Dysplasia: A Systematic Review with Meta-Analysis. Oncotarget 2017, 8, 1031–1045. [Google Scholar] [CrossRef] [PubMed]
- Ullman, T.A.; Itzkowitz, S.H. Intestinal Inflammation and Cancer. Gastroenterology 2011, 140, 1807–1816.e1. [Google Scholar] [CrossRef] [PubMed]
- Baars, J.E.; Kuipers, E.J.; Van Haastert, M.; Nicolaï, J.J.; Poen, A.C.; Van Der Woude, C.J. Age at Diagnosis of Inflammatory Bowel Disease Influences Early Development of Colorectal Cancer in Inflammatory Bowel Disease Patients: A Nationwide, Long-Term Survey. J. Gastroenterol. 2012, 47, 1308–1322. [Google Scholar] [CrossRef] [PubMed]
- Lutgens, M.W.M.D.; Vleggaar, F.P.; Schipper, M.E.I.; Stokkers, P.C.F.; Van Der Woude, C.J.; Hommes, D.W.; De Jong, D.J.; Dijkstra, G.; Van Bodegraven, A.A.; Oldenburg, B.; et al. High Frequency of Early Colorectal Cancer in Inflammatory Bowel Disease. Gut 2008, 57, 1246–1251. [Google Scholar] [CrossRef] [PubMed]
- Moussata, D.; Allez, M.; Cazals-Hatem, D.; Treton, X.; Laharie, D.; Reimund, J.M.; Bertheau, P.; Bourreille, A.; Lavergne-Slove, A.; Brixi, H.; et al. Are Random Biopsies Still Useful for the Detection of Neoplasia in Patients with IBD Undergoing Surveillance Colonoscopy with Chromoendoscopy? Gut 2018, 67, 616–624. [Google Scholar] [CrossRef]
- Annese, V.; Daperno, M.; Rutter, M.D.; Amiot, A.; Bossuyt, P.; East, J.; Ferrante, M.; Götz, M.; Katsanos, K.H.; Kießlich, R.; et al. European Evidence Based Consensus for Endoscopy in Inflammatory Bowel Disease. J. Crohn’s Colitis 2013, 7, 982–1018. [Google Scholar] [CrossRef]
- Laine, L.; Kaltenbach, T.; Barkun, A.; McQuaid, K.R.; Subramanian, V.; Soetikno, R. SCENIC International Consensus Statement on Surveillance and Management of Dysplasia in Inflammatory Bowel Disease. Gastroenterology 2015, 148, 639–651.e28. [Google Scholar] [CrossRef]
- Gordon, H.; Biancone, L.; Fiorino, G.; Katsanos, K.H.; Kopylov, U.; Al Sulais, E.; Axelrad, J.E.; Balendran, K.; Burisch, J.; de Ridder, L.; et al. ECCO Guidelines on Inflammatory Bowel Disease and Malignancies. J. Crohn’s Colitis 2022. online ahead of print. [Google Scholar] [CrossRef]
- Jess, T.; Gamborg, M.; Matzen, P.; Munkholm, P.; Sørensen, T.I.A. Increased Risk of Intestinal Cancer in Crohn’s Disease: A Meta-Analysis of Population-Based Cohort Studies. Am. J. Gastroenterol. 2005, 100, 2724–2729. [Google Scholar] [CrossRef]
- Elriz, K.; Carrat, F.; Carbonnel, F.; Marthey, L.; Bouvier, A.M.; Beaugerie, L. Incidence, Presentation, and Prognosis of Small Bowel Adenocarcinoma in Patients with Small Bowel Crohn’s Disease: A Prospective Observational Study. Inflamm. Bowel Dis. 2013, 19, 1823–1826. [Google Scholar] [CrossRef]
- Weber, N.K.; Fletcher, J.G.; Fidler, J.L.; Barlow, J.M.; Pruthi, S.; Loftus, E.V.; Pardi, D.S.; Smyrk, T.C.; Becker, B.D.; Pasha, S.F.; et al. Clinical Characteristics and Imaging Features of Small Bowel Adenocarcinomas in Crohn’s Disease. Abdom. Imaging 2015, 40, 1060–1067. [Google Scholar] [CrossRef] [PubMed]
- Laukoetter, M.G.; Mennigen, R.; Hannig, C.M.; Osada, N.; Rijcken, E.; Vowinkel, T.; Krieglstein, C.F.; Senninger, N.; Anthoni, C.; Bruewer, M. Intestinal Cancer Risk in Crohn’s Disease: A Meta-Analysis. J. Gastrointest. Surg. 2011, 15, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Slesser, A.A.P.; Bhangu, A.; Bower, M.; Goldin, R.; Tekkis, P.P. A Systematic Review of Anal Squamous Cell Carcinoma in Inflammatory Bowel Disease. Surg. Oncol. 2013, 22, 230–237. [Google Scholar] [CrossRef]
- Boonstra, K.; Weersma, R.K.; van Erpecum, K.J.; Rauws, E.A.; Spanier, B.W.M.; Poen, A.C.; van Nieuwkerk, K.M.; Drenth, J.P.; Witteman, B.J.; Tuynman, H.A.; et al. Population-Based Epidemiology, Malignancy Risk, and Outcome of Primary Sclerosing Cholangitis. Hepatology 2013, 58, 2045–2055. [Google Scholar] [CrossRef] [PubMed]
- Lo, B.; Zhao, M.; Vind, I.; Burisch, J. The Risk of Extraintestinal Cancer in Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis of Population-Based Cohort Studies. Clin. Gastroenterol. Hepatol. 2021, 19, 1117–1138.e19. [Google Scholar] [CrossRef] [PubMed]
- Lemaitre, M.; Kirchgesner, J.; Rudnichi, A.; Carrat, F.; Zureik, M.; Carbonnel, F.; Dray-Spira, R. Association between Use of Thiopurines or Tumor Necrosis Factor Antagonists Alone or in Combination and Risk of Lymphoma in Patients with Inflammatory Bowel Disease. JAMA J. Am. Med. Assoc. 2017, 318, 1679–1686. [Google Scholar] [CrossRef]
- Beaugerie, L.; Brousse, N.; Marie Bouvier, A.; Frédéric Colombel, J.; Lémann, M.; Cosnes, J.; Hébuterne, X.; Cortot, A.; Bouhnik, Y.; Pierre Gendre, J.; et al. Lymphoproliferative Disorders in Patients Receiving Thiopurines for Infl Ammatory Bowel Disease: A Prospective Observational Cohort Study. Lancet 2009, 374, 1617–1625. [Google Scholar] [CrossRef]
- Huang, S.Z.; Liu, Z.C.; Liao, W.X.; Wei, J.X.; Huang, X.W.; Yang, C.; Xia, Y.H.; Li, L.; Ye, C.; Dai, S.X. Risk of Skin Cancers in Thiopurines-Treated and Thiopurines-Untreated Patients with Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. J. Gastroenterol. Hepatol. 2019, 34, 507–516. [Google Scholar] [CrossRef]
- Wu, Y.; Ghaly, S.; Kerr, S.; Jackson, B.; Hanigan, K.; Martins, D.; Krishnaprasad, K.; Mountifield, R.E.; Whiteman, D.C.; Bampton, P.A.; et al. Level of UV Exposure, Skin Type, and Age Are More Important than Thiopurine Use for Keratinocyte Carcinoma Development in IBD Patients. Dig. Dis. Sci. 2020, 65, 1172–1179. [Google Scholar] [CrossRef]
- Ariyaratnam, J.; Subramanian, V. Association between Thiopurine Use and Nonmelanoma Skin Cancers in Patients with Inflammatory Bowel Disease: A Meta-Analysis. Am. J. Gastroenterol. 2014, 109, 163–169. [Google Scholar] [CrossRef]
- Hazenberg, H.M.J.L.; De Boer, N.K.H.; Mulder, C.J.J.; Mom, S.H.; Van Bodegraven, A.A.; Tack, G.J. Neoplasia and Precursor Lesions of the Female Genital Tract in Ibd: Epidemiology, Role of Immunosuppressants, and Clinical Implications. Inflamm. Bowel Dis. 2018, 24, 510–531. [Google Scholar] [CrossRef] [PubMed]
- Kopylov, U.; Vutcovici, M.; Kezouh, A.; Seidman, E.; Bitton, A.; Afif, W. Risk of Lymphoma, Colorectal and Skin Cancer in Patients with IBD Treated with Immunomodulators and Biologics: A Quebec Claims Database Study. Inflamm. Bowel Dis. 2015, 21, 1847–1853. [Google Scholar] [CrossRef]
- Allegretti, J.R.; Barnes, E.L.; Cameron, A. Are Patients with Inflammatory Bowel Disease on Chronic Immunosuppressive Therapy at Increased Risk of Cervical High-Grade Dysplasia/Cancer? A Meta-Analysis. Inflamm. Bowel Dis. 2015, 21, 1089–1097. [Google Scholar] [CrossRef] [PubMed]
- Cohen, R.D.; Bhayat, F.; Blake, A.; Travis, S. The Safety Profile of Vedolizumab in Ulcerative Colitis and Crohn’s Disease: 4 Years of Global Post-Marketing Data. J. Crohn’s Colitis 2020, 14, 192–204. [Google Scholar] [CrossRef] [PubMed]
- Menter, A.; Papp, K.A.; Gooderham, M.; Pariser, D.M.; Augustin, M.; Kerdel, F.A.; Fakharzadeh, S.; Goyal, K.; Calabro, S.; Langholff, W.; et al. Drug Survival of Biologic Therapy in a Large, Disease-Based Registry of Patients with Psoriasis: Results from the Psoriasis Longitudinal Assessment and Registry (PSOLAR). J. Eur. Acad. Dermatol. Venereol. 2016, 30, 1148–1158. [Google Scholar] [CrossRef]
- Papp, K.A.; Langholff, W. Safety Surveillance for Ustekinumab and Other Psoriasis Treatments from the Psoriasis Longitudinal Assessment and Registry (PSOLAR) Errata. J. Drugs Dermatol. 2020, 19, 571–572. [Google Scholar]
- Turner, D.; Ricciuto, A.; Lewis, A.; D’Amico, F.; Dhaliwal, J.; Griffiths, A.M.; Bettenworth, D.; Sandborn, W.J.; Sands, B.E.; Reinisch, W.; et al. STRIDE-II: An Update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): Determining Therapeutic Goals for Treat-to-Target Strategies in IBD. Gastroenterology 2021, 160, 1570–1583. [Google Scholar] [CrossRef]
- Ytterberg, S.R.; Bhatt, D.L.; Mikuls, T.R.; Koch, G.G.; Fleischmann, R.; Rivas, J.L.; Germino, R.; Menon, S.; Sun, Y.; Wang, C.; et al. Cardiovascular and Cancer Risk with Tofacitinib in Rheumatoid Arthritis. N. Engl. J. Med. 2022, 386, 316–326. [Google Scholar] [CrossRef]
- Polesie, S.; Gillstedt, M.; Schmidt, S.A.J.; Egeberg, A.; Pottegård, A.; Kristensen, K. Use of Methotrexate and Risk of Skin Cancer: A Nationwide Case–Control Study. Br. J. Cancer 2023, 128, 1311–1319. [Google Scholar] [CrossRef]
- Ferrante, M.; Feagan, B.G.; Panés, J.; Baert, F.; Louis, E.; Dewit, O.; Kaser, A.; Duan, W.R.; Gustafson, D.; Liao, X.; et al. OP27 Long-Term Safety and Efficacy of Risankizumab Treatment in Patients with Crohn’s Disease: Results from the Phase 2 Open-Label Extension Study. J. Crohn’s Colitis 2021, 18, 1001–2020. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Feagan, B.G.; Hanauer, S.; Vermeire, S.; Ghosh, S.; Liu, W.J.; Petersen, A.; Charles, L.; Huang, V.; Usiskin, K.; et al. Long-Term Efficacy and Safety of Ozanimod in Moderately to Severely Active Ulcerative Colitis: Results From the Open-Label Extension of the Randomized, Phase 2 TOUCHSTONE Study. J. Crohn’s Colitis 2021, 15, 1120–1129. [Google Scholar] [CrossRef] [PubMed]
- Friedberg, S.; Choi, D.; Hunold, T.; Choi, N.K.; Garcia, N.M.; Picker, E.A.; Cohen, N.A.; Cohen, R.D.; Dalal, S.R.; Pekow, J.; et al. Upadacitinib Is Effective and Safe in Both Ulcerative Colitis and Crohn’s Disease: Prospective Real-World Experience. Clin. Gastroenterol. Hepatol. 2023. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Taleban, S.; Colombel, J.F.; Mohler, M.J.; Fain, M.J. Inflammatory Bowel Disease and the Elderly: A Review. J. Crohn’s Colitis 2015, 9, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Quinn, M.J.; d’Onofrio, A.; Møller, B.; Black, R.; Martinez-Garcia, C.; Møller, H.; Rahu, M.; Robertson, C.; Schouten, L.J.; La Vecchia, C.; et al. Cancer Mortality Trends in the EU and Acceding Countries up to 2015. Ann. Oncol. 2003, 14, 1148–1152. [Google Scholar] [CrossRef]
- Poullenot, F.; Laharie, D. Management of Inflammatory Bowel Disease in Patients with Current or Past Malignancy. Cancers 2023, 15, 1083. [Google Scholar] [CrossRef]
- Laharie, D. Previous Cancer and/or Lymphoma in Patients with Refractory IBD—Pro: Anti-TNF or Immunosuppressive Treatment. Dig. Dis. 2014, 32, 116–121. [Google Scholar] [CrossRef]
- Jauregui-Amezaga, A.; Vermeire, S.; Prenen, H. Use of Biologics and Chemotherapy in Patients with Inflammatory Bowel Diseases and Cancer. Ann. Gastroenterol. 2016, 29, 127–136. [Google Scholar] [CrossRef]
- Rajca, S.; Seksik, P.; Bourrier, A.; Sokol, H.; Nion-Larmurier, I.; Beaugerie, L.; Cosnes, J. Impact of the Diagnosis and Treatment of Cancer on the Course of Inflammatory Bowel Disease. J. Crohn’s Colitis 2014, 8, 819–824. [Google Scholar] [CrossRef]
- Curtis, R.E.; Freedman, D.M.; Ron, E.; Ries, L.A.G.; Hacker, D.G.; Edwards, B.K.; Tucker, M.A.; Fraumeni, J.F., Jr. New Malignancies among Cancer Survivors: SEER Cancer Registries, 1973–2000; National Cancer Institute: Bethesda, MD, USA, 2006. [Google Scholar]
- Penn, I. The Effect of Immunosuppression on Pre-Existing Cancers. Transplantation 1993, 55, 742–747. [Google Scholar] [CrossRef]
- Beaugerie, L.; Carrat, F.; Colombel, J.-F.; Bouvier, A.-M.; Sokol, H.; Babouri, A.; Carbonnel, F.; Laharie, D.; Faucheron, J.-L.; Simon, T.; et al. Risk of New or Recurrent Cancer under Immunosuppressive Therapy in Patients with IBD and Previous Cancer. Gut 2014, 63, 1416–1423. [Google Scholar] [CrossRef]
- Axelrad, J.; Bernheim, O.; Colombel, J.F.; Malerba, S.; Ananthakrishnan, A.; Yajnik, V.; Hoffman, G.; Agrawal, M.; Lukin, D.; Desai, A.; et al. Risk of New or Recurrent Cancer in Patients With Inflammatory Bowel Disease and Previous Cancer Exposed to Immunosuppressive and Anti-Tumor Necrosis Factor Agents. Clin. Gastroenterol. Hepatol. 2016, 14, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, G.R.; Feagan, B.G.; Cohen, R.D.; Salzberg, B.A.; Diamond, R.H.; Langholff, W.; Londhe, A.; Sandborn, W.J. Drug Therapies and the Risk of Malignancy in Crohn’s Disease: Results from the TREATTM Registry. Am. J. Gastroenterol. 2014, 109, 212–223. [Google Scholar] [CrossRef] [PubMed]
- Axelrad, J.; Colombel, J.F.; Scherl, E.; Lukin, D.; Chang, S.; Chen, L.; Kwah, J.; Swaminath, A.; Sultan, K.; Lawlor, G.; et al. P729 The SAPPHIRE Registry: Safety of Immunosuppression in a Prospective Cohort of Inflammatory Bowel Disease Patients with a HIstoRy of CancEr. J. Crohn’s Colitis 2020, 14 (Suppl. S1), S585–S587. [Google Scholar] [CrossRef]
- Shelton, E.; Laharie, D.; Scott, F.I.; Mamtani, R.; Lewis, J.D.; Colombel, J.F.; Ananthakrishnan, A.N. Cancer Recurrence Following Immune-Suppressive Therapies in Patients With Immune-Mediated Diseases: A Systematic Review and Meta-Analysis. Gastroenterology 2016, 151, 97–109.e4. [Google Scholar] [CrossRef]
- Dixon, W.G.; Watson, K.D.; Lunt, M.; Mercer, L.K.; Hyrich, K.L.; Symmons, D.P.M.; Maiden, N.; Price, T.; Hopkinson, N.; O’Reilly, S.; et al. Influence of Anti-Tumor Necrosis Factor Therapy on Cancer Incidence in Patients with Rheumatoid Arthritis Who Have Had a Prior Malignancy: Results from the British Society for Rheumatology Biologics Register. Arthritis Care Res. (Hoboken) 2010, 62, 755–763. [Google Scholar] [CrossRef]
- Strangfeld, A.; Hierse, F.; Rau, R.; Burmester, G.R.; Krummel-Lorenz, B.; Demary, W.; Listing, J.; Zink, A. Risk of Incident or Recurrent Malignancies among Patients with Rheumatoid Arthritis Exposed to Biologic Therapy in the German Biologics Register RABBIT. Arthritis Res. Ther. 2010, 12, R5. [Google Scholar] [CrossRef]
- Hong, S.J.; Zenger, C.; Pecoriello, J.; Pang, A.; Vallely, M.; Hudesman, D.P.; Chang, S.; Axelrad, J.E. Ustekinumab and Vedolizumab Are Not Associated With Subsequent Cancer in IBD Patients with Prior Malignancy. Inflamm. Bowel Dis. 2022, 28, 1826–1832. [Google Scholar] [CrossRef]
- Vedamurthy, A.; Gangasani, N.; Ananthakrishnan, A.N. Vedolizumab or Tumor Necrosis Factor Antagonist Use and Risk of New or Recurrent Cancer in Patients with Inflammatory Bowel Disease with Prior Malignancy: A Retrospective Cohort Study. Clin. Gastroenterol. Hepatol. 2022, 20, 88–95. [Google Scholar] [CrossRef]
- Grimsdottir, S.; Attauabi, M.; Dahl, E.K.; Burisch, J.; Seidelin, J.B. Systematic Review with Meta-Analysis: The Impact of Cancer Treatments on the Disease Activity of Inflammatory Bowel Diseases. J. Crohn’s Colitis 2023. online ahead of print. [Google Scholar] [CrossRef]
- Axelrad, J.E.; Fowler, S.A.; Friedman, S.; Ananthakrishnan, A.N.; Yajnik, V. Effects of Cancer Treatment on Inflammatory Bowel Disease Remission and Reactivation. Clin. Gastroenterol. Hepatol. 2012, 10, 1021–1027.e1. [Google Scholar] [CrossRef]
- Axelrad, J.E.; Bazarbashi, A.; Zhou, J.; Castañeda, D.; Gujral, A.; Sperling, D.; Glass, J.; Agrawal, M.; Hong, S.; Lawlor, G.; et al. Hormone Therapy for Cancer Is a Risk Factor for Relapse of Inflammatory Bowel Diseases. Clin. Gastroenterol. Hepatol. 2020, 18, 872–880.e1. [Google Scholar] [CrossRef]
- Axelrad, J.; Kriplani, A.; Ozbek, U.; Harpaz, N.; Colombel, J.F.; Itzkowitz, S.; Holcombe, R.F.; Ang, C. Chemotherapy Tolerance and Oncologic Outcomes in Patients with Colorectal Cancer with and without Inflammatory Bowel Disease. Clin. Color. Cancer 2017, 16, e205–e210. [Google Scholar] [CrossRef]
- Stein, A.; Voigt, W.; Jordan, K. Review: Chemotherapy-Induced Diarrhea: Pathophysiology, Frequency and Guideline-Based Management. Ther. Adv. Med. Oncol. 2010, 2, 51–63. [Google Scholar] [CrossRef]
- O’Reilly, M.; Mellotte, G.; Ryan, B.; O’Connor, A. Gastrointestinal Side Effects of Cancer Treatments. Ther. Adv. Chronic Dis. 2020, 11, 2040622320970354. [Google Scholar] [CrossRef] [PubMed]
- Prieux-Klotz, C.; Dior, M.; Damotte, D.; Dreanic, J.; Brieau, B.; Brezault, C.; Abitbol, V.; Chaussade, S.; Coriat, R. Immune Checkpoint Inhibitor-Induced Colitis: Diagnosis and Management. Target. Oncol. 2017, 12, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Willett, C.G.; Ooi, C.-J.; Zietman, A.L.; Menon, V.; Goldberg, S.; Sands, B.E.; Podolsky, D.K. Acute and late toxicity of patients with inflammatory bowel disease undergoing irradiation for abdominal and pelvic neoplasms. Int. J. Radiat. Oncol. Biol. Phys. 2000, 46, 995–998. [Google Scholar] [CrossRef] [PubMed]
- Schaeffer, E.; Srinivas, S.; Antonarakis, E.S.; Armstrong, A.J.; Bekelman, J.E.; Cheng, H.; D’Amico, A.V.; Davis, B.J.; Desai, N.; Dorff, T.; et al. NCCN Guidelines Insights: Prostate Cancer, Version 1.2021. J. Natl. Compr. Cancer Netw. 2021, 19, 134–143. [Google Scholar] [CrossRef]
- Green, S.; Stock, R.G.; Greenstein, A.J. Rectal cancer and inflammatory bowel disease: Natural history and implications for radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 1999, 44, 835–840. [Google Scholar] [CrossRef]
- Kirk, P.S.; Govani, S.; Borza, T.; Hollenbeck, B.K.; Davis, J.; Shumway, D.; Waljee, A.K.; Skolarus, T.A. Implications of Prostate Cancer Treatment in Men With Inflammatory Bowel Disease. Urology 2017, 104, 131–136. [Google Scholar] [CrossRef]
- Bodofsky, S.; Freeman, R.H.; Hong, S.S.; Chundury, A.; Hathout, L.; Deek, M.P.; Jabbour, S.K. Inflammatory Bowel Disease-Associated Malignancies and Considerations for Radiation Impacting Bowel: A Scoping Review. J. Gastrointest. Oncol. 2022, 13, 2565–2582. [Google Scholar] [CrossRef]
- Sebastian, S.; Neilaj, S. Practical Guidance for the Management of Inflammatory Bowel Disease in Patients with Cancer. Which Treatment? Ther. Adv. Gastroenterol. 2019, 12, 1756284818817293. [Google Scholar] [CrossRef] [PubMed]
| Risk factors | Disease duration > 8 years |
| Disease extension | |
| Primary sclerosing cholangitis | |
| Family history of CRC | |
| Severity/persistence of inflammation | |
| Strictures | |
| Previous dysplasia | |
| Tubular colon | |
| Male sex | |
| Young age at UC diagnosis | |
| Post-inflammatory polyps |
| Drug | Malignancy |
|---|---|
| Thiopurines | Lymphomas Myeloproliferative syndromes Non-melanoma skin cancers Urinary tract cancers Cervical carcinoma |
| Anti-TNF | Lymphoma Melanoma |
| Anti-TNF + thiopurines | Lymphoma |
| Vedolizumab | None identified |
| Ustekinumab | None identified |
| JAK inhibitors | Unknown |
| Methotrexate | Non-melanoma skin cancers |
| IBD Treatment Management |
|---|
| Withhold immunomodulation during active cancer therapy |
| Withhold thiopurines if bone marrow suppression is expected from the planned chemotherapy |
| Considerer avoiding immunomodulation in the first 2 years after cancer |
| Avoid combination therapy in cancer patients |
| Avoid JAK inhibitors in cancer patients |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conceição, D.; Saraiva, M.R.; Rosa, I.; Claro, I. Inflammatory Bowel Disease Treatment in Cancer Patients—A Comprehensive Review. Cancers 2023, 15, 3130. https://doi.org/10.3390/cancers15123130
Conceição D, Saraiva MR, Rosa I, Claro I. Inflammatory Bowel Disease Treatment in Cancer Patients—A Comprehensive Review. Cancers. 2023; 15(12):3130. https://doi.org/10.3390/cancers15123130
Chicago/Turabian StyleConceição, Daniel, Margarida R. Saraiva, Isadora Rosa, and Isabel Claro. 2023. "Inflammatory Bowel Disease Treatment in Cancer Patients—A Comprehensive Review" Cancers 15, no. 12: 3130. https://doi.org/10.3390/cancers15123130
APA StyleConceição, D., Saraiva, M. R., Rosa, I., & Claro, I. (2023). Inflammatory Bowel Disease Treatment in Cancer Patients—A Comprehensive Review. Cancers, 15(12), 3130. https://doi.org/10.3390/cancers15123130






