Simple Summary
Electrochemotherapy has proven to be an efficient treatment for cutaneous metastases of various cancers including breast cancer (BC). The large number of patients collected within the INSPECT database provides the possibility of a differentiated analysis on BC with different receptor statuses (estrogen receptor and HER2 receptor). Patients with BC presenting cutaneous metastases were retrieved from the INSPECT database and divided by their receptor status: HER2+, HR+ (ER/PgR+), and TN (triple negative). ECT treatment is equally effective among groups, despite different conditions, age, time since diagnosis, previous or concomitant treatments, and treatment characteristics. Response and local tumor control seem to be better in multiple small lesions than in big armor-like lesions, suggesting that treating smaller, even multiple, lesions at the time of occurrence is more effective than treating bigger long-lasting armor-like cutaneous lesions.
Abstract
Electrochemotherapy has been proven to be an efficient treatment for cutaneous metastases of various cancers. Data on breast cancer (BC) patients with cutaneous metastases were retrieved from the INSPECT database. Patients were divided by their receptor status: HER2+, HR+ (ER/PgR+), and TN (triple negative). Groups were similar for histological subtype and location of the nodules. Most patients were previously treated with surgery/systemic therapy/radiotherapy. We found no differences in the three groups in terms of response ratio (OR per patient 86% HER2+, 80% HR+, 76% TN, p = 0.8664). The only factor positively affecting the complete response rate in all groups was small tumor size (<3 cm, p = 0.0105, p = 0.0001, p = 0.0266, respectively). Local progression-free survival was positively impacted by the achievement of complete response in HER2+ (p = 0.0297) and HR+ (p = 0.0094), while overall survival was affected by time to local progression in all groups (p = 0.0065 in HER2+, p < 0.0001 in HR+, p = 0.0363 in TN). ECT treatment is equally effective among groups, despite different receptor status. Response and local tumor control seem to be better in multiple small lesions than in big armor-like lesions, suggesting that treating smaller, even multiple, lesions at the time of occurrence is more effective than treating bigger long-lasting armor-like cutaneous lesions.
1. Introduction
Breast cancer is one of the most frequent cancers worldwide. Data from the 2019 US SEER (Surveillance, Epidemiology and End Result program) registry point out that the incidence of breast cancer among the US population is almost 134 new cases per 100.00 inhabitants per year, with an increasing trend and the prevalence of the female population affected is 2.3% of the total population [1]. Breast cancer is also one of the most frequent causes of cutaneous and subcutaneous metastases [2].
Clinically, skin and subcutaneous metastases could present themselves in different ways: they could be single or multiple, could be asymptomatic or could bleed, ulcerate, be painful, and lead to infections.
Treatment of these lesions varies accordingly. Surgery could be a first option in single small metastasis, while treatment of larger or multiple lesions spreading into a larger area could be challenging. Systemic therapies could be proposed according to tumor subtype (histology, immunohistochemistry, hormonal status, receptor status), while radiotherapy could be chosen if not previously delivered [3,4].
Electrochemotherapy is recognized as a safe and valid treatment option to manage these lesions; it could be performed in previously irradiated areas and could be repeated multiple times [5,6,7,8,9]. Various mono- and multicentric studies demonstrated high response rates in terms of local control, with the overall response rate up to about 80–90% and complete control rate up to about 60% [7,8,10].
It is also demonstrated in different cancer settings (e.g., melanoma) that ECT could be safely performed concurrently to other systemic treatments, leading to a cutaneous local control that can improve the patient’s quality of life and also have a benefit for their psychological well-being [11,12,13]. Moreover, some studies suggest that the combination of ECT with other systemic therapies could be beneficial, and this interaction is being explored [14,15]
Predictive factors to ECT response in breast cancer cutaneous and subcutaneous metastases have been previously investigated in small studies and some have been reported (e.g., small tumor size, absence of visceral metastases, estrogen receptor positivity, low Ki-67 index, lower body mass index, reduced body surface, absence of previous radiation treatment, concurrent systemic therapies) [7,8,10,16].
We present here the analyses from the INSPECT network on 171 patients treated with ECT for cutaneous and subcutaneous metastases from breast cancer from January 2010 to November 2020. Patients were divided into three groups regarding hormonal and immunohistochemical status: HER2+, HR+ (ER/PgR+), and TN (triple negative). The primary aim of the study was to evaluate any differences in terms of ECT response and to predict different factors to the treatment’s response, if any, related to hormonal status. The secondary goal was to evaluate survival (local progression-free survival, overall survival).
2. Materials and Methods
2.1. Patients
Patients were recruited and treated at institutions in the INSPECT network. Centers uploaded patient data prospectively in the International Network for Sharing Practices of ECT (InspECT) register (http://www.insp-ect.org). Approval from the ethics committee and data protection authority was according to guidelines of each institution and to the rules of Good Clinical Practice (Declaration of Helsinki).
Patients eligible for inclusion had histologically proven breast cancer with measurable cutaneous or subcutaneous metastases suitable for application of electric pulses. Patient selection was based on institutional preferences, including referral after multi-disciplinary discussion for patients with symptomatic cutaneous metastasis when other treatment modalities failed or were not possible. They were offered standard treatment options when possible, were 18 years old or older, had an Eastern Cooperative Oncology Group performance status ≤2, had life expectancy of at least 3 months and, where appropriate, were using adequate contraception. Patients were ineligible if they previously had allergic reactions to bleomycin or to any of the components required for anesthesia, if the cumulative dose of 250 mg (400,000 IU) bleomycin/m2 had previously been exceeded, and in case of chronic renal dysfunction (serum creatinine >150 mmol/L) or acute lung infection. Clinical information retrieved by the database included: demographic characteristics, number of treated lesions, site and size of the largest lesion, previous irradiation, and duration of follow-up.
Data on histology, hormone receptor status, HER2 receptor status, and previous treatments were also collected. Patients under concomitant systemic treatment or who started new systemic antineoplastic treatment after electrochemotherapy were included in the analysis.
2.2. Procedure
ECT was delivered based on European Standard Operating Procedures for Electrochemotherapy (ESOPE) updated guidelines [9]. Bleomycin was administrated in one of the following ways: intratumorally at a dose of 1000 IU/cm3 for tumor volume < 0.5 cm3, 500 IU/cm3 for tumor volume between 0.5 and 1 cm3, 250 IU/cm3 for tumor volume > 1 cm3, or intravenous at 15,000 IU m2 body surface. Electroporation was achieved using the Cliniporator (IGEA, Carpi, Italy), delivering 8 pulses of 100 ms at 1 kV/cm. Electrode choice was guided by the standard operation procedure (SOP) [9,17], which advocates parallel array electrodes for lesions less than 3 cm, with hexagonal array preferred for larger lesions, and subsequent analysis indicated that clinician preference dictates exceptions for this.
After electrochemotherapy, the treated metastases were covered with standard dressings where necessary.
2.3. Response Evaluation
Evaluation of the local tumor response was measured via dimensions of the treated lesions. The response was registered for each target lesion at each follow-up visit, and data of response at 1 and 2 months after electrochemotherapy were considered for local tumor response, according to the Modified Response Evaluation Criteria in Solid Tumors (RECIST) [18]: complete response (CR) was defined as disappearance of the target lesion; partial response (PR) with at least 30% decrease in the diameter of the target lesion; progressive disease (PD) with at least 20% increase in the diameter of the target lesion; and stable disease (SD) with neither sufficient shrinkage to qualify for PR nor sufficient increase to qualify for PD. In some cases with ulcerated tumors, evaluation was not possible because of crust formation. Data on local progression-free survival (LPFS) and overall survival (OS) were also collected.
2.4. Safety and Toxicity
Safety was reported in the form of adverse events using Common Toxicity Criteria version 5.1. Particular focus was put on local symptoms, such as odor, suppuration, hyperpigmentation, ulceration, and pain. Furthermore, patients were asked if they would potentially agree to another session as a measure of how patients felt about the treatment procedure. Symptomatology assessment was conducted before treatment and each follow-up visit and was specifically analyzed at 3 time points: before treatment, at 1 month from treatment, and at 2 months from treatment. Pain intensity was evaluated using the numeric rating scale (NRS) for pain. The NRS is a unidimensional 11-point numeric scale in which the patient is asked to indicate a whole number between “0” as “no pain” and “10” as “worst pain”.
2.5. Statistical Analysis
Descriptive methods were used for statistical analysis using NCSS version 9.16. Continuous variables were described using mean, standard deviation, median value and range, and categorical variables by absolute number and percentage. Comparison among groups was performed via ANOVA test (continuous) and contingency analysis with the χ2 test (categorical) for trend.
Univariate analysis was performed in each subgroup using a logistic regression model for complete response, using the investigated variables: oligometastatic disease, previous systemic treatment, concomitant systemic treatment, previous irradiation of treated lesions, lymphoedema, lesions’ size, lesions’ number, electrode type, and current.
Local tumor control was expressed as local progression-free survival, which was the time from electrochemotherapy up to the date of relapse or progression or last follow-up. Survival curves for local progression-free survival (LPFS) and overall survival (OS) were calculated using the Kaplan–Meier model. Cox regression analysis was performed to identify variables affecting LPFS and OS.
Significance of tests was reported with p-value, where a value <0.05 was considered as statistically significant.
3. Results
3.1. Patients
A cohort of 171 patients with a diagnosis of breast cancer and cutaneous or subcutaneous lesions were extracted from the INSPECT database. They were treated with ECT in the period January 2010–November 2020 in 16 European centers (Tuebingen, Padova, London (St. Georges, Guy’s, and St Thomas’ Hospitals) Copenhagen, Middlesbrough, Szeged, Cork, East Grinstead, Munchen, Rionero in Vulture (Potenza), Mirano (Venice), Munchen, Liverpool, Bristol, Castle Hill, Genova).
They were divided into three groups according to their receptor status: HER2+ (all patients with HER2 overexpression), HR+ (patients with either ER or PG expression (normal HER2), and TN (triple negative, without HER2+, ER, or PG expression). Descriptive characteristics of the patients are reported in Table 1.

Table 1.
Descriptive characteristics of the patients.
Almost all patients underwent previous treatments, as reported in Table 2.

Table 2.
Previous treatments in the analyzed groups.
The characteristics of the treated lesions and ECT parameters at the ECT session are reported in Table 3.

Table 3.
Characteristics of the treated lesions and ECT parameters.
3.2. Toxicity
Local symptoms were mild (grade I/II), and the percentage of patients suffering them showed a similar trend over time in the three groups, as reported in Figure 1. A slight increase in local symptoms can be observed 1 month after ECT, rapidly decreasing to pre-ECT values or even lower values, especially for what concerns odor, suppuration, ulceration, and pain.
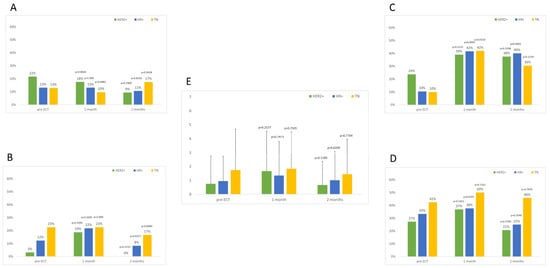
Figure 1.
Percentage of patients suffering local symptoms before and after ECT. (A) Odor, (B) suppuration, (C) hyperpigmentation, (D) ulceration, (E) pain VNS (mean values and standard deviation). p values reported are for comparisons within each group with pre-ECT values. Differences among groups are always non-significant.
3.3. Local Response to ECT
Response to ECT was assessed at around 2 months follow-up. Response per patient was: 49% CR, 37% PR, 7% SD, and 7% PD in the HER2+ group; 45% CR, 35% PR, 12% SD, and 9% PD in the HR+ group; 33% CR, 39% PR, 15% SD, and 9% PD in the TN group (p = 0.8664). Response per nodule was similar: 52% CR, 37% PR, 4% SD, and 7% PD in the HER2+ group; 55% CR, 31% PR, 10% SD, and 4% PD in the HR+ group; 49% CR, 34% PR, 5% SD, and 11% PD in the TN group (p = 0.3846).
Among all factors considered in the analysis (oligomestastatic condition, previous systemic treatment, concomitant systemic treatment, pre-irradiation of treated lesions, presence of lymphoedema, nodules’ size, nodules’ number, electrode used, current applied), the only significant factor affecting the achievement of complete response per patient was the size of the largest lesion in all three groups (Table S1). The CR rate in the HER2+ group for lesions smaller than 3 cm was 64% vs. 13% in those larger than 3 cm (p = 0.0018); in the HR+ group, the CR rate in small lesions was 57% vs. 27% of larger ones (p = 0.0039); in the TN group, the CR rate was 42% in smaller lesions and 8% in larger lesions (p = 0.0436), as reported in Figure 2.
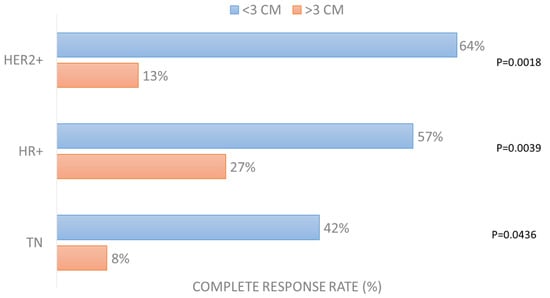
Figure 2.
Complete response rate (%) per patient according to largest lesion size (dichotomized at dimeter of 3 cm).
3.4. Local Progression-Free Survival
Patients were followed for different periods, depending on the center and availability of the patients themselves. The mean follow-up time was 12 ± 18 months (median 6.6, range 2–135) and was similar among groups (p = 0.0710).
During follow-up, 28 patients (16%) underwent local progression (12% in HER2+, 18% in HR+, 18% in TN) after a mean period of 13.7 ± 30.7 months. The local progression-free survival curve was significantly lower for the TN group in comparison with the HER2+ (p = 0.0390) and HR+ (p = 0.0151) groups (see Figure 3A). One-year local progression-free survival was 78% (C.I. 62–95%) in the HER2+ group, 81% (C.I. 72–91%) in the HR+ group, and 61% (C.I. 39–84%) in the TN group. In Figure 4, an example of local progression-free survival up to 3 years in a HER2+ patient is reported.
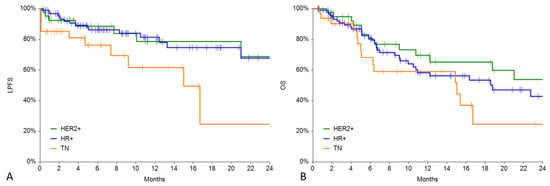
Figure 3.
(A) LPFS in the 3 groups. (B) Overall survival in the 3 groups.
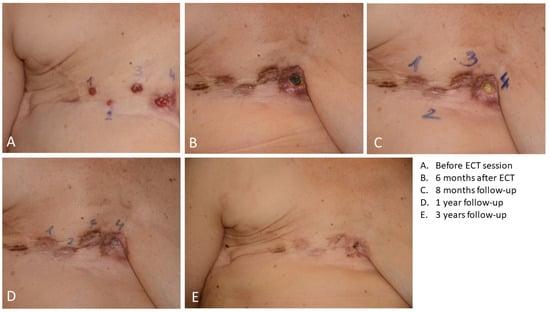
Figure 4.
Local progression-free survival up to 3 years in a HER2+ patient treated with ECT.
Cox regression analysis revealed that LPFS is positively affected by the achievement of complete response to ECT treatment in the HER2+ (p = 0.0297) and HR+ (p = 0.0094) groups (Figure 5). It is also positively affected by small lesions’ size in the HR+ group (p = 0.0260) and by the presence of concomitant systemic treatment (p = 0.0289) and treatment of multiple lesions (p = 0.0295) in the TN group (Table S2).
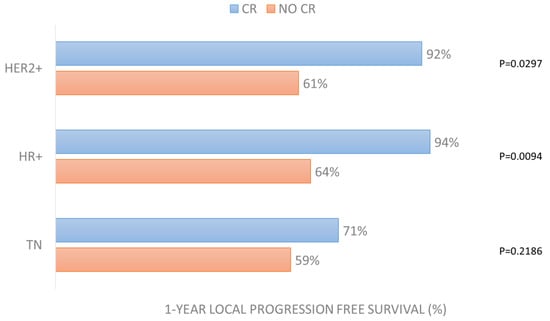
Figure 5.
One-year local progression-free survival in the 3 groups according to CR achievement after ECT treatment.
3.5. Overall Survival
During follow-up, 72 patients (42%) died; the distribution was similar among groups (44%, 40%, and 48%, respectively, p = 0.1255). Death occurred after a mean period of 12 ± 13 months (median 6.6, range 0.7–53).
The overall survival curve was significantly lower for the TN group in comparison with the HER2+ group (p = 0.0319) whilst not significantly different from the HR+ group (p = 0.1227) (see Figure 3B). One-year local progression-free survival was 65% (C.I. 48–82%) in the HER2+ group, 58% (C.I. 46–71%) in the HR+ group, and 59% (C.I. 39–79%) in the TN group.
Cox regression analysis revealed that overall survival was affected by time to local progression in all groups (p = 0.0065 in HER2+, p < 0.0001 in HR+, p = 0.0363 in TN). It was also affected by the oligometastatic condition in the HER2+ (p < 0.0001) and HR+ (p = 0.0202) groups (Table S3).
4. Discussion
This is, to our knowledge, the largest cohort study on breast cancer patients treated with ECT for cutaneous metastases to date and even the longest in terms of follow-up information.
The results in terms of local response to treatment are similar to the data already published in the literature [19], where the CR rate for treated nodules ranged from 40% to 74% in cohorts larger than 20 patients, and in a recent study [7], a CR rate of 58% was obtained in cutaneous lesions in the first INSPECT data collection on breast cancer patients. In our cohort study, a CR rate of 52% in the HER2+, 55% in HR+, and 49% in TN groups was very homogeneous and in the median region of the results available in the literature.
In this series, all patients showed a similar response to ECT, regardless of their receptor status. At the current time, no studies have been conducted to specifically evaluate if there is a difference between receptor status and response to ECT, but some studies have evaluated that amongst other parameters and have come to slightly different conclusions. In 2015, Cabula et al. [10] performed a retrospective multicenter cohort analysis where they evaluated 113 patients (and 214 tumors) treated between 2010 and 2013 in 13 Italian institutions. In their analysis, tumor size was the most powerful predictor of CR, together with absence of visceral metastases, ER positivity, and low Ki-67. In 2019, Wichtowski et al. [20] found that positivity to estrogen receptor better correlates to ECT response, while in 2021, Russano et al. [16] found that negativity to estrogen and progesterone receptor and to HER2 correlates better with ECT response. These discrepancies could be explained by the fact that all these studies were conducted retrospectively with a relatively small subset of patients. Further studies are needed to fully address this controversy.
ECT has been repeatedly demonstrated to be a safe procedure [6,21,22,23] and, in this series, we confirm this finding. All toxicities were mild, and all recovered within 2 months from the procedures. ECT could be performed not only as a palliative procedure but also as an alternative treatment to surgery in the case of patients not eligible for surgery [6] or to other standard treatments, even in the case of primary tumors, where surgery could be very mutilating, as new studies are investigating [21].
Skin involvement represents a relatively common event in the metastatic pattern of BC, with up to 30% of advanced cases in different series [24,25]. Furthermore, skin metastases are continuously under the patient’s eye, causing strong psychological distress [26]. An effective local treatment is, thus, mandatory to preserve the quality of life of patients. Surgical resection and/or radiotherapy can only be offered to a limited number of patients because of multifocality or previously irradiated tissues and on lesions that have spread to a wide area. At any rate, ECT is repeatable and can even be performed in an outpatient setting. In this context, a high responsiveness, together with the relative acceptability of the treatment in terms of pain, side effects, and discomfort, was observed in elderly patients in various studies [6,27]. Furthermore, ECT has demonstrated, in a cohort of melanoma patients, that quality of life is preserved in patients achieving a complete response to local treatment [12].
We evaluated many patient, tumor, and therapy characteristics, and we found out that ECT treatment was equally effective among all our three groups, despite their differences. Patient’s age, time since diagnosis, other treatments (both previously and concomitant), and ECT characteristics (such as electrodes used or current applied) did not result in different response to treatment. The only parameter that affected CR in all groups was lesion size. Our findings confirm what has been previously described, that tumor size is one of the main parameters or the main parameter that affects CR to ECT in these tumors [7,28]. In our study, in the HR+ group, lesion size also affected LPFS (p = 0.0260).
As for survival, LPFS was significatively lower in the TN group vs. HER2+ (p = 0.0390) and HR+ (p = 0.0151) groups. This could be due to different tumor biology, since TN tumors are known to be more aggressive and to have a somewhat smaller choice of systemic treatments (since both antiHER2 and hormone therapies are not indicated). In the TN group, concomitant systemic treatment (p = 0.0289) and treatment of multiple lesions (p = 0.0295) were associated with a better LPFS.
What is interesting to know is that in the “more favorable” groups, LPFS was affected by CR (in HER2+ group with p = 0.0297 and in HR+ group with p = 0.0094). Similarly, overall survival was affected by time to local progression in all groups (p = 0.0065 in HER2+, p < 0.0001 in HR+, p = 0.0363 in TN).
Our study shows that obtaining a CR to ECT impacts LPFS (in HER2+ and HR+ groups) and that OS is impacted by time to local progression. This correlation seems to demonstrate that a better response to a locoregional treatment could produce a benefit in overall survival. It is important to note that ECT is not a systemic treatment, but in the last few years, some studies have found a correlation between CR to ECT and OS, especially if other treatments are involved [29]. This is not the first time that a locoregional treatment seems to have produced a more systemic impact (e.g., radiotherapy and abscopal effect) [30].
The role and correlation of the immune system, of the concomitant or prior therapies and ECT, are not yet well established, and more studies are needed to better understand these phenomena. Since every correlation in our study (and in many others) was linked to lesion size, it is crucial that ECT is performed on smaller lesions to improve the probability to obtain a better CR and even better LPFS and OS. A multidisciplinary approach is needed to plan a therapeutic scenario where ECT could be performed on smaller lesions, in a time where its response could be at its best.
5. Conclusions
This study confirms a high local response to electrochemotherapy, which is mostly correlated to lesion size. When smaller lesions are treated, better complete responses are expected, and when complete responses are achieved, this could benefit not only the patient’s physical and psychological well-being but also their local progression-free survival, and it could also improve overall survival.
Electrochemotherapy should be considered as a therapeutical option for cutaneous and subcutaneous metastases in breast cancer patients, and its application should be discussed in a multidisciplinary team in an early referral setting.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers15123116/s1, Table S1. Factors affecting complete response to ECT treatment. Table S2. Cox regression analyses for local progression free survival in the 3 groups. Table S3. Cox regression analyses for overall survival (OS) in the 3 groups.
Author Contributions
Conceptualization, C.D.P., J.G. and E.-M.G.; methodology, J.G.; formal analysis, F.d.T., C.D.P., J.G. and E.-M.G.; data curation, all; writing—original draft preparation, C.D.P., J.G., E.-M.G., A.M.R. and B.S.; writing—review and editing, C.D.P., M.M., A.M.R., B.S., E.K., J.O., T.F., R.P.J., C.K., A.O., J.C., S.K., F.R., P.M., T.M., F.d.T., J.G. and E.-M.G.; visualization, all; supervision, J.G. and E.-M.G.; project administration, F.d.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the National Ethics Committee of the Republic of Slovenia (102/09/14), the Padua Veneto Institute of Oncology, Institutional Approval Prot. No. 006264—09/04/2018, the Clinical Research Ethics Committee, University College Cork, Ireland ECM 4 (IIIII) 07/05/13 & ECM 4 (qq) 29/03/18, the Institutional Review Board of the Artemed Fachklinik, the Regional and Institutional Review Board of Human Investigations in University of Szeged, the Comitato Etico Interaziandale AOU Città della Salute e della Scienza di Torino, the Internal approval at St Helens & Knowsley Teaching hospital NHS Trust, and the Comitato etico Policlinico Umberto I, Roma.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patient(s) to publish this paper.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy.
Conflicts of Interest
The authors declare no conflict of interest.
References
- SEER. Annual Report to the Nation 2022: Overall Cancer Statistics; SEER: Bethesda, MD, USA, 2022. [Google Scholar]
- Wong, C.Y.B.; Helm, M.A.; Helm, T.N.; Zeitouni, N. Patterns of Skin Metastases: A Review of 25 Years’ Experience at a Single Cancer Center: Patterns of Skin Metastases. Int. J. Dermatol. 2014, 53, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Sittenfeld, S.M.C.; Murray, E.; Guo, B.; Tendulkar, R.; Xia, P.; Shah, C. Treatment of Diffuse Cutaneous Metastases from Breast Cancer. Breast. J. 2020, 26, 2444–2446. [Google Scholar] [CrossRef] [PubMed]
- La Verde, N.; Moretti, A.; Farina, G.; Dazzani, M.C.; Gamucci, T.; Borgonovo, K.; Botta, M.; Salesi, N.; Zuradelli, M.; Pavese, I.; et al. Eribulin in Cutaneous Breast Cancer Metastasis Treatment: Clinical Activity and Symptom Control. Future Oncol. 2013, 9, 1841–1848. [Google Scholar] [CrossRef]
- Campana, L.G.; Valpione, S.; Falci, C.; Mocellin, S.; Basso, M.; Corti, L.; Balestrieri, N.; Marchet, A.; Rossi, C.R. The Activity and Safety of Electrochemotherapy in Persistent Chest Wall Recurrence from Breast Cancer after Mastectomy: A Phase-II Study. Breast. Cancer Res. Treat. 2012, 134, 1169–1178. [Google Scholar] [CrossRef] [PubMed]
- Sersa, G.; Mascherini, M.; Di Prata, C.; Odili, J.; de Terlizzi, F.; McKenzie, G.A.G.; Clover, A.J.P.; Bertino, G.; Spina, R.; Groselj, A.; et al. Outcomes of Older Adults Aged 90 and over with Cutaneous Malignancies after Electrochemotherapy with Bleomycin: A Matched Cohort Analysis from the InspECT Registry. Eur. J. Surg. Oncol. 2021, 47, 902–912. [Google Scholar] [CrossRef] [PubMed]
- Matthiessen, L.W.; Keshtgar, M.; Curatolo, P.; Kunte, C.; Grischke, E.-M.; Odili, J.; Muir, T.; Mowatt, D.; Clover, J.P.; Liew, S.H.; et al. Electrochemotherapy for Breast Cancer—Results from the INSPECT Database. Clin. Breast Cancer 2018, 18, e909–e917. [Google Scholar] [CrossRef]
- Clover, A.J.P.; de Terlizzi, F.; Bertino, G.; Curatolo, P.; Odili, J.; Campana, L.G.; Kunte, C.; Muir, T.; Brizio, M.; Sersa, G.; et al. Electrochemotherapy in the Treatment of Cutaneous Malignancy: Outcomes and Subgroup Analysis from the Cumulative Results from the Pan-European International Network for Sharing Practice in Electrochemotherapy Database for 2482 Lesions in 987 Patients (2008–2019). Eur. J. Cancer 2020, 138, 30–40. [Google Scholar] [CrossRef]
- Gehl, J.; Sersa, G.; Matthiessen, L.W.; Muir, T.; Soden, D.; Occhini, A.; Quaglino, P.; Curatolo, P.; Campana, L.G.; Kunte, C.; et al. Updated Standard Operating Procedures for Electrochemotherapy of Cutaneous Tumours and Skin Metastases. Acta Oncol. 2018, 57, 874–882. [Google Scholar] [CrossRef]
- Cabula, C.; Campana, L.G.; Grilz, G.; Galuppo, S.; Bussone, R.; De Meo, L.; Bonadies, A.; Curatolo, P.; De Laurentiis, M.; Renne, M.; et al. Electrochemotherapy in the Treatment of Cutaneous Metastases from Breast Cancer: A Multicenter Cohort Analysis. Ann. Surg. Oncol. 2015, 22, 442–450. [Google Scholar] [CrossRef]
- Moore, S. Cutaneous Metastatic Breast Cancer. Clin. J. Oncol. Nurs. 2002, 6, 255–260. [Google Scholar] [CrossRef]
- Campana, L.G.; Quaglino, P.; de Terlizzi, F.; Mascherini, M.; Brizio, M.; Spina, R.; Bertino, G.; Kunte, C.; Odili, J.; Matteucci, P.; et al. Health-related Quality of Life Trajectories in Melanoma Patients after Electrochemotherapy: Real-world Insights from the InspECT Register. Acad. Derm. Venereol. 2022, 36, 2352–2363. [Google Scholar] [CrossRef] [PubMed]
- Vestergaard, K.; Vissing, M.; Gehl, J.; Lindhardt, C.L. Qualitative Investigation of Experience and Quality of Life in Patients Treated with Calcium Electroporation for Cutaneous Metastases. Cancers 2023, 15, 599. [Google Scholar] [CrossRef] [PubMed]
- Bosnjak, M.; Jesenko, T.; Markelc, B.; Cerovsek, A.; Sersa, G.; Cemazar, M. Sunitinib Potentiates the Cytotoxic Effect of Electrochemotherapy in Pancreatic Carcinoma Cells. Radiol. Oncol. 2022, 56, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Heppt, M.V.; Eigentler, T.K.; Kähler, K.C.; Herbst, R.A.; Göppner, D.; Gambichler, T.; Ulrich, J.; Dippel, E.; Loquai, C.; Schell, B.; et al. Immune Checkpoint Blockade with Concurrent Electrochemotherapy in Advanced Melanoma: A Retrospective Multicenter Analysis. Cancer Immunol. Immunother. 2016, 65, 951–959. [Google Scholar] [CrossRef] [PubMed]
- Russano, F.; Del Fiore, P.; Di Prata, C.; Pasqual, A.; Marconato, R.; Campana, L.G.; Spina, R.; Gianesini, C.M.; Collodetto, A.; Tropea, S.; et al. The Role of Electrochemotherapy in the Cutaneous and Subcutaneous Metastases from Breast Cancer: Analysis of Predictive Factors to Treatment from an Italian Cohort of Patients. Front. Oncol. 2021, 11, 772144. [Google Scholar] [CrossRef]
- Mir, L.M.; Gehl, J.; Sersa, G.; Collins, C.G.; Garbay, J.-R.; Billard, V.; Geertsen, P.F.; Rudolf, Z.; O’Sullivan, G.C.; Marty, M. Standard Operating Procedures of the Electrochemotherapy: Instructions for the Use of Bleomycin or Cisplatin Administered Either Systemically or Locally and Electric Pulses Delivered by the CliniporatorTM by Means of Invasive or Non-Invasive Electrodes. Eur. J. Cancer Suppl. 2006, 4, 14–25. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New Response Evaluation Criteria in Solid Tumours: Revised RECIST Guideline (Version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Banerjee, S.; Keshtgar, M.R.S. Electrochemotherapy for Treatment of Cutaneous Breast Cancer Metastases: A Review. Arch. Breast Cancer 2016, 3, 108–117. [Google Scholar] [CrossRef]
- Wichtowski, M.; Murawa, D.; Czarnecki, R.; Piechocki, J.; Nowecki, Z.; Witkiewicz, W. Electrochemotherapy in the Treatment of Breast Cancer Metastasis to the Skin and Subcutaneous Tissue—Multicenter Experience. Oncol. Res. Treat. 2019, 42, 47–51. [Google Scholar] [CrossRef]
- Farricha, V.; Quaglino, P.; Brizio, M.; de Terlizzi, F.; Bartolo, J.; Carvalhal, S.; Caracò, C.; Di Monta, G. Safety and Efficacy of Electrochemotherapy in a Series of Patients with Nonmetastasized Primary or Recurrent Anorectal Malignant Melanoma. Melanoma Res. 2021, 31, 76–80. [Google Scholar] [CrossRef]
- Curatolo, P.; Quaglino, P.; Marenco, F.; Mancini, M.; Nardò, T.; Mortera, C.; Rotunno, R.; Calvieri, S.; Bernengo, M.G. Electrochemotherapy in the Treatment of Kaposi Sarcoma Cutaneous Lesions: A Two-Center Prospective Phase II Trial. Ann. Surg. Oncol. 2012, 19, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Claussen, C.S.; Moir, G.; Bechara, F.G.; Orlando, A.; Matteucci, P.; Mowatt, D.; Clover, A.J.P.; Mascherini, M.; Gehl, J.; Muir, T.; et al. Prospective Cohort Study by InspECT on Safety and Efficacy of Electrochemotherapy for Cutaneous Tumors and Metastases Depending on Ulceration. J. Dtsch. Derma Gesell. 2022, 20, 470–481. [Google Scholar] [CrossRef] [PubMed]
- Zagar, T.M.; Higgins, K.A.; Miles, E.F.; Vujaskovic, Z.; Dewhirst, M.W.; Clough, R.W.; Prosnitz, L.R.; Jones, E.L. Durable Palliation of Breast Cancer Chest Wall Recurrence with Radiation Therapy, Hyperthermia, and Chemotherapy. Radiother. Oncol. 2010, 97, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Oldenborg, S.; Van Os, R.M.; Van rij, C.M.; Crezee, J.; Van de kamer, J.B.; Rutgers, E.J.T.; Geijsen, E.D.; Zum vörde sive vörding, P.J.; Koning, C.C.E.; Van tienhoven, G. Elective Re-Irradiation and Hyperthermia Following Resection of Persistent Locoregional Recurrent Breast Cancer: A Retrospective Study. Int. J. Hyperth. 2010, 26, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Campana, L.G.; Mocellin, S.; Basso, M.; Puccetti, O.; De Salvo, G.L.; Chiarion-Sileni, V.; Vecchiato, A.; Corti, L.; Rossi, C.R.; Nitti, D. Bleomycin-Based Electrochemotherapy: Clinical Outcome from a Single Institution’s Experience with 52 Patients. Ann. Surg. Oncol. 2009, 16, 191–199. [Google Scholar] [CrossRef]
- Campana, L.G.; Galuppo, S.; Valpione, S.; Brunello, A.; Ghiotto, C.; Ongaro, A.; Rossi, C.R. Bleomycin Electrochemotherapy in Elderly Metastatic Breast Cancer Patients: Clinical Outcome and Management Considerations. J. Cancer Res. Clin. Oncol. 2014, 140, 1557–1565. [Google Scholar] [CrossRef]
- Bourke, M.G.; Salwa, S.P.; Sadadcharam, M.; Whelan, M.C.; Forde, P.F.; Larkin, J.O.; Collins, C.G.; O’Reilly, S.; O’Sullivan, G.C.; Clover, A.J.; et al. Effective Treatment of Intractable Cutaneous Metastases of Breast Cancer with Electrochemotherapy: Ten-Year Audit of Single Centre Experience. Breast. Cancer Res. Treat. 2017, 161, 289–297. [Google Scholar] [CrossRef]
- Campana, L.G.; Peric, B.; Mascherini, M.; Spina, R.; Kunte, C.; Kis, E.; Rozsa, P.; Quaglino, P.; Jones, R.P.; Clover, A.J.P.; et al. Combination of Pembrolizumab with Electrochemotherapy in Cutaneous Metastases from Melanoma: A Comparative Retrospective Study from the InspECT and Slovenian Cancer Registry. Cancers 2021, 13, 4289. [Google Scholar] [CrossRef]
- Falk, H.; Lambaa, S.; Johannesen, H.H.; Wooler, G.; Venzo, A.; Gehl, J. Electrochemotherapy and Calcium Electroporation Inducing a Systemic Immune Response with Local and Distant Remission of Tumors in a Patient with Malignant Melanoma—A Case Report. Acta Oncol. 2017, 56, 1126–1131. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).