Long Term Outcome and Quality of Life of Intracranial Meningioma Patients Treated with Pencil Beam Scanning Proton Therapy
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
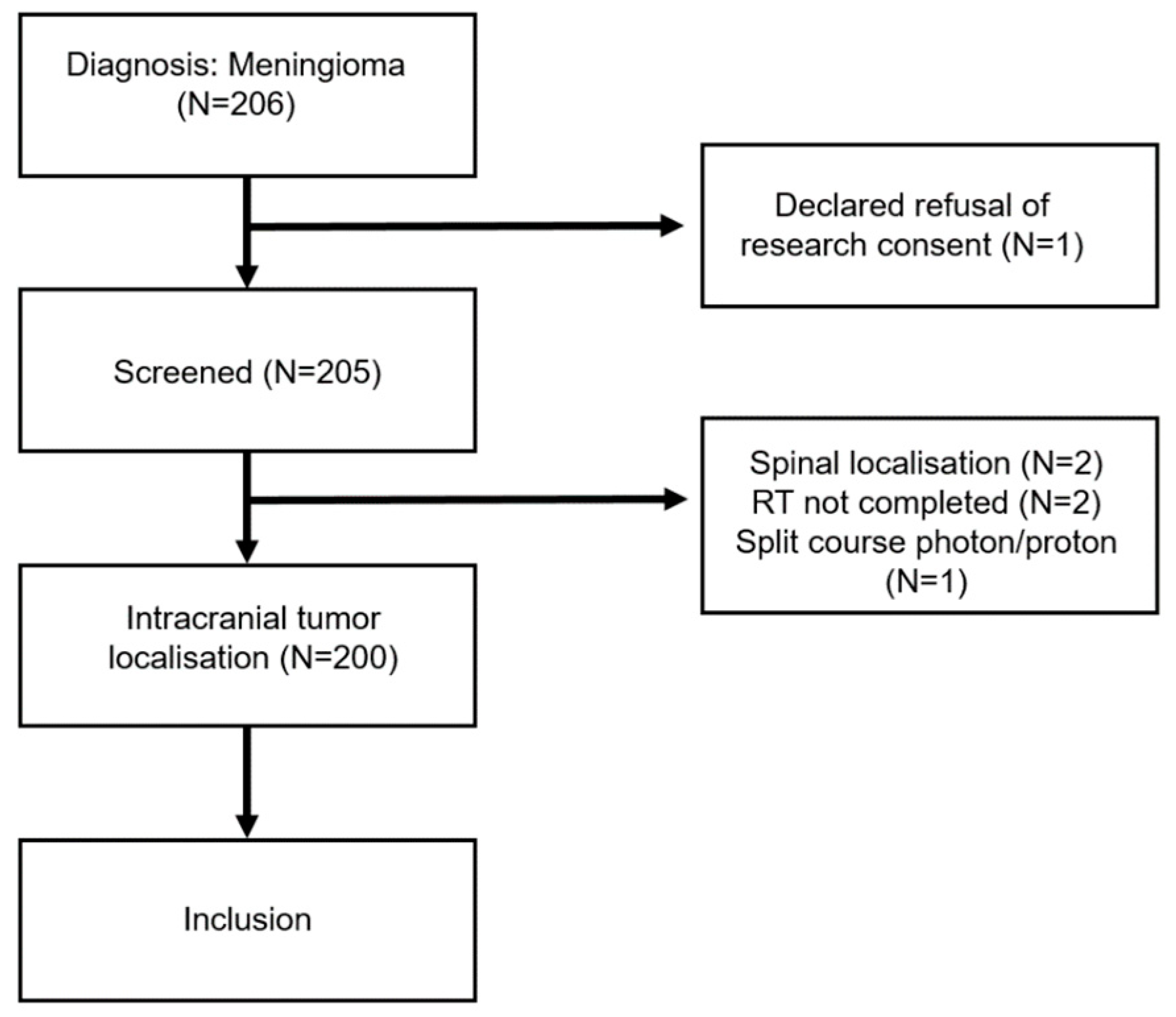
2.1. Patients
Pencil Beam Scanning Proton Therapy Delivery
2.2. Follow-Up Evaluation
2.3. Statistical Analysis
3. Results
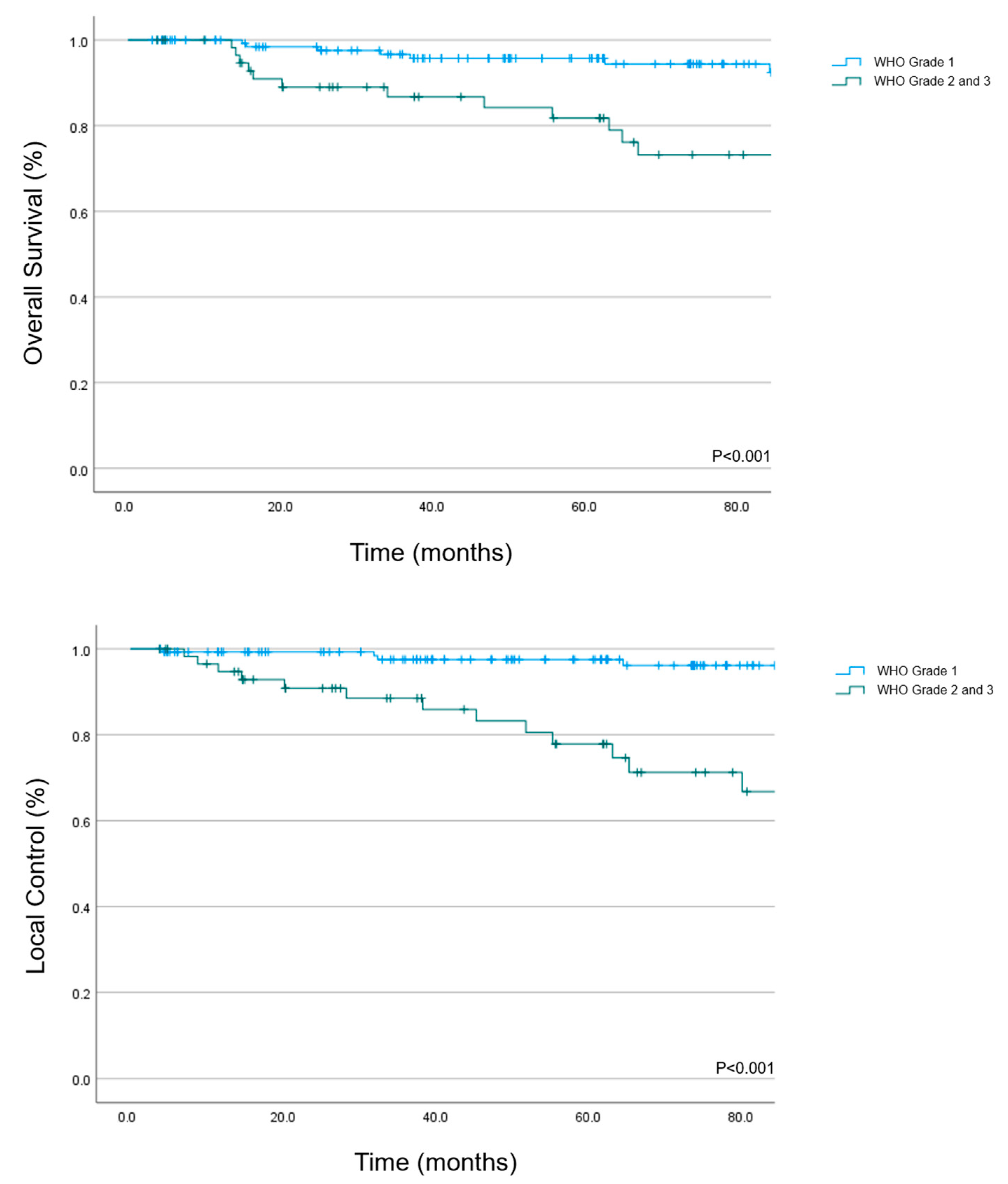
3.1. Overall Survival
3.2. Local Control
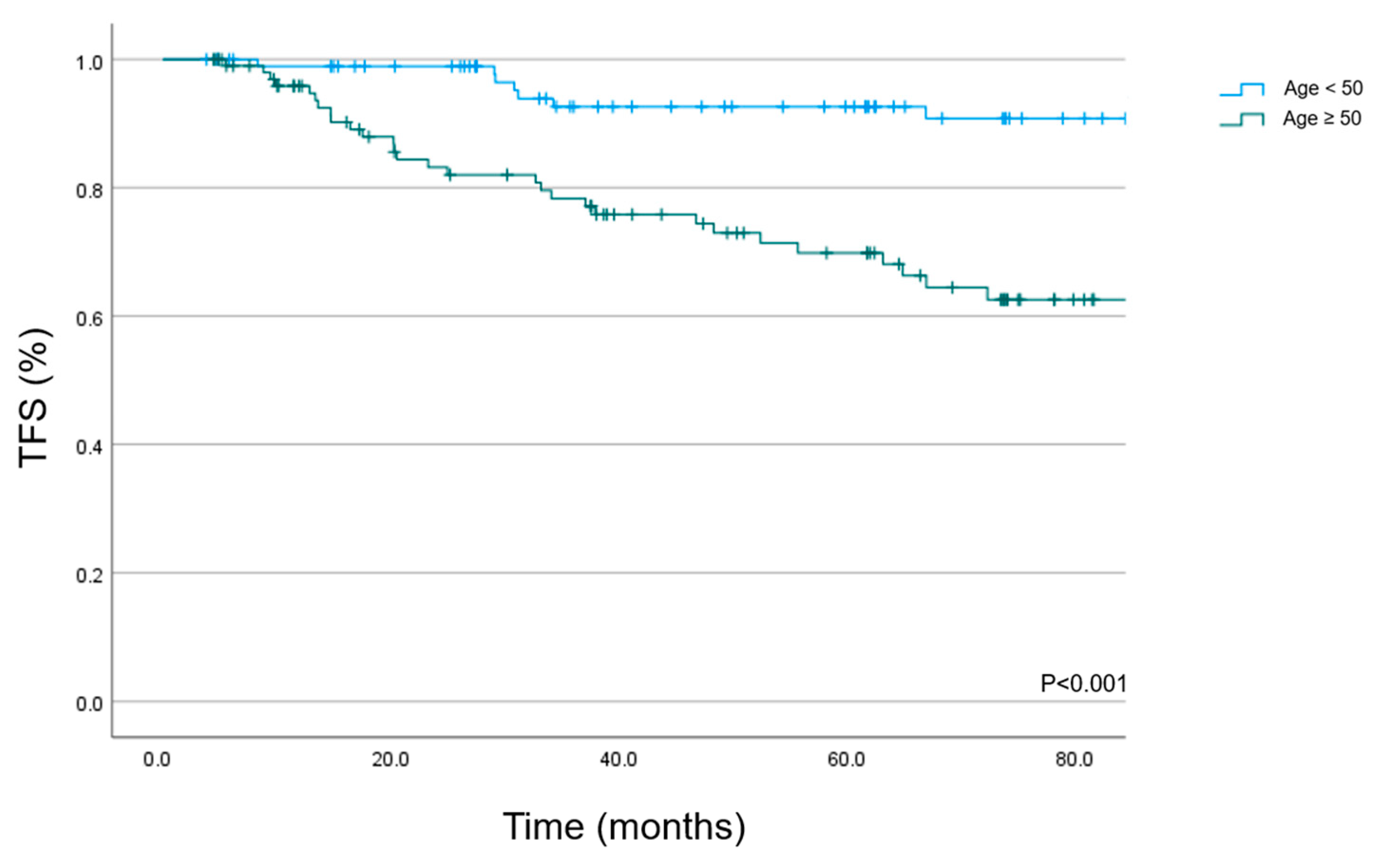
3.3. Toxicity
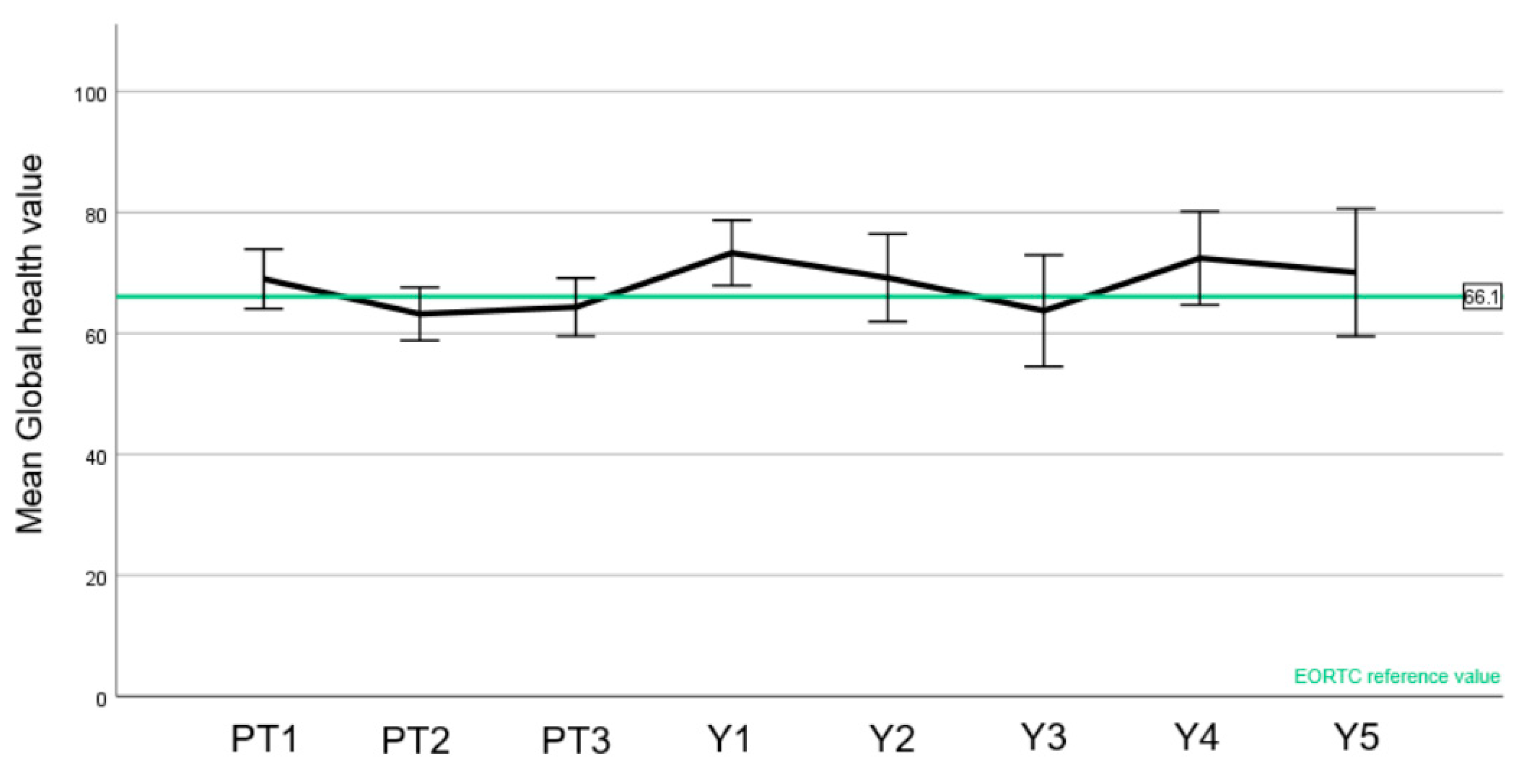
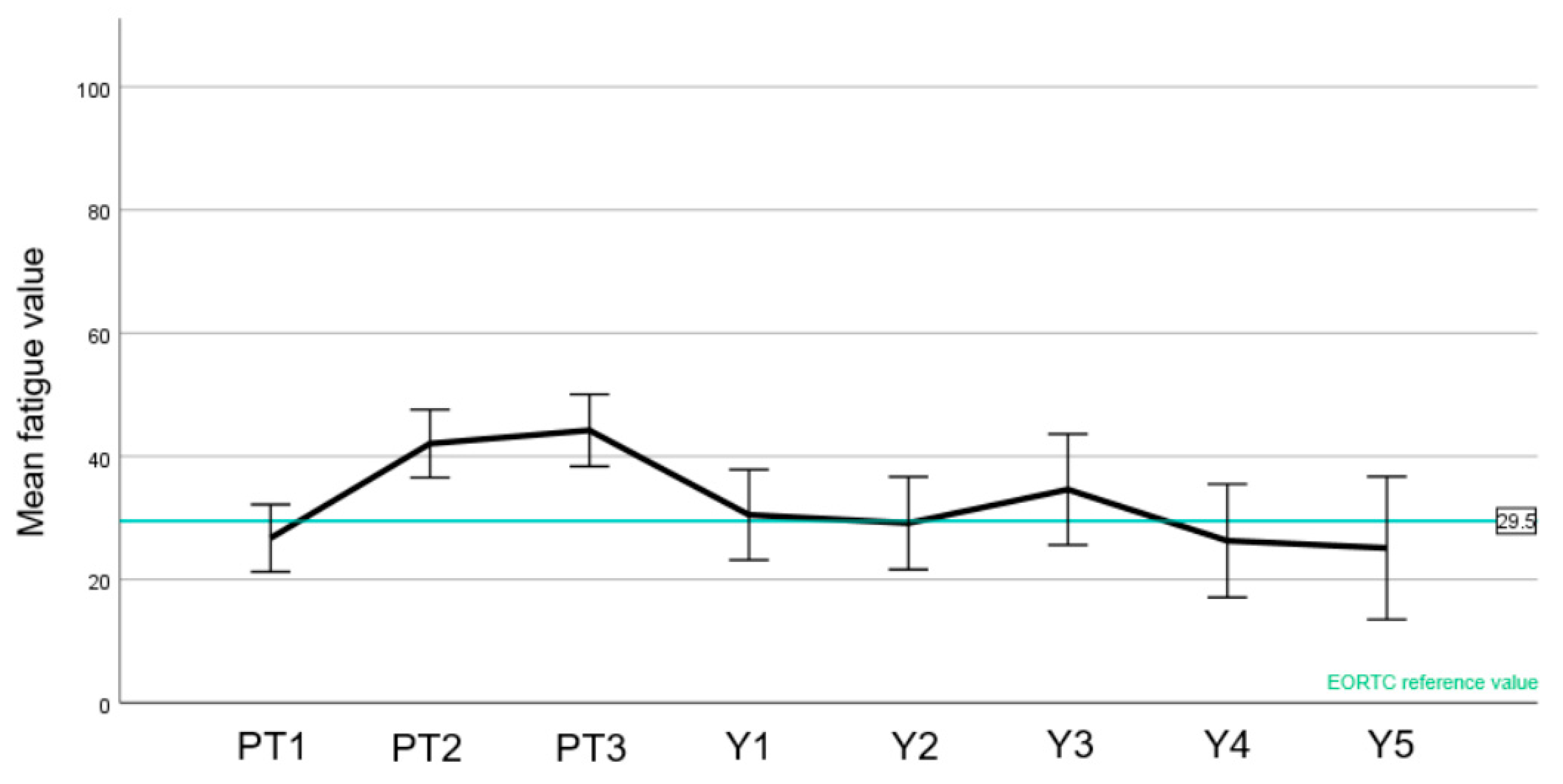
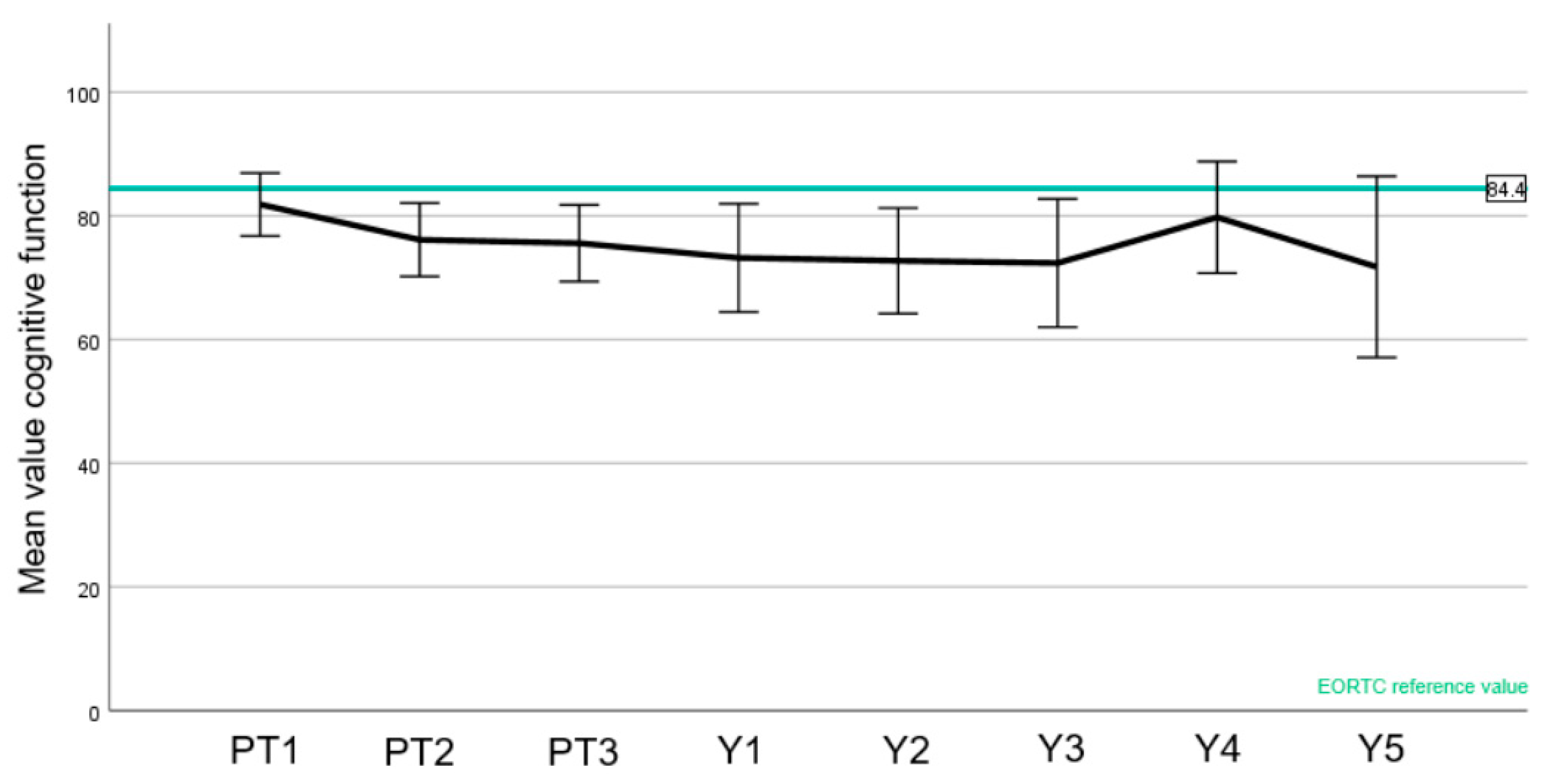
3.4. Quality of Life
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CPT | Centre for Proton Therapy |
| RBE | Relative Biological Effectiveness |
| CTCAE | Common Terminology Criteria for Adverse Events |
| CTV | Clinical Target Volume |
| EORTC | European Organization for Research and Treatment of Cancer |
| LF | Local Failure |
| LC | Local Control |
| GTR | Gross Total Resection |
| GTV | Gross Tumor Volume |
| OAR | Organs At Risk |
| OS | Overall Survival |
| PBS | Pencil Beam Scanning |
| PSI | Paul Scherrer Institute |
| PT | Proton Therapy |
| PTV | Planning Target Volume |
| RBE | Relative biological effectiveness |
| RT | Radiotherapy |
| QoL | Quality of Life |
| STR | Subtotal Resection |
| TFS | Toxicity Free Survival |
| WHO | World Health Organization |
References
- Ostrom, Q.T.; Cioffi, G.; Gittleman, H.; Patil, N.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2012–2016. Neuro-Oncology 2019, 21 (Suppl. S5), v1–v100. [Google Scholar] [CrossRef] [PubMed]
- Goldbrunner, R.; Stavrinou, P.; Jenkinson, M.D.; Sahm, F.; Mawrin, C.; Weber, D.C.; Preusser, M.; Minniti, G.; Lund-Johansen, M.; Lefranc, F.; et al. EANO guideline on the diagnosis and management of meningiomas. Neuro-Oncology 2021, 23, 1821–1834. [Google Scholar] [CrossRef]
- Kshettry, V.R.; Ostrom, Q.T.; Kruchko, C.; Al-Mefty, O.; Barnett, G.H.; Barnholtz-Sloan, J.S. Descriptive epidemiology of World Health Organization grades II and III intracranial meningiomas in the United States. Neuro-Oncology 2015, 17, 1166–1173. [Google Scholar] [CrossRef] [PubMed]
- Whittle, I.R.; Smith, C.; Navoo, P.; Collie, D. Meningiomas. Lancet 2004, 363, 1535–1543. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, N.; Khosla, D.; Gupta, S.; Radotra, B.; Sharma, S. Long term outcome analysis of role of radiotherapy in Grade I meningiomas: A single centre experience from North India. Int. J. Appl. Basic Med. Res. 2015, 5, 128. [Google Scholar] [CrossRef] [PubMed]
- Combs, S.E.; Ganswindt, U.; Foote, R.L.; Kondziolka, D.; Tonn, J.-C. State-of-the-art treatment alternatives for base of skull meningiomas: Complementing and controversial indications for neurosurgery, stereotactic and robotic based radiosurgery or modern fractionated radiation techniques. Radiat. Oncol. 2012, 7, 226. [Google Scholar] [CrossRef]
- Song, J.; Aljabab, S.; Abduljabbar, L.; Tseng, Y.D.; Rockhill, J.K.; Fink, J.R.; Chang, L.; Halasz, L.M. Radiation-induced brain injury in patients with meningioma treated with proton or photon therapy. J. Neurooncol. 2021, 153, 169–180. [Google Scholar] [CrossRef]
- Hu, M.; Jiang, L.; Cui, X.; Zhang, J.; Yu, J. Proton beam therapy for cancer in the era of precision medicine. J. Hematol. Oncol. 2018, 11, 136. [Google Scholar] [CrossRef]
- Lomax, A.J.; Böhringer, T.; Bolsi, A.; Coray, D.; Emert, F.; Goitein, G.; Jermann, M.; Lin, S.; Pedroni, E.; Rutz, H.; et al. Treatment planning and verification of proton therapy using spot scanning: Initial experiences. Med. Phys. 2004, 31, 3150–3157. [Google Scholar] [CrossRef]
- Pedroni, E.; Bacher, R.; Blattmann, H.; Böhringer, T.; Coray, A.; Lomax, A.; Lin, S.; Scheib, S.; Schneider, U. The 200-MeV proton therapy project at the Paul Scherrer Institute: Conceptual design and practical realization. Med. Phys. 1995, 22, 37–53. [Google Scholar] [CrossRef]
- Weber, D.C.; Lomax, A.J.; Peter Rutz, H.; Stadelmann, O.; Egger, E.; Timmermann, B.; Pedroni, E.S.; Verwey, J.; Miralbell, R.; Goitein, G.; et al. Spot-scanning proton radiation therapy for recurrent, residual or untreated intracranial meningiomas. Radiother. Oncol. 2004, 71, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Murray, F.R.; Snider, J.W.; Bolsi, A.; Lomax, A.J.; Walser, M.; Kliebsch, U.; Schneider, R.A.; Weber, D.C. Long-Term Clinical Outcomes of Pencil Beam Scanning Proton Therapy for Benign and Non-benign Intracranial Meningiomas. Int. J. Radiat. Oncol. 2017, 99, 1190–1198. [Google Scholar] [CrossRef] [PubMed]
- Simpson, D. The Recurrence of Intracranlal Meningiomas after Surgical Treatment. J. Neurol. Neurosurg. Psychiatry 1957, 20, 22–39. [Google Scholar] [CrossRef] [PubMed]
- Kroeze, S.G.C.; Mackeprang, P.-H.; De Angelis, C.; Pica, A.; Bachtiary, B.; Kliebsch, U.L.; Weber, D.C. A Prospective Study on Health-Related Quality of Life and Patient-Reported Outcomes in Adult Brain Tumor Patients Treated with Pencil Beam Scanning Proton Therapy. Cancers 2021, 13, 4892. [Google Scholar] [CrossRef] [PubMed]
- Nolte, S.; Liegl, G.; Petersen, M.A.; Aaronson, N.K.; Costantini, A.; Fayers, P.M.; Groenvold, M.; Holzner, B.; Johnson, C.D.; Kemmler, G.; et al. General population normative data for the EORTC QLQ-C30 health-related quality of life questionnaire based on 15,386 persons across 13 European countries, Canada and the Unites States. Eur. J. Cancer 2019, 107, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Holtzman, A.L.; Glassman, G.E.; Dagan, R.; Rao, D.; Fiester, P.J.; Tavanaieour, D.; Morris, C.G.; Indelicato, D.J.; Mendenhall, W.M. Long-term outcomes of fractionated proton beam therapy for benign or radiographic intracranial meningioma. J. Neurooncol. 2023, 161, 481–489. [Google Scholar] [CrossRef]
- Hage, R.; Alapetite, C.; Brisse, H.; Zuber, K.; Lecler, A.; Lot, G.; Le Guerinel, C.; Vignal-Clermont, C.; Boissonnet, H. Efficacy and Safety of Proton Beam Therapy for Primary Optic Nerve Sheath Meningioma. Eye Brain 2021, 13, 219–229. [Google Scholar] [CrossRef]
- Sato, H.; Mizumoto, M.; Okumura, T.; Sakurai, H.; Sakamoto, N.; Akutsu, H.; Ishikawa, E.; Tsuboi, K. Long-term outcomes of patients with unresectable benign meningioma treated with proton beam therapy. J. Radiat. Res. 2021, 62, 427–437. [Google Scholar] [CrossRef]
- Champeaux-Depond, C.; Weller, J. Outcome After Protontherapy for Progression or Recurrence of Surgically Treated Meningioma. Brain Tumor Res. Treat. 2021, 9, 46. [Google Scholar] [CrossRef]
- El Shafie, R.A.; Czech, M.; Kessel, K.A.; Habermehl, D.; Weber, D.; Rieken, S.; Bougatf, N.; Jäkel, O.; Debus, J.; Combs, S.E. Clinical outcome after particle therapy for meningiomas of the skull base: Toxicity and local control in patients treated with active rasterscanning. Radiat. Oncol. 2018, 13, 54. [Google Scholar] [CrossRef]
- Vlachogiannis, P.; Gudjonsson, O.; Montelius, A.; Grusell, E.; Isacsson, U.; Nilsson, K.; Blomquist, E. Hypofractionated high-energy proton-beam irradiation is an alternative treatment for WHO grade I meningiomas. Acta Neurochir. 2017, 159, 2391–2400. [Google Scholar] [CrossRef] [PubMed]
- Sanford, N.N.; Yeap, B.Y.; Larvie, M.; Daartz, J.; Munzenrider, J.E.; Liebsch, N.J.; Fullerton, B.; Pan, E.; Loeffler, J.S.; Shih, H.A. Prospective, Randomized Study of Radiation Dose Escalation with Combined Proton-Photon Therapy for Benign Meningiomas. Int. J. Radiat. Oncol. 2017, 99, 787–796. [Google Scholar] [CrossRef] [PubMed]
- McDonald, M.W.; Plankenhorn, D.A.; McMullen, K.P.; Henderson, M.A.; Dropcho, E.J.; Shah, M.V.; Cohen-Gadol, A.A. Proton therapy for atypical meningiomas. J. Neurooncol. 2015, 123, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Slater, J.D.; Loredo, L.N.; Chung, A.; Bush, D.A.; Patyal, B.; Johnson, W.D.; Hsu, F.P.K.; Slater, J.M. Fractionated Proton Radiotherapy for Benign Cavernous Sinus Meningiomas. Int. J. Radiat. Oncol. 2012, 83, e633–e637. [Google Scholar] [CrossRef]
- Halasz, L.M.; Bussière, M.R.; Dennis, E.R.; Niemierko, A.; Chapman, P.H.; Loeffler, J.S.; Shih, H.A. Proton Stereotactic Radiosurgery for the Treatment of Benign Meningiomas. Int. J. Radiat. Oncol. 2011, 81, 1428–1435. [Google Scholar] [CrossRef]
- Weber, D.C.; Bizzocchi, N.; Bolsi, A.; Jenkinson, M.D. Proton Therapy for Intracranial Meningioma for the Treatment of Primary/Recurrent Disease Including Re-Irradiation. Front. Oncol. 2020, 10, 558845. [Google Scholar] [CrossRef]
- Amichetti, M.; Amelio, D.; Minniti, G. Radiosurgery with photons or protons for benign and malignant tumours of the skull base: A review. Radiat. Oncol. 2012, 7, 210. [Google Scholar] [CrossRef]
- Baumert, B.G.; Norton, I.A.; Lomax, A.J.; Davis, J.B. Dose conformation of intensity-modulated stereotactic photon beams, proton beams, and intensity-modulated proton beams for intracranial lesions. Int. J. Radiat. Oncol. 2004, 60, 1314–1324. [Google Scholar] [CrossRef]
- Rombi, B.; Ruggi, A.; Sardi, I.; Zucchelli, M.; Scagnet, M.; Toni, F.; Cammelli, S.; Giulietti, G.; Fabbri, V.P.; Gianno, F.; et al. Proton therapy: A therapeutic opportunity for aggressive pediatric meningioma. Pediatr. Blood Cancer 2021, 68, e28919. [Google Scholar] [CrossRef]
- Tommasino, F.; Durante, M. Proton radiobiology. Cancers 2015, 7, 353–381. [Google Scholar] [CrossRef]
- Matani, H.; Abel, S.; Yu, A.; Karlovits, S.M.; Wegner, R.E. Trends in the use of radiation for meningioma across the United States. Radiat. Oncol. J. 2022, 40, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Weber, D.C.; Ares, C.; Villa, S.; Peerdeman, S.M.; Renard, L.; Baumert, B.G.; Lucas, A.; Veninga, T.; Pica, A.; Jefferies, S.; et al. Adjuvant postoperative high-dose radiotherapy for atypical and malignant meningioma: A phase-II parallel non-randomized and observation study (EORTC 22042-26042). Radiother. Oncol. 2018, 128, 260–265. [Google Scholar] [CrossRef]
- Carroll, R.S.; Zhang, J.; Black, P.M. Expression of Estrogen Receptors Alpha and Beta in Human Meningiomas. J. Neuro-Oncol. 1999, 42, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Wolfsberger, S.; Doostkam, S.; Boecher-Schwarz, H.-G.; Roessler, K.; van Trotsenburg, M.; Hainfellner, J.A.; Knosp, E. Progesterone-receptor index in meningiomas: Correlation with clinico-pathological parameters and review of the literature. Neurosurg. Rev. 2004, 27, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Le Rhun, E.; Weller, M. Sex-specific aspects of epidemiology, molecular genetics and outcome: Primary brain tumours. ESMO Open 2020, 5, e001034. [Google Scholar] [CrossRef]
- Zhu, H.; Bi, W.L.; Aizer, A.; Hua, L.; Tian, M.; Den, J.; Tang, H.; Chen, H.; Wang, Y.; Mao, Y.; et al. Efficacy of adjuvant radiotherapy for atypical and anaplastic meningioma. Cancer Med. 2019, 8, 13–20. [Google Scholar] [CrossRef]
- Endo, T.; Narisawa, A.; Ali, H.S.M.; Murakami, K.; Watanabe, T.; Watanabe, M.; Jokura, H.; Endo, H.; Fujimura, M.; Sonoda, Y.; et al. A study of prognostic factors in 45 cases of atypical meningioma. Acta Neurochir. 2016, 158, 1661–1667. [Google Scholar] [CrossRef]
- Gallagher, M.J.; Jenkinson, M.D.; Brodbelt, A.R.; Mills, S.J.; Chavredakis, E. WHO grade 1 meningioma recurrence: Are location and Simpson grade still relevant? Clin. Neurol. Neurosurg. 2016, 141, 117–121. [Google Scholar] [CrossRef]
- Silva, S.R.; Sethi, A.; Prabhu, V.C.; Anderson, D.; Melian, E. Prognostic factors affecting overall survival and local control in meningioma patients treated with radiotherapy or combined radiotherapy and surgery. J. Radiat. Oncol. 2018, 7, 27–35. [Google Scholar] [CrossRef]
- Goutagny, S.; Kalamarides, M. Meningiomas and neurofibromatosis. J. Neurooncol. 2010, 99, 341–347. [Google Scholar] [CrossRef]
- Champeaux-Depond, C.; Weller, J.; Constantinou, P.; Tuppin, P.; Froelich, S. Five-year cause-specific survival after meningioma surgery. A nationwide population-based study. Neurochirurgie 2022, 68, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Pou, P.; Biau, J.; Verrelle, P.; Lemaire, J.J.; El Ouadih, Y.; Chassin, V.; Magnier, F.; Dedieu, V.; Lapeyre, M.; Dupic, G.; et al. Long-Term Outcomes After Linac Radiosurgery for Benign Meningiomas. Clin. Oncol. 2020, 32, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Maclean, J.; Fersht, N.; Short, S. Controversies in Radiotherapy for Meningioma. Clin. Oncol. 2014, 26, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, N.; Rabo, C.S.; Okita, Y.; Kinoshita, M.; Kagawa, N.; Fujimoto, Y.; Morii, E.; Kishima, H.; Maruno, M.; Kato, A.; et al. Slower growth of skull base meningiomas compared with non–skull base meningiomas based on volumetric and biological studies: Clinical article. J. Neurosurg. 2012, 116, 574–580. [Google Scholar] [CrossRef]
- McGovern, S.L.; Aldape, K.D.; Munsell, M.F.; Mahajan, A.; DeMonte, F.; Woo, S.Y. A comparison of World Health Organization tumor grades at recurrence in patients with non–skull base and skull base meningiomas: Clinical article. J. Neurosurg. 2010, 112, 925–933. [Google Scholar] [CrossRef]
- Bagshaw, H.P.; Burt, L.M.; Jensen, R.L.; Suneja, G.; Palmer, C.A.; Couldwell, W.T.; Shrieve, D.C. Adjuvant radiotherapy for atypical meningiomas. J. Neurosurg. 2016, 126, 1822–1828. [Google Scholar] [CrossRef]
- Kaur, G.; Sayegh, E.T.; Larson, A.; Bloch, O.; Madden, M.; Sun, M.Z.; Barani, I.J.; James, C.D.; Parsa, A.T. Adjuvant radiotherapy for atypical and malignant meningiomas: A systematic review. Neuro-Oncol. 2014, 16, 628–636. [Google Scholar] [CrossRef]
- Chun, S.-W.; Kim, K.M.; Kim, M.-S.; Kang, H.; Dho, Y.-S.; Seo, Y.; Kim, J.W.; Kim, Y.H.; Park, C.-K. Adjuvant radiotherapy versus observation following gross total resection for atypical meningioma: A systematic review and meta-analysis. Radiat. Oncol. 2021, 16, 34. [Google Scholar] [CrossRef]
- Jenkinson, M.D.; Javadpour, M.; Haylock, B.J.; Young, B.; Gillard, H.; Vinten, J.; Bulbeck, H.; Das, K.; Farrell, M.; Looby, S.; et al. The ROAM/EORTC-1308 trial: Radiation versus Observation following surgical resection of Atypical Meningioma: Study protocol for a randomised controlled trial. Trials 2015, 16, 519. [Google Scholar] [CrossRef]
- Kocher, M.; Treuer, H.; Hoevels, M.; Semrau, R.; Sturm, V.; Mueller, R.-P. Endocrine and visual function after fractionated stereotactic radiotherapy of perioptic tumors. Strahlenther. Onkol. 2013, 189, 137–141. [Google Scholar] [CrossRef]
- Pollock, B.E.; Stafford, S.L.; Link, M.J.; Garces, Y.I.; Foote, R.L. Single-Fraction Radiosurgery for Presumed Intracranial Meningiomas: Efficacy and Complications from a 22-Year Experience. Int. J. Radiat. Oncol. 2011, 81, S36. [Google Scholar] [CrossRef]
- Pinzi, V.; Biagioli, E.; Roberto, A.; Galli, F.; Rizzi, M.; Chiappa, F.; Brenna, G.; Fariselli, L.; Floriani, I. Radiosurgery for intracranial meningiomas: A systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 2017, 113, 122–134. [Google Scholar] [CrossRef]
- Rogers, C.L.; Won, M.; Vogelbaum, M.A.; Perry, A.; Ashby, L.S.; Modi, J.M.; Alleman, A.M.; Galvin, J.; Fogh, S.E.; Youssef, E.; et al. High-risk Meningioma: Initial Outcomes from NRG Oncology/RTOG 0539. Int. J. Radiat. Oncol. 2020, 106, 790–799. [Google Scholar] [CrossRef] [PubMed]
- Köthe, A.; Feuvret, L.; Weber, D.C.; Safai, S.; Lomax, A.J.; Fattori, G. Assessment of Radiation-Induced Optic Neuropathy in a Multi-Institutional Cohort of Chordoma and Chondrosarcoma Patients Treated with Proton Therapy. Cancers 2021, 13, 5327. [Google Scholar] [CrossRef] [PubMed]
- Lisowski, D.; Trömel, J.; Lutyj, P.; Lewitzki, V.; Hartrampf, P.E.; Polat, B.; Flentje, M.; Tamihardja, J. Health-related quality of life and clinical outcome after radiotherapy of patients with intracranial meningioma. Sci. Rep. 2022, 12, 19730. [Google Scholar] [CrossRef]
- Shin, Y.; Kim, J. Validation of the Korean version of the European Organization for Research and Treatment of Cancer brain cancer module (EORTC QLQ-BN20) in patients with brain tumors. Health Qual. Life Outcomes 2013, 11, 145. [Google Scholar] [CrossRef]
- Budrukkar, A.; Jalali, R.; Dutta, D.; Sarin, R.; Devlekar, R.; Parab, S.; Kakde, A. Prospective assessment of quality of life in adult patients with primary brain tumors in routine neurooncology practice. J. Neurooncol. 2009, 95, 413–419. [Google Scholar] [CrossRef]
- Erharter, A.; Giesinger, J.; Kemmler, G.; Schauer-Maurer, G.; Stockhammer, G.; Muigg, A.; Hutterer, M.; Rumpold, G.; Sperner-Unterweger, B.; Holzner, B. Implementation of Computer-Based Quality-of-Life Monitoring in Brain Tumor Outpatients in Routine Clinical Practice. J. Pain Symptom Manag. 2010, 39, 219–229. [Google Scholar] [CrossRef]
- Henzel, M.; Fokas, E.; Sitter, H.; Wittig, A.; Engenhart-Cabillic, R. Quality of life after stereotactic radiotherapy for meningioma: A prospective non-randomized study. J. Neurooncol. 2013, 113, 135–141. [Google Scholar] [CrossRef]
- Gondar, R.; Patet, G.; Schaller, K.; Meling, T.R. Meningiomas and Cognitive Impairment after Treatment: A Systematic and Narrative Review. Cancers 2021, 13, 1846. [Google Scholar] [CrossRef]
- van Nieuwenhuizen, D.; Douw, L.; Klein, M.; Peerdeman, S.M.; Heimans, J.J.; Reijneveld, J.C.; Stam, C.J.; Hillebrand, A. Cognitive functioning and functional brain networks in postoperative WHO grade I meningioma patients. J. Neurooncol. 2018, 140, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncology 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]

| N (%) or Median (Range) | |
|---|---|
| Gender | |
| Male | 55 (27.5%) |
| Female | 145 (72.5%) |
| Age (years) | 50.4 (3.2–79.8) |
| Histology | |
| WHO Grade 1 | 140 (70%) |
| WHO Grade 2 | 55 (27.5%) |
| WHO Grade 3 | 5 (2.5%) |
| Tumor site | |
| Skull Base | 140 (70%) |
| Non-Skull Base | 60 (30%) |
| Type of resection | |
| No resection | 38 (19%) |
| Simpson 1–3 | 30 (15%) |
| Simpson 4/5 | 132 (66%) |
| Timing of PT | |
| Initial Treatment | 111 (55.5%) |
| Recurrence/Progressive Disease | 89 (44.5%) |
| Largest PTV (cm3) | 102.3 (4.6–1142) |
| 5 Year Local Control | 5 Year Overall Survival | ||||||
|---|---|---|---|---|---|---|---|
| (%) | 95% CI | p-Value | (%) | 95% CI | p-Value | ||
| Age | 0.423 | <0.001 | |||||
| <50 y | 91.8 | 85.3–98.3 | 100 | ||||
| ≥50 y | 91.8 | 85.9–97.7 | 83 | 74.8–91.2 | |||
| Gender | 0.005 | 0.016 | |||||
| Male | 81.5 | 69–94 | 86.5 | 76.3–96.7 | |||
| Female | 95.4 | 91.7–99.1 | 93.3 | 88.8–97.8 | |||
| Histology | <0.001 | <0.001 | |||||
| WHO Grade 1 | 97.5 | 94.8–100 | 95.7 | 92–99.4 | |||
| WHO Grade 2/3 | 77.8 | 65.3–90.3 | 81.8 | 70.8–92.8 | |||
| Timing of PT | 0.011 | 0.002 | |||||
| Initial | 95 | 90.1–99.9 | 96.7 | 93–100 | |||
| Relapse/Progression | 87.2 | 79.4–95 | 84.8 | 76.4–93.2 | |||
| Grade of resection | 0.551 | 0.036 | |||||
| GTR | 92.1 | 81.5–100 | 80.5 | 65.0–96.0 | |||
| STR | 89.5 | 83.6–95.4 | 91.6 | 86.3–96.9 | |||
| Skull Base * | 0.03 | 0.016 | |||||
| Yes | 94.7 | 90.6–98.8 | 93.7 | 89.2–98.2 | |||
| No | 84.2 | 73.2–95.2 | 86.2 | 76.6–95.8 | |||
| Multiple Meningiomas | 0.005 | 0.256 | |||||
| Yes | 82.5 | 69.6–95.4 | 88.2 | 77.2–94.8 | |||
| No | 94.4 | 90.3–98.5 | 92.3 | 87.6–97.0 | |||
| PET/CT before PT | 0.31 | 0.547 | |||||
| Yes | 92.1 | 83.1–100 | 93.1 | 83.7–100 | |||
| No | 92.3 | 87.6–97 | 91.2 | 86.5–95.9 | |||
| Local Failure | <0.001 | ||||||
| Yes | 73 | 51.4–94.6 | |||||
| No | 93.8 | 89.9–87.7 | |||||
| Acute Toxicity All Grades | N (%) Patients * |
| Alopecia | 109 (54.5%) |
| Dermatitis | 101 (50.5%) |
| Fatigue | 59 (29.5%) |
| Headache | 45 (22.5%) |
| Nausea | 28 (14%) |
| Late Toxicity ≥Grade 3 | N (%) Patients |
| Visual toxicity | 10 (5%) |
| Cataract | 4 (2%) |
| Brain necrosis | 3 (1.5%) |
| Ear and labyrinth disorder | 2 (1%) |
| Stroke | 2 (1%) |
| Brain edema | 1 (0.5%) |
| Pain | 1 (0.5%) |
| Pituitary dysfunction | 1 (0.5%) |
| Author | Year | Patients | WHO Grade | F/U (Months) | Outcome | PT Modality |
|---|---|---|---|---|---|---|
| The present study | 2023 | 200 | 1–3 | 65 | 5y LC WHO 1/n.h.: 97.5% WHO 2/3: 77.8% | PBS |
| Holtzman [16] | 2023 | 59 | 1 | 75.6 | 5y LC: 94% | PSPT |
| Hage [17] | 2021 | 60 | 1 | 48 | LC: 100% | PSPT |
| Sato [18] | 2021 | 27 | 1 | 301 | 5y LC: 100% | PSPT |
| Champeaux-Depond [19] | 2021 | 193 | 1–3 | 52.8 | 5y PFS WHO 1: 71.5%; WHO 2: 55.6%; WHO 3: 35.6% | PSPT/PBS |
| El Shafie [20] | 2018 | 110 | 1–3 | 46.8 | 5y PFS WHO 1: 96.6%; WHO 2/3: 75% | Raster scanning |
| Vlachogiannis [21] | 2017 | 170 | 1 | 84 | 5y PFS: 93% | PSPT |
| Sanford [22] | 2017 | 47 | 1 | 205.2 | 10y LC: 98% | PSPT + Photon |
| McDonald [23] | 2015 | 22 | 2 | 39 | 5y LC: 71.1% | PSPT |
| Slater [24] | 2012 | 72 | 1–2 | 74 | 5y LC WHO 1/n.h.: 99%; WHO 2: 50% | PSPT |
| Halasz [25] | 2011 | 50 | 1 | 32 | 3y LC: 94% | PBS |
| Total | Sum 1010 (range: 22–200) | Median 65 (range: 32–301) | PSPT = 6 PBS/raster only = 3 PSPT/PBS = 1 PSPT/photon = 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krcek, R.; Leiser, D.; García-Marqueta, M.; Bolsi, A.; Weber, D.C. Long Term Outcome and Quality of Life of Intracranial Meningioma Patients Treated with Pencil Beam Scanning Proton Therapy. Cancers 2023, 15, 3099. https://doi.org/10.3390/cancers15123099
Krcek R, Leiser D, García-Marqueta M, Bolsi A, Weber DC. Long Term Outcome and Quality of Life of Intracranial Meningioma Patients Treated with Pencil Beam Scanning Proton Therapy. Cancers. 2023; 15(12):3099. https://doi.org/10.3390/cancers15123099
Chicago/Turabian StyleKrcek, Reinhardt, Dominic Leiser, Marta García-Marqueta, Alessandra Bolsi, and Damien Charles Weber. 2023. "Long Term Outcome and Quality of Life of Intracranial Meningioma Patients Treated with Pencil Beam Scanning Proton Therapy" Cancers 15, no. 12: 3099. https://doi.org/10.3390/cancers15123099
APA StyleKrcek, R., Leiser, D., García-Marqueta, M., Bolsi, A., & Weber, D. C. (2023). Long Term Outcome and Quality of Life of Intracranial Meningioma Patients Treated with Pencil Beam Scanning Proton Therapy. Cancers, 15(12), 3099. https://doi.org/10.3390/cancers15123099





