Population-Attributable Fractions of Personal Comorbidities for Liver, Gallbladder, and Bile Duct Cancers
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population
2.3. Data Sources
2.4. Outcome Measures
2.5. Statistical Analyses and Adjustment Variables
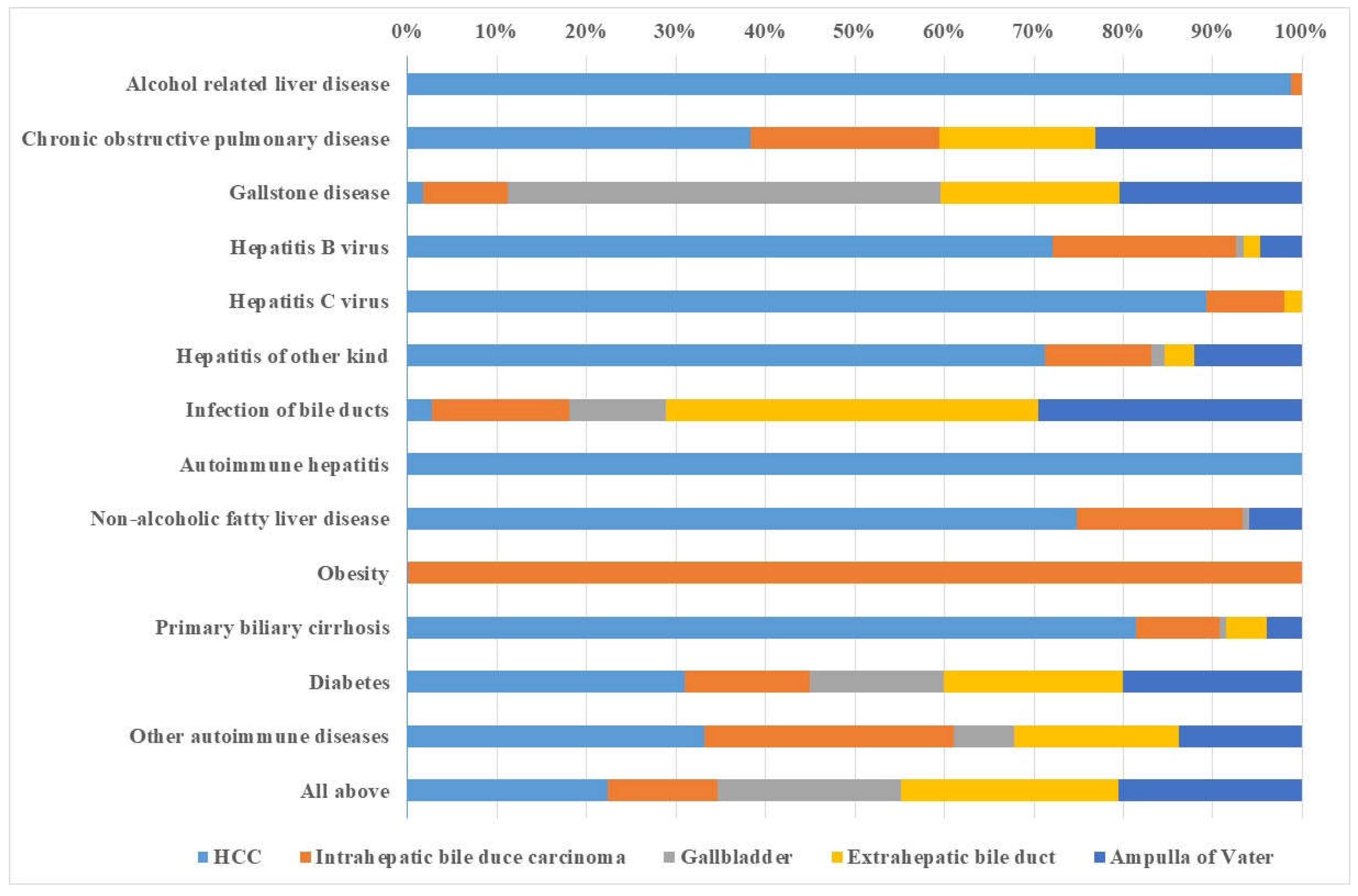
3. Results
4. Discussion
Strengths and Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CCAW | cholangiocarcinoma |
| CI | confidence interval |
| GBC | gallbladder cancer |
| HBV | hepatitis B virus |
| HCC | hepatocellular carcinoma |
| HCV | hepatitis C virus |
| HR | hazard ratio |
| ICD | International Classification of Diseases |
| NAFLD | non-alcoholic fatty liver disease |
| O | observed number |
| PAF | population attributable fraction |
| SIR | standardized incidence ratio |
References
- Valle, J.W.; Kelley, R.K.; Nervi, B.; Oh, D.Y.; Zhu, A.X. Biliary tract cancer. Lancet 2021, 397, 428–444. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, A. Hepatocellular Carcinoma. N. Engl. J. Med. 2019, 380, 1450–1462. [Google Scholar] [CrossRef]
- Leone, V.; Ali, A.; Weber, A.; Tschaharganeh, D.F.; Heikenwalder, M. Liver Inflammation and Hepatobiliary Cancers. Trends Cancer 2021, 7, 606–623. [Google Scholar] [CrossRef] [PubMed]
- Koshiol, J.; Yu, B.; Kabadi, S.M.; Baria, K.; Shroff, R.T. Epidemiologic patterns of biliary tract cancer in the United States: 2001–2015. BMC Cancer 2022, 22, 1178. [Google Scholar] [CrossRef] [PubMed]
- Hemminki, K.; Försti, A.; Hemminki, O.; Liska, V.; Hemminki, A. Long-term incidence and survival trends in cancer of the gallbladder and extrahepatic bile ducts in Denmark, Finland, Norway and Sweden with etiological implications related to Thorotrast. Int. J. Cancer 2022, 151, 200–208. [Google Scholar] [CrossRef]
- Hemminki, K.; Tichanek, F.; Försti, A.; Hemminki, O.; Liska, V.; Hemminki, A. Long-term incidence in hepatocellular carcinoma and intrahepatic bile duct cancer in Denmark, Finland, Norway and Sweden, role of Thorotrast? Int. J. Cancer 2022, 151, 510–517. [Google Scholar] [CrossRef]
- Lazarus, J.V.; Mark, H.E.; Anstee, Q.M.; Arab, J.P.; Batterham, R.L.; Castera, L.; Cortez-Pinto, H.; Crespo, J.; Cusi, K.; Dirac, M.A.; et al. Advancing the global public health agenda for NAFLD: A consensus statement. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 60–78. [Google Scholar] [CrossRef]
- Hemminki, K.; Sundquist, K.; Sundquist, J.; Försti, A.; Liska, V.; Hemminki, A.; Li, X. Personal comorbidities and their subsequent risks for liver, gallbladder and bile duct cancers. Int. J. Cancer 2023, 152, 1107–1114. [Google Scholar] [CrossRef]
- Hemminki, K.; Sundquist, K.; Sundquist, J.; Försti, A.; Liska, V.; Hemminki, A.; Li, X. Familial Risks for Liver, Gallbladder and Bile Duct Cancers and for Their Risk Factors in Sweden, a Low-Incidence Country. Cancers 2022, 14, 1938. [Google Scholar] [CrossRef]
- Liu, X.; Hemminki, K.; Forsti, A.; Sundquist, K.; Sundquist, J.; Ji, J. Cancer risk in patients with type 2 diabetes mellitus and their relatives. Int. J. Cancer 2015, 137, 903–910. [Google Scholar] [CrossRef]
- Rawla, P.; Sunkara, T.; Thandra, K.C.; Barsouk, A. Epidemiology of gallbladder cancer. Clin. Exp. Hepatol. 2019, 5, 93–102. [Google Scholar] [CrossRef]
- Biological Agents. Agents. A review of human carcinogens. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; Internactional Agency for Research on Cancers: Paris, France, 2012; Volume 100, pp. 1–441. [Google Scholar]
- McGee, E.E.; Jackson, S.S.; Petrick, J.L.; Van Dyke, A.L.; Adami, H.O.; Albanes, D.; Andreotti, G.; Beane-Freeman, L.E.; Berrington de Gonzalez, A.; Buring, J.E.; et al. Smoking, Alcohol, and Biliary Tract Cancer Risk: A Pooling Project of 26 Prospective Studies. J. Natl. Cancer Inst. 2019, 111, 1263–1278. [Google Scholar] [CrossRef]
- Barner-Rasmussen, N.; Pukkala, E.; Hadkhale, K.; Färkkilä, M. Risk factors, epidemiology and prognosis of cholangiocarcinoma in Finland. United Eur. Gastroenterol. J. 2021, 9, 1128–1135. [Google Scholar] [CrossRef]
- Clements, O.; Eliahoo, J.; Kim, J.U.; Taylor-Robinson, S.D.; Khan, S.A. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma: A systematic review and meta-analysis. J. Hepatol. 2020, 72, 95–103. [Google Scholar] [CrossRef]
- Hemminki, J.; Försti, A.; Hemminki, A.; Hemminki, K. Survival trends in solid cancers in the Nordic countries through 50 years. Eur. J. Cancer 2022, 175, 77–85. [Google Scholar] [CrossRef]
- Hemminki, K.; Försti, A.; Hemminki, O.; Liska, V.; Hemminki, A. Long-term survival trends for primary liver and pancreatic cancers in the Nordic countries. JHEP Rep. 2022, 4, 100602. [Google Scholar] [CrossRef]
- Rutherford, M.J.; Arnold, M.; Bardot, A.; Ferlay, J.; De, P.; Tervonen, H.; Little, A.; Bucher, O.; St. Jacques, N.; Gavin, A.; et al. Comparison of liver cancer incidence and survival by subtypes across seven high-income countries. Int. J. Cancer 2021, 149, 2020–2031. [Google Scholar] [CrossRef]
- Hemminki, K.; Försti, A.; Liska, V.; Kanerva, A.; Hemminki, O.; Hemminki, A. Long-term survival trends in solid cancers in the Nordic countries marking timing of improvements. Int. J. Cancer 2023, 152, 1837–1846. [Google Scholar] [CrossRef]
- Nakagawa, H.; Maeda, S. Inflammation- and stress-related signaling pathways in hepatocarcinogenesis. World J. Gastroenterol. 2012, 18, 4071–4081. [Google Scholar] [CrossRef]
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Sangro, B.; Schwartz, M.; Sherman, M.; Gores, G. Hepatocellular carcinoma. Nat. Rev. Disease Primers 2021, 7, 6. [Google Scholar] [CrossRef]
- Li, X.; Ramadori, P.; Pfister, D.; Seehawer, M.; Zender, L.; Heikenwalder, M. The immunological and metabolic landscape in primary and metastatic liver cancer. Nat. Rev. Cancer 2021, 21, 541–557. [Google Scholar] [CrossRef] [PubMed]
- Miettinen, O. Proportion of disease caused or prevented by a given exposure, trait or intervention. Am. J. Epidemiol. 1974, 99, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos Silva, I. Cancer Epidemiology: Principles and Methods; IARC: Lyon, France, 1999; 442p. [Google Scholar]
- Brown, K.F.; Rumgay, H.; Dunlop, C.; Ryan, M.; Quartly, F.; Cox, A.; Deas, A.; Elliss-Brookes, L.; Gavin, A.; Hounsome, L.; et al. The fraction of cancer attributable to modifiable risk factors in England, Wales, Scotland, Northern Ireland, and the United Kingdom in 2015. Br. J. Cancer 2018, 118, 1130–1141. [Google Scholar] [CrossRef] [PubMed]
- Henriksson, M.; Björnsson, B.; Sternby Eilard, M.; Lindell, G.; Strömberg, C.; Hemmingsson, O.; Isaksson, B.; Rizell, M.; Sandström, P. Treatment patterns and survival in patients with hepatocellular carcinoma in the Swedish national registry SweLiv. BJS Open 2020, 4, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Lindberg, A.; Bjerg, A.; Rönmark, E.; Larsson, L.G.; Lundbäck, B. Prevalence and underdiagnosis of COPD by disease severity and the attributable fraction of smoking Report from the Obstructive Lung Disease in Northern Sweden Studies. Respir. Med. 2006, 100, 264–272. [Google Scholar] [CrossRef]
- Thomsen, H.; Li, X.; Sundquist, K.; Sundquist, J.; Försti, A.; Hemminki, K. Familial risks between Graves disease and Hashimoto thyroiditis and other autoimmune diseases in the population of Sweden. J. Transl. Autoimmun. 2020, 3, 100058. [Google Scholar] [CrossRef]
- Rothman, K.; Greenland, S. Modern Epidemiology, 2nd ed.; Lippincott-Raven: Philadelphia, PA, USA, 1998. [Google Scholar]
- Rückinger, S.; von Kries, R.; Toschke, A.M. An illustration of and programs estimating attributable fractions in large scale surveys considering multiple risk factors. BMC Med. Res. Methodol. 2009, 9, 7. [Google Scholar] [CrossRef]
- Whiteman, D.C.; Wilson, L.F. The fractions of cancer attributable to modifiable factors: A global review. Cancer Epidemiol. 2016, 44, 203–221. [Google Scholar] [CrossRef]
- Baecker, A.; Liu, X.; La Vecchia, C.; Zhang, Z.F. Worldwide incidence of hepatocellular carcinoma cases attributable to major risk factors. Eur. J. Cancer Prev. 2018, 27, 205–212. [Google Scholar] [CrossRef]
- Makarova-Rusher, O.V.; Altekruse, S.F.; McNeel, T.S.; Ulahannan, S.; Duffy, A.G.; Graubard, B.I.; Greten, T.F.; McGlynn, K.A. Population attributable fractions of risk factors for hepatocellular carcinoma in the United States. Cancer 2016, 122, 1757–1765. [Google Scholar] [CrossRef]
- Hemminki, K.; Hemminki, O.; Försti, A.; Sundquist, K.; Sundquist, J.; Li, X. Surveillance bias in cancer risk after unrelated medical conditions: Example urolithiasis. Sci. Rep. 2017, 7, 8073. [Google Scholar] [CrossRef]
- Jepsen, P.; Andersen, M.W.; Villadsen, G.E.; Ott, P.; Vilstrup, H. Time-trends in incidence and prognosis of hepatocellular carcinoma in Denmark: A nationwide register-based cohort study. Liver Int. 2017, 37, 871–878. [Google Scholar] [CrossRef]
- Rigopoulou, E.I.; Dalekos, G.N. Current Trends and Characteristics of Hepatocellular Carcinoma in Patients with Autoimmune Liver Diseases. Cancers 2021, 13, 1023. [Google Scholar] [CrossRef]
- Facciorusso, A. The influence of diabetes in the pathogenesis and the clinical course of hepatocellular carcinoma: Recent findings and new perspectives. Curr. Diabetes Rev. 2013, 9, 382–386. [Google Scholar] [CrossRef]
- Yi, M.; Feng, X.; Peng, W.; Teng, F.; Tang, Y.; Chen, Z. Aspirin for the prevention of hepatocellular carcinoma: An updated meta-analysis with particular focus on patients with chronic liver disease. Eur. J. Clin. Pharmacol. 2022, 78, 647–656. [Google Scholar] [CrossRef]
- Lapumnuaypol, K.; Tiu, A.; Thongprayoon, C.; Wijarnpreecha, K.; Ungprasert, P.; Mao, M.A.; Cheungpasitporn, W. Effects of aspirin and non-steroidal anti-inflammatory drugs on the risk of cholangiocarcinoma: A meta-analysis. QJM 2019, 112, 421–427. [Google Scholar] [CrossRef]
- Prasai, K.; Tella, S.H.; Yadav, S.; Kommalapati, A.; Mara, K.; Mady, M.; Hassan, M.A.; Wongjarupong, N.; Rodriguez-Payan, N.; Borad, M.; et al. Aspirin and Statin Use and the Risk of Gallbladder Cancer. Cancers 2021, 13, 1186. [Google Scholar] [CrossRef]
- Facciorusso, A.; Abd El Aziz, M.A.; Singh, S.; Pusceddu, S.; Milione, M.; Giacomelli, L.; Sacco, R. Statin Use Decreases the Incidence of Hepatocellular Carcinoma: An Updated Meta-Analysis. Cancers 2020, 12, 874. [Google Scholar] [CrossRef]
- Batyrbekova, N.; Aleman, S.; Lybeck, C.; Montgomery, S.; Duberg, A.S. Hepatitis C Virus Infection and the Temporal Trends in the Risk of Liver Cancer: A National Register-Based Cohort Study in Sweden. Cancer Epidemiol. Biomark. Prev. 2020, 29, 63–70. [Google Scholar] [CrossRef]



| HCC | Intrahepatic Bile Duct Carcer | GBC | Extrahepatic Bile Duct Cancer | Ampullary Cancer | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diagnosis of Comorbidities | O | HR | 95% CI | O | HR | 95% CI | O | HR | 95% CI | O | HR | 95% CI | O | HR | 95% CI | |||||
| Alcohol-related LD | 943 | 6.70 | 6.24 | 7.19 | 63 | 2.02 | 1.56 | 2.61 | 24 | 0.87 | 0.58 | 1.31 | 30 | 1.04 | 0.73 | 1.50 | 17 | 0.96 | 0.59 | 1.55 |
| COPD | 323 | 1.63 | 1.45 | 1.83 | 57 | 1.88 | 1.43 | 2.48 | 57 | 1.34 | 1.02 | 1.75 | 48 | 1.59 | 1.19 | 2.14 | 39 | 1.53 | 1.09 | 2.16 |
| Gallstone disease | 317 | 1.06 | 0.95 | 1.19 | 95 | 1.89 | 1.53 | 2.34 | 317 | 7.17 | 6.29 | 8.17 | 136 | 2.85 | 2.38 | 3.41 | 108 | 3.45 | 2.81 | 4.25 |
| Hepatitis B virus | 163 | 39.27 | 33.57 | 45.95 | 13 | 14.78 | 8.55 | 25.56 | 1 | 1.55 | 0.22 | 10.91 | 3 | 3.99 | 1.28 | 12.38 | 0 | |||
| Hepatitis C virus | 740 | 67.42 | 62.25 | 73.02 | 19 | 7.34 | 4.65 | 11.56 | 2 | 0.99 | 0.03 | 3.97 | 4 | 1.77 | 0.66 | 7.71 | 4 | 2.96 | 1.11 | 7.93 |
| Hepatitis of other kinds | 113 | 31.19 | 25.89 | 37.58 | 12 | 17.13 | 9.69 | 30.26 | 4 | 6.25 | 2.34 | 16.66 | 5 | 7.82 | 3.26 | 18.80 | 1 | 2.44 | 0.34 | 17.34 |
| Infection of bile ducts | 81 | 3.72 | 2.98 | 4.63 | 167 | 56.35 | 47.64 | 66.65 | 135 | 33.83 | 28.21 | 40.58 | 275 | 108.51 | 94.22 | 124.97 | 121 | 57.81 | 47.34 | 70.60 |
| Autoimmune hepatitis | 5 | 8.75 | 3.64 | 21.01 | 1 | 8.61 | 1.21 | 61.20 | 0 | 0 | 0 | |||||||||
| NAFLD | 60 | 17.63 | 13.67 | 22.74 | 4 | 6.00 | 2.25 | 16.02 | 3 | 5.17 | 1.66 | 16.05 | 1 | 1.64 | 0.23 | 11.66 | 1 | 2.64 | 0.37 | 18.73 |
| Obesity | 65 | 1.39 | 1.09 | 1.78 | 14 | 1.50 | 0.89 | 2.55 | 12 | 1.71 | 0.97 | 3.02 | 14 | 1.74 | 1.03 | 2.96 | 2 | 0.42 | 0.11 | 1.68 |
| Primary biliary cirrhosis | 17 | 33.42 | 20.46 | 54.60 | 0 | 0 | 0 | 0 | ||||||||||||
| Diabetes | 1357 | 5.39 | 5.05 | 5.74 | 180 | 4.42 | 3.74 | 5.23 | 122 | 1.86 | 1.53 | 2.26 | 163 | 3.80 | 3.19 | 4.53 | 115 | 3.47 | 2.82 | 4.28 |
| Other AID | 893 | 2.95 | 2.74 | 3.18 | 240 | 5.07 | 4.35 | 5.91 | 166 | 2.42 | 2.02 | 2.88 | 139 | 2.72 | 2.25 | 3.29 | 66 | 1.75 | 1.35 | 2.28 |
| All of above | 5077 | 6.25 | 5.91 | 6.61 | 865 | 4.03 | 3.57 | 4.55 | 843 | 4.37 | 3.86 | 4.94 | 818 | 5.47 | 4.80 | 6.24 | 474 | 4.47 | 3.81 | 5.24 |
| HCC | Intrahepatic Bile Duct Carcer | GBC | Extrahepatic Bile Duct Cancer | Ampullary Cancer | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diagnosis of Comorbidities | O | HR | 95% CI | O | HR | 95% CI | O | HR | 95% CI | O | HR | 95% CI | O | HR | 95% CI | |||||
| Alcohol-related LD | 137 | 5.65 | 4.74 | 6.73 | 15 | 1.05 | 0.63 | 1.75 | 19 | 0.65 | 0.41 | 1.02 | 7 | 0.62 | 0.30 | 1.31 | 4 | 0.66 | 0.25 | 1.77 |
| COPD | 190 | 2.06 | 1.77 | 2.40 | 63 | 1.67 | 1.28 | 2.18 | 128 | 0.99 | 0.83 | 1.19 | 54 | 1.52 | 1.14 | 2.02 | 35 | 1.64 | 1.15 | 2.34 |
| Gallstone disease | 195 | 1.16 | 1.00 | 1.34 | 146 | 1.89 | 1.59 | 2.25 | 1097 | 7.36 | 6.83 | 7.92 | 193 | 3.06 | 2.61 | 3.57 | 109 | 3.09 | 2.51 | 3.81 |
| Hepatitis B virus | 49 | 42.69 | 32.07 | 56.83 | 7 | 10.64 | 5.06 | 22.38 | 2 | 1.59 | 0.40 | 6.32 | 1 | 1.99 | 0.28 | 14.08 | 1 | 3.69 | 0.52 | 26.24 |
| Hepatitis C virus | 185 | 71.59 | 61.39 | 83.48 | 10 | 6.13 | 3.29 | 11.44 | 1 | 0.31 | 0.04 | 2.18 | 3 | 2.35 | 0.76 | 7.31 | 0 | |||
| Hepatitis of other kinds | 67 | 37.79 | 29.64 | 48.19 | 6 | 6.67 | 3.00 | 14.87 | 4 | 1.76 | 0.66 | 4.68 | 2 | 2.58 | 0.65 | 10.34 | 3 | 6.91 | 2.23 | 21.44 |
| Infection of bile ducts | 49 | 4.76 | 3.59 | 6.32 | 104 | 25.85 | 21.09 | 31.67 | 201 | 15.23 | 13.20 | 17.59 | 245 | 80.60 | 69.60 | 93.34 | 97 | 49.25 | 39.47 | 61.45 |
| Autoimmune hepatitis | 13 | 20.63 | 11.96 | 35.57 | 0 | 0 | 0 | 0 | ||||||||||||
| NAFLD | 39 | 31.53 | 22.98 | 43.27 | 5 | 7.75 | 3.22 | 18.64 | 2 | 1.30 | 0.33 | 5.19 | 0 | 1 | 3.30 | 0.46 | 23.47 | |||
| Obesity | 23 | 0.76 | 0.50 | 1.15 | 17 | 1.01 | 0.63 | 1.64 | 33 | 0.99 | 0.70 | 1.40 | 9 | 0.69 | 0.36 | 1.34 | 3 | 0.43 | 0.14 | 1.34 |
| Primary biliary cirrhosis | 67 | 54.08 | 41.83 | 69.92 | 4 | 8.42 | 3.15 | 22.45 | 3 | 1.29 | 0.32 | 5.18 | 2 | 4.48 | 1.12 | 17.93 | 1 | 3.82 | 0.54 | 27.17 |
| Diabetes | 362 | 3.77 | 3.35 | 4.24 | 96 | 2.43 | 1.94 | 3.04 | 276 | 2.43 | 1.94 | 3.04 | 109 | 2.93 | 2.38 | 3.62 | 61 | 2.88 | 2.19 | 3.78 |
| Other AID | 413 | 2.49 | 2.23 | 2.78 | 172 | 2.29 | 1.92 | 2.72 | 310 | 1.25 | 1.11 | 1.41 | 126 | 1.83 | 1.50 | 2.22 | 64 | 1.59 | 1.22 | 2.08 |
| All of above | 1789 | 3.62 | 3.34 | 3.93 | 645 | 2.08 | 1.85 | 2.34 | 2076 | 3.57 | 3.32 | 3.84 | 751 | 4.60 | 4.03 | 5.26 | 379 | 3.67 | 3.09 | 4.37 |
| HCC | Intrahepatic Bile Duct Cancer | GBC | Extrahepatic Bile Duct | Cancer Ampullary Cancer | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diagnosis of Comorbidities | PAF | 95% CI | PAF | 95% CI | PAF | 95% CI | PAF | 95% CI | PAF | 95% CI | |||||
| Alcohol-related LD | 12.93 | 11.51 | 14.35 | 2.81 | 0.46 | 5.16 | 0.00 | 0.11 | 0.00 | 2.37 | 0.00 | ||||
| COPD | 2.01 | 0.39 | 3.64 | 2.36 | 0.05 | 4.66 | 1.27 | 0.00 | 4.16 | 1.64 | 0.00 | 3.98 | 1.92 | 0.00 | 5.21 |
| Gallstone disease | 0.29 | 0.00 | 2.58 | 3.96 | 0.64 | 7.28 | 23.87 | 19.50 | 28.24 | 8.11 | 4.59 | 11.63 | 10.90 | 6.55 | 15.25 |
| Hepatitis B virus | 2.56 | 2.15 | 2.97 | 1.07 | 0.44 | 1.70 | 0.03 | 0.00 | 0.20 | 0.21 | 0.00 | 0.53 | |||
| Hepatitis C virus | 11.75 | 10.83 | 12.66 | 1.45 | 0.66 | 2.23 | 0.00 | 0.16 | 0.00 | 0.57 | 0.38 | 0.00 | 0.97 | ||
| Hepatitis of other kinds | 1.76 | 1.42 | 2.10 | 1.00 | 0.39 | 1.60 | 0.29 | 0.00 | 0.64 | 0.40 | 0.00 | 0.81 | 0.08 | 0.00 | 0.36 |
| Infection of bile ducts | 0.95 | 0.59 | 1.31 | 14.49 | 12.06 | 16.92 | 11.46 | 9.33 | 13.60 | 25.04 | 21.58 | 28.50 | 16.89 | 13.52 | 20.26 |
| Autoimmune hepatitis | 0.07 | 0.00 | 0.14 | 0.08 | 0.00 | 0.25 | |||||||||
| NAFLD | 0.91 | 0.66 | 1.16 | 0.29 | 0.00 | 0.65 | 0.21 | 0.00 | 0.51 | 0.04 | 0.00 | 0.22 | 0.09 | 0.00 | 0.37 |
| Obesity | 0.29 | 0.00 | 0.85 | 0.41 | 0.00 | 1.38 | 0.44 | 0.00 | 1.25 | 0.55 | 0.00 | 1.49 | 0.00 | ||
| Primary biliary cirrhosis | 0.27 | 0.14 | 0.40 | ||||||||||||
| Diabetes | 17.80 | 15.64 | 19.97 | 12.31 | 8.91 | 15.70 | 4.94 | 0.87 | 9.01 | 11.04 | 7.52 | 14.56 | 11.63 | 7.03 | 16.23 |
| Autoimmune diseases | 9.51 | 7.25 | 11.78 | 17.02 | 12.98 | 21.05 | 8.51 | 4.08 | 12.93 | 8.08 | 4.34 | 11.83 | 4.03 | 0.00 | 8.47 |
| All of above | 68.71 | 67.96 | 69.41 | 57.44 | 54.99 | 59.62 | 56.86 | 54.66 | 58.81 | 61.45 | 41.24 | 81.66 | 52.25 | 32.24 | 72.27 |
| HCC | Intrahepatic Bile Duct Cancer | GBC | Extrahepatic Bile Duct Cancer | Ampullary Cancer | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diagnosis of Comorbidities | PAF | 95% CI | PAF | 95% CI | PAF | 95% CI | PAF | 95% CI | PAF | 95% CI | |||||
| Alcohol-related LD | 4.57 | 3.45 | 5.69 | 0.06 | 0.00 | 1.32 | 0.00 | 0.00 | 0.00 | ||||||
| COPD | 3.96 | 1.62 | 6.30 | 2.19 | 0.00 | 4.84 | 0.00 | 1.80 | 0.00 | 4.65 | 2.40 | 0.00 | 5.99 | ||
| Gallstone disease | 1.07 | 0.00 | 4.81 | 5.97 | 1.43 | 10.50 | 30.57 | 26.90 | 34.24 | 12.64 | 7.88 | 17.41 | 12.94 | 7.07 | 18.81 |
| Hepatitis B virus | 1.94 | 1.38 | 2.50 | 0.55 | 0.10 | 1.00 | 0.02 | 0.00 | 0.12 | 0.05 | 0.00 | 0.24 | 0.13 | 0.00 | 0.47 |
| Hepatitis C virus | 7.39 | 6.27 | 8.52 | 0.73 | 0.17 | 1.28 | 0.00 | 0.17 | 0.00 | 0.52 | |||||
| Hepatitis of other kinds | 2.64 | 1.98 | 3.31 | 0.44 | 0.02 | 0.87 | 0.06 | 0.00 | 0.20 | 0.12 | 0.00 | 0.40 | 0.45 | 0.00 | 1.05 |
| Infection of bile ducts | 1.57 | 0.94 | 2.20 | 8.67 | 6.84 | 10.50 | 6.06 | 5.10 | 7.01 | 23.56 | 20.13 | 26.99 | 16.67 | 12.95 | 20.40 |
| Autoimmune hepatitis | 0.50 | 0.21 | 0.79 | ||||||||||||
| NAFLD | 1.53 | 1.03 | 2.03 | 0.38 | 0.00 | 0.76 | 0.01 | 0.00 | 0.11 | 0.12 | 0.00 | 0.47 | |||
| Obesity | 0.00 | 0.02 | 0.00 | 1.44 | 0.00 | 0.00 | 0.00 | ||||||||
| Primary biliary cirrhosis | 2.67 | 2.01 | 3.33 | 0.31 | 0.00 | 0.65 | 0.02 | 0.00 | 0.16 | 0.15 | 0.00 | 0.42 | 0.13 | 0.00 | 0.47 |
| Diabetes | 10.78 | 8.17 | 13.39 | 4.89 | 2.03 | 7.75 | 5.23 | 2.48 | 7.98 | 7.00 | 3.83 | 10.16 | 6.98 | 3.05 | 10.92 |
| Autoimmune diseases | 10.01 | 6.20 | 13.83 | 8.39 | 3.71 | 13.07 | 2.02 | 0.00 | 6.04 | 5.55 | 0.78 | 10.33 | 4.18 | 0.00 | 9.95 |
| All of above | 52.50 | 50.78 | 54.08 | 29.06 | 25.69 | 32.07 | 48.18 | 46.75 | 49.51 | 57.24 | 36.32 | 78.16 | 48.39 | 25.68 | 71.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hemminki, K.; Sundquist, K.; Sundquist, J.; Försti, A.; Liska, V.; Hemminki, A.; Li, X. Population-Attributable Fractions of Personal Comorbidities for Liver, Gallbladder, and Bile Duct Cancers. Cancers 2023, 15, 3092. https://doi.org/10.3390/cancers15123092
Hemminki K, Sundquist K, Sundquist J, Försti A, Liska V, Hemminki A, Li X. Population-Attributable Fractions of Personal Comorbidities for Liver, Gallbladder, and Bile Duct Cancers. Cancers. 2023; 15(12):3092. https://doi.org/10.3390/cancers15123092
Chicago/Turabian StyleHemminki, Kari, Kristina Sundquist, Jan Sundquist, Asta Försti, Vaclav Liska, Akseli Hemminki, and Xinjun Li. 2023. "Population-Attributable Fractions of Personal Comorbidities for Liver, Gallbladder, and Bile Duct Cancers" Cancers 15, no. 12: 3092. https://doi.org/10.3390/cancers15123092
APA StyleHemminki, K., Sundquist, K., Sundquist, J., Försti, A., Liska, V., Hemminki, A., & Li, X. (2023). Population-Attributable Fractions of Personal Comorbidities for Liver, Gallbladder, and Bile Duct Cancers. Cancers, 15(12), 3092. https://doi.org/10.3390/cancers15123092







