Simple Summary
We conducted a systematic review and meta-analysis to investigate the relationship between pre-operative vitamin D (VD) levels and time-to-outcome in stage III colorectal cancer (CRC) patients. Four articles were included in the analysis, with pooled data from 2628 patients for overall survival and 2024 patients for disease-free survival. The results showed that patients with lower levels of VD had a 38% and 13% increased risk of death and recurrence, respectively, according to random-effects models. These findings suggest that a low VD concentration negatively impacts the time-to-outcome in stage III CRC.
Abstract
Background: Vitamin D (VD) has been implicated in several diseases, including colorectal cancer (CRC). This study aimed to determine whether there is an association between VD levels and time-to-outcome in stage III CRC patients through a systematic review and meta-analysis. Methods: The study adhered to the PRISMA 2020 statement. Articles were searched in PubMed/MEDLINE and Scopus/ELSEVIER. Four articles were selected, with the primary objective of providing a pooled estimate of the risk of death specifically in stage III CRC patients based on pre-operative VD levels. Study heterogeneity and publication bias were analyzed using Tau2 statistics and funnel plots. Results: The selected studies showed significant heterogeneity regarding time-to-outcome, technical assessments, and serum VD concentration measures. The pooled analysis of 2628 and 2024 patients revealed a 38% and 13% increase in the risk of death (HR: 1.38, 95% CI: 0.71–2.71) and recurrence (HR: 1.13; 95% CI: 0.84–1.53), respectively, for random-effects models among patients with lower levels of VD. Conclusions: Our findings suggest that a low concentration of VD has a significant negative impact on time-to-outcome in stage III CRC.
1. Introduction
Colorectal cancer (CRC) is a neoplasm commonly diagnosed worldwide, ranking third in incidence (1,931,590 new cases per year) and second in mortality (935,180 deaths per year) []. Roughly 70% of CRC cases are diagnosed as non-metastatic at stages I–III []. Stage I disease typically requires clinical follow-up only, while the use of adjuvant chemotherapy for stage II colon cancer patients remains controversial. According to the current guidelines, chemotherapy should be reserved for high-risk patients with biologic features, such as T4, inadequate lymph node sampling, bowel obstruction, MSS, etc. [,]. Treatment decisions in these cases should consider various clinical, personal, and molecular factors, and be based on individualized risk assessments. However, the benefits of adjuvant chemotherapy for this patient population still remain uncertain. On the other hand, adjuvant chemotherapy should be administered to stage III colon cancer patients with loco-regional lymph nodes involved. This therapeutic approach, involving the use of fluoropyrimidines and oxaliplatin after radical surgical removal of the primary tumor, has been shown to be effective in achieving a cure, reducing the risk of death by 20–30% compared to placebo or observation [,,].
Despite this success, CRC can still progress locally and/or distantly, particularly to the liver, thereby reducing the chances of a cure and the patient’s prognosis. Therefore, it is crucial to identify patients within this group who would benefit most from different therapeutic approaches or monitoring strategies. Several innovative biomarkers have been associated with the risk of relapse and death after radical surgery, including cancer gene signatures, protein expression, and soluble molecules [,,]. For example, the concomitant overexpression of CXCR4 and VEGF has been strongly associated with early relapse in stage II–III CRC []. However, their use in clinical practice is limited by heterogeneous expression and technical issues, and none of these biomarkers have been adopted for routine use.
Among the soluble molecules, vitamin D3 (VD) is a fat-soluble substance that originates from 7-dehydrocholesterol and is subsequently activated by ultraviolet light []. After entering the bloodstream, VD is converted to 25-hydroxyvitamin D (25(OH)D), also known as calcidiol, by the enzyme 25-hydroxylase in the liver. This is the major circulating form of vitamin D and is commonly used to determine individual levels. Subsequently, 25(OH)D is transported to the kidneys, where it undergoes a second hydroxylation step by the enzyme 1α-hydroxylase to form 1,25(OH)2D, also known as calcitriol. Calcitriol binds to different cytosolic receptors and elicits its effects by translocating to the nucleus, essentially modulating gene expression []. This reprogramming leads to the regulation of calcium homeostasis. Despite its critical role in calcium absorption and bone resorption [], VD is also involved in other clinical entities, such as cancer and cardiovascular diseases [,].
Previous meta-analyses have reported a favorable impact of VD on cancer-specific survival in colorectal cancer (CRC). However, some studies exploring CRC susceptibility [] included patients who received external VD supplementation [,], or included patients with all stages of the disease without differentiating the prognostic effect of low vs. high VD levels in non-metastatic, stage III CRC patients [,].
In this systematic review and meta-analysis, we assessed whether VD levels are associated with time-to-outcome in stage III CRC patients, which is the typical setting of adjuvant systemic interventions. We provide a pooled and updated estimate of the risk of death and progression in stage III CRC patients according to VD levels.
2. Methods
This manuscript presents a systematic review and meta-analysis that investigates the association between the circulating level of VD (intended as 25(OH)D) at the time of diagnosis and survival outcomes in patients with stage III CRC. The study was conducted in accordance with the PRISMA statement 2020, and a structured protocol registered in PROSPERO (CRD42023401378) was established at the outset. The selection criteria and methods were explicitly defined in this protocol.
2.1. Selection Criteria
To identify the studies, three independent researchers searched two major international paper databases, PubMed/MEDLINE and Scopus/ELSEVIER, using the following keywords: “colorectal cancer” OR “colon” OR “rectal” AND “vitamin D” OR “cholecalciferol” OR “calcidiol”. Articles published between 2002 and 2022 were searched (accessed on 31 July 2022). The selection criteria for study enrollment were: 1. articles written in English to avoid language and publishing biases, 2. patients aged 18 years and older, 3. histologically diagnosed with stage III CRC, 4. VD dosed at the time of diagnosis, before surgery (post-operative cohorts were excluded from the analysis), and 5. an explicit indication of the hazard ratio (HR) (according to VD levels) for overall survival (including 95% confidence intervals, 95% CIs) specifically calculated for stage III CRC patients. The information about HR was sought anywhere in the selected articles. There were no limitations on VD supplementation, study design, or possible adjuvant therapy administered. Preclinical articles that focused on in vitro and/or animal experiments were excluded. Articles that evaluated VD and other biomarkers were included only if VD was independently studied. The complete flowchart for the selection process is presented in Figure 1.
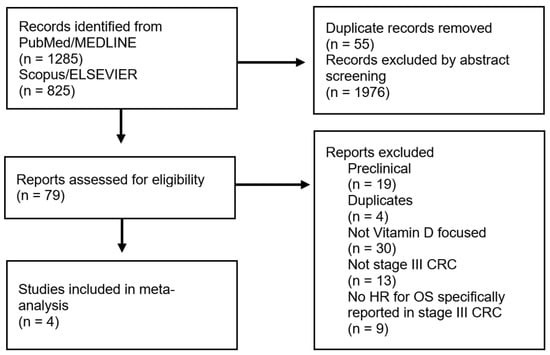
Figure 1.
PRISMA flowchart reporting the criteria for the study selection (left side) and exclusion (right side).
2.2. Data Extraction
For each study, we extracted the following information: the first author, year of publication, study design, clinical–pathological characteristics of patients, methodology for assessing VD levels, number of enrolled patients, follow-up time, time-to-outcome including HRs and 95% CIs. To minimize the risk of confounding effects, we extracted the maximally adjusted HR from each paper. Three investigators (A.O., S.F., M.B.) independently reviewed all data, and any criticisms or discrepancies were discussed with all authors.
2.3. Principal Endpoint
The primary objective of this meta-analysis was to estimate the pooled risk of death in patients with stage III CRC based on their circulating VD levels before surgery.
2.4. Quality Assessment
To ensure a formal assessment of the quality and risk of bias, four authors (A.O., S.F., M.S., and M.B.) were responsible for managing the rating of the methodologies and results of the selected studies. We used the Methodological Index for Non-Randomized Studies (MINORS) criteria [] and the Newcastle–Ottawa Scale [] for evaluation. The final scores were independently rated by D.I. and M.C. with blinding to previous results. Any discrepancies were resolved through consensus discussions involving all authors.
2.5. Statistical Methods
A meta-analysis was conducted to examine the correlation between VD levels and the risk of death in stage III CRC patients. Disease-free survival (DFS) was also examined as a secondary outcome. A random-effects model was utilized due to the heterogeneity observed among the studies, utilizing the Hartung–Knapp–Sidik–Jonkman approach []. Under this model, true effects are believed to differ between the studies, and the summary effect is the weighted average of the effects reported in different studies. This method produces a more cautious estimate of the pooled HR and is favored in the presence of heterogeneity. Results were presented using Forest plots, displaying HRs and 95% CIs, as well as a final pooled HR. An HR of 1.0 indicates that the event probability (EP) is the same in both high- and low-VD-level groups (EP VD low/EP VD high). However, if the HR is greater than 1.0, it implies that the low VD level group is at a higher risk of death or progression. In situations where the HR reports high VD levels in the numerator (VD high vs. low), the HRs and CIs were recalculated based on Altman et al. [] to ensure that the comparison trajectory is consistent between VD low vs. VD high (calculated HR VD low vs. high was 1/HR VD high vs. low), making it easier for readers to comprehend. The degree of variation among the studies (heterogeneity) was assessed using I2 and Tau2 statistics. I2 calculates the proportion of observed variation that is due to genuine differences rather than chance. I2 = 100% × (Q − DF)/Q, where Q represents Cochran’s heterogeneity statistic and DF represents the degrees of freedom. Negative I2 values are set to zero to ensure that I2 falls between 0% and 100%. A value of 0% suggests no observed heterogeneity, while larger values indicate increasing heterogeneity. In addition to I2, Tau2, which is a measure of the between-study variance that takes into account the size of the individual studies, was also used to assess the degree of heterogeneity among the studies in this meta-analysis. Unlike I2, which provides a relative measure of heterogeneity, Tau2 estimates the absolute magnitude of the between-study variance. The formula for Tau2 is Tau2 = (Q − DF)/[(k − 1) × sum of inverse variances], where k is the number of studies included in the meta-analysis. Tau2 is especially useful when the studies have varying sample sizes and effect estimates []. Potential publication bias was evaluated graphically using a funnel plot []. The statistical analyses were conducted using the MedCalc Statistical Software (MedCalc®® Statistical Software version 19.6, MedCalc Software Ltd., Ostend, Belgium) and R studio software version 4.1.1 (R studio Inc. Company, Boston, MA, USA).
3. Results
Four studies were included in this meta-analysis [,,,]. Figure 1 shows the selection flowchart.
The studies selected for analysis demonstrated substantial heterogeneity in terms of OS, as indicated by the Tau2 values (Tau2: 0.16; Cis: 0.012–3.2). Funnel plots for OS in two of the studies (the second cohort of Bao et al. [] and Zgaga et al. []) revealed clear asymmetries, suggesting a significant publication bias in these articles (Figure 2A). Conversely, no clear asymmetries were observed in the funnel plots for DFS (Figure 2B).
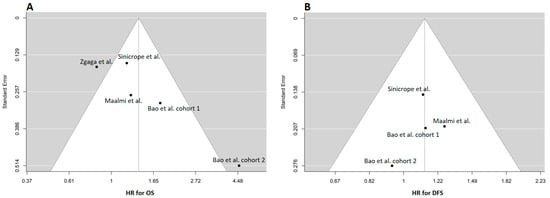
Figure 2.
Funnel plots of selected studies for overall survival (OS) (A) and disease-free survival (DFS) [,,,]. (B) The estimate of the outcome is reported in the x-axis, while the y-axis typically represents the standard error [,,]. The funnel plot in OS (A) shows two studies that are not symmetrically distributed (this asymmetry suggests the possibility of publication bias). In (B), which depicts PFS, there is no observed asymmetry in the funnel plot. The studies appear to be distributed symmetrically around the overall estimate, indicating that publication bias or small-study effects may not be present in this particular analysis.
Table 1 reports the technical and methodological characteristics of the studies. Three out of the four studies were retrospective analyses. All studies met the principal characteristic of presenting an independent HR analysis for stage III CRC patients. Technical assessments of serum VD level determination were heterogeneous. VD concentration was adjusted per season in two studies. Information about eventual VD supplementation was reported only in one study. Furthermore, VD concentration was expressed through different measurement units. Two articles expressed concentration in nmol/L, while two expressed it in ng/mL. Concentration cutoffs (low vs. high) were also heterogeneous. When expressed in nmol/L, the cutoff definition for the low level ranged from 45.2 to 75 nmol/L. A similar variability was observed in the ng/mL subgroup (range 13.25–30 ng/mL). The enrolled articles’ quality scores were equal to or greater than 5 for the MINORS and NOS scales, respectively.

Table 1.
Study characteristics and quality.
Table 2 reports the clinical–pathological characteristics of patients included in the selected articles. The median age ranged from 62.5 to 69 years and was similar across all papers. The cutoffs for age dichotomization into elderly and non-elderly patients were heterogeneous. The male gender was predominant, and when reported, T3 was the predominant diagnosed T-stage across the studies. The primary tumor side was not specified in two articles.

Table 2.
Clinical–pathological characteristics of entire patients cohorts included in the selected articles.
Table 3 presents the time-to-outcome estimates. The median follow-up was adequate in three studies (>36 months), but was not specified in one study. An adjuvant chemotherapy regimen was administered (as declared in the Patients and Methods sections of the articles), but not detailed in the studies (timing, regimens, toxicities, etc.).

Table 3.
Detailed risk of death and progression in the selected articles.
The effect size of the VD levels on prognosis was defined by the HR. Forest plots of the VD effects on OS and DFS are shown in Figure 3. The pooled analysis resulted in a 38% increase in the risk of death in 2628 patients for the random-effects models (HR: 1.38, 95% CI: 0.71–2.71). The pooled progression risk increase in 2024 patients was 13% (HR: 1.13; 95% CI: 0.84–1.53). Based on our findings, a low concentration of circulating VD before surgery has a negative effect on OS and DFS in individuals with stage III CRC.
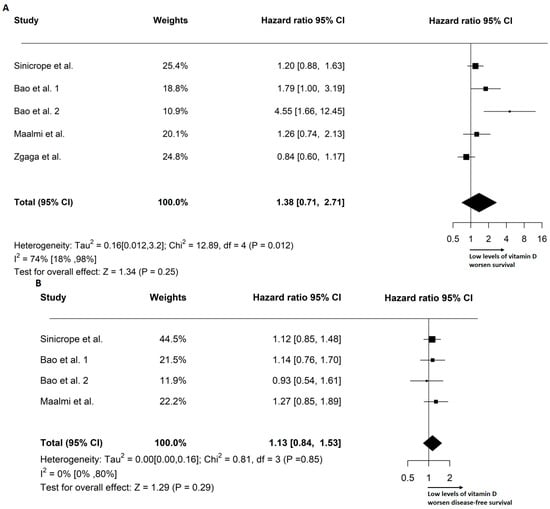
Figure 3.
Forest plots depicting the overall survival (A) and disease-free survival [,,,] (B) outcomes, stratified by pre-operative circulating levels of VD [,,]. The plots report Tau2, I2 statistics, and the pooled hazard ratio (HR) based on the random-effects model.
4. Discussion
Although there are some controversial aspects in stage II, adjuvant chemotherapy is currently considered the standard of care for patients with stage III colorectal cancer who have undergone radical surgery. The goal of adjuvant therapy is to eliminate residual cancer cells and achieve definitive cure. Several studies have suggested a positive association between VD levels and cancer outcomes, although results have not been differentiated for stages or the initial tumor burden []. In our study, we focused on the effect of VD circulating levels on the prognosis of colorectal cancer stage III patients by selecting studies [,,,] that reported this effect. Our aim was to investigate the correlation between VD and CRC in terms of influencing the OS and DFS in a clinical context where adjuvant chemotherapy is considered a benchmark, and to provide a more comprehensive and convincing evidence base than what is currently available in fragmented form.
Interestingly, VD supplementation is widely used to treat and prevent VD deficiency, which is prevalent worldwide []. The recommended daily intake of VD varies by age and health status, and can be obtained from dietary sources such as fortified foods or supplements []. Additionally, adequate sunlight exposure can contribute to VD synthesis in the skin. VD supplements are available in different forms, including cholecalciferol (vitamin D3) and ergocalciferol (vitamin D2), and can be easily administered orally []. VD is a fat-soluble vitamin that plays a crucial role in maintaining bone health and calcium homeostasis. Its role in other physiological functions, such as immune response and cellular proliferation, has also been extensively studied []. However, only one study included in our meta-analysis provided a description of VD supplementation, mentioning the dietary intake and supplements at different dosage ranges (<5, 5–10, >10 μg/day) []. Nevertheless, it was unclear whether the supplementation was regulated or controlled, and the reasons for supplementation were not reported. This information was obtained through the administration of “frequency questionnaires” to patients. Moreover, it was not specified whether the VD intake was self-managed by the patients or prescribed by a doctor, either through food or supplements. These details could shed light on the methodological aspects of the selected articles. Regrettably, controlled and prospective studies assessing the potential benefits of VD supplements (type, dosage, timing, etc.) on the time-to-outcome, achievable with adjuvant therapies for stage III CRC, are entirely lacking. Therefore, a direct piece of evidence indicating the importance of maintaining high levels of VD to prevent cancer recurrence in CRC patients is also absent.
Recently, a growing body of literature has focused on the association between VD and cancer []. Its active form, 1,25-dihydroxyvitamin D, binds to the VD receptor (VDR) in various tissues, including the bone, intestine, and immune cells. VDR activation regulates calcium and phosphate metabolism, which is essential for skeletal health. Additionally, it influences cell differentiation and proliferation, and has anti-inflammatory and anti-carcinogenic properties []. In recent years, research has suggested that VD has a protective effect against several types of cancer. Mechanistically, VD exerts an anti-proliferative effect on cancer cells by inducing cell cycle arrest and apoptosis, and reducing angiogenesis and metastasis []. Moreover, VD modulates the immune response, leading to the activation of natural killer cells and the inhibition of pro-inflammatory cytokines [], which can have positive impacts on chemotherapy efficacy and toxicity [,].
Furthermore, VD has garnered considerable attention for its potential role in potentiating and/or synergizing the efficacy of chemotherapy in the context of CRC treatment. Both in vivo and in vitro studies have provided insights into the anti-tumor effects observed when combining VD with anti-cancer treatments. In vitro investigations have demonstrated that VD can enhance the cytotoxic effects of chemotherapy agents, leading to increased cancer cell death []. It has been suggested that it can modulate molecular pathways involved in apoptosis, cell cycle regulation, thereby sensitizing cancer cells to chemotherapy-induced cytotoxicity. In vivo studies using animal models of CRC have provided additional evidence supporting the potential benefits of combining VD with chemotherapy. These studies have shown that VD supplementation can enhance the anti-tumor activity of chemotherapeutic agents, leading to reduced tumor growth, improved survival rates, and decreased metastasis [,]. While the exact mechanisms underlying the synergistic effects of VD and chemotherapy remain to be fully elucidated, the accumulating evidence suggests a promising therapeutic strategy for CRC treatment [,]. However, future clinical trials are needed to validate these findings and determine the optimal dosing, timing, and patient selection for this combined approach.
Our meta-analysis reveals an increased risk of death and progression in stage III patients with low circulating levels of VD before surgery, suggesting potential beneficial effects of VD supplementation in this clinical setting. Very interestingly, a recent study suggests that VD can prompt and support immune responses in the early stages of CRC, by modulating T-regulatory-cells (Treg) function [].
Some limitations of our study need to be highlighted and discussed. First of all, despite our meta-analysis being methodologically sound and rigorous, it is based on a relatively small number of studies, specifically four. This is due to the limited number of studies that have specifically evaluated the prognostic effect of VD in stage III CRC patients. It is worth noting, however, that this represents an exceptionally interesting clinical setting as it pertains to the treatments (adjuvant chemotherapy) associated with patient recovery. In this regard, we hope that the scientific community will generate further prospective findings regarding the role of VD levels in this clinical scenario. Although the included studies had large sample sizes, long follow-up times, and good quality scores according to the MINORS and NOS scales, they demonstrated heterogeneity in methodological issues, such as VD assessment methods and cutoffs used to distinguish between low and high VD levels. Additionally, all but one study were retrospective. Lastly, the selected studies reported that stage III patients received adjuvant chemotherapy based on standard guidelines, but they did not specify the number of patients who were actually treated or the type of adjuvant therapies administered. Together, these factors may introduce unknown biases that could negatively impact the reliability of pooled data, and they should be taken into account when interpreting our results.
5. Conclusions
It has been well-established that VD possesses anti-carcinogenic properties and modulates the immune response, which can enhance the effectiveness of chemotherapy. Based on the results of our meta-analysis, future studies should investigate whether VD levels can serve as an additional prognostic stratification factor, and if supplementation in this clinical setting could be an innovative approach to improving clinical outcomes.
Author Contributions
Conceptualization: A.O., S.F. and M.B.; Methodology A.O., S.F., D.I. and M.B.; Validation: all authors; Resources, data curation and formal analysis: A.O., S.F., L.R., V.Q. and M.B.; Original draft preparation and writing: A.O., S.F. and M.B.; Review and Editing: all authors; Supervision: all authors. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The R script for conducting the meta-analysis is available at https://zenodo.org/record/7907127#.ZFjEFHZBxD8 last accessed on 19 April 2023.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Goodarzi, E.; Beiranvand, R.; Naemi, H.; Momenabadi, V.; Khazaei, Z. Worldwide incidence and mortality of colorectal cancer and human development index (HDI): An ecological study. World Cancer Res. J. 2019, 6, e1433. [Google Scholar] [CrossRef]
- Chen, V.W.; Hsieh, M.C.; Charlton, M.E.; Ruiz, B.A.; Karlitz, J.; Altekruse, S.F.; Ries, L.A.; Jessup, J.M. Analysis of stage and clinical/prognostic factors for colon and rectal cancer from SEER registries: AJCC and collaborative stage data collection system. Cancer 2014, 120, 3793–3806. [Google Scholar] [CrossRef] [PubMed]
- Taieb, J.; Karoui, M.; Basile, D. How I treat stage II colon cancer patients. ESMO Open 2021, 6, 100184. [Google Scholar] [CrossRef]
- Hu, C.; Shi, F.; Zhang, Z.; Zhang, L.; Liu, R.; Sun, X.; Zheng, L.; She, J. Development and validation of a new stage-specific nomogram model for predicting cancer-specific survival in patients in different stages of colon cancer: A SEER population-based study and external validation. Front. Oncol. 2022, 12, 1024467. [Google Scholar] [CrossRef]
- Oneda, E.; Zaniboni, A. Adjuvant treatment of colon cancer with microsatellite instability—The state of the art. Crit. Rev. Oncol. Hematol. 2022, 169, 103537. [Google Scholar] [CrossRef]
- Taieb, J.; André, T.; Auclin, E. Refining adjuvant therapy for non-metastatic colon cancer, new standards and perspectives. Cancer Treat. Rev. 2019, 75, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bender, U.; Rho, Y.S.; Barrera, I.; Aghajanyan, S.; Acoba, J.; Kavan, P. Adjuvant therapy for stages II and III colon cancer: Risk stratification, treatment duration, and future directions. Curr. Oncol. 2019, 26, S43–S52. [Google Scholar] [CrossRef]
- Luo, X.J.; Zhao, Q.; Liu, J.; Zheng, J.B.; Qiu, M.Z.; Ju, H.Q.; Xu, R.H. Novel Genetic and Epigenetic Biomarkers of Prognostic and Predictive Significance in Stage II/III Colorectal Cancer. Mol. Ther. 2021, 29, 587–596. [Google Scholar] [CrossRef]
- Parent, P.; Cohen, R.; Rassy, E.; Svrcek, M.; Taieb, J.; André, T.; Turpin, A. A comprehensive overview of promising biomarkers in stage II colorectal cancer. Cancer Treat. Rev. 2020, 88, 102059. [Google Scholar] [CrossRef]
- Stanisavljević, L.; Myklebust, M.P.; Leh, S.; Dahl, O. LGR5 and CD133 as prognostic and predictive markers for fluoropyrimidine-based adjuvant chemotherapy in colorectal cancer. Acta Oncol. 2016, 55, 1425–1433. [Google Scholar] [CrossRef]
- Ottaiano, A.; Franco, R.; Aiello Talamanca, A.; Liguori, G.; Tatangelo, F.; Delrio, P.; Nasti, G.; Barletta, E.; Facchini, G.; Daniele, B.; et al. Overexpression of both CXC chemokine receptor 4 and vascular endothelial growth factor proteins predicts early distant relapse in stage II-III colorectal cancer patients. Clin. Cancer Res. 2006, 12, 2795–2803. [Google Scholar] [CrossRef] [PubMed]
- Bollen, S.E.; Bass, J.J.; Fujita, S.; Wilkinson, D.; Hewison, M.; Atherton, P.J. The Vitamin D/Vitamin D receptor (VDR) axis in muscle atrophy and sarcopenia. Cell Signal. 2022, 96, 110355. [Google Scholar] [CrossRef] [PubMed]
- Khayami, R.; Goltzman, D.; Rabbani, S.A.; Kerachian, M.A. Epigenomic effects of vitamin D in colorectal cancer. Epigenomics 2022, 14, 1213–1228. [Google Scholar] [CrossRef] [PubMed]
- van Driel, M.; van Leeuwen, J.P.T.M. Vitamin D and Bone: A Story of Endocrine and Auto/Paracrine Action in Osteoblasts. Nutrients 2023, 15, 480. [Google Scholar] [CrossRef] [PubMed]
- Lawler, T.; Warren Andersen, S. Serum 25-Hydroxyvitamin D and Cancer Risk: A Systematic Review of Mendelian Randomization Studies. Nutrients 2023, 15, 422. [Google Scholar] [CrossRef]
- Berretta, M.; Quagliariello, V.; Bignucolo, A.; Facchini, S.; Maurea, N.; Di Francia, R.; Fiorica, F.; Sharifi, S.; Bressan, S.; Richter, S.N.; et al. The Multiple Effects of Vitamin D against Chronic Diseases: From Reduction of Lipid Peroxidation to Updated Evidence from Clinical Studies. Antioxidants 2022, 11, 1090. [Google Scholar] [CrossRef]
- Zhang, L.; Zou, H.; Zhao, Y.; Hu, C.; Atanda, A.; Qin, X.; Jia, P.; Jiang, Y.; Qi, Z. Association between blood circulating vitamin D and colorectal cancer risk in Asian countries: A systematic review and dose-response meta-analysis. BMJ Open 2019, 9, e030513. [Google Scholar] [CrossRef]
- Emmanouilidou, G.; Kalopitas, G.; Bakaloudi, D.R.; Karanika, E.; Theocharidou, E.; Germanidis, G.; Chourdakis, M. Vitamin D as a chemopreventive agent in colorectal neoplasms. A systematic review and meta-analysis of randomized controlled trials. Pharmacol. Ther. 2022, 237, 108252. [Google Scholar] [CrossRef]
- Lopez-Caleya, J.F.; Ortega-Valín, L.; Fernández-Villa, T.; Delgado-Rodríguez, M.; Martín-Sánchez, V.; Molina, A.J. The role of calcium and vitamin D dietary intake on risk of colorectal cancer: Systematic review and meta-analysis of case-control studies. Cancer Causes Control 2022, 33, 167–182. [Google Scholar] [CrossRef]
- Maalmi, H.; Walter, V.; Jansen, L.; Boakye, D.; Schöttker, B.; Hoffmeister, M.; Brenner, H. Association between Blood 25-Hydroxyvitamin D Levels and Survival in Colorectal Cancer Patients: An Updated Systematic Review and Meta-Analysis. Nutrients 2018, 10, 896. [Google Scholar] [CrossRef]
- Xu, J.; Yuan, X.; Tao, J.; Yu, N.; Wu, R.; Zhang, Y. Association of Circulating 25-Hydroxyvitamin D Levels with Colorectal Cancer: An Updated Meta-Analysis. J. Nutr. Sci. Vitaminol. 2018, 64, 432–444. [Google Scholar] [CrossRef] [PubMed]
- Slim, K.; Nini, E.; Forestier, D.; Kwiatkowski, F.; Panis, Y.; Chipponi, J. Methodological index for non-randomized studies (MINORS): Development and validation of a new instrument. ANZ J. Surg. 2003, 73, 712–716. [Google Scholar] [CrossRef] [PubMed]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef]
- IntHout, J.; Ioannidis, J.P.; Borm, G.F. The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC Med. Res. Methodol. 2014, 14, 25. [Google Scholar] [CrossRef]
- Altman, D.G.; Machin, D.; Bryant, T.N.; Gardner, M.J. (Eds.) Statistics with Confidence, 2nd ed.; BMJ USA: London, UK, 2000. [Google Scholar]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. Br. Med. J. 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. Br. Med. J. 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Sinicrope, F.A.; Shi, Q.; Smyrk, T.C.; Goldberg, R.M.; Cohen, S.J.; Gill, S.; Kahlenberg, M.S.; Nair, S.; Shield, A.F.; Jahagirdar, B.N.; et al. Association of Adiponectin and Vitamin D With Tumor Infiltrating Lymphocytes and Survival in Stage III Colon Cancer. JNCI Cancer Spectr. 2021, 5, pkab070. [Google Scholar] [CrossRef]
- Bao, Y.; Li, Y.; Gong, Y.; Huang, Q.; Cai, S.; Peng, J. Vitamin D Status and Survival in Stage II-III Colorectal Cancer. Front. Oncol. 2020, 10, 581597. [Google Scholar] [CrossRef]
- Maalmi, H.; Walter, V.; Jansen, L.; Chang-Claude, J.; Owen, R.W.; Ulrich, A.; Schöttker, B.; Hoffmeister, M.; Brenner, H. Relationship of very low serum 25-hydroxyvitamin D3 levels with long-term survival in a large cohort of colorectal cancer patients from Germany. Eur. J. Epidemiol. 2017, 32, 961–971. [Google Scholar] [CrossRef]
- Zgaga, L.; Theodoratou, E.; Farrington, S.M.; Din, F.V.; Ooi, L.Y.; Glodzik, D.; Johnston, S.; Tenesa, A.; Campbell, H.; Dunlop, M.G. Plasma vitamin D concentration influences survival outcome after a diagnosis of colorectal cancer. J. Clin. Oncol. 2014, 32, 2430–2439. [Google Scholar] [CrossRef]
- Vaughan-Shaw, P.G.; O’Sullivan, F.; Farrington, S.M.; Theodoratou, E.; Campbell, H.; Dunlop, M.G.; Zgaga, L. The impact of vitamin D pathway genetic variation and circulating 25-hydroxyvitamin D on cancer outcome: Systematic review and meta-analysis. Br. J. Cancer. 2017, 116, 1092–1110. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Sánchez, L.; Clark, P.; Winzenberg, T.M.; Tugwell, P.; Correa-Burrows, P.; Costello, R. Calcium and vitamin D for increasing bone mineral density in premenopausal women. Cochrane Database Syst. Rev. 2023, 1, CD012664. [Google Scholar] [CrossRef]
- Bertoldo, F.; Cianferotti, L.; Di Monaco, M.; Falchetti, A.; Fassio, A.; Gatti, D.; Gennari, L.; Giannini, S.; Girasole, G.; Gonnelli, S.; et al. Definition, Assessment, and Management of Vitamin D Inadequacy: Suggestions, Recommendations, and Warnings from the Italian Society for Osteoporosis, Mineral Metabolism and Bone Diseases (SIOMMMS). Nutrients 2022, 14, 4148. [Google Scholar] [CrossRef] [PubMed]
- Stanescu, A.M.A.; Simionescu, A.A.; Diaconu, C.C. Oral Vitamin D Therapy in Patients with Psoriasis. Nutrients 2021, 13, 163. [Google Scholar] [CrossRef] [PubMed]
- Charoenngam, N.; Holick, M.F. Immunologic Effects of Vitamin D on Human Health and Disease. Nutrients 2020, 12, 2097. [Google Scholar] [CrossRef]
- Marigoudar, J.B.; Sarkar, D.; Yuguda, Y.M.; Abutayeh, R.F.; Kaur, A.; Pati, A.; Mitra, D.; Ghosh, A.; Banerjee, D.; Borah, S.; et al. Role of vitamin D in targeting cancer and cancer stem cell populations and its therapeutic implications. Med. Oncol. 2022, 40, 2. [Google Scholar] [CrossRef]
- Carlberg, C.; Muñoz, A. An update on vitamin D signaling and cancer. Semin. Cancer Biol. 2022, 79, 217–230. [Google Scholar] [CrossRef]
- Negri, M.; Gentile, A.; de Angelis, C.; Montò, T.; Patalano, R.; Colao, A.; Pivonello, R.; Pivonello, C. Vitamin D-Induced Molecular Mechanisms to Potentiate Cancer Therapy and to Reverse Drug-Resistance in Cancer Cells. Nutrients 2020, 12, 1798. [Google Scholar] [CrossRef]
- Skrobot, A.; Demkow, U.; Wachowska, M. Immunomodulatory Role of Vitamin D: A Review. Adv. Exp. Med. Biol. 2018, 1108, 13–23. [Google Scholar] [CrossRef]
- Fink, M. Vitamin D deficiency is a cofactor of chemotherapy-induced mucocutaneous toxicity and dysgeusia. J. Clin. Oncol. 2011, 29, e81–e82. [Google Scholar] [CrossRef]
- Kitchen, D.; Hughes, B.; Gill, I.; O’Brien, M.; Rumbles, S.; Ellis, P.; Harper, P.; Stebbing, J.; Rohatgi, N. The relationship between vitamin D and chemotherapy-induced toxicity—A pilot study. Br. J. Cancer. 2012, 107, 158–160. [Google Scholar] [CrossRef] [PubMed]
- Milczarek, M.; Filip-Psurska, B.; Swiętnicki, W.; Kutner, A.; Wietrzyk, J. Vitamin D analogs combined with 5-fluorouracil in human HT-29 colon cancer treatment. Oncol. Rep. 2014, 32, 491–504. [Google Scholar] [CrossRef] [PubMed]
- Idris, S.; Refaat, B.; Almaimani, R.A.; Ahmed, H.G.; Ahmad, J.; Alhadrami, M.; El-Readi, M.Z.; Elzubier, M.E.; Alaufi, H.A.A.; Al-Amin, B.; et al. Enhanced in vitro tumoricidal effects of 5-Fluorouracil, thymoquinone, and active vitamin D3 triple therapy against colon cancer cells by attenuating the PI3K/AKT/mTOR pathway. Life Sci. 2022, 296, 120442. [Google Scholar] [CrossRef] [PubMed]
- Vaughan-Shaw, P.G.; Blackmur, J.P.; Grimes, G.; Ooi, L.Y.; Ochocka-Fox, A.M.; Dunbar, K.; von Kriegsheim, A.; Rajasekaran, V.; Timofeeva, M.; Walker, M.; et al. Vitamin D treatment induces in vitro and ex vivo transcriptomic changes indicating anti-tumor effects. FASEB J. 2022, 36, e22082. [Google Scholar] [CrossRef]
- Bintintan, V.V. Vitamin D as a Potential Therapeutic Target and Prognostic Marker for Colorectal Cancer. EBioMedicine 2018, 31, 11–12. [Google Scholar] [CrossRef]
- Duffy, M.J.; Murray, A.; Synnott, N.C.; O’Donovan, N.; Crown, J. Vitamin D analogues: Potential use in cancer treatment. Crit. Rev. Oncol. Hematol. 2017, 112, 190–197. [Google Scholar] [CrossRef]
- Srichomchey, P.; Sukprasert, S.; Khulasittijinda, N.; Voravud, N.; Sahakitrungruang, C.; Lumjiaktase, P. Vitamin D3 Supplementation Promotes Regulatory T-Cells to Maintain Immune Homeostasis After Surgery for Early Stages of Colorectal Cancer. In Vivo 2023, 37, 286–293. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).