Combination of EGFR-Directed Tyrosine Kinase Inhibitors (EGFR-TKI) with Radiotherapy in Brain Metastases from Non-Small Cell Lung Cancer: A 2010–2019 Retrospective Cohort Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Study Design, Patient Population, and Selection
2.2. EGFR-TKI Data
2.3. SRS Data
2.4. WBRT Data
2.5. SRS + WBRT Data
2.6. Data Analysis
3. Results
3.1. Patient Characteristics
3.2. Single Therapies
3.3. Combination Therapies
4. Discussion
4.1. Relevance to Literature and Clinical Practice
4.2. Limitations
4.3. Potential Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gelatti, A.C.Z.; Drilon, A.; Santini, F.C. Optimizing the sequencing of tyrosine kinase inhibitors (TKIs) in epidermal growth factor receptor (EGFR) mutation-positive non-small cell lung cancer (NSCLC). Lung Cancer 2019, 137, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.E.; Nam, E.M.; Lee, H.J.; Nam, S.H.; Kim, D.Y.; Im, S.A.; Seong, C.M.; Lee, S.N.; Lee, K.J. Clinical Features and Prognosis of Lung Cancer with Brain Metastasis. Cancer Res. Treat. 2001, 33, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Ernani, V.; Stinchcombe, T.E. Management of Brain Metastases in Non-Small-Cell Lung Cancer. J. Oncol. Pract. 2019, 15, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Saad, A.G.; Yeap, B.Y.; Thunnissen, F.B.; Pinkus, G.S.; Pinkus, J.L.; Loda, M.; Sugarbaker, D.J.; Johnson, B.E.; Chirieac, L.R. Immunohistochemical markers associated with brain metastases in patients with nonsmall cell lung carcinoma. Cancer 2008, 113, 2129–2138. [Google Scholar] [CrossRef] [PubMed]
- Rangachari, D.; Yamaguchi, N.; VanderLaan, P.A.; Folch, E.; Mahadevan, A.; Floyd, S.R.; Uhlmann, E.J.; Wong, E.T.; Dahlberg, S.E.; Huberman, M.S.; et al. Brain metastases in patients with EGFR-mutated or ALK-rearranged non-small-cell lung cancers. Lung Cancer 2015, 88, 108–111. [Google Scholar] [CrossRef]
- Aiko, N.; Shimokawa, T.; Miyazaki, K.; Misumi, Y.; Agemi, Y.; Ishii, M.; Nakamura, Y.; Yamanaka, T.; Okamoto, H. Comparison of the efficacies of first-generation epidermal growth factor receptor tyrosine kinase inhibitors for brain metastasis in patients with advanced non-small-cell lung cancer harboring EGFR mutations. BMC Cancer 2018, 18, 1012. [Google Scholar] [CrossRef]
- Rosell, R.; Carcereny, E.; Gervais, R.; Vergnenegre, A.; Massuti, B.; Felip, E.; Palmero, R.; Garcia-Gomez, R.; Pallares, C.; Sanchez, J.M.; et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012, 13, 239–246. [Google Scholar] [CrossRef]
- Mitsudomi, T.; Morita, S.; Yatabe, Y.; Negoro, S.; Okamoto, I.; Tsurutani, J.; Seto, T.; Satouchi, M.; Tada, H.; Hirashima, T.; et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): An open label, randomised phase 3 trial. Lancet Oncol. 2010, 11, 121–128. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, M.; Zhong, W.; Zhang, L.; Li, L.; Xiao, Y.; Nie, L.; Hu, P.; Wang, M. Cerebrospinal fluid concentrations of gefitinib in patients with lung adenocarcinoma. Clin. Lung Cancer 2013, 14, 188–193. [Google Scholar] [CrossRef]
- Remon, J.; Steuer, C.E.; Ramalingam, S.S.; Felip, E. Osimertinib and other third-generation EGFR TKI in EGFR-mutant NSCLC patients. Ann. Oncol. 2018, 29, i20–i27. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Lee, S.H.; Ahn, J.S.; Ahn, M.J.; Park, K.; Sun, J.M. Efficacy and Safety of Afatinib for EGFR-mutant Non-small Cell Lung Cancer, Compared with Gefitinib or Erlotinib. Cancer Res. Treat. 2019, 51, 502–509. [Google Scholar] [CrossRef]
- Ballard, P.; Yates, J.W.; Yang, Z.; Kim, D.W.; Yang, J.C.; Cantarini, M.; Pickup, K.; Jordan, A.; Hickey, M.; Grist, M.; et al. Preclinical Comparison of Osimertinib with Other EGFR-TKIs in EGFR-Mutant NSCLC Brain Metastases Models, and Early Evidence of Clinical Brain Metastases Activity. Clin. Cancer Res. 2016, 22, 5130–5140. [Google Scholar] [CrossRef]
- Soria, J.C.; Ohe, Y.; Vansteenkiste, J.; Reungwetwattana, T.; Chewaskulyong, B.; Lee, K.H.; Dechaphunkul, A.; Imamura, F.; Nogami, N.; Kurata, T.; et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 113–125. [Google Scholar] [CrossRef]
- Mok, T.S.; Wu, Y.L.; Ahn, M.J.; Garassino, M.C.; Kim, H.R.; Ramalingam, S.S.; Shepherd, F.A.; He, Y.; Akamatsu, H.; Theelen, W.S.; et al. Osimertinib or Platinum-Pemetrexed in EGFR T790M-Positive Lung Cancer. N. Engl. J. Med. 2017, 376, 629–640. [Google Scholar] [CrossRef]
- Fan, Y.; Xu, X.; Xie, C. EGFR-TKI therapy for patients with brain metastases from non-small-cell lung cancer: A pooled analysis of published data. Onco Targets Ther. 2014, 7, 2075–2084. [Google Scholar] [CrossRef] [PubMed]
- Welsh, J.W.; Komaki, R.; Amini, A.; Munsell, M.F.; Unger, W.; Allen, P.K.; Chang, J.Y.; Wefel, J.S.; McGovern, S.L.; Garland, L.L.; et al. Phase II trial of erlotinib plus concurrent whole-brain radiation therapy for patients with brain metastases from non-small-cell lung cancer. J. Clin. Oncol. 2013, 31, 895–902. [Google Scholar] [CrossRef]
- Gerber, N.K.; Yamada, Y.; Rimner, A.; Shi, W.; Riely, G.J.; Beal, K.; Yu, H.A.; Chan, T.A.; Zhang, Z.; Wu, A.J. Erlotinib versus radiation therapy for brain metastases in patients with EGFR-mutant lung adenocarcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2014, 89, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Niranjan, A.; Monaco, E.; Flickinger, J.; Lunsford, L.D. Guidelines for Multiple Brain Metastases Radiosurgery. Prog. Neurol. Surg. 2019, 34, 100–109. [Google Scholar] [CrossRef]
- Achrol, A.S.; Rennert, R.C.; Anders, C.; Soffietti, R.; Ahluwalia, M.S.; Nayak, L.; Peters, S.; Arvold, N.D.; Harsh, G.R.; Steeg, P.S.; et al. Brain metastases. Nat. Rev. Dis. Prim. 2019, 5, 5. [Google Scholar] [CrossRef]
- Specht, H.M.; Combs, S.E. Stereotactic radiosurgery of brain metastases. J. Neurosurg. Sci. 2016, 60, 357–366. [Google Scholar] [PubMed]
- Dong, K.; Liang, W.; Zhao, S.; Guo, M.; He, Q.; Li, C.; Song, H.; He, J.; Xia, X. EGFR-TKI plus brain radiotherapy versus EGFR-TKI alone in the management of EGFR-mutated NSCLC patients with brain metastases. Transl. Lung Cancer Res. 2019, 8, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wei, J.; Cai, J.; Liu, A. Combination therapy of brain radiotherapy and EGFR-TKIs is more effective than TKIs alone for EGFR-mutant lung adenocarcinoma patients with asymptomatic brain metastasis. BMC Cancer 2019, 19, 793. [Google Scholar] [CrossRef] [PubMed]
- Magnuson, W.J.; Lester-Coll, N.H.; Wu, A.J.; Yang, T.J.; Lockney, N.A.; Gerber, N.K.; Beal, K.; Amini, A.; Patil, T.; Kavanagh, B.D.; et al. Management of Brain Metastases in Tyrosine Kinase Inhibitor-Naïve Epidermal Growth Factor Receptor-Mutant Non-Small-Cell Lung Cancer: A Retrospective Multi-Institutional Analysis. J. Clin. Oncol. 2017, 35, 1070–1077. [Google Scholar] [CrossRef] [PubMed]
- Sperduto, P.W.; Yang, T.J.; Beal, K.; Pan, H.; Brown, P.D.; Bangdiwala, A.; Shanley, R.; Yeh, N.; Gaspar, L.E.; Braunstein, S.; et al. Estimating Survival in Patients With Lung Cancer and Brain Metastases: An Update of the Graded Prognostic Assessment for Lung Cancer Using Molecular Markers (Lung-molGPA). JAMA Oncol. 2017, 3, 827–831. [Google Scholar] [CrossRef] [PubMed]
- Jia, F.; Cheng, X.; Zeng, H.; Miao, J.; Hou, M. Clinical research on stereotactic radiosurgery combined with epithermal growth factor tyrosine kinase inhibitors in the treatment of brain metastasis of non-small cell lung cancer. J. Buon 2019, 24, 578–584. [Google Scholar]
- Cheng, W.C.; Shen, Y.C.; Chien, C.R.; Liao, W.C.; Chen, C.H.; Hsia, T.C.; Tu, C.Y.; Chen, H.J. The optimal therapy strategy for epidermal growth factor receptor-mutated non-small cell lung cancer patients with brain metastasis: A real-world study from Taiwan. Thorac. Cancer 2022, 13, 1505–1512. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, Y.; Li, R.; Yisikandaer, A.; Ren, B.; Sun, J.; Li, J.; Chen, L.; Zhao, R.; Zhang, J.; et al. Whole-brain radiotherapy with and without concurrent erlotinib in NSCLC with brain metastases: A multicenter, open-label, randomized, controlled phase III trial. Neuro-Oncology 2021, 23, 967–978. [Google Scholar] [CrossRef]
- Zhai, X.; Li, W.; Li, J.; Jia, W.; Jing, W.; Tian, Y.; Xu, S.; Li, Y.; Zhu, H.; Yu, J. Therapeutic effect of osimertinib plus cranial radiotherapy compared to osimertinib alone in NSCLC patients with EGFR-activating mutations and brain metastases: A retrospective study. Radiat. Oncol. 2021, 16, 233. [Google Scholar] [CrossRef]
- He, Z.Y.; Li, M.F.; Lin, J.H.; Lin, D.; Lin, R.J. Comparing the efficacy of concurrent EGFR-TKI and whole-brain radiotherapy vs EGFR-TKI alone as a first-line therapy for advanced EGFR-mutated non-small-cell lung cancer with brain metastases: A retrospective cohort study. Cancer Manag. Res. 2019, 11, 2129–2138. [Google Scholar] [CrossRef]
- Chiou, G.Y.; Chiang, C.L.; Yang, H.C.; Shen, C.I.; Wu, H.M.; Chen, Y.W.; Chen, C.J.; Luo, Y.H.; Hu, Y.S.; Lin, C.J.; et al. Combined stereotactic radiosurgery and tyrosine kinase inhibitor therapy versus tyrosine kinase inhibitor therapy alone for the treatment of non-small cell lung cancer patients with brain metastases. J. Neurosurg. 2022, 137, 563–570. [Google Scholar] [CrossRef]
- Yang, J.J.; Zhou, C.; Huang, Y.; Feng, J.; Lu, S.; Song, Y.; Huang, C.; Wu, G.; Zhang, L.; Cheng, Y.; et al. Icotinib versus whole-brain irradiation in patients with EGFR-mutant non-small-cell lung cancer and multiple brain metastases (BRAIN): A multicentre, phase 3, open-label, parallel, randomised controlled trial. Lancet Respir. Med. 2017, 5, 707–716. [Google Scholar] [CrossRef]
- Fan, Y.; Xu, Y.; Gong, L.; Fang, L.; Lu, H.; Qin, J.; Han, N.; Xie, F.; Qiu, G.; Huang, Z. Effects of icotinib with and without radiation therapy on patients with EGFR mutant non-small cell lung cancer and brain metastases. Sci. Rep. 2017, 7, 45193. [Google Scholar] [CrossRef] [PubMed]
- An, N.; Wang, H.; Li, J.; Zhai, X.; Jing, W.; Jia, W.; Kong, L.; Zhu, H.; Yu, J. Therapeutic Effect Of First-Line EGFR-TKIs Combined With Concurrent Cranial Radiotherapy On NSCLC Patients With EGFR Activating Mutation And Brain Metastasis: A Retrospective Study. Onco Targets Ther. 2019, 12, 8311–8318. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Sun, Y.; Cui, Y.; Ye, K.; Yang, C.; Yang, D.; Ma, J.; Liu, X.; Yu, J.; Ge, H. Clinical outcome of tyrosine kinase inhibitors alone or combined with radiotherapy for brain metastases from epidermal growth factor receptor (EGFR) mutant non small cell lung cancer (NSCLC). Oncotarget 2017, 8, 13304–13311. [Google Scholar] [CrossRef] [PubMed]
- Planchard, D.; Boyer, M.J.; Lee, J.S.; Dechaphunkul, A.; Cheema, P.K.; Takahashi, T.; Gray, J.E.; Tiseo, M.; Ramalingam, S.S.; Todd, A.; et al. Postprogression Outcomes for Osimertinib versus Standard-of-Care EGFR-TKI in Patients with Previously Untreated EGFR-mutated Advanced Non-Small Cell Lung Cancer. Clin. Cancer Res. 2019, 25, 2058–2063. [Google Scholar] [CrossRef]
- Johung, K.L.; Yao, X.; Li, F.; Yu, J.B.; Gettinger, S.N.; Goldberg, S.; Decker, R.H.; Hess, J.A.; Chiang, V.L.; Contessa, J.N. A clinical model for identifying radiosensitive tumor genotypes in non-small cell lung cancer. Clin. Cancer Res. 2013, 19, 5523–5532. [Google Scholar] [CrossRef] [PubMed]
- Spano, J.P.; Fagard, R.; Soria, J.C.; Rixe, O.; Khayat, D.; Milano, G. Epidermal growth factor receptor signaling in colorectal cancer: Preclinical data and therapeutic perspectives. Ann. Oncol. 2005, 16, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.J.; Saad, S.; Qureshi, Y.H.; Jani, A.; Nanda, T.; Yaeh, A.M.; Rozenblat, T.; Sisti, M.B.; Bruce, J.N.; McKhann, G.M.; et al. Does lung cancer mutation status and targeted therapy predict for outcomes and local control in the setting of brain metastases treated with radiation? Neuro-Oncology 2015, 17, 1022–1028. [Google Scholar] [CrossRef]
- Yamamoto, M.; Serizawa, T.; Shuto, T.; Akabane, A.; Higuchi, Y.; Kawagishi, J.; Yamanaka, K.; Sato, Y.; Jokura, H.; Yomo, S.; et al. Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): A multi-institutional prospective observational study. Lancet Oncol. 2014, 15, 387–395. [Google Scholar] [CrossRef]
- Habets, E.J.; Dirven, L.; Wiggenraad, R.G.; Verbeek-de Kanter, A.; Lycklama À Nijeholt, G.J.; Zwinkels, H.; Klein, M.; Taphoorn, M.J. Neurocognitive functioning and health-related quality of life in patients treated with stereotactic radiotherapy for brain metastases: A prospective study. Neuro-Oncology 2016, 18, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kim, W.S.; Kwon, D.H.; Cho, Y.H.; Choi, C.M. Effects of an Epithelial Growth Factor Receptor-Tyrosine Kinase Inhibitor Add-on in Stereotactic Radiosurgery for Brain Metastases Originating from Non-Small-Cell Lung Cancer. J. Korean Neurosurg. Soc. 2015, 58, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Okada, H.; Weller, M.; Huang, R.; Finocchiaro, G.; Gilbert, M.R.; Wick, W.; Ellingson, B.M.; Hashimoto, N.; Pollack, I.F.; Brandes, A.A.; et al. Immunotherapy response assessment in neuro-oncology: A report of the RANO working group. Lancet Oncol. 2015, 16, e534–e542. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.U.; Lee, E.Q.; Aoyama, H.; Barani, I.J.; Barboriak, D.P.; Baumert, B.G.; Bendszus, M.; Brown, P.D.; Camidge, D.R.; Chang, S.M.; et al. Response assessment criteria for brain metastases: Proposal from the RANO group. Lancet Oncol. 2015, 16, e270–e278. [Google Scholar] [CrossRef]
- Wang, W.; Song, Z.; Zhang, Y. Efficacy of brain radiotherapy plus EGFR-TKI for EGFR-mutated non-small cell lung cancer patients who develop brain metastasis. Arch. Med. Sci. 2018, 14, 1298–1307. [Google Scholar] [CrossRef]
| Characteristics | WBRT | SRS | WBRT + SRS | EGFR-TKIs + WBRT | EGFR-TKIs + SRS |
|---|---|---|---|---|---|
| Population (N) | 32 | 51 | 36 | 29 | 18 |
| Average age in years (N, range) | 68.36 (38.91, 84. 95) | 62.66 (38.42, 89.50) | 64.76 (40.79, 89.88) | 61.49 (43.66, 83.25) | 70.27 (28.44, 90.34) |
| Female (%) | 20 (62.5) | 36 (70.6) | 20 (55.6) | 17 (58.6) | 9 (50.0) |
| Multiple brain metastases (N, %) | 20 (83.3) | 27 (55.1) | 20 (69.0) | 23 (88.5) | 15 (83.3) |
| Single brain metastases (N, %) | 4 (16.7) | 22 (44.9) | 9 (31.0) | 3 (11.5) | 3 (16.7) |
| Extracranial metastases (N, %) | 25 (80.6) | 28 (54.9) | 21 (60.0) | 23 (82.1) | 13 (72.2) |
| Leptomeningeal spread (N, %) | 5 (16.7) | 4 (8.3) | 10 (29.4) | 4 (14.3) | 0 (0.0) |
| Symptomatic at time of brain metastases (N, %) | 22 (81.5) | 23 (53.5) | 24 (70.6) | 12 (60.0) | 10 (58.8) |
| Type of EGFR-TKI | |||||
| Erlotinib/gefitinib | 19 (65.5) | 8 (44.4) | |||
| Osimertinib/afatinib | 10 (34.5) | 10 (55.6) |
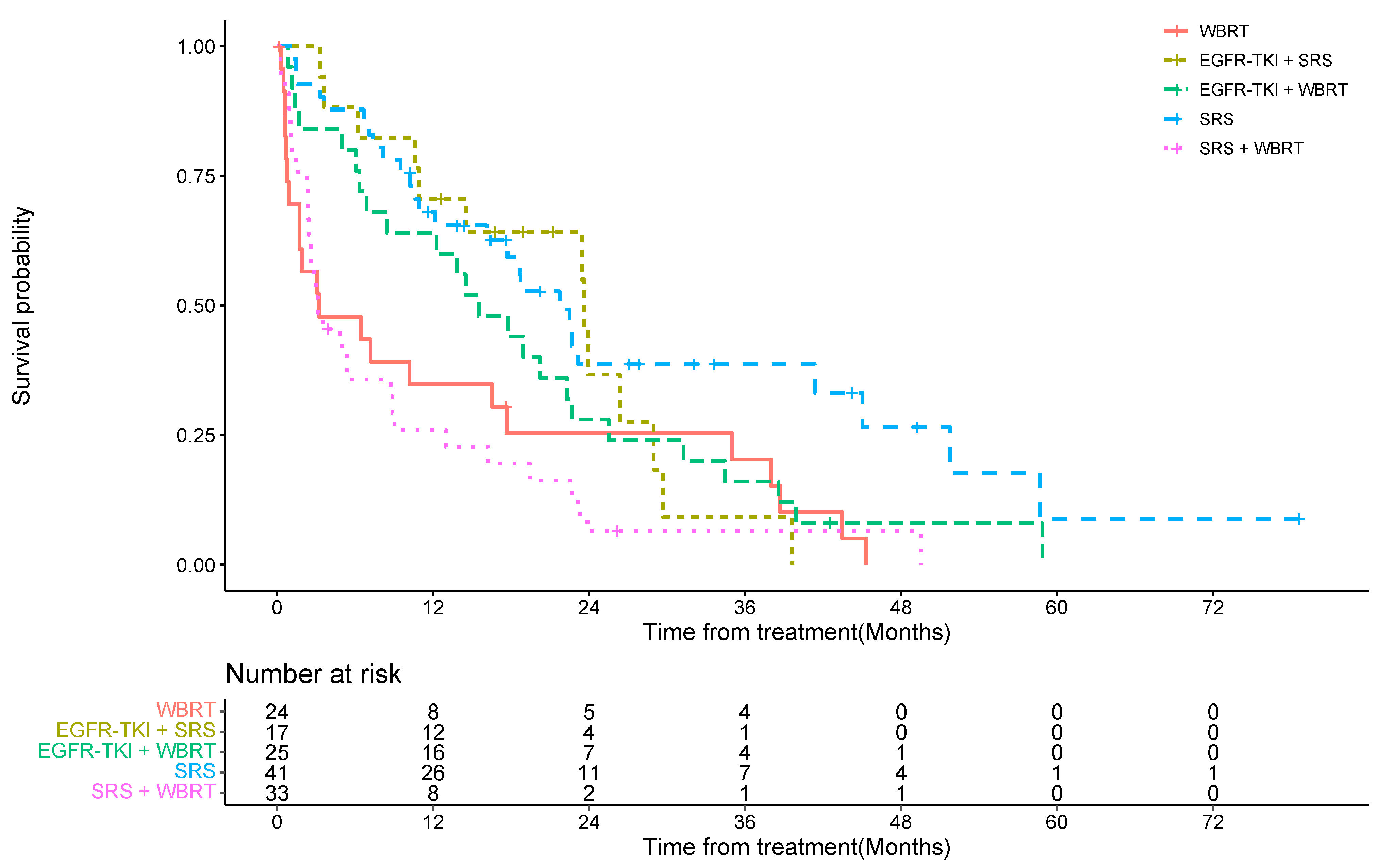
| Cohort | Median OS (Months) | 1-Year OS (95%CI) | 2-Year OS Rate (95%CI) |
|---|---|---|---|
| WBRT only | 3.23 | 35% (17%, 54%) | 25% (10%, 44%) |
| SRS only | 21.73 | 68% (51%, 80%) | 39% (22%, 55%) |
| WBRT + SRS | 3.17 | 26% (12%, 42%) | 6% (1%, 19%) |
| EGFR-TKI + WBRT | 15.50 | 64% (42%, 79%) | 28% (12%, 46%) |
| EGFR-TKI + SRS | 23.63 | 71% (43%, 87%) | 37% (12%, 62%) |
| Cohort | Hazard Ratio (95% CI) | p-Value |
|---|---|---|
| Treatment | ||
| WBRT only | Reference | |
| SRS only | 0.38 (0.17, 0.84) | 0.017 |
| WBRT + SRS | 1.30 (0.60, 2.82) | 0.50 |
| EGFR-TKI + WBRT | 0.93 (0.41, 2.08) | 0.85 |
| EGFR-TKI + SRS | 0.46 (0.20, 1.09) | 0.077 |
| Age | 1.02 (1.00, 1.04) | 0.03 |
| Time from brain metastases to treatment | 1.00 (1.00, 1.00) | 0.012 |
| KPS | ||
| Greater than 70 | Reference | |
| Lesser than 70 | 2.49 (1.10, 5.66) | 0.029 |
| Symptomatic at time of brain metastases | ||
| Asymptomatic | Reference | |
| Symptomatic | 1.42 (0.89, 2.27) | 0.14 |
| Number of brain metastases | ||
| Multiple | Reference | |
| Single | 0.92 (0.53, 1.62) | 0.78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tatineni, V.; O’Shea, P.J.; Saxena, S.; Khosla, A.A.; Ozair, A.; Kotecha, R.R.; Jia, X.; Rauf, Y.; Murphy, E.S.; Chao, S.T.; et al. Combination of EGFR-Directed Tyrosine Kinase Inhibitors (EGFR-TKI) with Radiotherapy in Brain Metastases from Non-Small Cell Lung Cancer: A 2010–2019 Retrospective Cohort Study. Cancers 2023, 15, 3015. https://doi.org/10.3390/cancers15113015
Tatineni V, O’Shea PJ, Saxena S, Khosla AA, Ozair A, Kotecha RR, Jia X, Rauf Y, Murphy ES, Chao ST, et al. Combination of EGFR-Directed Tyrosine Kinase Inhibitors (EGFR-TKI) with Radiotherapy in Brain Metastases from Non-Small Cell Lung Cancer: A 2010–2019 Retrospective Cohort Study. Cancers. 2023; 15(11):3015. https://doi.org/10.3390/cancers15113015
Chicago/Turabian StyleTatineni, Vineeth, Patrick J. O’Shea, Shreya Saxena, Atulya A. Khosla, Ahmad Ozair, Rupesh R. Kotecha, Xuefei Jia, Yasmeen Rauf, Erin S. Murphy, Samuel T. Chao, and et al. 2023. "Combination of EGFR-Directed Tyrosine Kinase Inhibitors (EGFR-TKI) with Radiotherapy in Brain Metastases from Non-Small Cell Lung Cancer: A 2010–2019 Retrospective Cohort Study" Cancers 15, no. 11: 3015. https://doi.org/10.3390/cancers15113015
APA StyleTatineni, V., O’Shea, P. J., Saxena, S., Khosla, A. A., Ozair, A., Kotecha, R. R., Jia, X., Rauf, Y., Murphy, E. S., Chao, S. T., Suh, J. H., Peereboom, D. M., & Ahluwalia, M. S. (2023). Combination of EGFR-Directed Tyrosine Kinase Inhibitors (EGFR-TKI) with Radiotherapy in Brain Metastases from Non-Small Cell Lung Cancer: A 2010–2019 Retrospective Cohort Study. Cancers, 15(11), 3015. https://doi.org/10.3390/cancers15113015








