Preoperative EUS vs. PET-CT Evaluation of Response to Neoadjuvant Therapy for Esophagogastric Cancer and Its Correlation with Survival
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Collection
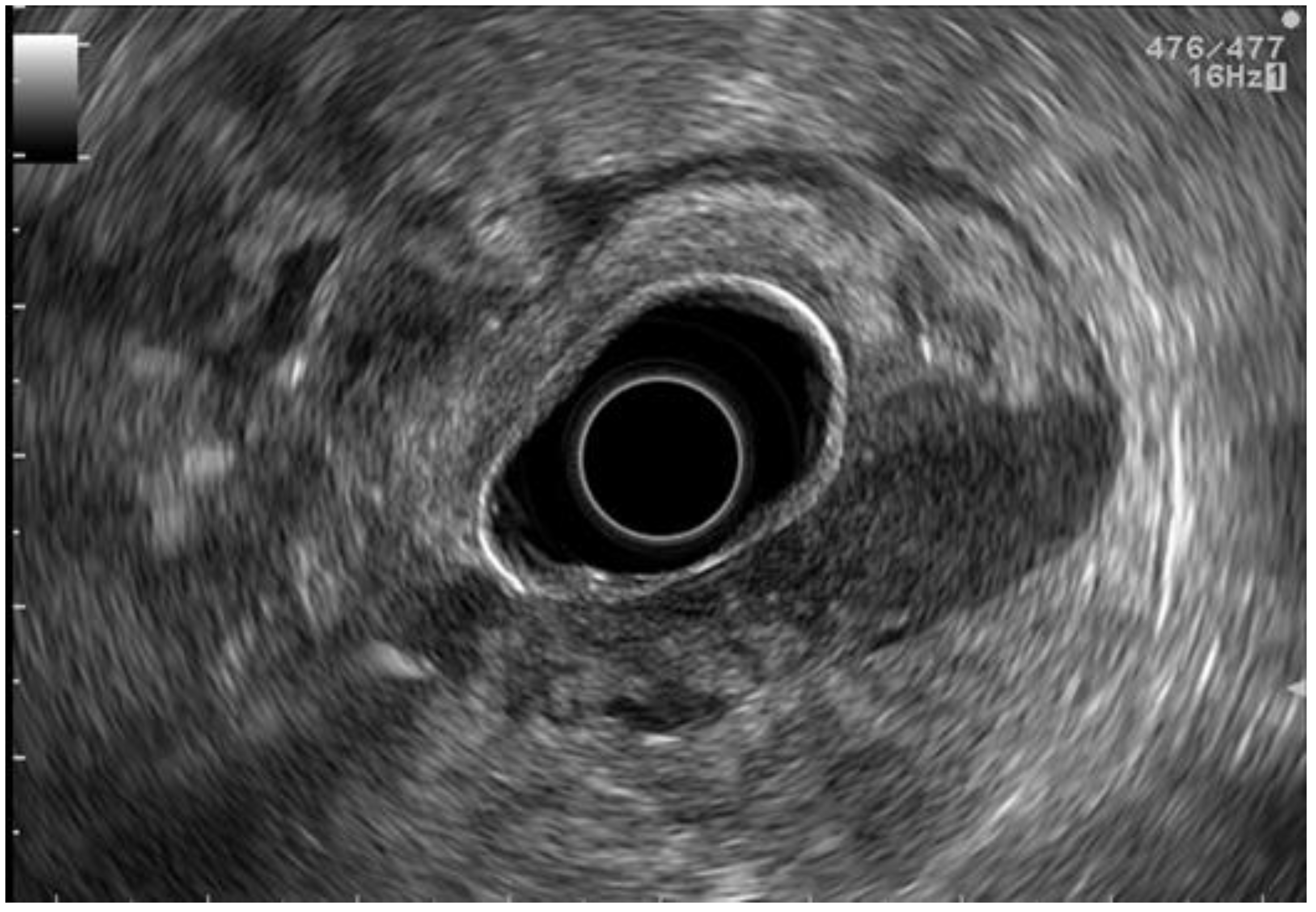
2.3. EUS Staging
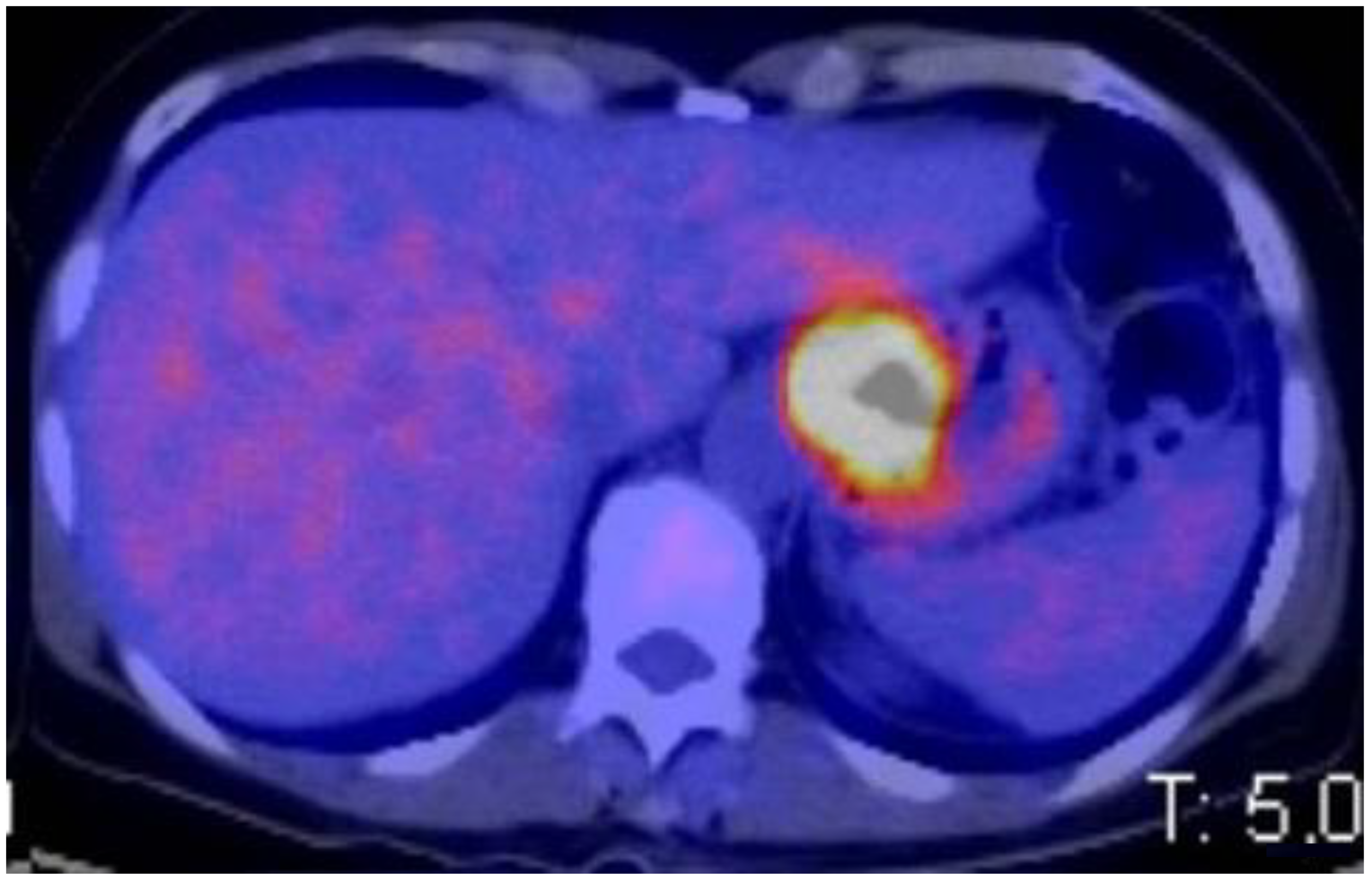
2.4. PET-CT Staging
2.5. Follow Up
2.6. Outcomes
2.7. Statistical Analysis
2.8. Ethics
3. Results
3.1. Tumor Characteristics
3.2. Neoadjuvant Therapy
3.3. Surgical Treatment
3.4. Accuracy of EUS and PET-CT in Restaging
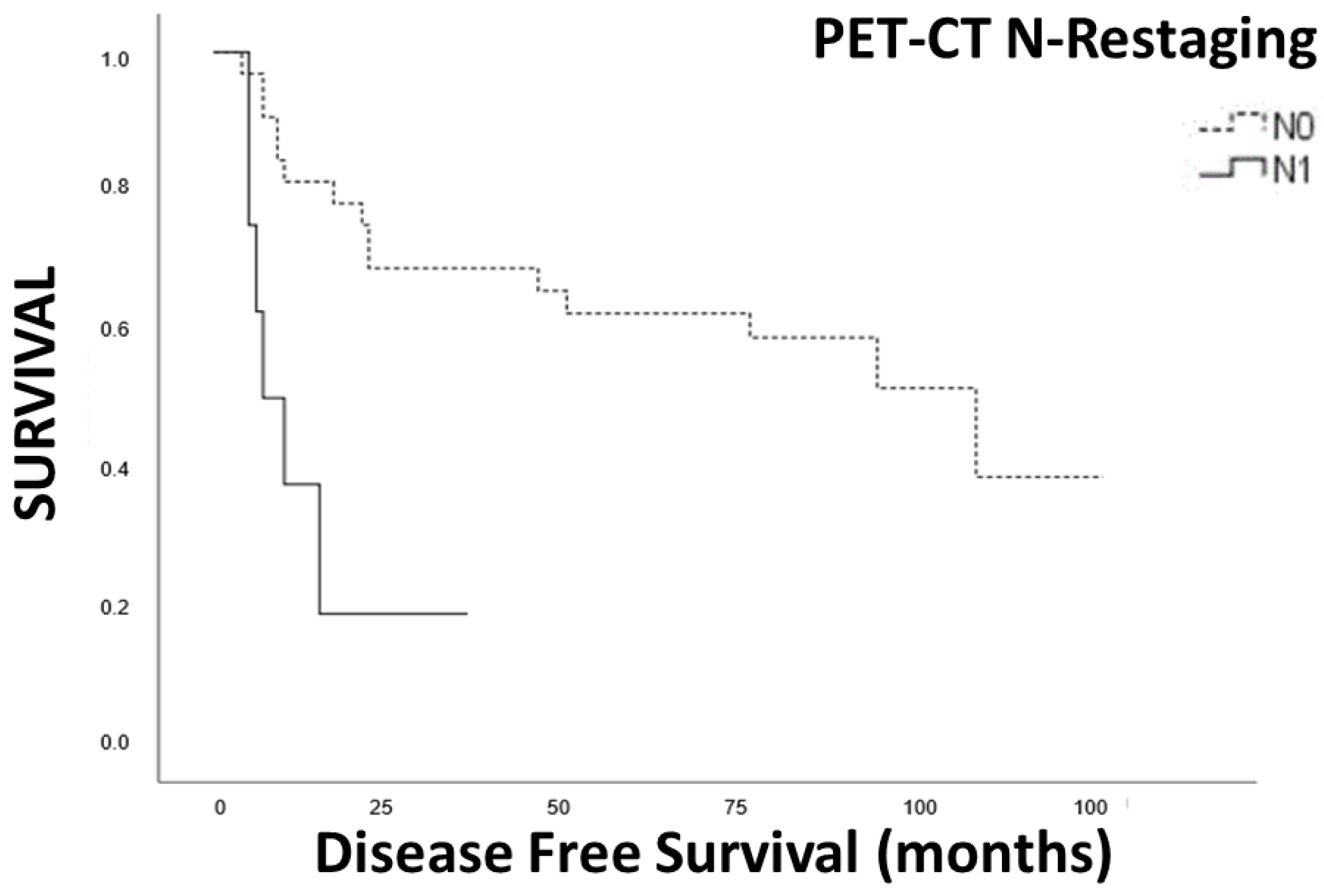
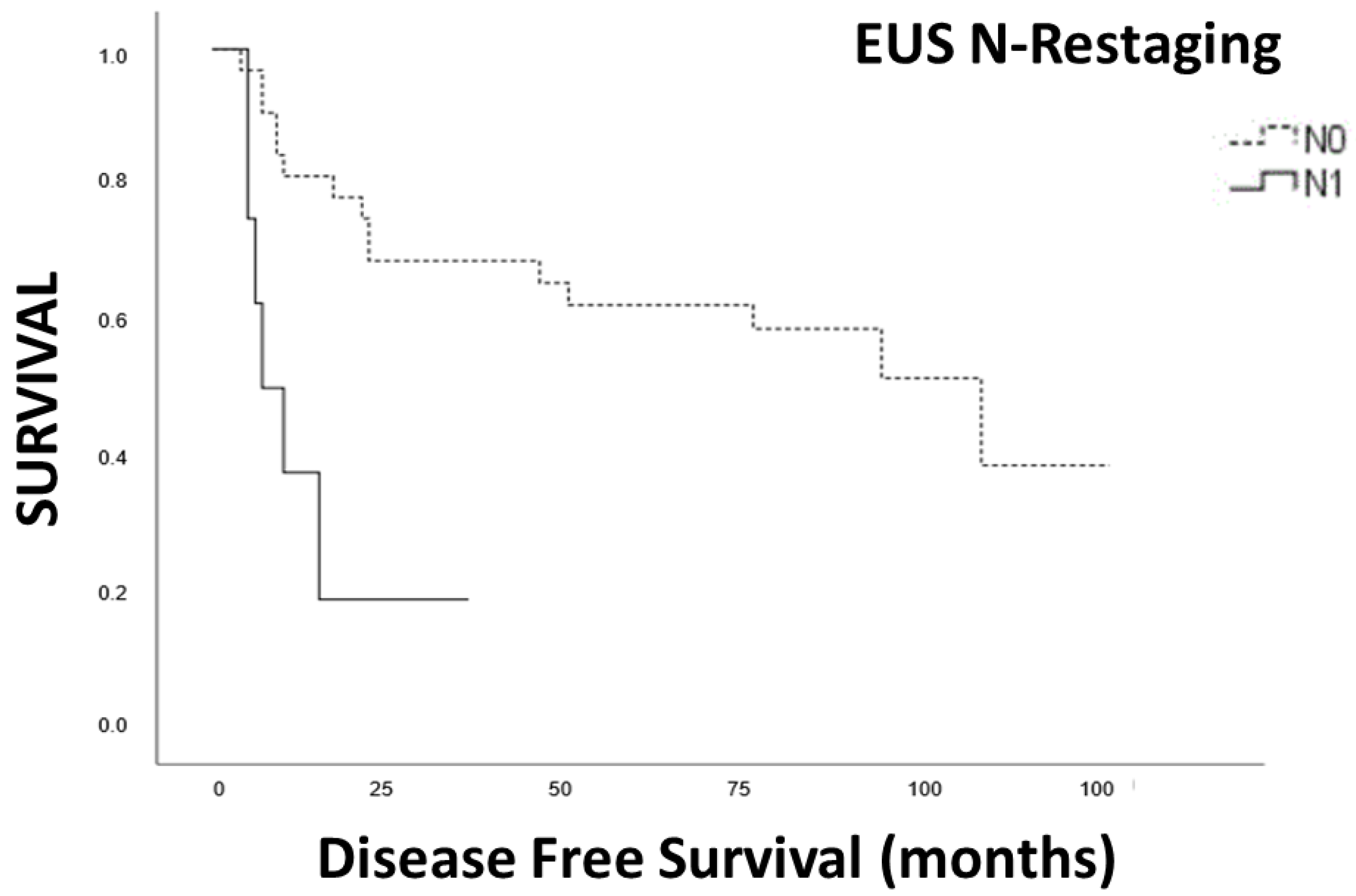
3.5. Survival Analysis
3.6. Disease-Free Survival
3.7. Overall Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siewert, J.R.; Stein, H.J. Classification of adenocarcinoma of the oesophagogastric junction. Br. J. Surg. 1998, 85, 1457–1459. [Google Scholar] [CrossRef]
- Torre, L.A.; Siegel, R.L.; Ward, E.M.; Jemal, A. Global Cancer Incidence and Mortality Rates and Trends--An Update. Cancer Epidemiol. Biomarkers Prev. 2016, 25, 16–27. [Google Scholar] [CrossRef]
- Marrelli, D.; Polom, K.; de Manzoni, G.; Morgagni, P.; Baiocchi, G.L.; Roviello, F. Multimodal treatment of gastric cancer in the west: Where are we going? World J. Gastroenterol. 2015, 21, 7954–7969. [Google Scholar] [CrossRef]
- Ju, M.R.; Karalis, J.D.; Blackwell, J.M.; Mansour, J.C.; Polanco, P.M.; Augustine, M.; Yopp, A.C.; Zeh, H.J., 3rd; Wang, S.C.; Porembka, M.R. Inaccurate Clinical Stage Is Common for Gastric Adenocarcinoma and Is Associated with Undertreatment and Worse Outcomes. Ann. Surg. Oncol. 2021, 28, 2831–2843. [Google Scholar] [CrossRef]
- Dumonceau, J.M.; Polkowski, M.; Larghi, A.; Vilmann, P.; Giovannini, M.; Frossard, J.L.; Heresbach, D.; Pujol, B.; Fernandez-Esparrach, G.; Vazquez-Sequeiros, E.; et al. Indications, results, and clinical impact of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy 2011, 43, 897–912. [Google Scholar] [CrossRef]
- Hoibian, S.; Giovannini, M.; Autret, A.; Pesenti, C.; Bories, E.; Ratone, J.P.; Dahel, Y.; Dermeche, S.; Meillat, H.; Guiramand, J.; et al. Preoperative EUS evaluation of the response to neoadjuvant therapy for gastric and esophagogastric junction cancer is correlated with survival: A single retrospective study of 97 patients. Endosc. Ultrasound 2021, 10, 103–110. [Google Scholar] [CrossRef]
- NCCN. Esophageal and Esophagogastric Junction Cancers (Version 2.2021). Available online: https://www.nccn.org/professionals/physician_gls/pdf/esophageal.pdf (accessed on 1 May 2021).
- NCCN. Gastric Cancer (Version 2.2021). Available online: https://www.nccn.org/professionals/physician_gls/pdf/gastric.pdf (accessed on 1 May 2021).
- de Gouw, D.; Klarenbeek, B.R.; Driessen, M.; Bouwense, S.A.W.; van Workum, F.; Futterer, J.J.; Rovers, M.M.; Ten Broek, R.P.G.; Rosman, C. Detecting Pathological Complete Response in Esophageal Cancer after Neoadjuvant Therapy Based on Imaging Techniques: A Diagnostic Systematic Review and Meta-Analysis. J. Thorac. Oncol. 2019, 14, 1156–1171. [Google Scholar] [CrossRef]
- Catalano, M.F.; Sivak, M.V., Jr.; Rice, T.; Gragg, L.A.; Van Dam, J. Endosonographic features predictive of lymph node metastasis. Gastrointest. Endosc. 1994, 40, 442–446. [Google Scholar] [CrossRef]
- Faige, D.O. EUS in patients with benign and malignant lymphadenopathy. Gastrointest. Endosc. 2001, 53, 593–598. [Google Scholar] [CrossRef]
- van der Bogt, R.D.; van der Wilk, B.J.; Poley, J.W.; Krishnadath, K.K.; Schoon, E.J.; Oostenbrug, L.E.; Siersema, P.D.; Vleggaar, F.P.; Bruno, M.J.; Biermann, K.; et al. Endoscopic ultrasound and fine-needle aspiration for the detection of residual nodal disease after neoadjuvant chemoradiotherapy for esophageal cancer. Endoscopy 2020, 52, 186–192. [Google Scholar] [CrossRef]
- Redondo-Cerezo, E.; Martinez-Cara, J.G.; Jimenez-Rosales, R.; Valverde-Lopez, F.; Caballero-Mateos, A.; Jervez-Puente, P.; Ariza-Fernandez, J.L.; Ubeda-Munoz, M.; Lopez-de-Hierro, M.; de Teresa, J. Endoscopic ultrasound in gastric cancer staging before and after neoadjuvant chemotherapy. A comparison with PET-CT in a clinical series. United Eur. Gastroenterol. J. 2017, 5, 641–647. [Google Scholar] [CrossRef]
- Mesenas, S.; Vu, C.; McStay, M.; Forshaw, M.; Doig, L.; Mason, R.; Boyle, N.; Meenan, J. A large series, resection controlled study to assess the value of radial EUS in restaging gastroesophageal cancer following neoadjuvant chemotherapy. Dis. Esophagus 2008, 21, 37–42. [Google Scholar] [CrossRef]
- Guo, T.; Yao, F.; Yang, A.M.; Li, X.Y.; Zhong, D.R.; Wu, D.S.; Wu, X.; Lu, X.H. Endoscopic ultrasound in restaging and predicting pathological response for advanced gastric cancer patients after neoadjuvant chemotherapy. Asia Pac. J. Clin. Oncol. 2014, 10, e28–e32. [Google Scholar] [CrossRef]
- Misra, S.; Choi, M.; Livingstone, A.S.; Franceschi, D. The role of endoscopic ultrasound in assessing tumor response and staging after neoadjuvant chemotherapy for esophageal cancer. Surg. Endosc. 2012, 26, 518–522. [Google Scholar] [CrossRef]
- Bohle, W.; Zachmann, R.; Zoller, W.G. Sequential endoscopic ultrasound identifies predictive variables for relapse-free follow-up after neoadjuvant chemotherapy in gastric cancer. Scand. J. Gastroenterol. 2017, 52, 754–761. [Google Scholar] [CrossRef]
- Cunningham, D.; Allum, W.H.; Stenning, S.P.; Thompson, J.N.; Van de Velde, C.J.; Nicolson, M.; Scarffe, J.H.; Lofts, F.J.; Falk, S.J.; Iveson, T.J.; et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N. Engl. J. Med. 2006, 355, 11–20. [Google Scholar] [CrossRef]
- Xu, X.; Zheng, G.; Zhang, T.; Zhao, Y.; Zheng, Z. Is pathologic tumor regression grade after neo-adjuvant chemotherapy a promising prognostic indicator for patients with locally advanced gastric cancer? A cohort study evaluating tumor regression response. Cancer Chemother. Pharmacol. 2019, 84, 635–646. [Google Scholar] [CrossRef]
- Davies, A.R.; Gossage, J.A.; Zylstra, J.; Mattsson, F.; Lagergren, J.; Maisey, N.; Smyth, E.C.; Cunningham, D.; Allum, W.H.; Mason, R.C. Tumor stage after neoadjuvant chemotherapy determines survival after surgery for adenocarcinoma of the esophagus and esophagogastric junction. J. Clin. Oncol. 2014, 32, 2983–2990. [Google Scholar] [CrossRef]
- Lopci, E.; Kauppi, J.; Lugaresi, M.; Mattioli, B.; Daddi, N.; Fortunato, F.; Rasanen, J.; Mattioli, S. Siewert type I and II oesophageal adenocarcinoma: Sensitivity/specificity of computed tomography, positron emission tomography and endoscopic ultrasound for assessment of lymph node metastases in groups of thoracic and abdominal lymph node stations. Interact. Cardiovasc Thorac. Surg. 2019, 28, 518–525. [Google Scholar] [CrossRef]
- Swisher, S.G.; Maish, M.; Erasmus, J.J.; Correa, A.M.; Ajani, J.A.; Bresalier, R.; Komaki, R.; Macapinlac, H.; Munden, R.F.; Putnam, J.B.; et al. Utility of PET, CT, and EUS to identify pathologic responders in esophageal cancer. Ann Thorac. Surg. 2004, 78, 1152–1160; discussion 1152–1160. [Google Scholar] [CrossRef]
- Schwameis, K.; Zehetner, J.; Hagen, J.A.; Oh, D.S.; Worrell, S.G.; Rona, K.; Cheng, N.; Samaan, J.; Green, K.M.; Lipham, J.C. Esophageal adenocarcinoma stage III: Survival based on pathological response to neoadjuvant treatment. Surg. Oncol. 2017, 26, 522–526. [Google Scholar] [CrossRef]
- Sultan, J.; Robinson, S.; Hayes, N.; Griffin, S.M.; Richardson, D.L.; Preston, S.R. Endoscopic ultrasonography-detected low-volume ascites as a predictor of inoperability for oesophagogastric cancer. Br. J. Surg. 2008, 95, 1127–1130. [Google Scholar] [CrossRef]
- Lee, Y.T.; Ng, E.K.; Hung, L.C.; Chung, S.C.; Ching, J.Y.; Chan, W.Y.; Chu, W.C.; Sung, J.J. Accuracy of endoscopic ultrasonography in diagnosing ascites and predicting peritoneal metastases in gastric cancer patients. Gut 2005, 54, 1541–1545. [Google Scholar] [CrossRef]
- Chen, C.H.; Yang, C.C.; Yeh, Y.H. Preoperative staging of gastric cancer by endoscopic ultrasound: The prognostic usefulness of ascites detected by endoscopic ultrasound. J. Clin. Gastroenterol. 2002, 35, 321–327. [Google Scholar] [CrossRef]
- Al-Batran, S.E.; Homann, N.; Pauligk, C.; Goetze, T.O.; Meiler, J.; Kasper, S.; Kopp, H.G.; Mayer, F.; Haag, G.M.; Luley, K.; et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): A randomised, phase 2/3 trial. Lancet 2019, 393, 1948–1957. [Google Scholar] [CrossRef]
| Parameter | n | % |
|---|---|---|
| Age (median. Years) | 67 | |
| Gender | ||
| Male | 139 | 62.5 |
| Female | 46 | 21.4 |
| Localization | ||
| Distal esophagus | 15 | 8.1 |
| Gastroesophageal junction | 41 | 22 |
| Upper stomach | 129 | 69.4 |
| Grading | ||
| G1 | 9 | 4.9 |
| G2 | 104 | 56.5 |
| G3 | 71 | 38.6 |
| Lauren’s classification | ||
| Intestinal | 97 | 52.4 |
| Mixed | 15 | 8.3 |
| Diffuse | 73 | 39.3 |
| Staging | ||
| Depth of invasion (EUS) | ||
| T1 | 2 | 1.1 |
| T2 | 8 | 4.5 |
| T3 | 54 | 30.2 |
| T4 | 117 | 65.4 |
| Node detected (EUS) | ||
| N0 | 49 | 28.7 |
| N1 | 48 | 28.1 |
| N2 | 55 | 32.2 |
| N3 | 19 | 11.1 |
| Node detected (PET-CT) | ||
| N (+) | 58 | 38.2 |
| N (−) | 94 | 61.8 |
| Restaging | ||
| Depth of invasión | ||
| T1 | 1 | 0.54% |
| T2 | 44 | 23.91 |
| T3 | 65 | 35.4 |
| T4 | 74 | 40 |
| Node detected (EUS) | ||
| N0 | 96 | 52 |
| N1 | 43 | 23.2 |
| N2 | 43 | 23.2 |
| N3 | 2 | 1.4 |
| Node detected (PET-CT) | ||
| N (+) | 49 | 26.7 |
| N (−) | 136 | 73.3 |
| Histopathologic T category (ypT) | ||
| ypT0 | 20 | 11.4 |
| ypT1 | 15 | 7.9 |
| ypT2 | 29 | 15.8 |
| ypT3 | 80 | 43 |
| ypT4 | 41 | 21.9 |
| Histopathologic N category (ypN) | ||
| ypN0 | 96 | 51.8 |
| ypN1 | 41 | 21.9 |
| ypN2 | 27 | 14.9 |
| ypN3 | 21 | 11.4 |
| EUS | PET-CT | |
|---|---|---|
| % [95% CI] | % [95% CI] | |
| Sensitivity | 60 [38.7–78.1] | 27.3 [13.2–48.2] |
| Specificity | 78.6 [60.5–89.8] | 88.5 [71–96] |
| PPV | 73.3 [55.6–85.8] | 59.0 [43.4–72.9] |
| NPV | 66.7 [43.7–83.7] | 66.7 [35.4–87.9] |
| Accuracy | 70.8 [1.54–19.6] | 60.4 [46.3–73] |
| n | Median (CI 95%) (Months) | p | |
|---|---|---|---|
| EUS restaging | |||
| N+ | 98 | 22 (15.47–28.53) | 0.001 |
| N0 | 72 | 108 (26.34–189.65) | |
| PET-CT restaging | |||
| N+ | 34 | 13.69 (5.25–22.11) | 0.001 |
| N0 | 136 | 79.13 (61.44–96.81) | |
| Disease Free Survival (DFS) | |||
|---|---|---|---|
| Hazard Ratio | 95% CI | p | |
| Age | 0.95 | 0.89–1.01 | 0.12 |
| Sex | 0.768 | 0.20–2.93 | 0.69 |
| Charlson | 2.51 | 1.25–5.04 | 0.009 |
| N+ restaging PET-CT | 20.91 | 3.39–129.08 | 0.001 |
| N+ restaging EUS | 4.37 | 1.09–17.54 | 0.037 |
| N+ pathology | 4.68 | 0.92–23.91 | 0.063 |
| Overall Survival (OS) | |||
| Hazard Ratio | 95% CI | p | |
| Age | 1.004 | 0.93–1.08 | 0.29 |
| Sex (female) | 0.24 | 1.09–3.09 | 0.12 |
| Charlson | 1.83 | 1.21–6.57 | 0.023 |
| N response to NT on EUS | 3.02 | 0.88–11.62 | 0.145 |
| T response to NT on EUS | 0.09 | 0.02–0.46 | 0.004 |
| N+ restaging EUS | 0.85 | 0.22–3.21 | 0.44 |
| N+ restaging PET-CT | 0.34 | 0.08–1.40 | 0.20 |
| N+ pathology | 10.72 | 1.56–77.77 | 0.016 |
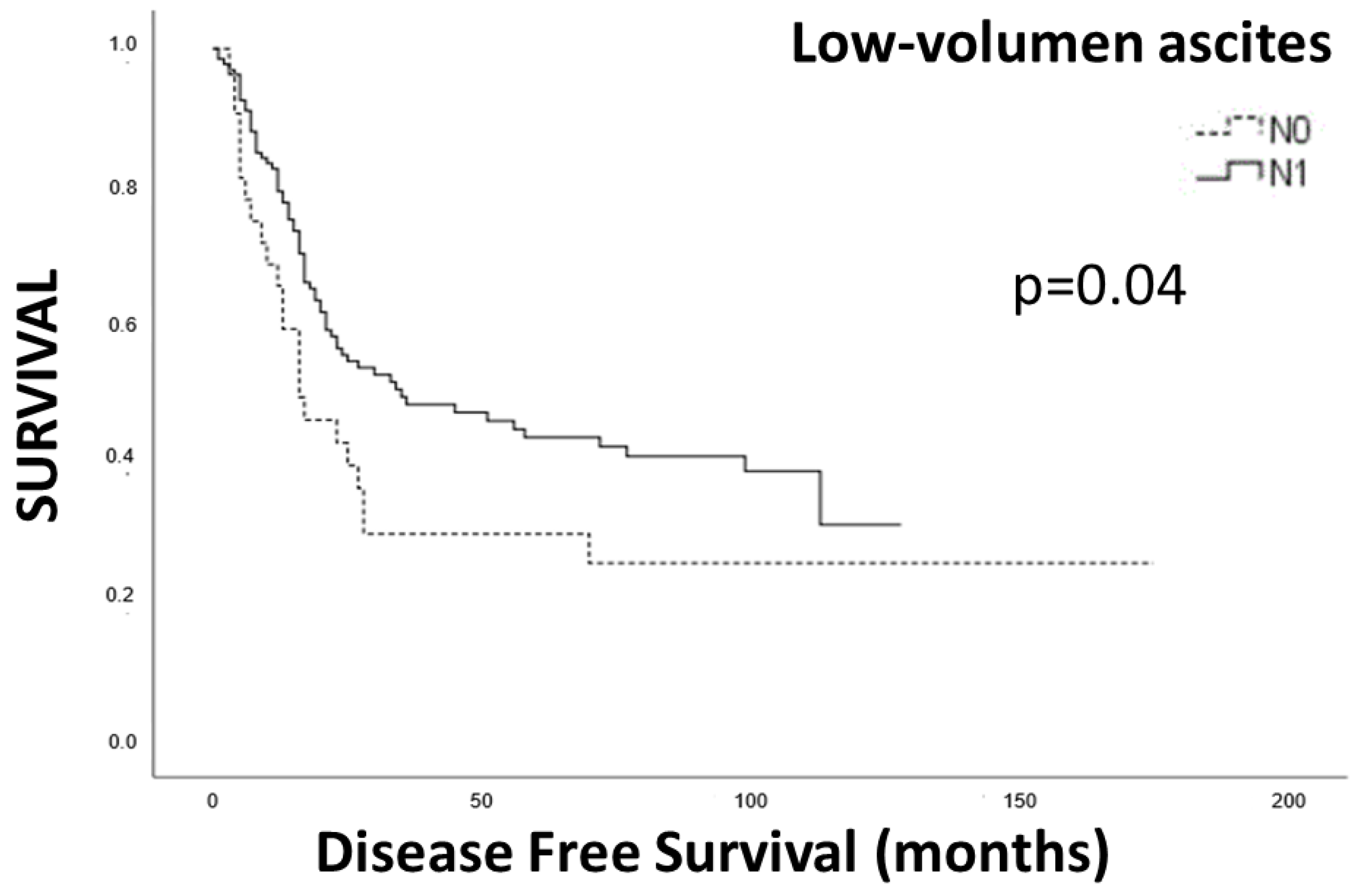
| Low volumen ascites | 1.374 | 0.51–3.68 | 0.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amezcua-Hernandez, V.; Jimenez-Rosales, R.; Martinez-Cara, J.G.; Garcia-Garcia, J.; Valverde Lopez, F.; Redondo-Cerezo, E. Preoperative EUS vs. PET-CT Evaluation of Response to Neoadjuvant Therapy for Esophagogastric Cancer and Its Correlation with Survival. Cancers 2023, 15, 2941. https://doi.org/10.3390/cancers15112941
Amezcua-Hernandez V, Jimenez-Rosales R, Martinez-Cara JG, Garcia-Garcia J, Valverde Lopez F, Redondo-Cerezo E. Preoperative EUS vs. PET-CT Evaluation of Response to Neoadjuvant Therapy for Esophagogastric Cancer and Its Correlation with Survival. Cancers. 2023; 15(11):2941. https://doi.org/10.3390/cancers15112941
Chicago/Turabian StyleAmezcua-Hernandez, Victor, Rita Jimenez-Rosales, Juan Gabriel Martinez-Cara, Javier Garcia-Garcia, Francisco Valverde Lopez, and Eduardo Redondo-Cerezo. 2023. "Preoperative EUS vs. PET-CT Evaluation of Response to Neoadjuvant Therapy for Esophagogastric Cancer and Its Correlation with Survival" Cancers 15, no. 11: 2941. https://doi.org/10.3390/cancers15112941
APA StyleAmezcua-Hernandez, V., Jimenez-Rosales, R., Martinez-Cara, J. G., Garcia-Garcia, J., Valverde Lopez, F., & Redondo-Cerezo, E. (2023). Preoperative EUS vs. PET-CT Evaluation of Response to Neoadjuvant Therapy for Esophagogastric Cancer and Its Correlation with Survival. Cancers, 15(11), 2941. https://doi.org/10.3390/cancers15112941






