Endoscopic Ultrasound-Guided Gallbladder Drainage for Malignant Biliary Obstruction: A Systematic Review
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Review
2.2. Inclusion Criteria
3. Results
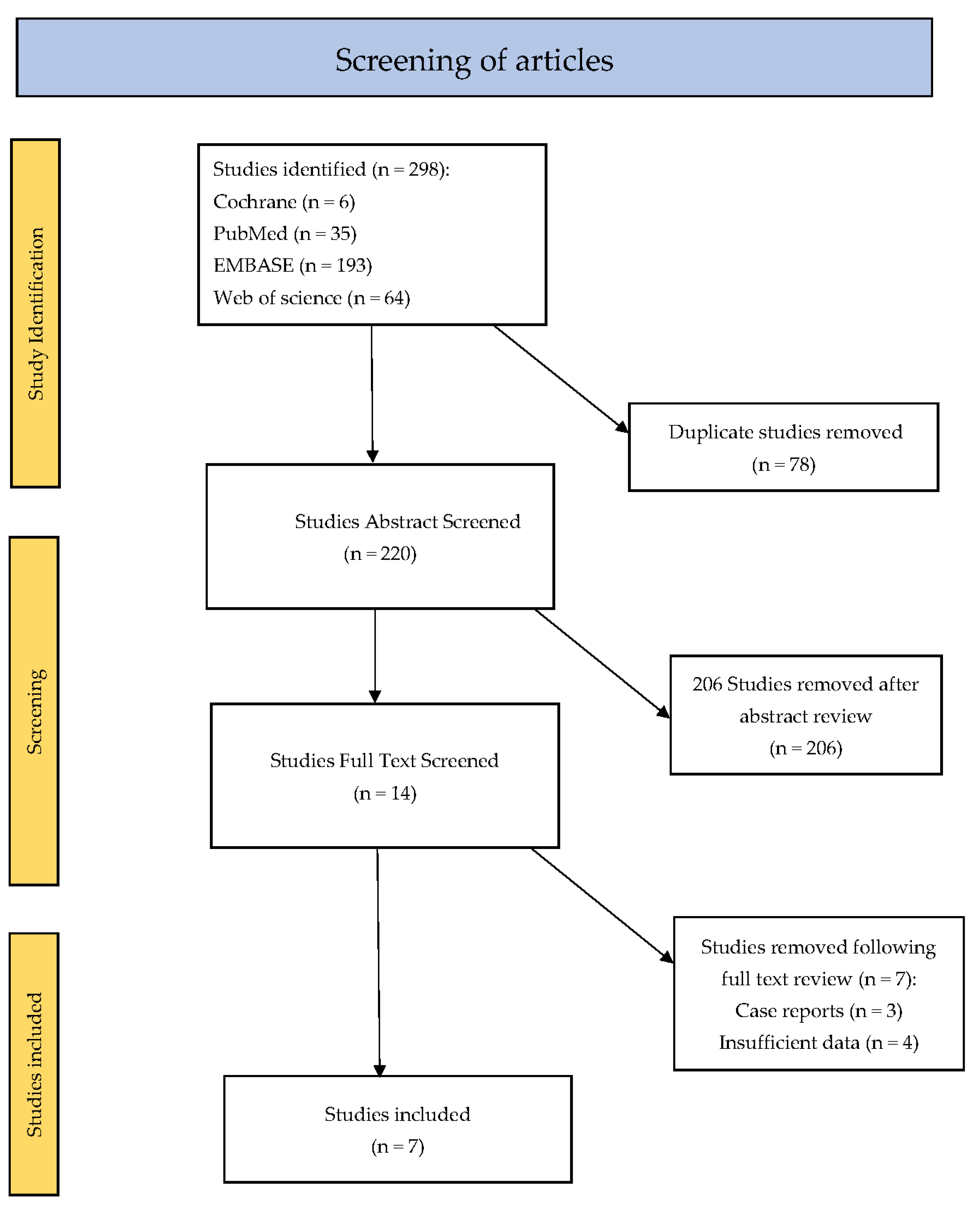
3.1. Literature Search
3.2. Clinical Details
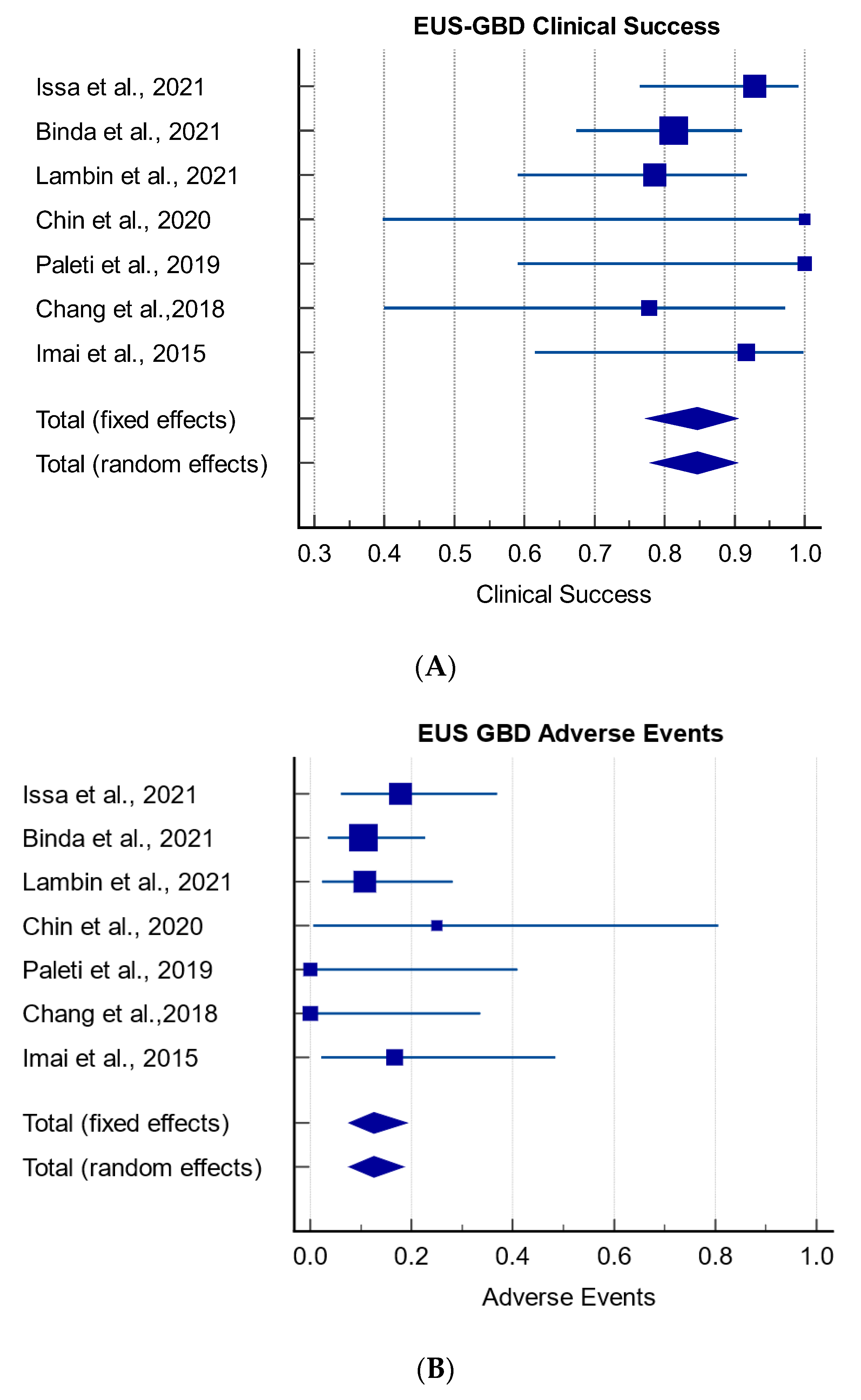
3.3. Meta-Analysis of Clinical Success and Adverse Events
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- O’reilly, D.; Fou, L.; Hasler, E.; Hawkins, J.; O’connell, S.; Pelone, F.; Callaway, M.; Campbell, F.; Capel, M.; Charnley, R.; et al. Diagnosis and management of pancreatic cancer in adults: A summary of guidelines from the UK National Institute for Health and Care Excellence. Pancreatology 2018, 18, 962–970. [Google Scholar] [CrossRef]
- Lorenz, J.M. Management of malignant biliary obstruction. Semin. Interv. Radiol. 2016, 33, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Udd, M.; Kylänpää, L.; Halttunen, J. Management of difficult bile duct cannulation in ERCP. World J. Gastrointest. Endosc. 2010, 2, 97. [Google Scholar] [CrossRef] [PubMed]
- Balik, E.; Eren, T.; Keskin, M.; Ziyade, S.; Bulut, T.; Buyukuncu, Y.; Yamaner, S. Parameters that may be used for predicting failure during endoscopic retrograde cholangiopancreatography. J. Oncol. 2013, 2013, 201681. [Google Scholar] [CrossRef] [PubMed]
- On, W.; Paranandi, B.; Smith, A.M.; Venkatachalapathy, S.V.; James, M.W.; Aithal, G.P.; Varbobitis, I.; Cheriyan, D.; McDonald, C.; Leeds, J.S.; et al. EUS-guided choledochoduodenostomy with electrocautery-enhanced lumen-apposing metal stents in patients with malignant distal biliary obstruction: Multicenter collaboration from the United Kingdom and Ireland. Gastrointest. Endosc. 2022, 95, 432–442. [Google Scholar] [CrossRef]
- Ginestet, C.; Sanglier, F.; Hummel, V.; Rouchaud, A.; Legros, R.; Lepetit, H.; Dahan, M.; Carrier, P.; Loustaud-Ratti, V.; Sautereau, D.; et al. EUS-guided biliary drainage with electrocautery-enhanced lumen-apposing metal stent placement should replace PTBD after ERCP failure in patients with distal tumoral biliary obstruction: A large real-life study. Surg. Endosc. 2021, 36, 3365–3373. [Google Scholar] [CrossRef]
- Tyberg, A.; Sarkar, A.; Shahid, H.M.; Shah-Khan, S.M.; Gaidhane, M.; Simon, A.; Eisenberg, I.A.; Lajin, M.; Karagyozov, P.; Liao, K.; et al. EUS-guided biliary drainage versus ERCP in malignant biliary obstruction prior to hepatobiliary surgery: An international multicenter comparative study. Gastrointest. Endosc. 2022, 95, AB520. [Google Scholar] [CrossRef]
- Hayat, U.; Bakker, C.; Dirweesh, A.; Khan, M.Y.; Adler, D.G.; Okut, H.; Leul, N.; Bilal, M.; Siddiqui, A. EUS-guided versus percutaneous transhepatic cholangiography biliary drainage for obstructed distal malignant biliary strictures in patients who have failed endoscopic retrograde cholangiopancreatography: A systematic review and meta-analysis. Endosc. Ultrasound 2022, 11, 4. [Google Scholar] [CrossRef]
- Fabbri, C.; Binda, C.; Sbrancia, M.; Dajti, E.; Coluccio, C.; Ercolani, G.; Anderloni, A.; Cucchetti, A. Determinants of outcomes of transmural EUS-guided gallbladder drainage: Systematic review with proportion meta-analysis and meta-regression. Surg. Endosc. 2022, 36, 7974–7985. [Google Scholar] [CrossRef]
- Maire, F.; Sauvanet, A. Palliation of biliary and duodenal obstruction in patients with unresectable pancreatic cancer: Endoscopy or surgery? J. Visc. Surg. 2013, 150, S27–S31. [Google Scholar] [CrossRef]
- Pu, L.Z.C.T.; Singh, R.; Loong, C.K.; de Moura, E.G.H. Malignant biliary obstruction: Evidence for best practice. Gastroenterol. Res. Pract. 2016, 2016, 3296801. [Google Scholar] [CrossRef] [PubMed]
- Baron, T.H.; Topazian, M.D. Endoscopic transduodenal drainage of the gallbladder: Implications for endoluminal treatment of gallbladder disease. Gastrointest. Endosc. 2007, 65, 735–737. [Google Scholar] [CrossRef] [PubMed]
- Imai, H.; Kitano, M.; Omoto, S.; Kadosaka, K.; Kamata, K.; Miyata, T.; Yamao, K.; Sakamoto, H.; Harwani, Y.; Kudo, M. EUS-guided gallbladder drainage for rescue treatment of malignant distal biliary obstruction after unsuccessful ERCP. Gastrointest. Endosc. 2016, 84, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Mussetto, A.; Fugazza, A.; Fuccio, L.; Triossi, O.; Repici, A.; Anderloni, A. Current uses and outcomes of lumen-apposing metal stents. Ann. Gastroenterol. 2018, 31, 535. [Google Scholar] [PubMed]
- Anderloni, A.; Fugazza, A.; Troncone, E.; Auriemma, F.; Carrara, S.; Semeraro, R.; Maselli, R.; Di Leo, M.; D’amico, F.; Sethi, A.; et al. Single-stage EUS-guided choledochoduodenostomy using a lumen-apposing metal stent for malignant distal biliary obstruction. Gastrointest. Endosc. 2019, 89, 69–76. [Google Scholar] [CrossRef]
- Van Wanrooij, R.L.; Bronswijk, M.; Kunda, R.; Everett, S.M.; Lakhtakia, S.; Rimbas, M.; Hucl, T.; Badaoui, A.; Law, R.; Arcidiacono, P.G.; et al. Therapeutic endoscopic ultrasound: European Society of Gastrointestinal Endoscopy (ESGE) technical review. Endoscopy 2022, 54, 310–332. [Google Scholar] [CrossRef]
- Higa, J.T.; Irani, S.S. Endoscopic methods for gallbladder drainage. Curr. Treat. Options Gastroenterol. 2019, 17, 357–366. [Google Scholar] [CrossRef]
- Irani, S.S.; Sharzehi, K.; Siddiqui, U.D. AGA Clinical Practice Update on Role of EUS-Guided Gallbladder Drainage in Acute Cholecystitis: Commentary. Clin. Gastroenterol. Hepatol. 2023, 21, 1141–1147. [Google Scholar] [CrossRef]
- Paranandi, B.; Nayar, M.; Scott, J.; Charnley, R.; Wilson, C.; Oppong, K. Endoscopic cholecystogastrostomy in a patient with gallbladder empyema secondary to cholangiocarcinoma. Endoscopy 2016, 48 (Suppl. 1), E163. [Google Scholar] [CrossRef]
- Teoh, A.Y.; Kitano, M.; Itoi, T.; Pérez-Miranda, M.; Ogura, T.; Chan, S.M.; Serna-Higuera, C.; Omoto, S.; Torres-Yuste, R.; Tsuichiya, T.; et al. Endosonography-guided gallbladder drainage versus percutaneous cholecystostomy in very high-risk surgical patients with acute cholecystitis: An international randomised multicentre controlled superiority trial (DRAC 1). Gut 2020, 69, 1085–1091. [Google Scholar] [CrossRef] [PubMed]
- Issa, D.; Irani, S.; Law, R.; Shah, S.; Bhalla, S.; Mahadev, S.; Hajifathalian, K.; Sampath, K.; Mukewar, S.; Carr-Locke, D.L.; et al. Endoscopic ultrasound-guided gallbladder drainage as a rescue therapy for unresectable malignant biliary obstruction: A multicenter experience. Endoscopy 2021, 53, 827–831. [Google Scholar] [CrossRef] [PubMed]
- McInnes, M.D.; Moher, D.; Thombs, B.D.; McGrath, T.A.; Bossuyt, P.M.; Clifford, T.; Cohen, J.F.; Deeks, J.J.; Gatsonis, C.; Hooft, L.; et al. Preferred reporting items for a systematic review and meta-analysis of diagnostic test accuracy studies: The PRISMA-DTA statement. JAMA 2018, 319, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.I.; Dong, E.; Kwok, K.K. Endoscopic ultrasound-guided transmural gallbladder drainage in malignant obstruction using a novel lumen-apposing stent: A case series (with video). Endosc. Int. Open 2019, 7, E655–E661. [Google Scholar] [CrossRef] [PubMed]
- Chin, J.Y.-L.; Seleq, S.; Weilert, F. Safety and outcomes of endoscopic ultrasound-guided drainage for malignant biliary obstruction using cautery-enabled lumen-apposing metal stent. Endosc. Int. Open 2020, 8, E1633–E1638. [Google Scholar] [CrossRef] [PubMed]
- Paleti, S.; Gulati, R.; Sánchez-Luna, S.A.; Ling, C.; Rustagi, T. Su1191 EUS-guided gall bladder drainage using lumen apposing metal stent for malignant biliary obstruction. Gastrointest. Endosc. 2019, 89, AB308. [Google Scholar] [CrossRef]
- Binda, C.; Anderloni, A.; Fugazza, A.; Amato, A.; De Nucci, G.; Redaelli, A.; Di Mitri, R.; Cugia, L.; Pollino, V.; Macchiarelli, R.; et al. EUS-guided gallbladder drainage using lumen-apposing metal stent as rescue treatment for malignant distal biliary obstruction: A large multicenter experience. Endoscopy 2021, 53 (Suppl. 1), eP427. [Google Scholar]
- Lambin, T.; Branche, J.; Privat, J.; Bourgaux, J.; Koch, S.; Palazzo, M.; Chaput, U.; Ponchon, T.; Pioche, M.; Grandval, P.; et al. Efficacy and Safety of Eus-Guided Transmural Gallbladder Drainage In Malignant Biliary Obstruction Using an Electrocautery-Enhanced Lumen Apposing Metal Stent: A French Multicenter Study. Endoscopy 2021, 53 (Suppl. 1), OP94. [Google Scholar]
- Singh, S.M.; Reber, H.A. Surgical palliation for pancreatic cancer. Surg. Clin. N. Am. 1989, 69, 599–611. [Google Scholar] [CrossRef]
- Speer, A.; Christopher, R.; Russell, G.; Hatfield, A.W.; Macrae, K.; Cotton, P.; Mason, R.; Leung, J.; Houghton, J.; Lennon, C. Randomised trial of endoscopic versus percutaneous stent insertion in malignant obstructive jaundice. Lancet 1987, 330, 57–62. [Google Scholar] [CrossRef]
- Moss, A.C.; Morris, E.; MacMathuna, P. Palliative biliary stents for obstructing pancreatic carcinoma. Cochrane Database Syst. Rev. 2006, 2006, CD004200. [Google Scholar]
- Anderloni, A.; Buda, A.; Vieceli, F.; Khashab, M.A.; Hassan, C.; Repici, A. Endoscopic ultrasound-guided transmural stenting for gallbladder drainage in high-risk patients with acute cholecystitis: A systematic review and pooled analysis. Surg. Endosc. 2016, 30, 5200–5208. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Atiq, O.; Kubiliun, N.; Ali, B.; Kamal, F.; Nollan, R.; Ismail, M.K.; Tombazzi, C.; Kahaleh, M.; Baron, T.H. Efficacy and safety of endoscopic gallbladder drainage in acute cholecystitis: Is it better than percutaneous gallbladder drainage? Gastrointest. Endosc. 2017, 85, 76–87.e3. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Sun, S.; Guo, J.; Wang, S.; Ge, N.; Liu, X.; Wang, G. Retrievable puncture anchor traction method for EUS-guided gallbladder drainage: A porcine study. Gastrointest. Endosc. 2018, 88, 957–963. [Google Scholar] [CrossRef]
- Dhindsa, B.S.; Mashiana, H.S.; Dhaliwal, A.; Mohan, B.P.; Jayaraj, M.; Sayles, H.; Singh, S.; Ohning, G.; Bhat, I. EUS-guided biliary drainage: A systematic review and meta-analysis. Endosc. Ultrasound 2020, 9, 101. [Google Scholar] [PubMed]
- Park, J.K.; Woo, Y.S.; Noh, D.H.; Yang, J.-I.; Bae, S.Y.; Yun, H.S.; Lee, J.K.; Lee, K.T.; Lee, K.H. Efficacy of EUS-guided and ERCP-guided biliary drainage for malignant biliary obstruction: Prospective randomized controlled study. Gastrointest. Endosc. 2018, 88, 277–282. [Google Scholar] [CrossRef]
- McKay, A.; Abulfaraj, M.; Lipschitz, J. Short-and long-term outcomes following percutaneous cholecystostomy for acute cholecystitis in high-risk patients. Surg. Endosc. 2012, 26, 1343–1351. [Google Scholar] [CrossRef]

| N | Study Type | No. of Males (%) | Stent Type | Route of Access | Adverse Events | Clinical Success | |
|---|---|---|---|---|---|---|---|
| Issa et al., 2021 [21] | 28 | Retrospective | 15 (54) | LAMS: 26 (92%), SEMS: 2 (7%) | Transduodenal: 15, transgastric: 13 | 5 (18%) | 26 (93%) |
| Binda et al., 2021 [26] | 48 | Retrospective | 23 (48) | LAMS: 48 (100%) | Transduodenal: 20, transgastric: 28 | 5 (10%) | 39 (81%) |
| Lambin et al., 2021 [27] | 28 | Retrospective | N/A | LAMS: 28 (100%) | N/A | 3 (11%) | 22 (79%) |
| Chin et al., 2020 [24] | 4 | Retrospective | N/A | LAMS: 4 (100%) | N/A | 1 (25%) | 4 (100%) |
| Paleti et al., 2019 [25] | 7 | Retrospective | 5 (71) | LAMS: 7 (100%) | N/A | 0 | 7 (100%) |
| Chang et al., 2018 [23] | 9 | Retrospective | 5 (56) | LAMS: 9 (100%) | Transduodenal: 5, transgastric 4 | 0 | 7 (77.8%) |
| Imai et al., 2015 [13] | 12 | Retrospective | 8 (67) | SEMS: 12 (100%) | Transduodenal: 5, transgastric: 7 | 2 (17%) | (91.7%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McDonagh, P.; Awadelkarim, B.; Leeds, J.S.; Nayar, M.K.; Oppong, K.W. Endoscopic Ultrasound-Guided Gallbladder Drainage for Malignant Biliary Obstruction: A Systematic Review. Cancers 2023, 15, 2988. https://doi.org/10.3390/cancers15112988
McDonagh P, Awadelkarim B, Leeds JS, Nayar MK, Oppong KW. Endoscopic Ultrasound-Guided Gallbladder Drainage for Malignant Biliary Obstruction: A Systematic Review. Cancers. 2023; 15(11):2988. https://doi.org/10.3390/cancers15112988
Chicago/Turabian StyleMcDonagh, Padraic, Bidour Awadelkarim, John S. Leeds, Manu K. Nayar, and Kofi W. Oppong. 2023. "Endoscopic Ultrasound-Guided Gallbladder Drainage for Malignant Biliary Obstruction: A Systematic Review" Cancers 15, no. 11: 2988. https://doi.org/10.3390/cancers15112988
APA StyleMcDonagh, P., Awadelkarim, B., Leeds, J. S., Nayar, M. K., & Oppong, K. W. (2023). Endoscopic Ultrasound-Guided Gallbladder Drainage for Malignant Biliary Obstruction: A Systematic Review. Cancers, 15(11), 2988. https://doi.org/10.3390/cancers15112988






