Modeling the Tumor Microenvironment and Cancer Immunotherapy in Next-Generation Humanized Mice
Abstract
Simple Summary
Abstract
1. Introduction
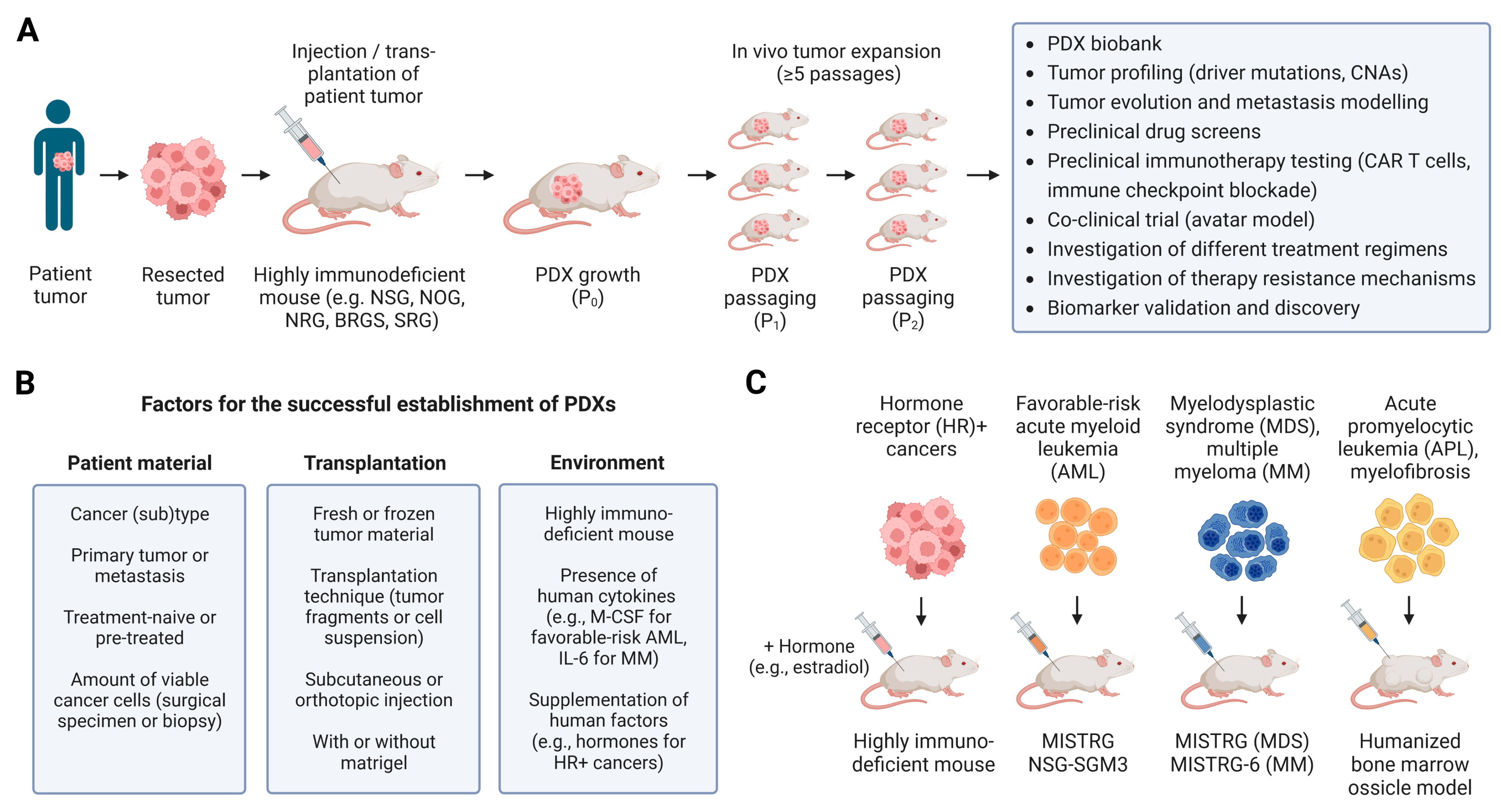
2. Establishing and Modeling Patient-Derived Tumors in Humanized Mice
| Mouse Model | Type of PDX | Number of Patients | PDX Engraftment Rate | References |
|---|---|---|---|---|
| Nude | CRC | 85 | 63.5% | [61] |
| NOD-SCID, nude | CRC | 48 | 71–74% | [96] |
| SCID | Uveal melanoma | 90 | 28% | [97] |
| NOD-SCID | Cervical cancer | 22 | 30.7% | [98] |
| CRC (metastatic) | 85 | 87% | [99] | |
| Esophageal squamous cell carcinoma | 25 | 13.3% | [100] | |
| Gastric cancer | 185 | 34.1% | [101] | |
| Leukemia (T-ALL) | 19 | 52% | [102] | |
| Liposarcoma | 56 | 44.6% | [103] | |
| NSCLC | 75 | 49% | [104] | |
| NOD-SCID, NSG, NRG | CRC | 10 | 46% (NOD-SCID) 90–91% (NSG, NRG) | [105] |
| Ovarian cancer (high-grade serous) | 43 | 76.7% | [106] | |
| NOD-SCID, NSG | PDAC | 35 | 90% (NSG) 40% (NOD-SCID) | [66] |
| NSG | 16 tumor types, such as bladder cancer, breast cancer, CRC, gastric cancer, glioblastoma, HCC, HNSCC, lung cancer, melanoma, ovarian cancer, PDAC, RCC, sarcoma | 324 PDXs | [70] | |
| Breast cancer | 83 | - | [56] | |
| CRC | 50 | 54% | [107] | |
| HNSCC | 115 | 45.2% | [108] | |
| Nasopharyngeal carcinoma | 37 | 18.9% | [109] | |
| Testicular cancer | 8 | 38% | [110] | |
| Melanoma | 694 | 62% | [76] | |
| Leukemia, lymphoma | 138 PDXs | 67.5% (B-ALL), 46.7% (T-ALL), 23.2% (AML) | [79] | |
| NSG, NRG | Breast cancer | 102 | 25% (P), 36% (M); 9% (ER+ P), 16% (ER+ M); 25% (HER2+ P), 33% (HER2+ M); 29% (TNBC P) | [69] |
| NSG, NOG | Prostate cancer | 48 | 0% | [111] |
| NOG | 10 tumor types, such as breast cancer, CRC, lung cancer, PDAC and RCC | 116 | 53% | [112] |
| Gastric cancer | 62 | 24.2% | [113] | |
| CLL | 7 PDXs | - | [114] | |
| ALL | 60 | 93.3% | [115] | |
| NOG, NOG-IL2 | Metastatic melanoma | 21 | 95% (lower engraftment in NOG-IL2) | [81] |
| NOG-EXL | AML | 26 | 62% | [116] |
| NSG, NSG-SGM3 | AML | 77 | 82% (NSG-SGM3) 50% (NSG) | [86] |
| AML | 8 | 62.5% (NSG-SGM3) 37.5% (NSG) | [117] | |
| NSG, MISTRG | Favorable-risk AML | 9 | 68% (MISTRG) 0–20% (NSG) | [11] |
| MDS | 31 | Higher engraftment in MISTRG | [17] | |
| SRG-6, MISTRG-6 | MM | 30 | Higher engraftment in MISTRG-6 | [16] |
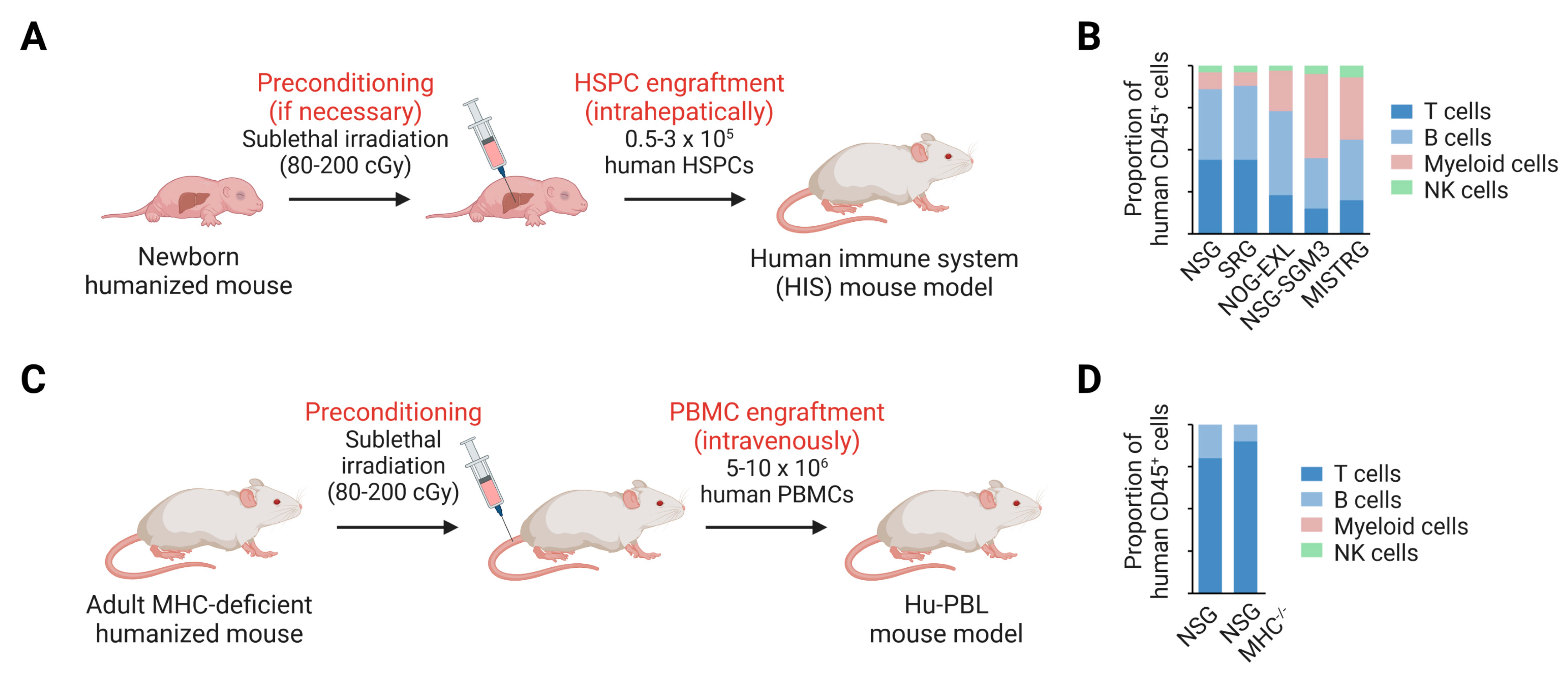
3. Human Immune System Development and Function in Next-Generation Humanized Mice
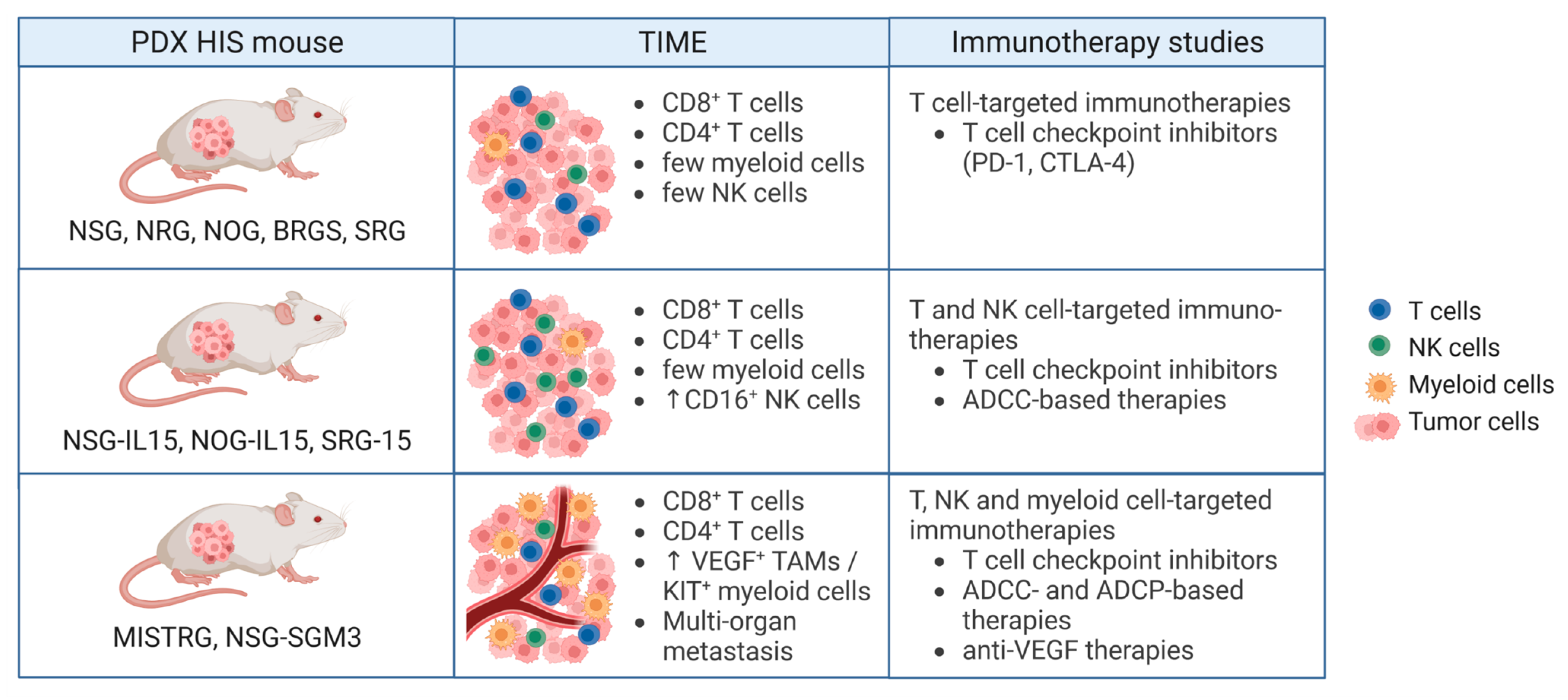
4. Humanized PDX Mouse Models for Preclinical Testing of Cancer Immunotherapies
| Mouse Model | Type of PDX | Type of Immunotherapy | Human Immune System Reconstitution | References |
|---|---|---|---|---|
| NOD-SCID | NSCLC | Non-autologous PBMCs + anti-PD-L1 | PBMCs | [104] |
| NSG | ALL | Anti-CD19 antibody; anti-CD19-TRAIL fusion antibody | No | [168,169] |
| Bladder cancer, NSCLC, sarcoma, TNBC | Partially HLA-matched HSPCs + anti-PD-1 antibody | HSPCs | [170] | |
| CLL | Autologous PBMCs + anti-CD38 antibody | PBMCs | [171] | |
| Dedifferentiated liposarcoma | Anti-PD-1 antibody | HSPCs | [172] | |
| Gastric cancer | Mesothelin-specific CAR NK cells | No | [173] | |
| HCC | Partially HLA-A/B-matched HSPCs + anti-PD-1 or anti-CTLA-4 | HSPCs | [174] | |
| HNSCC | Adoptive transfer of NK cells + anti-PCNA antibody | No | [175] | |
| Nasopharyngeal carcinoma | Anti-PD-1, anti-CTLA-4 | HSPCs | [109] | |
| Neuroblastoma | Adoptive transfer of activated NK cells + anti-GD2 antibody | No | [176] | |
| Ovarian cancer | Autologous TILs + anti-PD-1 antibody | TILs | [177] | |
| TNBC | ACT (cytokine-induced killer cells) | No | [178] | |
| NRG | HNSCC | Radio-immunotherapy: anti-EGFR Ab labeled with 177Lu | No | [179] |
| BRG | Osteosarcoma | GD2- or HER2-targeting BiTE antibody ± anti-PD-1 or anti-PD-L1 antibody | No | [180] |
| BRGS | ACC, CRC, melanoma, PDAC, SCLC, TNBC | Anti-PD-1 therapy ± TKIs/WNTi/ VEGFi/HDACi | HSPCs | [132] |
| CRC | Anti-PD-1 ± cabozantinib, anti-PD-L1 + cobimetinib | HSPCs | [181] | |
| CRC | Anti-PD-1 antibody | HSPCs | [182] | |
| ACC | Anti-PD-1 antibody | HSPCs | [183] | |
| NOG-IL2 | Metastatic melanoma | ACT | Patient TILs | [184] |
| NOG, NOG-IL2 | Metastatic melanoma | ACT, anti-PD-1 antibody | Patient TILs | [81] |
| NOG-IL2 | Uveal melanoma | Anti-HER2 CAR T cells | No | [185] |
| NOG-EXL | AML | Co-clinical trial with BETi mivebresib | No | [116] |
| NOG-EXL, NSG-SGM3 | Breast cancer | TLR7/8 agonist activates tumor-infiltrating pDCs | HSPCs | [186] |
| NSG-SGM3 | B-ALL | Anti-PD-1 + anti-CD19 bi-specific T cell engager | HSPCs | [187] |
| MISTRG | Melanoma (CDX) | Anti-VEGF antibody | [18] | |
| AML | Anti-CD117 CAR T cells | HSPCs | [188] | |
| AML | Adoptive transfer of CBFB-MYH11-specific T cells | No | [189] | |
| HLA-deficient neuroblastoma | Anti-GD2 antibody | HSPCs | [190] | |
| MISTRG-6 | DLBCL | Anti-IL-6R antibody | HSPCs | [191] |
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, Z.; Denton, P.W.; Estes, J.D.; Othieno, F.A.; Wei, B.L.; Wege, A.K.; Melkus, M.W.; Padgett-Thomas, A.M.; Zupancic, A.; Haase, T.; et al. Intrarectal Transmission, Systemic Infection, and Cd4+ T Cell Depletion in Humanized Mice Infected with Hiv-1. J. Exp. Med. 2007, 204, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Deng, K.; Pertea, M.; Rongvaux, A.; Wang, L.; Durand, C.M.; Ghiaur, G.; Lai, J.; McHugh, H.L.; Hao, H.; Zhang, H.; et al. Broad Ctl Response Is Required to Clear Latent Hiv-1 Due to Dominance of Escape Mutations. Nature 2015, 517, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Sefik, E.; Israelow, B.; Mirza, H.; Zhao, J.; Qu, R.; Kaffe, E.; Song, E.; Halene, S.; Meffre, E.; Kluger, Y.; et al. A Humanized Mouse Model of Chronic COVID-19. Nat. Biotechnol. 2022, 40, 906–920. [Google Scholar] [CrossRef] [PubMed]
- Sefik, E.; Qu, R.; Junqueira, C.; Kaffe, E.; Mirza, H.; Zhao, J.; Brewer, J.R.; Han, A.; Steach, H.R.; Israelow, B.; et al. Inflammasome Activation in Infected Macrophages Drives Covid-19 Pathology. Nature 2022, 606, 585–593. [Google Scholar] [CrossRef]
- Bissig, K.-D.; Wieland, S.F.; Tran, P.; Isogawa, M.; Le, T.T.; Chisari, F.; Verma, I.M. Human Liver Chimeric Mice Provide a Model for Hepatitis B and C Virus Infection and Treatment. J. Clin. Investig. 2010, 120, 924–930. [Google Scholar] [CrossRef]
- Dorner, M.; Horwitz, J.A.; Donovan, B.M.; Labitt, R.N.; Budell, W.C.; Friling, T.; Vogt, A.; Catanese, M.T.; Satoh, T.; Kawai, T.; et al. Completion of the Entire Hepatitis C Virus Life Cycle in Genetically Humanized Mice. Nature 2013, 501, 237–241. [Google Scholar] [CrossRef]
- Traggiai, E.; Chicha, L.; Mazzucchelli, L.; Bronz, L.; Piffaretti, J.-C.; Lanzavecchia, A.; Manz, M.G. Development of a Human Adaptive Immune System in Cord Blood Cell-Transplanted Mice. Science 2004, 304, 104–107. [Google Scholar] [CrossRef]
- Jaiswal, S.; Pearson, T.; Friberg, H.; Shultz, L.D.; Greiner, D.L.; Rothman, A.L.; Mathew, A. Dengue Virus Infection and Virus-Specific Hla-A2 Restricted Immune Responses in Humanized Nod-Scid Il2rgammanull Mice. PLoS ONE 2009, 4, e7251. [Google Scholar] [CrossRef]
- Song, Y.; Shan, L.; Gbyli, R.; Liu, W.; Strowig, T.; Patel, A.; Fu, X.; Wang, X.; Xu, M.L.; Gao, Y.; et al. Combined Liver-Cytokine Humanization Comes to the Rescue of Circulating Human Red Blood Cells. Science 2021, 371, 1019–1025. [Google Scholar] [CrossRef]
- Barabé, F.; Kennedy, J.A.; Hope, K.J.; Dick, J.E. Modeling the Initiation and Progression of Human Acute Leukemia in Mice. Science 2007, 316, 600–604. [Google Scholar] [CrossRef]
- Ellegast, J.M.; Rauch, P.J.; Kovtonyuk, L.V.; Müller, R.; Wagner, U.; Saito, Y.; van Wijk, N.W.-V.; Fritz, C.; Rafiei, A.; Lysenko, V.; et al. Inv(16) and Npm1mut Amls Engraft Human Cytokine Knock-in Mice. Blood 2016, 128, 2130–2134. [Google Scholar] [CrossRef]
- Saito, Y.; Mochizuki, Y.; Ogahara, I.; Watanabe, T.; Hogdal, L.; Takagi, S.; Sato, K.; Kaneko, A.; Kajita, H.; Uchida, N.; et al. Overcoming Mutational Complexity in Acute Myeloid Leukemia by Inhibition of Critical Pathways. Sci. Transl. Med. 2017, 9, eaao1214. [Google Scholar] [CrossRef]
- Dobrovolsky, D.; Wang, E.S.; Morrow, S.; Leahy, C.; Faust, T.; Nowak, R.P.; Donovan, K.A.; Yang, G.; Li, Z.; Fischer, E.S.; et al. Bruton Tyrosine Kinase Degradation as a Therapeutic Strategy for Cancer. Blood 2019, 133, 952–961. [Google Scholar] [CrossRef] [PubMed]
- Herndler-Brandstetter, D.; Shan, L.; Yao, Y.; Stecher, C.; Plajer, V.; Lietzenmayer, M.; Strowig, T.; de Zoete, M.R.; Palm, N.W.; Chen, J.; et al. Humanized Mouse Model Supports Development, Function, and Tissue Residency of Human Natural Killer Cells. Proc. Natl. Acad. Sci. USA 2017, 114, E9626–E9634. [Google Scholar] [CrossRef] [PubMed]
- Matsui, W.; Huff, C.A.; Wang, Q.; Malehorn, M.T.; Barber, J.; Tanhehco, Y.; Smith, B.D.; Civin, C.I.; Jones, R.J. Characterization of Clonogenic Multiple Myeloma Cells. Blood 2004, 103, 2332–2336. [Google Scholar] [CrossRef]
- Das, R.; Strowig, T.; Verma, R.; Koduru, S.; Hafemann, A.; Hopf, S.; Kocoglu, M.H.; Borsotti, C.; Zhang, L.; Branagan, A.; et al. Microenvironment-Dependent Growth of Preneoplastic and Malignant Plasma Cells in Humanized Mice. Nat. Med. 2016, 22, 1351–1357. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Rongvaux, A.; Taylor, A.; Jiang, T.; Tebaldi, T.; Balasubramanian, K.; Bagale, A.; Terzi, Y.K.; Gbyli, R.; Wang, X.; et al. A Highly Efficient and Faithful Mds Patient-Derived Xenotransplantation Model for Pre-Clinical Studies. Nat. Commun. 2019, 10, 366. [Google Scholar] [CrossRef]
- Rongvaux, A.; Willinger, T.; Martinek, J.; Strowig, T.; Gearty, S.; Teichmann, L.L.; Saito, Y.; Marches, F.; Halene, S.; Palucka, A.K.; et al. Development and Function of Human Innate Immune Cells in a Humanized Mouse Model. Nat. Biotechnol. 2014, 32, 364–372. [Google Scholar] [CrossRef]
- Voillet, V.; Berger, T.R.; McKenna, K.M.; Paulson, K.G.; Tan, W.H.; Smythe, K.S.; Hunter, D.S.; Valente, W.J.; Weaver, S.; Campbell, J.S.; et al. An In Vivo Model of Human Macrophages in Metastatic Melanoma. J. Immunol. 2022, 209, 606–620. [Google Scholar] [CrossRef]
- Odunsi, A.; McGray, A.J.R.; Miliotto, A.; Zhang, Y.; Wang, J.; Abiola, A.; Eppolito, C.; Huang, R.-Y. Fidelity of Human Ovarian Cancer Patient-Derived Xenografts in a Partially Humanized Mouse Model for Preclinical Testing of Immunotherapies. J. Immunother. Cancer 2020, 8, e001237. [Google Scholar] [CrossRef]
- Norelli, M.; Camisa, B.; Barbiera, G.; Falcone, L.; Purevdorj, A.; Genua, M.; Sanvito, F.; Ponzoni, M.; Doglioni, C.; Cristofori, P.; et al. Monocyte-Derived Il-1 and Il-6 Are Differentially Required for Cytokine-Release Syndrome and Neurotoxicity Due to Car T Cells. Nat. Med. 2018, 24, 739–748. [Google Scholar] [CrossRef]
- Ganesh, K.; Stadler, Z.K.; Cercek, A.; Mendelsohn, R.B.; Shia, J.; Segal, N.H.; Diaz, L.A., Jr. Immunotherapy in Colorectal Cancer: Rationale, Challenges and Potential. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 361–375. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.H.; Siah, K.W.; Lo, A.W. Estimation of Clinical Trial Success Rates and Related Parameters. Biostatistics 2019, 20, 273–286. [Google Scholar] [CrossRef] [PubMed]
- De La Rochere, P.; Guil-Luna, S.; Decaudin, D.; Azar, G.; Sidhu, S.S.; Piaggio, E. Humanized Mice for the Study of Immuno-Oncology. Trends Immunol. 2018, 39, 748–763. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, S.P. ‘Nude’, a New Hairless Gene with Pleiotropic Effects in the Mouse. Genet. Res. 1966, 8, 295–309. [Google Scholar] [CrossRef]
- Bosma, G.C.; Custer, R.P.; Bosma, M.J. A Severe Combined Immunodeficiency Mutation in the Mouse. Nature 1983, 301, 527–530. [Google Scholar] [CrossRef] [PubMed]
- Shultz, L.D.; Schweitzer, P.A.; Christianson, S.W.; Gott, B.; Schweitzer, I.B.; Tennent, B.; McKenna, S.; Mobraaten, L.; Rajan, T.V.; Greiner, D.L. Multiple Defects in Innate and Adaptive Immunologic Function in Nod/Ltsz-Scid Mice. J. Immunol. 1950, 154, 180–191. [Google Scholar] [CrossRef]
- Ito, M.; Hiramatsu, H.; Kobayashi, K.; Suzue, K.; Kawahata, M.; Hioki, K.; Ueyama, Y.; Koyanagi, Y.; Sugamura, K.; Tsuji, K.; et al. Nod/Scid/Gamma(C)(Null) Mouse: An Excellent Recipient Mouse Model for Engraftment of Human Cells. Blood 2002, 100, 3175–3182. [Google Scholar] [CrossRef]
- Shultz, L.D.; Lyons, B.L.; Burzenski, L.M.; Gott, B.; Chen, X.; Chaleff, S.; Kotb, M.; Gillies, S.D.; King, M.; Mangada, J.; et al. Human Lymphoid and Myeloid Cell Development in Nod/Ltsz-Scid Il2r Gamma Null Mice Engrafted with Mobilized Human Hemopoietic Stem Cells. J. Immunol. 2005, 174, 6477–6489. [Google Scholar] [CrossRef]
- Dewan, M.Z.; Terashima, K.; Taruishi, M.; Hasegawa, H.; Ito, M.; Tanaka, Y.; Mori, N.; Sata, T.; Koyanagi, Y.; Maeda, M.; et al. Rapid Tumor Formation of Human T-Cell Leukemia Virus Type 1-Infected Cell Lines in Novel Nod-Scid/Gammac(Null) Mice: Suppression by an Inhibitor against Nf-Kappab. J. Virol. 2003, 77, 5286–5294. [Google Scholar] [CrossRef]
- Pearson, T.; Shultz, L.D.; Miller, D.; King, M.; Laning, J.; Fodor, W.; Cuthbert, A.; Burzenski, L.; Gott, B.; Lyons, B.; et al. Non-Obese Diabetic-Recombination Activating Gene-1 (Nod-Rag1 Null) Interleukin (Il)-2 Receptor Common Gamma Chain (Il2r Gamma Null) Null Mice: A Radioresistant Model for Human Lymphohaematopoietic Engraftment. Clin. Exp. Immunol. 2008, 154, 270–284. [Google Scholar] [CrossRef] [PubMed]
- Oettinger, M.A.; Schatz, D.G.; Gorka, C.; Baltimore, D. RAG-1 and RAG-2, Adjacent Genes That Synergistically Activate V(D)J Recombination. Science 1990, 248, 1517–1523. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, K.; Prasolava, T.K.; Wang, J.C.; Mortin-Toth, S.M.; Khalouei, S.; Gan, O.I.; Dick, J.E.; Danska, J.S. Polymorphism in Sirpa Modulates Engraftment of Human Hematopoietic Stem Cells. Nat. Immunol. 2007, 8, 1313–1323. [Google Scholar] [CrossRef]
- Strowig, T.; Rongvaux, A.; Rathinam, C.; Takizawa, H.; Borsotti, C.; Philbrick, W.; Eynon, E.E.; Manz, M.G.; Flavell, R.A. Transgenic Expression of Human Signal Regulatory Protein Alpha in Rag2-/-Gamma(C)-/- Mice Improves Engraftment of Human Hematopoietic Cells in Humanized Mice. Proc. Natl. Acad. Sci. USA 2011, 108, 13218–13223. [Google Scholar] [CrossRef]
- Yamauchi, T.; Takenaka, K.; Urata, S.; Shima, T.; Kikushige, Y.; Tokuyama, T.; Iwamoto, C.; Nishihara, M.; Iwasaki, H.; Miyamoto, T.; et al. Polymorphic Sirpa Is the Genetic Determinant for Nod-Based Mouse Lines to Achieve Efficient Human Cell Engraftment. Blood 2013, 121, 1316–1325. [Google Scholar] [CrossRef] [PubMed]
- Mian, S.A.; Anjos-Afonso, F.; Bonnet, D. Advances in Human Immune System Mouse Models for Studying Human Hematopoiesis and Cancer Immunotherapy. Front. Immunol. 2021, 11, 619236. [Google Scholar] [CrossRef]
- Devoy, A.; Bunton-Stasyshyn, R.K.A.; Tybulewicz, V.L.J.; Smith, A.J.H.; Fisher, E.M.C. Genomically Humanized Mice: Technologies and Promises. Nat. Rev. Genet. 2011, 13, 14–20. [Google Scholar] [CrossRef]
- Zhu, F.; Nair, R.R.; Fisher, E.M.C.; Cunningham, T.J. Humanising the Mouse Genome Piece by Piece. Nat. Commun. 2019, 10, 1845. [Google Scholar] [CrossRef]
- Coppin, E.; Sundarasetty, B.S.; Rahmig, S.; Blume, J.; Verheyden, N.A.; Bahlmann, F.; Ravens, S.; Schubert, U.; Schmid, J.; Ludwig, S.; et al. Enhanced Differentiation of Functional Human T Cells in Nsgw41 Mice with Tissue-Specific Expression of Human Interleukin-7. Leukemia 2021, 35, 3561–3567. [Google Scholar] [CrossRef]
- Matsuda, M.; Ono, R.; Iyoda, T.; Endo, T.; Iwasaki, M.; Tomizawa-Murasawa, M.; Saito, Y.; Kaneko, A.; Shimizu, K.; Yamada, D.; et al. Human Nk Cell Development in Hil-7 and Hil-15 Knockin Nod/Scid/Il2rgko Mice. Life Sci. Alliance 2019, 2, e201800195. [Google Scholar] [CrossRef]
- Aryee, K.E.; Burzenski, L.M.; Yao, L.C.; Keck, J.G.; Greiner, D.L.; Shultz, L.D.; Brehm, M.A. Enhanced Development of Functional Human Nk Cells in Nod-Scid-Il2rg(Null) Mice Expressing Human Il15. FASEB J. 2022, 36, e22476. [Google Scholar] [CrossRef] [PubMed]
- Katano, I.; Nishime, C.; Ito, R.; Kamisako, T.; Mizusawa, T.; Ka, Y.; Ogura, T.; Suemizu, H.; Kawakami, Y.; Ito, M.; et al. Long-Term Maintenance of Peripheral Blood Derived Human Nk Cells in a Novel Human Il-15- Transgenic Nog Mouse. Sci. Rep. 2017, 7, 17230. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Masse-Ranson, G.; Garcia, Z.; Bruel, T.; Kök, A.; Strick-Marchand, H.; Jouvion, G.; Serafini, N.; Lim, A.I.; Dusseaux, M.; et al. A Human Immune System Mouse Model with Robust Lymph Node Development. Nat. Methods 2018, 15, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Shultz, L.D.; Saito, Y.; Najima, Y.; Tanaka, S.; Ochi, T.; Tomizawa, M.; Ishikawa, F. Generation of Functional Human T-Cell Subsets with Hla-Restricted Immune Responses in Hla Class I Expressing Nod/Scid/Il2r Gamma(Null) Humanized Mice. Proc. Natl. Acad. Sci. USA 2010, 107, 13022–13027. [Google Scholar] [CrossRef]
- Mori, A.; Murata, S.; Tashiro, N.; Tadokoro, T.; Okamoto, S.; Otsuka, R.; Wada, H.; Murata, T.; Takahashi, T.; Seino, K.-I.; et al. Establishment of Human Leukocyte Antigen-Mismatched Immune Responses after Transplantation of Human Liver Bud in Humanized Mouse Models. Cells 2021, 10, 476. [Google Scholar] [CrossRef]
- Majji, S.; Wijayalath, W.; Shashikumar, S.; Pow-Sang, L.; Villasante, E.; Brumeanu, T.D.; Casares, S. Differential Effect of Hla Class-I Versus Class-Ii Transgenes on Human T and B Cell Reconstitution and Function in Nrg Mice. Sci. Rep. 2016, 6, 28093. [Google Scholar] [CrossRef]
- Labarthe, L.; Henriquez, S.; Lambotte, O.; Di Santo, J.P.; Le Grand, R.; Pflumio, F.; Arcangeli, M.-L.; Legrand, N.; Bourgeois, C. Frontline Science: Exhaustion and Senescence Marker Profiles on Human T Cells in Brgsf-A2 Humanized Mice Resemble Those in Human Samples. J. Leukoc. Biol. 2020, 107, 27–42. [Google Scholar] [CrossRef]
- Masse-Ranson, G.; Dusséaux, M.; Fiquet, O.; Darche, S.; Boussand, M.; Li, Y.; Lopez-Lastra, S.; Legrand, N.; Corcuff, E.; Toubert, A.; et al. Accelerated Thymopoiesis and Improved T-Cell Responses in Hla-A2/-Dr2 Transgenic Brgs-Based Human Immune System Mice. Eur. J. Immunol. 2019, 49, 954–965. [Google Scholar] [CrossRef]
- Billerbeck, E.; Horwitz, J.A.; Labitt, R.N.; Donovan, B.M.; Vega, K.; Budell, W.C.; Koo, G.C.; Rice, C.M.; Ploss, A. Characterization of Human Antiviral Adaptive Immune Responses during Hepatotropic Virus Infection in HLA-Transgenic Human Immune System Mice. J. Immunol. 2013, 191, 1753–1764. [Google Scholar] [CrossRef]
- Suzuki, M.; Takahashi, T.; Katano, I.; Ito, R.; Ito, M.; Harigae, H.; Ishii, N.; Sugamura, K. Induction of Human Humoral Immune Responses in a Novel Hla-Dr-Expressing Transgenic Nod/Shi-Scid/Gammacnull Mouse. Int. Immunol. 2012, 24, 243–252. [Google Scholar] [CrossRef]
- Serr, I.; Fürst, R.W.; Achenbach, P.; Scherm, M.G.; Gökmen, F.; Haupt, F.; Sedlmeier, E.-M.; Knopff, A.; Shultz, L.; Willis, R.A.; et al. Type 1 Diabetes Vaccine Candidates Promote Human Foxp3(+)Treg Induction in Humanized Mice. Nat. Commun. 2016, 7, 10991. [Google Scholar] [CrossRef]
- Johnson, J.I.; Decker, S.; Zaharevitz, D.; Rubinstein, L.V.; Venditti, J.M.; Schepartz, S.; Kalyandrug, S.; Christian, M.; Arbuck, S.; Hollingshead, M.; et al. Relationships between Drug Activity in Nci Preclinical in Vitro and in Vivo Models and Early Clinical Trials. Br. J. Cancer 2001, 84, 1424–1431. [Google Scholar] [CrossRef]
- Gillet, J.-P.; Calcagno, A.M.; Varma, S.; Marino, M.; Green, L.J.; Vora, M.I.; Patel, C.; Orina, J.N.; Eliseeva, T.A.; Singal, V.; et al. Redefining the Relevance of Established Cancer Cell Lines to the Study of Mechanisms of Clinical Anti-Cancer Drug Resistance. Proc. Natl. Acad. Sci. USA 2011, 108, 18708–18713. [Google Scholar] [CrossRef] [PubMed]
- Dangles-Marie, V.; Pocard, M.; Richon, S.; Weiswald, L.-B.; Assayag, F.; Saulnier, P.; Judde, J.-G.; Janneau, J.-L.; Auger, N.; Validire, P.; et al. Establishment of Human Colon Cancer Cell Lines from Fresh Tumors versus Xenografts: Comparison of Success Rate and Cell Line Features. Cancer Res. 2007, 67, 398–407. [Google Scholar] [CrossRef] [PubMed]
- Rückert, F.; Aust, D.; Böhme, I.; Werner, K.; Brandt, A.; Diamandis, E.P.; Krautz, C.; Hering, S.; Saeger, H.-D.; Grützmann, R.; et al. Five Primary Human Pancreatic Adenocarcinoma Cell Lines Established by the Outgrowth Method. J. Surg. Res. 2012, 172, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Bruna, A.; Rueda, O.M.; Greenwood, W.; Batra, A.S.; Callari, M.; Batra, R.N.; Pogrebniak, K.; Sandoval, J.; Cassidy, J.W.; Tufegdzic-Vidakovic, A.; et al. A Biobank of Breast Cancer Explants with Preserved Intra-tumor Heterogeneity to Screen Anticancer Compounds. Cell 2016, 167, 260–274.e22. [Google Scholar] [CrossRef]
- Qiu, Z.; Li, H.; Zhang, Z.; Zhu, Z.; He, S.; Wang, X.; Wang, P.; Qin, J.; Zhuang, L.; Wang, W.; et al. A Pharmacogenomic Landscape in Human Liver Cancers. Cancer Cell 2019, 36, 179–193.e11. [Google Scholar] [CrossRef]
- Weeber, F.; Ooft, S.N.; Dijkstra, K.K.; Voest, E.E. Tumor Organoids as a Pre-clinical Cancer Model for Drug Discovery. Cell Chem. Biol. 2017, 24, 1092–1100. [Google Scholar] [CrossRef]
- Marusyk, A.; Janiszewska, M.; Polyak, K. Intratumor Heterogeneity: The Rosetta Stone of Therapy Resistance. Cancer Cell 2020, 37, 471–484. [Google Scholar] [CrossRef]
- Pearson, A.T.; Finkel, K.A.; Warner, K.A.; Nör, F.; Tice, D.; Martins, M.D.; Jackson, T.L.; Nör, J.E. Patient-Derived Xenograft (Pdx) Tumors Increase Growth Rate with Time. Oncotarget 2016, 7, 7993–8005. [Google Scholar] [CrossRef]
- Julien, S.; Merino-Trigo, A.; Lacroix, L.; Pocard, M.; Goéré, D.; Mariani, P.; Landron, S.; Bigot, L.; Nemati, F.; Dartigues, P.; et al. Characterization of a Large Panel of Patient-Derived Tumor Xenografts Representing the Clinical Heterogeneity of Human Colorectal Cancer. Clin. Cancer Res. 2012, 18, 5314–5328. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Li, L.; Lu, T.; Yoo, H.; Zhu, J.; Gopal, P.; Wang, S.C.; Porembka, M.R.; Rich, N.E.; Kagan, S.; et al. Uncovering Biological Factors That Regulate Hepatocellular Carcinoma Growth Using Patient-Derived Xenograft Assays. Hepatology 2020, 72, 1085–1101. [Google Scholar] [CrossRef] [PubMed]
- Byrne, A.T.; Alférez, D.G.; Amant, F.; Annibali, D.; Arribas, J.; Biankin, A.V.; Bruna, A.; Budinská, E.; Caldas, C.; Chang, D.K.; et al. Interrogating Open Issues in Cancer Precision Medicine with Patient-Derived Xenografts. Nat. Rev. Cancer 2017, 17, 254–268. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.; Jiang, Z.; Li, G.-X.; Xiao, Y.; Lin, S.; Lai, Y.; Wang, S.; Li, B.; Jia, B.; Li, Y.; et al. Quantitative Evaluation of the Immunodeficiency of a Mouse Strain by Tumor Engraftments. J. Hematol. Oncol. 2015, 8, 59. [Google Scholar] [CrossRef] [PubMed]
- Okada, S.; Vaeteewoottacharn, K.; Kariya, R. Application of Highly Immunocompromised Mice for the Establishment of Patient-Derived Xenograft (Pdx) Models. Cells 2019, 8, 889. [Google Scholar] [CrossRef] [PubMed]
- Magouliotis, D.E.; Lafazanis, K.; Koutsougianni, F.; Sakellaridis, N.; Ioannou, M.; Zacharoulis, D.; Dimas, K. Establishment of Patient-derived Orthotopic Xenografts (PDX) as Models for Pancreatic Ductal Adenocarcinoma. In Vivo 2022, 36, 1114–1119. [Google Scholar] [CrossRef]
- Hidalgo, M.; Amant, F.; Biankin, A.V.; Budinská, E.; Byrne, A.T.; Caldas, C.; Clarke, R.B.; de Jong, S.; Jonkers, J.; Mælandsmo, G.M.; et al. Patient-Derived Xenograft Models: An Emerging Platform for Translational Cancer Research. Cancer Discov. 2014, 4, 998–1013. [Google Scholar] [CrossRef]
- Koga, Y.; Ochiai, A. Systematic Review of Patient-Derived Xenograft Models for Preclinical Studies of Anti-Cancer Drugs in Solid Tumors. Cells 2019, 8, 418. [Google Scholar] [CrossRef]
- Guillen, K.P.; Fujita, M.; Butterfield, A.J.; Scherer, S.D.; Bailey, M.H.; Chu, Z.; DeRose, Y.S.; Zhao, L.; Cortes-Sanchez, E.; Yang, C.-H.; et al. A Human Breast Cancer-Derived Xenograft and Organoid Platform for Drug Discovery and Precision Oncology. Nat. Cancer 2022, 3, 232–250. [Google Scholar] [CrossRef]
- Woo, X.Y.; Giordano, J.; Srivastava, A.; Zhao, Z.M.; Lloyd, M.W.; de Bruijn, R.; Suh, Y.S.; Patidar, R.; Chen, L.; Scherer, S.; et al. Conservation of Copy Number Profiles During Engraftment and Passaging of Patient-Derived Cancer Xenografts. Nat. Genet. 2021, 53, 86–99. [Google Scholar] [CrossRef]
- Zanella, E.R.; Grassi, E.; Trusolino, L. Towards Precision Oncology with Patient-Derived Xenografts. Nat. Rev. Clin. Oncol. 2022, 19, 719–732. [Google Scholar] [CrossRef] [PubMed]
- Abdirahman, S.M.; Christie, M.; Preaudet, A.; Burstroem, M.C.U.; Mouradov, D.; Lee, B.; Sieber, O.M.; Putoczki, T.L. A Biobank of Colorectal Cancer Patient-Derived Xenografts. Cancers 2020, 12, 2340. [Google Scholar] [CrossRef]
- Gao, H.; Korn, J.M.; Ferretti, S.; Monahan, J.E.; Wang, Y.; Singh, M.; Zhang, C.; Schnell, C.; Yang, G.; Zhang, Y.; et al. High-Throughput Screening Using Patient-Derived Tumor Xenografts to Predict Clinical Trial Drug Response. Nat. Med. 2015, 21, 1318–1325. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Claerhout, S.; Prat, A.; Dobrolecki, L.E.; Petrovic, I.; Lai, Q.; Landis, M.D.; Wiechmann, L.; Schiff, R.; Giuliano, M.; et al. A Renewable Tissue Resource of Phenotypically Stable, Biologically and Ethnically Diverse, Patient-Derived Human Breast Cancer Xenograft Models. Cancer Res. 2013, 73, 4885–4897. [Google Scholar] [CrossRef] [PubMed]
- Savage, P.; Pacis, A.; Kuasne, H.; Liu, L.; Lai, D.; Wan, A.; Dankner, M.; Martinez, C.; Muñoz-Ramos, V.; Pilon, V.; et al. Chemogenomic Profiling of Breast Cancer Patient-Derived Xenografts Reveals Targetable Vulnerabilities for Difficult-to-Treat Tumors. Commun. Biol. 2020, 3, 310. [Google Scholar] [CrossRef]
- Krepler, C.; Sproesser, K.; Brafford, P.; Beqiri, M.; Garman, B.; Xiao, M.; Shannan, B.; Watters, A.; Perego, M.; Zhang, G.; et al. A Comprehensive Patient-Derived Xenograft Collection Representing the Heterogeneity of Melanoma. Cell Rep. 2017, 21, 1953–1967. [Google Scholar] [CrossRef]
- Caeser, R.; Egger, J.V.; Chavan, S.; Socci, N.D.; Jones, C.B.; Kombak, F.E.; Asher, M.; Roehrl, M.H.; Shah, N.S.; Allaj, V.; et al. Genomic and Transcriptomic Analysis of a Library of Small Cell Lung Cancer Patient-Derived Xenografts. Nat. Commun. 2022, 13, 2144. [Google Scholar] [CrossRef]
- Risbridger, G.P.; Clark, A.K.; Porter, L.H.; Toivanen, R.; Bakshi, A.; Lister, N.L.; Pook, D.; Pezaro, C.J.; Sandhu, S.; Keerthikumar, S.; et al. The Mural Collection of Prostate Cancer Patient-Derived Xenografts Enables Discovery through Preclinical Models of Uro-Oncology. Nat. Commun. 2021, 12, 5049. [Google Scholar] [CrossRef]
- Townsend, E.C.; Murakami, M.A.; Christodoulou, A.; Christie, A.L.; Koster, J.; DeSouza, T.A.; Morgan, E.A.; Kallgren, S.P.; Liu, H.; Wu, S.C.; et al. The Public Repository of Xenografts Enables Discovery and Randomized Phase Ii-Like Trials in Mice. Cancer Cell 2016, 30, 183. [Google Scholar] [CrossRef]
- Wang, Y.; Revelo, M.P.; Sudilovsky, D.; Cao, M.; Chen, W.G.; Goetz, L.; Xue, H.; Sadar, M.; Shappell, S.B.; Cunha, G.R.; et al. Development and Characterization of Efficient Xenograft Models for Benign and Malignant Human Prostate Tissue. Prostate 2005, 64, 149–159. [Google Scholar] [CrossRef]
- Ny, L.; Rizzo, L.Y.; Belgrano, V.; Karlsson, J.; Jespersen, H.; Carstam, L.; Bagge, R.O.; Nilsson, L.M.; Nilsson, J.A. Supporting Clinical Decision Making in Advanced Melanoma by Preclinical Testing in Personalized Immune-Humanized Xenograft Mouse Models. Ann. Oncol. 2020, 31, 266–273. [Google Scholar] [CrossRef] [PubMed]
- DeRose, Y.S.; Wang, G.; Lin, Y.C.; Bernard, P.S.; Buys, S.S.; Ebbert, M.T.; Factor, R.; Matsen, C.; Milash, B.A.; Nelson, E.; et al. Tumor Grafts Derived from Women with Breast Cancer Authentically Reflect Tumor Pathology, Growth, Metastasis and Disease Outcomes. Nat. Med. 2011, 17, 1514–1520. [Google Scholar] [CrossRef]
- Scherer, S.D.; Riggio, A.I.; Haroun, F.; DeRose, Y.S.; Ekiz, H.A.; Fujita, M.; Toner, J.; Zhao, L.; Li, Z.; Oesterreich, S.; et al. An Immune-Humanized Patient-Derived Xenograft Model of Estrogen-Independent, Hormone Receptor Positive Metastatic Breast Cancer. Breast Cancer Res. 2021, 23, 100. [Google Scholar] [CrossRef] [PubMed]
- Petrosyan, V.; Dobrolecki, L.E.; LaPlante, E.L.; Srinivasan, R.R.; Bailey, M.H.; Welm, A.L.; Welm, B.E.; Lewis, M.T.; Milosavljevic, A. Immunologically "Cold" Triple Negative Breast Cancers Engraft at a Higher Rate in Patient Derived Xenografts. npj Breast Cancer 2022, 8, 104. [Google Scholar] [CrossRef] [PubMed]
- de la Guardia, R.D.; Velasco-Hernandez, T.; Gutiérrez-Agüera, F.; Roca-Ho, H.; Molina, O.; Nombela-Arrieta, C.; Bataller, A.; Fuster, J.L.; Anguita, E.; Vives, S.; et al. Engraftment Characterization of Risk-Stratified Aml in Nsgs Mice. Blood Adv. 2021, 5, 4842–4854. [Google Scholar] [CrossRef]
- Krevvata, M.; Shan, X.; Zhou, C.; Dos Santos, C.; Ndikuyeze, G.H.; Secreto, A.; Glover, J.; Trotman, W.; Brake-Silla, G.; Nunez-Cruz, S.; et al. Cytokines Increase Engraftment of Human Acute Myeloid Leukemia Cells in Immunocompromised Mice but Not Engraftment of Human Myelodysplastic Syndrome Cells. Haematologica 2018, 103, 959–971. [Google Scholar] [CrossRef]
- Shlush, L.I.; Mitchell, A.; Heisler, L.; Abelson, S.; Ng, S.W.K.; Trotman-Grant, A.; Medeiros, J.J.F.; Rao-Bhatia, A.; Jaciw-Zurakowsky, I.; Marke, R.; et al. Tracing the Origins of Relapse in Acute Myeloid Leukaemia to Stem Cells. Nature 2017, 547, 104–108. [Google Scholar] [CrossRef]
- Reinisch, A.; Thomas, D.; Corces, M.R.; Zhang, X.; Gratzinger, D.; Hong, W.-J.; Schallmoser, K.; Strunk, D.; Majeti, R. A Humanized Bone Marrow Ossicle Xenotransplantation Model Enables Improved Engraftment of Healthy and Leukemic Human Hematopoietic Cells. Nat. Med. 2016, 22, 812–821. [Google Scholar] [CrossRef]
- Medyouf, H.; Mossner, M.; Jann, J.-C.; Nolte, F.; Raffel, S.; Herrmann, C.; Lier, A.; Eisen, C.; Nowak, V.; Zens, B.; et al. Myelodysplastic Cells in Patients Reprogram Mesenchymal Stromal Cells to Establish a Transplantable Stem Cell Niche Disease Unit. Cell Stem Cell 2014, 14, 824–837. [Google Scholar] [CrossRef]
- Rouault-Pierre, K.; Mian, S.A.; Goulard, M.; Abarrategi, A.; Di Tulio, A.; Smith, A.E.; Mohamedali, A.; Best, S.; Nloga, A.-M.; Kulasekararaj, A.G.; et al. Preclinical Modeling of Myelodysplastic Syndromes. Leukemia 2017, 31, 2702–2708. [Google Scholar] [CrossRef]
- Nicolini, F.E.; Cashman, J.D.; Hogge, D.E.; Humphries, R.K.; Eaves, C.J. Nod/Scid Mice Engineered to Express Human Il-3, Gm-Csf and Steel Factor Constitutively Mobilize Engrafted Human Progenitors and Compromise Human Stem Cell Regeneration. Leukemia 2004, 18, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Billerbeck, E.; Barry, W.T.; Mu, K.; Dorner, M.; Rice, C.M.; Ploss, A. Development of Human Cd4+Foxp3+ Regulatory T Cells in Human Stem Cell Factor-, Granulocyte-Macrophage Colony-Stimulating Factor-, and Interleukin-3-Expressing Nod-Scid Il2rgamma(Null) Humanized Mice. Blood 2011, 117, 3076–3086. [Google Scholar] [CrossRef] [PubMed]
- Svoboda, D.S.; Barrasa, M.I.; Shu, J.; Rietjens, R.; Zhang, S.; Mitalipova, M.; Berube, P.; Fu, D.; Shultz, L.D.; Bell, G.W.; et al. Human Ipsc-Derived Microglia Assume a Primary Microglia-Like State after Transplantation into the Neonatal Mouse Brain. Proc. Natl. Acad. Sci. USA 2019, 116, 25293–25303. [Google Scholar] [CrossRef] [PubMed]
- Sandén, C.; Lilljebjörn, H.; Pietras, C.O.; Henningsson, R.; Saba, K.H.; Landberg, N.; Thorsson, H.; von Palffy, S.; Peña-Martinez, P.; Högberg, C.; et al. Clonal Competition within Complex Evolutionary Hierarchies Shapes Aml over Time. Nat. Commun. 2020, 11, 579. [Google Scholar]
- Eirew, P.; Steif, A.; Khattra, J.; Ha, G.; Yap, D.; Farahani, H.; Gelmon, K.; Chia, S.; Mar, C.; Wan, A.; et al. Dynamics of Genomic Clones in Breast Cancer Patient Xenografts at Single-Cell Resolution. Nature 2015, 518, 422–426. [Google Scholar] [CrossRef]
- Linnebacher, M.; Maletzki, C.; Ostwald, C.; Klier, U.; Krohn, M.; Klar, E.; Prall, F. Cryopreservation of Human Colorectal Carcinomas Prior to Xenografting. BMC Cancer 2010, 10, 362. [Google Scholar] [CrossRef]
- Nemati, F.; Sastre-Garau, X.; Laurent, C.; Couturier, J.; Mariani, P.; Desjardins, L.; Piperno-Neumann, S.; Lantz, O.; Asselain, B.; Plancher, C.; et al. Establishment and Characterization of a Panel of Human Uveal Melanoma Xenografts Derived from Primary and/or Metastatic Tumors. Clin. Cancer Res. 2010, 16, 2352–2362. [Google Scholar] [CrossRef]
- Zou, S.; Ye, M.; Zhang, J.-A.; Ji, H.; Chen, Y.; Zhu, X. Establishment and Genetically Characterization of Patient-Derived Xenograft Models of Cervical Cancer. BMC Med. Genom. 2022, 15, 191. [Google Scholar] [CrossRef]
- Bertotti, A.; Migliardi, G.; Galimi, F.; Sassi, F.; Torti, D.; Isella, C.; Cora, D.; Di Nicolantonio, F.; Buscarino, M.; Petti, C.; et al. A Molecularly Annotated Platform of Patient-Derived Xenografts (“Xenopatients”) Identifies Her2 as an Effective Therapeutic Target in Cetuximab-Resistant Colorectal Cancer. Cancer Discov. 2011, 1, 508–523. [Google Scholar] [CrossRef]
- Zou, J.; Liu, Y.; Wang, J.; Liu, Z.; Lu, Z.; Chen, Z.; Li, Z.; Dong, B.; Huang, W.; Li, Y.; et al. Establishment and Genomic Characterizations of Patient-Derived Esophageal Squamous Cell Carcinoma Xenograft Models Using Biopsies for Treatment Optimization. J. Transl. Med. 2018, 16, 15. [Google Scholar] [CrossRef]
- Zhu, Y.; Tian, T.; Li, Z.; Tang, Z.; Wang, L.; Wu, J.; Li, Y.; Dong, B.; Li, Y.; Li, N.; et al. Establishment and Characterization of Patient-Derived Tumor Xenograft Using Gastroscopic Biopsies in Gastric Cancer. Sci. Rep. 2015, 5, 8542. [Google Scholar] [CrossRef] [PubMed]
- Agnusdei, V.; Minuzzo, S.A.; Frasson, C.; Grassi, A.; Axelrod, F.; Satyal, S.; Gurney, A.; Hoey, T.; Seganfreddo, E.; Basso, G.; et al. Therapeutic Antibody Targeting of Notch1 in T-Acute Lymphoblastic Leukemia Xenografts. Leukemia 2014, 28, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Yan, L.; An, Q.; Zhang, S.; Guan, X.; Wang, Z.; Lv, A.; Liu, D.; Liu, F.; Dong, B.; et al. Establishment and Evaluation of Retroperitoneal Liposarcoma Patient-Derived Xenograft Models: An Ideal Model for Preclinical Study. Int. J. Med. Sci. 2022, 19, 1241–1253. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Shen, C.; Wei, Z.; Zhang, R.; Wang, Y.; Jiang, L.; Chen, K.; Qiu, S.; Zhang, Y.; Zhang, T.; et al. Patient-Derived Non-Small Cell Lung Cancer Xenograft Mirrors Complex Tumor Heterogeneity. Cancer Biol. Med. 2021, 18, 184–198. [Google Scholar] [CrossRef]
- Maykel, J.; Liu, J.H.; Li, H.; Shultz, L.D.; Greiner, D.L.; Houghton, J. Nod-Scidil2rg (Tm1wjl) and Nod-Rag1 (Null) Il2rg (Tm1wjl): A Model for Stromal Cell-Tumor Cell Interaction for Human Colon Cancer. Dig. Dis. Sci. 2014, 59, 1169–1179. [Google Scholar] [CrossRef] [PubMed]
- Cybula, M.; Wang, L.; Wang, L.; Drumond-Bock, A.L.; Moxley, K.M.; Benbrook, D.M.; Gunderson-Jackson, C.; Ruiz-Echevarria, M.J.; Bhattacharya, R.; Mukherjee, P.; et al. Patient-Derived Xenografts of High-Grade Serous Ovarian Cancer Subtype as a Powerful Tool in Pre-Clinical Research. Cancers 2021, 13, 628. [Google Scholar] [CrossRef] [PubMed]
- Chou, J.; FitzGibbon, M.P.; Mortales, C.-L.L.; Towlerton, A.M.H.; Upton, M.P.; Yeung, R.S.; McIntosh, M.W.; Warren, E.H. Phenotypic and Transcriptional Fidelity of Patient-Derived Colon Cancer Xenografts in Immune-Deficient Mice. PLoS ONE 2013, 8, e79874. [Google Scholar] [CrossRef]
- Klinghammer, K.; Raguse, J.-D.; Plath, T.; Albers, A.E.; Joehrens, K.; Zakarneh, A.; Brzezicha, B.; Wulf-Goldenberg, A.; Keilholz, U.; Hoffmann, J.; et al. A Comprehensively Characterized Large Panel of Head and Neck Cancer Patient-Derived Xenografts Identifies the Mtor Inhibitor Everolimus as Potential New Treatment Option. Int. J. Cancer 2015, 136, 2940–2948. [Google Scholar] [CrossRef]
- Liu, W.N.; Fong, S.Y.; Tan, W.W.S.; Tan, S.Y.; Liu, M.; Cheng, J.Y.; Lim, S.; Suteja, L.; Huang, E.K.; Chan, J.K.Y.; et al. Establishment and Characterization of Humanized Mouse Npc-Pdx Model for Testing Immunotherapy. Cancers 2020, 12, 1025. [Google Scholar] [CrossRef]
- de Vries, G.; Rosas-Plaza, X.; Meersma, G.J.; Leeuwenburgh, V.C.; Kok, K.; Suurmeijer, A.J.H.; van Vugt, M.A.T.M.; Gietema, J.A.; de Jong, S. Establishment and Characterisation of Testicular Cancer Patient-Derived Xenograft Models for Preclinical Evaluation of Novel Therapeutic Strategies. Sci. Rep. 2020, 10, 18938. [Google Scholar] [CrossRef]
- Wetterauer, C.; Vlajnic, T.; Schuler, J.; Gsponer, J.R.; Thalmann, G.N.; Cecchini, M.; Schneider, J.; Zellweger, T.; Pueschel, H.; Bachmann, A.; et al. Early Development of Human Lymphomas in a Prostate Cancer Xenograft Program Using Triple Knock-out Immunocompromised Mice. Prostate 2015, 75, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Chijiwa, T.; Kawai, K.; Noguchi, A.; Sato, H.; Hayashi, A.; Cho, H.; Shiozawa, M.; Kishida, T.; Morinaga, S.; Yokose, T.; et al. Establishment of Patient-Derived Cancer Xenografts in Immunodeficient Nog Mice. Int. J. Oncol. 2015, 47, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.Y.; Lee, J.E.; Kim, H.; Sim, M.H.; Kim, K.-K.; Lee, G.; Kim, H.I.; An, J.Y.; Hyung, W.J.; Kim, C.-B.; et al. Establishment and Characterisation of Patient-Derived Xenografts as Paraclinical Models for Gastric Cancer. Sci. Rep. 2016, 6, 22172. [Google Scholar] [CrossRef]
- Davies, N.J.; Kwok, M.; Gould, C.; Oldreive, C.E.; Mao, J.; Parry, H.; Smith, E.; Agathanggelou, A.; Pratt, G.; Taylor, A.M.R.; et al. Dynamic Changes in Clonal Cytogenetic Architecture During Progression of Chronic Lymphocytic Leukemia in Patients and Patient-Derived Murine Xenografts. Oncotarget 2017, 8, 44749–44760. [Google Scholar] [CrossRef]
- Tanaka, K.; Kato, I.; Dobashi, Y.; Imai, J.; Mikami, T.; Kubota, H.; Ueno, H.; Ito, M.; Ogawa, S.; Nakahata, T.; et al. The First Japanese Biobank of Patient-Derived Pediatric Acute Lymphoblastic Leukemia Xenograft Models. Cancer Sci. 2022, 113, 3814–3825. [Google Scholar] [CrossRef] [PubMed]
- Albert, D.H.; Goodwin, N.C.; Davies, A.M.; Rowe, J.; Feuer, G.; Boyiadzis, M.; Dorritie, K.A.; Mancini, M.; Gandour-Edwards, R.; Jonas, B.A.; et al. Co-Clinical Modeling of the Activity of the Bet Inhibitor Mivebresib (Abbv-075) in Aml. In Vivo 2022, 36, 1615–1627. [Google Scholar] [CrossRef]
- Wunderlich, M.; Chou, F.-S.; Link, K.A.; Mizukawa, B.; Perry, R.L.; Carroll, M.; Mulloy, J.C. Aml Xenograft Efficiency Is Significantly Improved in Nod/Scid-Il2rg Mice Constitutively Expressing Human Scf, Gm-Csf and Il-3. Leukemia 2010, 24, 1785–1788. [Google Scholar] [CrossRef]
- Zanella, E.R.; Galimi, F.; Sassi, F.; Migliardi, G.; Cottino, F.; Leto, S.M.; Lupo, B.; Erriquez, J.; Isella, C.; Comoglio, P.M.; et al. Igf2 Is an Actionable Target That Identifies a Distinct Subpopulation of Colorectal Cancer Patients with Marginal Response to Anti-Egfr Therapies. Sci. Transl. Med. 2015, 7, 272ra12. [Google Scholar] [CrossRef]
- Yoshida, G.J. Applications of Patient-Derived Tumor Xenograft Models and Tumor Organoids. J. Hematol. Oncol. 2020, 13, 4. [Google Scholar] [CrossRef]
- Genta, S.; Coburn, B.; Cescon, D.W.; Spreafico, A. Patient-Derived Cancer Models: Valuable Platforms for Anticancer Drug Testing. Front. Oncol. 2022, 12, 976065. [Google Scholar] [CrossRef]
- Fiebig, H.H.; Schuchhardt, C.; Henss, H.; Fiedler, L.; Löhr, G.W. Comparison of Tumor Response in Nude Mice and in the Patients. Behring Inst. Mitteilungen 1984, 74, 343–352. [Google Scholar]
- Stewart, E.L.; Mascaux, C.; Pham, N.-A.; Sakashita, S.; Sykes, J.; Kim, L.; Yanagawa, N.; Allo, G.; Ishizawa, K.; Wang, D.; et al. Clinical Utility of Patient-Derived Xenografts to Determine Biomarkers of Prognosis and Map Resistance Pathways in EGFR-Mutant Lung Adenocarcinoma. J. Clin. Oncol. 2015, 33, 2472–2480. [Google Scholar] [CrossRef]
- Hidalgo, M.; Bruckheimer, E.; Rajeshkumar, N.; Garrido-Laguna, I.; De Oliveira, E.; Rubio-Viqueira, B.; Strawn, S.; Wick, M.J.; Martell, J.; Sidransky, D. A Pilot Clinical Study of Treatment Guided by Personalized Tumorgrafts in Patients with Advanced Cancer. Mol. Cancer Ther. 2011, 10, 1311–1316. [Google Scholar] [CrossRef] [PubMed]
- Stebbing, J.; Paz, K.; Schwartz, G.K.; Wexler, L.H.; Maki, R.; Pollock, R.E.; Morris, R.; Cohen, R.; Shankar, A.; Blackman, G.; et al. Patient-Derived Xenografts for Individualized Care in Advanced Sarcoma. Cancer 2014, 120, 2006–2015. [Google Scholar] [CrossRef] [PubMed]
- Sorokin, A.V.; Marie, P.K.; Bitner, L.; Syed, M.; Woods, M.; Manyam, G.; Kwong, L.N.; Johnson, B.; Morris, V.K.; Jones, P.; et al. Targeting Ras Mutant Colorectal Cancer with Dual Inhibition of Mek and Cdk4/6. Cancer Res. 2022, 82, 3335–3344. [Google Scholar] [CrossRef]
- Juric, D.; Castel, P.; Griffith, M.; Griffith, O.L.; Won, H.H.; Ellis, H.; Ebbesen, S.H.; Ainscough, B.J.; Ramu, A.; Iyer, G.; et al. Convergent Loss of Pten Leads to Clinical Resistance to a Pi(3)Kalpha Inhibitor. Nature 2015, 518, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Rehman, S.K.; Haynes, J.; Collignon, E.; Brown, K.R.; Wang, Y.; Nixon, A.M.; Bruce, J.P.; Wintersinger, J.A.; Mer, A.S.; Lo, E.B.; et al. Colorectal Cancer Cells Enter a Diapause-like DTP State to Survive Chemotherapy. Cell 2021, 184, 226–242.e21. [Google Scholar] [CrossRef]
- Ocana, A.; Pandiella, A.; Siu, L.L.; Tannock, I.F. Preclinical Development of Molecular-Targeted Agents for Cancer. Nat. Rev. Clin. Oncol. 2010, 8, 200–209. [Google Scholar] [CrossRef]
- Patton, E.E.; Mueller, K.L.; Adams, D.J.; Anandasabapathy, N.; Aplin, A.E.; Bertolotto, C.; Bosenberg, M.; Ceol, C.J.; Burd, C.E.; Chi, P.; et al. Melanoma Models for the Next Generation of Therapies. Cancer Cell 2021, 39, 610–631. [Google Scholar] [CrossRef]
- Chuprin, J.; Buettner, H.; Seedhom, M.O.; Greiner, D.L.; Keck, J.G.; Ishikawa, F.; Shultz, L.D.; Brehm, M.A. Humanized Mouse Models for Immuno-Oncology Research. Nat. Rev. Clin. Oncol. 2023, 20, 192–206. [Google Scholar] [CrossRef]
- Choi, Y.; Lee, S.; Kim, K.; Kim, S.-H.; Chung, Y.-J.; Lee, C. Studying Cancer Immunotherapy Using Patient-Derived Xenografts (Pdxs) in Humanized Mice. Exp. Mol. Med. 2018, 50, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Marin-Jimenez, J.A.; Capasso, A.; Lewis, M.S.; Bagby, S.M.; Hartman, S.J.; Shulman, J.; Navarro, N.M.; Yu, H.; Rivard, C.J.; Wang, X.; et al. Testing Cancer Immunotherapy in a Human Immune System Mouse Model: Correlating Treatment Responses to Human Chimerism, Therapeutic Variables and Immune Cell Phenotypes. Front. Immunol. 2021, 12, 607282. [Google Scholar] [CrossRef] [PubMed]
- Martinov, T.; McKenna, K.M.; Tan, W.H.; Collins, E.J.; Kehret, A.R.; Linton, J.D.; Olsen, T.M.; Shobaki, N.; Rongvaux, A. Building the Next Generation of Humanized Hemato-Lymphoid System Mice. Front. Immunol. 2021, 12, 643852. [Google Scholar] [CrossRef] [PubMed]
- Legrand, N.; Huntington, N.D.; Nagasawa, M.; Bakker, A.Q.; Schotte, R.; Strick-Marchand, H.; de Geus, S.J.; Pouw, S.M.; Bohne, M.; Voordouw, A.; et al. Functional Cd47/Signal Regulatory Protein Alpha (Sirp(Alpha)) Interaction Is Required for Optimal Human T- and Natural Killer- (Nk) Cell Homeostasis in Vivo. Proc. Natl. Acad. Sci. USA 2011, 108, 13224–13229. [Google Scholar] [CrossRef] [PubMed]
- Jinnouchi, F.; Yamauchi, T.; Yurino, A.; Nunomura, T.; Nakano, M.; Iwamoto, C.; Obara, T.; Miyawaki, K.; Kikushige, Y.; Kato, K.; et al. A Human Sirpa Knock-in Xenograft Mouse Model to Study Human Hematopoietic and Cancer Stem Cells. Blood 2020, 135, 1661–1672. [Google Scholar] [CrossRef]
- Lavender, K.J.; Pang, W.W.; Messer, R.J.; Duley, A.K.; Race, B.; Phillips, K.; Scott, D.; Peterson, K.E.; Chan, C.K.; Dittmer, U.; et al. Blt-Humanized C57bl/6 Rag2-/-Gammac-/-Cd47-/- Mice Are Resistant to Gvhd and Develop B- and T-Cell Immunity to Hiv Infection. Blood 2013, 122, 4013–4020. [Google Scholar] [CrossRef]
- Hayakawa, J.; Hsieh, M.M.; Uchida, N.; Phang, O.; Tisdale, J.F. Busulfan Produces Efficient Human Cell Engraftment in Nod/Ltsz-Scid Il2rgamma(Null) Mice. Stem Cells 2009, 27, 175–182. [Google Scholar] [CrossRef]
- McIntosh, B.E.; Brown, M.E.; Duffin, B.M.; Maufort, J.P.; Vereide, D.T.; Slukvin; Thomson, J.A. Nonirradiated Nod,B6.Scid Il2rgamma-/- Kit(W41/W41) (Nbsgw) Mice Support Multilineage Engraftment of Human Hematopoietic Cells. Stem Cell Rep. 2015, 4, 171–180. [Google Scholar] [CrossRef]
- Rahmig, S.; Kronstein-Wiedemann, R.; Fohgrub, J.; Kronstein, N.; Nevmerzhitskaya, A.; Bornhäuser, M.; Gassmann, M.; Platz, A.; Ordemann, R.; Tonn, T.; et al. Improved Human Erythropoiesis and Platelet Formation in Humanized NSGW41 Mice. Stem Cell Rep. 2016, 7, 591–601. [Google Scholar] [CrossRef]
- Yurino, A.; Takenaka, K.; Yamauchi, T.; Nunomura, T.; Uehara, Y.; Jinnouchi, F.; Miyawaki, K.; Kikushige, Y.; Kato, K.; Miyamoto, T.; et al. Enhanced Reconstitution of Human Erythropoiesis and Thrombopoiesis in an Immunodeficient Mouse Model with Kit Wv Mutations. Stem Cell Rep. 2016, 7, 425–438. [Google Scholar] [CrossRef]
- Shan, L.; Flavell, R.A.; Herndler-Brandstetter, D. Development of Humanized Mouse Models for Studying Human NK Cells in Health and Disease. Methods Mol. Biol. 2022, 2463, 53–66. [Google Scholar]
- Rongvaux, A.; Takizawa, H.; Strowig, T.; Willinger, T.; Eynon, E.E.; Flavell, R.A.; Manz, M.G. Human Hemato-Lymphoid System Mice: Current Use and Future Potential for Medicine. Annu. Rev. Immunol. 2013, 31, 635–674. [Google Scholar] [CrossRef] [PubMed]
- Holyoake, T.L.; Nicolini, F.E.; Eaves, C.J. Functional Differences between Transplantable Human Hematopoietic Stem Cells from Fetal Liver, Cord Blood, and Adult Marrow. Exp. Hematol. 1999, 27, 1418–1427. [Google Scholar] [CrossRef] [PubMed]
- Brehm, M.A.; Kenney, L.L.; Wiles, M.V.; Low, B.E.; Tisch, R.M.; Burzenski, L.; Mueller, C.; Greiner, D.L.; Shultz, L.D. Lack of Acute Xenogeneic Graft- Versus-Host Disease, but Retention of T-Cell Function Following Engraftment of Human Peripheral Blood Mononuclear Cells in Nsg Mice Deficient in Mhc Class I and Ii Expression. FASEB J. 2019, 33, 3137–3151. [Google Scholar] [CrossRef] [PubMed]
- Yaguchi, T.; Kobayashi, A.; Inozume, T.; Morii, K.; Nagumo, H.; Nishio, H.; Iwata, T.; Ka, Y.; Katano, I.; Ito, R.; et al. Human Pbmc-Transferred Murine Mhc Class I/Ii-Deficient Nog Mice Enable Long-Term Evaluation of Human Immune Responses. Cell Mol. Immunol. 2018, 15, 953–962. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Liu, B.; Rubio, M.T.; Wang, X.; Ojcius, D.M.; Tang, R.; Durrbach, A.; Ru, Z.; Zhou, Y.; Lone, Y.C. Creation of an Immunodeficient Hla-Transgenic Mouse (Humamice) and Functional Validation of Human Immunity after Transfer of Hla-Matched Human Cells. PLoS ONE 2017, 12, e0173754. [Google Scholar] [CrossRef]
- Holguin, L.; Echavarria, L.; Burnett, J.C. Novel Humanized Peripheral Blood Mononuclear Cell Mouse Model with Delayed Onset of Graft-Versus-Host Disease for Preclinical Hiv Research. J. Virol. 2022, 96, e0139421. [Google Scholar] [CrossRef]
- Strowig, T.; Gurer, C.; Ploss, A.; Liu, Y.F.; Arrey, F.; Sashihara, J.; Koo, G.; Rice, C.M.; Young, J.W.; Chadburn, A.; et al. Priming of Protective T Cell Responses against Virus-Induced Tumors in Mice with Human Immune System Components. J. Exp. Med. 2009, 206, 1423–1434. [Google Scholar] [CrossRef]
- Sippel, T.R.; Radtke, S.; Olsen, T.M.; Kiem, H.-P.; Rongvaux, A. Human Hematopoietic Stem Cell Maintenance and Myeloid Cell Development in Next-Generation Humanized Mouse Models. Blood Adv. 2019, 3, 268–274. [Google Scholar] [CrossRef]
- Ito, R.; Takahashi, T.; Katano, I.; Kawai, K.; Kamisako, T.; Ogura, T.; Ida-Tanaka, M.; Suemizu, H.; Nunomura, S.; Ra, C.; et al. Establishment of a Human Allergy Model Using Human Il-3/Gm-Csf-Transgenic Nog Mice. J. Immunol. 2013, 191, 2890–2899. [Google Scholar] [CrossRef]
- Yu, H.; Borsotti, C.; Schickel, J.N.; Zhu, S.; Strowig, T.; Eynon, E.E.; Frleta, D.; Gurer, C.; Murphy, A.J.; Yancopoulos, G.D.; et al. A Novel Humanized Mouse Model with Significant Improvement of Class-Switched, Antigen-Specific Antibody Production. Blood 2017, 129, 959–969. [Google Scholar] [CrossRef] [PubMed]
- Ono, R.; Watanabe, T.; Kawakami, E.; Iwasaki, M.; Tomizawa-Murasawa, M.; Matsuda, M.; Najima, Y.; Takagi, S.; Fujiki, S.; Sato, R.; et al. Co-Activation of Macrophages and T Cells Contribute to Chronic Gvhd in Human Il-6 Transgenic Humanised Mouse Model. Ebiomedicine 2019, 41, 584–596. [Google Scholar] [CrossRef] [PubMed]
- Lang, J.; Zhang, B.; Kelly, M.; Peterson, J.N.; Barbee, J.; Freed, B.M.; Di Santo, J.P.; Matsuda, J.L.; Torres, R.M.; Pelanda, R. Replacing Mouse Baff with Human Baff Does Not Improve B-Cell Maturation in Hematopoietic Humanized Mice. Blood Adv. 2017, 1, 2729–2741. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.-C.; Liang, Q.; Ali, H.; Bayliss, L.; Beasley, A.; Bloomfield-Gerdes, T.; Bonoli, L.; Brown, R.; Campbell, J.; Carpenter, A.; et al. Complete Humanization of the Mouse Immunoglobulin Loci Enables Efficient Therapeutic Antibody Discovery. Nat. Biotechnol. 2014, 32, 356–363. [Google Scholar] [CrossRef]
- Kennedy, M.K.; Glaccum, M.; Brown, S.N.; Butz, E.A.; Viney, J.L.; Embers, M.; Matsuki, N.; Charrier, K.; Sedger, L.; Willis, C.R.; et al. Reversible Defects in Natural Killer and Memory Cd8 T Cell Lineages in Interleukin 15–Deficient Mice. J. Exp. Med. 2000, 191, 771–780. [Google Scholar] [CrossRef]
- Katano, I.; Takahashi, T.; Ito, R.; Kamisako, T.; Mizusawa, T.; Ka, Y.; Ogura, T.; Suemizu, H.; Kawakami, Y.; Ito, M. Predominant Development of Mature and Functional Human NK Cells in a Novel Human IL-2–Producing Transgenic NOG Mouse. J. Immunol. 2015, 194, 3513–3525. [Google Scholar] [CrossRef]
- Wunderlich, M.; Brooks, R.A.; Panchal, R.; Rhyasen, G.W.; Danet-Desnoyers, G.; Mulloy, J.C. Okt3 Prevents Xenogeneic Gvhd and Allows Reliable Xenograft Initiation from Unfractionated Human Hematopoietic Tissues. Blood 2014, 123, e134–e144. [Google Scholar] [CrossRef]
- Shultz, L.D.; Keck, J.; Burzenski, L.; Jangalwe, S.; Vaidya, S.; Greiner, D.L.; Brehm, M.A. Humanized Mouse Models of Immunological Diseases and Precision Medicine. Mamm. Genome 2019, 30, 123–142. [Google Scholar] [CrossRef]
- Wunderlich, M.; Chou, F.-S.; Sexton, C.; Presicce, P.; Chougnet, C.A.; Aliberti, J.; Mulloy, J.C. Improved Multilineage Human Hematopoietic Reconstitution and Function in Nsgs Mice. PLoS ONE 2018, 13, e0209034. [Google Scholar] [CrossRef]
- Lopez-Lastra, S.; Masse-Ranson, G.; Fiquet, O.; Darche, S.; Serafini, N.; Li, Y.; Dusséaux, M.; Strick-Marchand, H.; Di Santo, J.P. A Functional Dc Cross Talk Promotes Human Ilc Homeostasis in Humanized Mice. Blood Adv. 2017, 1, 601–614. [Google Scholar] [CrossRef]
- Li, Y.; Mention, J.-J.; Court, N.; Masse-Ranson, G.; Toubert, A.; Spits, H.; Legrand, N.; Corcuff, E.; Strick-Marchand, H.; Di Santo, J.P. A Novel Flt3-Deficient His Mouse Model with Selective Enhancement of Human Dc Development. Eur. J. Immunol. 2016, 46, 1291–1299. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Sefik, E.; Astle, J.; Karatepe, K.; Öz, H.H.; Solis, A.G.; Jackson, R.; Luo, H.R.; Bruscia, E.M.; Halene, S.; et al. Human Neutrophil Development and Functionality Are Enabled in a Humanized Mouse Model. Proc. Natl. Acad. Sci. USA 2022, 119, e2121077119. [Google Scholar] [CrossRef] [PubMed]
- Ito, R.; Katano, I.; Kwok, I.W.; Ng, L.G.; Ida-Tanaka, M.; Ohno, Y.; Mu, Y.; Morita, H.; Nishinaka, E.; Nishime, C.; et al. Efficient Differentiation of Human Neutrophils with Recapitulation of Emergency Granulopoiesis in Human G-Csf Knockin Humanized Mice. Cell Rep. 2022, 41, 111841. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Khoury, M.; Chen, J. Expression of Human Cytokines Dramatically Improves Reconstitution of Specific Human-Blood Lineage Cells in Humanized Mice. Proc. Natl. Acad. Sci. USA 2009, 106, 21783–21788. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, S.; Yang, Z.; Algazi, A.P.; Lomeli, S.H.; Wang, Y.; Othus, M.; Hong, A.; Wang, X.; Randolph, C.E.; et al. Anti-Pd-1/L1 Lead-in before Mapk Inhibitor Combination Maximizes Antitumor Immunity and Efficacy. Cancer Cell 2021, 39, 1375–1387.e6. [Google Scholar] [CrossRef]
- Haas, L.; Elewaut, A.; Gerard, C.L.; Umkehrer, C.; Leiendecker, L.; Pedersen, M.; Krecioch, I.; Hoffmann, D.; Novatchkova, M.; Kuttke, M.; et al. Acquired Resistance to Anti-Mapk Targeted Therapy Confers an Immune-Evasive Tumor Microenvironment and Cross-Resistance to Immunotherapy in Melanoma. Nat. Cancer 2021, 2, 693–708. [Google Scholar] [CrossRef]
- Barrett, D.M.; Zhao, Y.; Liu, X.; Jiang, S.; Carpenito, C.; Carroll, R.G.; June, C.H.; Grupp, S.A.; Ramos, C.A.; Heslop, H.E.; et al. Treatment of Advanced Leukemia in Mice with mRNA Engineered T Cells. Hum. Gene Ther. 2011, 22, 1575–1586. [Google Scholar] [CrossRef]
- Schewe, D.M.; Alsadeq, A.; Sattler, C.; Lenk, L.; Vogiatzi, F.; Cario, G.; Vieth, S.; Valerius, T.; Rosskopf, S.; Meyersieck, F.; et al. An Fc-Engineered Cd19 Antibody Eradicates Mrd in Patient-Derived Mll-Rearranged Acute Lymphoblastic Leukemia Xenografts. Blood 2017, 130, 1543–1552. [Google Scholar] [CrossRef]
- Winterberg, D.; Lenk, L.; Oßwald, M.; Vogiatzi, F.; Gehlert, C.L.; Frielitz, F.-S.; Klausz, K.; Rösner, T.; Valerius, T.; Trauzold, A.; et al. Engineering of CD19 Antibodies: A CD19-TRAIL Fusion Construct Specifically Induces Apoptosis in B-Cell Precursor Acute Lymphoblastic Leukemia (BCP-ALL) Cells In Vivo. J. Clin. Med. 2021, 10, 2634. [Google Scholar] [CrossRef]
- Wang, M.; Yao, L.C.; Cheng, M.; Cai, D.; Martinek, J.; Pan, C.X.; Shi, W.; Ma, A.H.; De Vere White, R.W.; Airhart, S.; et al. Humanized Mice in Studying Efficacy and Mechanisms of Pd-1-Targeted Cancer Immunotherapy. FASEB J. 2018, 32, 1537–1549. [Google Scholar] [CrossRef]
- Matas-Cespedes, A.; Vidal-Crespo, A.; Rodriguez, V.; Villamor, N.; Delgado, J.; Gine, E.; Roca-Ho, H.; Menendez, P.; Campo, E.; Lopez-Guillermo, A.; et al. The Human Cd38 Monoclonal Antibody Daratumumab Shows Antitumor Activity and Hampers Leukemia-Microenvironment Interactions in Chronic Lymphocytic Leukemia. Clin. Cancer Res. 2017, 23, 1493–1505. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.; Lee, J.S.; Kim, S.J.; Hong, D.; Park, J.B.; Lee, K.-Y. Anti-Tumor Effects of Anti-Pd-1 Antibody, Pembrolizumab, in Humanized Nsg Pdx Mice Xenografted with Dedifferentiated Liposarcoma. Cancer Lett. 2020, 478, 56–69. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Liu, M.; Huang, J.; Zhou, J.; Li, J.; Lian, H.; Huang, W.; Guo, Y.; Yang, S.; Lin, L.; et al. Development of Mesothelin-Specific Car Nk-92 Cells for the Treatment of Gastric Cancer. Int. J. Biol. Sci. 2021, 17, 3850–3861. [Google Scholar] [CrossRef]
- Zhao, Y.; Shuen, T.W.H.; Toh, T.B.; Chan, X.Y.; Liu, M.; Tan, S.Y.; Fan, Y.; Yang, H.; Lyer, S.G.; Bonney, G.K.; et al. Development of a New Patient-Derived Xenograft Humanised Mouse Model to Study Human-Specific Tumour Microenvironment and Immunotherapy. Gut 2018, 67, 1845–1854. [Google Scholar] [CrossRef] [PubMed]
- Kundu, K.; Ghosh, S.; Sarkar, R.; Edri, A.; Brusilovsky, M.; Gershoni-Yahalom, O.; Yossef, R.; Shemesh, A.; Soria, J.C.; Lazar, V.; et al. Inhibition of the Nkp44-Pcna Immune Checkpoint Using a Mab to Pcna. Cancer Immunol. Res. 2019, 7, 1120–1134. [Google Scholar] [CrossRef] [PubMed]
- Barry, W.E.; Jackson, J.R.; Asuelime, G.E.; Wu, H.W.; Sun, J.; Wan, Z.; Malvar, J.; Sheard, M.A.; Wang, L.; Seeger, R.C.; et al. Activated Natural Killer Cells in Combination with Anti-Gd2 Antibody Dinutuximab Improve Survival of Mice after Surgical Resection of Primary Neuroblastoma. Clin. Cancer Res. 2019, 25, 325–333. [Google Scholar] [CrossRef]
- Gitto, S.B.; Kim, H.; Rafail, S.; Omran, D.K.; Medvedev, S.; Kinose, Y.; Rodriguez-Garcia, A.; Flowers, A.J.; Xu, H.; Schwartz, L.E.; et al. An Autologous Humanized Patient-Derived-Xenograft Platform to Evaluate Immunotherapy in Ovarian Cancer. Gynecol. Oncol. 2020, 156, 222–232. [Google Scholar] [CrossRef]
- Sommaggio, R.; Cappuzzello, E.; Pietà, A.D.; Tosi, A.; Palmerini, P.; Carpanese, D.; Nicolè, L.; Rosato, A. Adoptive Cell Therapy of Triple Negative Breast Cancer with Redirected Cytokine-Induced Killer Cells. Oncoimmunology 2020, 9, 1777046. [Google Scholar] [CrossRef]
- Ku, A.; Kondo, M.; Cai, Z.; Meens, J.; Li, M.R.; Ailles, L.; Reilly, R.M. Dose Predictions for [(177)Lu]Lu-Dota-Panitumumab F(Ab’)(2) in Nrg Mice with Hnscc Patient-Derived Tumour Xenografts Based on [(64)Cu]Cu-Dota-Panitumumab F(Ab’)(2)—Implications for a Pet Theranostic Strategy. EJNMMI Radiopharm. Chem. 2021, 6, 25. [Google Scholar] [CrossRef]
- Park, J.A.; Cheung, N.-K.V. Gd2 or Her2 Targeting T Cell Engaging Bispecific Antibodies to Treat Osteosarcoma. J. Hematol. Oncol. 2020, 13, 172. [Google Scholar] [CrossRef]
- Lang, J.; Leal, A.D.; Marín-Jiménez, J.A.; Hartman, S.J.; Shulman, J.; Navarro, N.M.; Lewis, M.S.; Capasso, A.; Bagby, S.M.; Yacob, B.W.; et al. Cabozantinib Sensitizes Microsatellite Stable Colorectal Cancer to Immune Checkpoint Blockade by Immune Modulation in Human Immune System Mouse Models. Front. Oncol. 2022, 12, 877635. [Google Scholar] [CrossRef]
- Capasso, A.; Lang, J.; Pitts, T.M.; Jordan, K.R.; Lieu, C.H.; Davis, S.L.; Diamond, J.R.; Kopetz, S.; Barbee, J.; Peterson, J.; et al. Characterization of Immune Responses to Anti-Pd-1 Mono and Combination Immunotherapy in Hematopoietic Humanized Mice Implanted with Tumor Xenografts. J. Immunother. Cancer 2019, 7, 37. [Google Scholar] [CrossRef] [PubMed]
- Lang, J.; Capasso, A.; Jordan, K.R.; French, J.D.; Kar, A.; Bagby, S.M.; Barbee, J.; Yacob, B.W.; Head, L.S.; Tompkins, K.D.; et al. Development of an Adrenocortical Cancer Humanized Mouse Model to Characterize Anti-PD1 Effects on Tumor Microenvironment. J. Clin. Endocrinol. Metab. 2020, 105, 26–42. [Google Scholar] [CrossRef]
- Jespersen, H.; Lindberg, M.F.; Donia, M.; Söderberg, E.M.V.; Andersen, R.; Keller, U.; Ny, L.; Svane, I.M.; Nilsson, L.M.; Nilsson, J.A. Clinical Responses to Adoptive T-Cell Transfer Can Be Modeled in an Autologous Immune-Humanized Mouse Model. Nat. Commun. 2017, 8, 707. [Google Scholar] [CrossRef]
- Forsberg, E.M.; Lindberg, M.F.; Jespersen, H.; Alsén, S.; Bagge, R.O.; Donia, M.; Svane, I.M.; Nilsson, O.; Ny, L.; Nilsson, L.M.; et al. HER2 CAR-T Cells Eradicate Uveal Melanoma and T-cell Therapy–Resistant Human Melanoma in IL2 Transgenic NOD/SCID IL2 Receptor Knockout Mice. Cancer Res 2019, 79, 899–904. [Google Scholar] [CrossRef]
- Maser, I.-P.; Hoves, S.; Bayer, C.; Heidkamp, G.; Nimmerjahn, F.; Eckmann, J.; Ries, C.H. The Tumor Milieu Promotes Functional Human Tumor-Resident Plasmacytoid Dendritic Cells in Humanized Mouse Models. Front. Immunol. 2020, 11, 2082. [Google Scholar] [CrossRef] [PubMed]
- Wunderlich, M.; Manning, N.; Sexton, C.; O’brien, E.; Byerly, L.; Stillwell, C.; Perentesis, J.P.; Mulloy, J.C.; Mizukawa, B. PD-1 Inhibition Enhances Blinatumomab Response in a UCB/PDX Model of Relapsed Pediatric B-Cell Acute Lymphoblastic Leukemia. Front. Oncol. 2021, 11, 642466. [Google Scholar] [CrossRef]
- Myburgh, R.; Kiefer, J.D.; Russkamp, N.F.; Magnani, C.F.; Nuñez, N.; Simonis, A.; Pfister, S.; Wilk, C.M.; McHugh, D.; Friemel, J.; et al. Anti-Human Cd117 Car T-Cells Efficiently Eliminate Healthy and Malignant Cd117-Expressing Hematopoietic Cells. Leukemia 2020, 34, 2688–2703. [Google Scholar] [CrossRef] [PubMed]
- Biernacki, M.A.; Foster, K.A.; Woodward, K.B.; Coon, M.E.; Cummings, C.; Cunningham, T.M.; Dossa, R.G.; Brault, M.; Stokke, J.; Olsen, T.M.; et al. Cbfb-Myh11 Fusion Neoantigen Enables T Cell Recognition and Killing of Acute Myeloid Leukemia. J. Clin. Investig. 2020, 130, 5127–5141. [Google Scholar] [CrossRef]
- Nguyen, R.; Patel, A.; Griffiths, L.M.; Dapper, J.; Stewart, E.A.; Houston, J.; Johnson, M.; Akers, W.J.; Furman, W.L.; Dyer, M.A. Next-Generation Humanized Patient-Derived Xenograft Mouse Model for Pre-Clinical Antibody Studies in Neuroblastoma. Cancer Immunol. Immunother. 2021, 70, 721–732. [Google Scholar] [CrossRef]
- Hashwah, H.; Bertram, K.; Stirm, K.; Stelling, A.; Wu, C.T.; Kasser, S.; Manz, M.G.; Theocharides, A.P.; Tzankov, A.; Muller, A. The Il-6 Signaling Complex Is a Critical Driver, Negative Prognostic Factor, and Therapeutic Target in Diffuse Large B-Cell Lymphoma. EMBO Mol. Med. 2019, 11, e10576. [Google Scholar] [CrossRef] [PubMed]
- Gabrilovich, D.I.; Ostrand-Rosenberg, S.; Bronte, V. Coordinated Regulation of Myeloid Cells by Tumours. Nat. Rev. Immunol. 2012, 12, 253–268. [Google Scholar] [CrossRef] [PubMed]
- Pittet, M.J.; Michielin, O.; Migliorini, D. Clinical Relevance of Tumour-Associated Macrophages. Nat. Rev. Clin. Oncol. 2022, 19, 402–421. [Google Scholar] [CrossRef] [PubMed]
- Bruni, D.; Angell, H.K.; Galon, J. The Immune Contexture and Immunoscore in Cancer Prognosis and Therapeutic Efficacy. Nat. Rev. Cancer 2020, 20, 662–680. [Google Scholar] [CrossRef] [PubMed]
- Ruffell, B.; Coussens, L.M. Macrophages and Therapeutic Resistance in Cancer. Cancer Cell 2015, 27, 462–472. [Google Scholar] [CrossRef] [PubMed]
- Cassetta, L.; Pollard, J.W. Targeting Macrophages: Therapeutic Approaches in Cancer. Nat. Rev. Drug Discov. 2018, 17, 887–904. [Google Scholar] [CrossRef]
- Mantovani, A.; Allavena, P.; Marchesi, F.; Garlanda, C. Macrophages as Tools and Targets in Cancer Therapy. Nat. Rev. Drug Discov. 2022, 21, 799–820. [Google Scholar] [CrossRef]
- Yu, C.I.; Martinek, J.; Wu, T.-C.; Kim, K.I.; George, J.; Ahmadzadeh, E.; Maser, R.; Marches, F.; Metang, P.; Authie, P.; et al. Human Kit+ Myeloid Cells Facilitate Visceral Metastasis by Melanoma. J. Exp. Med. 2021, 218, e20182163. [Google Scholar] [CrossRef]
- Su, S.; Liu, Q.; Chen, J.; Chen, J.; Chen, F.; He, C.; Huang, D.; Wu, W.; Lin, L.; Huang, W.; et al. A Positive Feedback Loop between Mesenchymal-like Cancer Cells and Macrophages Is Essential to Breast Cancer Metastasis. Cancer Cell 2014, 25, 605–620. [Google Scholar] [CrossRef]
- Rosato, R.R.; Dávila-González, D.; Choi, D.S.; Qian, W.; Chen, W.; Kozielski, A.J.; Wong, H.; Dave, B.; Chang, J.C. Evaluation of Anti-Pd-1-Based Therapy against Triple-Negative Breast Cancer Patient-Derived Xenograft Tumors Engrafted in Humanized Mouse Models. Breast Cancer Res. 2018, 20, 108. [Google Scholar] [CrossRef]
- Yao, L.-C.; Aryee, K.-E.; Cheng, M.; Kaur, P.; Keck, J.G.; Brehm, M.A. Creation of PDX-Bearing Humanized Mice to Study Immuno-oncology. Methods Mol. Biol. 2019, 1953, 241–252. [Google Scholar] [PubMed]
- Hodis, E.; Triglia, E.T.; Kwon, J.Y.H.; Biancalani, T.; Zakka, L.R.; Parkar, S.; Hutter, J.C.; Buffoni, L.; Delorey, T.M.; Phillips, D.; et al. Stepwise-Edited, Human Melanoma Models Reveal Mutations’ Effect on Tumor and Microenvironment. Science 2022, 376, eabi8175. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, M.L.; Francescangeli, F.; Nicolazzo, C.; Xhelili, E.; La Torre, F.; Colace, L.; Bruselles, A.; Macchia, D.; Vitale, S.; Gazzaniga, P.; et al. An Orthotopic Patient-Derived Xenograft (PDX) Model Allows the Analysis of Metastasis-Associated Features in Colorectal Cancer. Front. Oncol. 2022, 12, 869485. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, S.; Jamieson, C.H.; Pang, W.W.; Park, C.Y.; Chao, M.P.; Majeti, R.; Traver, D.; van Rooijen, N.; Weissman, I.L. CD47 Is Upregulated on Circulating Hematopoietic Stem Cells and Leukemia Cells to Avoid Phagocytosis. Cell 2009, 138, 271–285. [Google Scholar] [CrossRef]
- Ring, N.G.; Herndler-Brandstetter, D.; Weiskopf, K.; Shan, L.; Volkmer, J.P.; George, B.M.; Lietzenmayer, M.; McKenna, K.M.; Naik, T.J.; McCarty, A.; et al. Anti-Sirpalpha Antibody Immunotherapy Enhances Neutrophil and Macrophage Antitumor Activity. Proc. Natl. Acad. Sci. USA 2017, 114, E10578–E10585. [Google Scholar] [CrossRef]
- Morton, J.J.; Alzofon, N.; Keysar, S.B.; Chimed, T.S.; Reisinger, J.; Perrenoud, L.; Le, P.N.; Nieto, C.; Gomez, K.; Miller, B.; et al. Studying Immunotherapy Resistance in a Melanoma Autologous Humanized Mouse Xenograft. Mol. Cancer Res. 2021, 19, 346–357. [Google Scholar] [CrossRef] [PubMed]
- Pyo, K.H.; Kim, J.H.; Lee, J.-M.; Kim, S.E.; Cho, J.S.; Lim, S.M.; Cho, B.C. Promising Preclinical Platform for Evaluation of Immuno-Oncology Drugs Using Hu-Pbl-Nsg Lung Cancer Models. Lung Cancer 2019, 127, 112–121. [Google Scholar] [CrossRef]
- Meraz, I.M.; Majidi, M.; Meng, F.; Shao, R.; Ha, M.J.; Neri, S.; Fang, B.; Lin, S.H.; Tinkey, P.T.; Shpall, E.J.; et al. An Improved Patient-Derived Xenograft Humanized Mouse Model for Evaluation of Lung Cancer Immune Responses. Cancer Immunol. Res. 2019, 7, 1267–1279. [Google Scholar] [CrossRef]
- Kang, Y.; Armstrong, A.J.; Hsu, D.S. An Autologous Humanized Patient-Derived Xenograft (Pdx) Model for Evaluation of Nivolumab Immunotherapy in Renal Cell Cancer: A Case Report. Stem Cell Investig. 2022, 9, 8. [Google Scholar] [CrossRef]
- Tentler, J.J.; Lang, J.; Capasso, A.; Kim, D.J.; Benaim, E.; Lee, Y.B.; Eisen, A.; Bagby, S.M.; Hartman, S.J.; Yacob, B.W.; et al. Rx-5902, a Novel Beta-Catenin Modulator, Potentiates the Efficacy of Immune Checkpoint Inhibitors in Preclinical Models of Triple-Negative Breast Cancer. BMC Cancer 2020, 20, 1063. [Google Scholar] [CrossRef]
- Somasundaram, R.; Connelly, T.; Choi, R.; Choi, H.; Samarkina, A.; Li, L.; Gregorio, E.; Chen, Y.; Thakur, R.; Abdel-Mohsen, M.; et al. Tumor-Infiltrating Mast Cells Are Associated with Resistance to Anti-Pd-1 Therapy. Nat. Commun. 2021, 12, 346. [Google Scholar] [CrossRef] [PubMed]
- Küçükköse, E.; Heesters, B.A.; Villaudy, J.; Verheem, A.; Cercel, M.; van Hal, S.; Boj, S.F.; Rinkes, I.H.M.B.; Punt, C.J.A.; Roodhart, J.M.L.; et al. Modeling Resistance of Colorectal Peritoneal Metastases to Immune Checkpoint Blockade in Humanized Mice. J. Immunother. Cancer 2022, 10, e005345. [Google Scholar] [CrossRef] [PubMed]
- Fourcade, J.; Sun, Z.; Benallaoua, M.; Guillaume, P.; Luescher, I.F.; Sander, C.; Kirkwood, J.M.; Kuchroo, V.; Zarour, H.M. Upregulation of Tim-3 and Pd-1 Expression Is Associated with Tumor Antigen-Specific Cd8+ T Cell Dysfunction in Melanoma Patients. J. Exp. Med. 2010, 207, 2175–2186. [Google Scholar] [CrossRef] [PubMed]
- Sakuishi, K.; Apetoh, L.; Sullivan, J.M.; Blazar, B.R.; Kuchroo, V.K.; Anderson, A.C. Targeting Tim-3 and Pd-1 Pathways to Reverse T Cell Exhaustion and Restore Anti-Tumor Immunity. J. Exp. Med. 2010, 207, 2187–2194. [Google Scholar] [CrossRef]
- Chhabra, Y.; Weeraratna, A.T. Fibroblasts in Cancer: Unity in Heterogeneity. Cell 2023, 186, 1580–1609. [Google Scholar] [CrossRef]
- Delitto, D.; Pham, K.; Vlada, A.C.; Sarosi, G.A.; Thomas, R.M.; Behrns, K.E.; Liu, C.; Hughes, S.J.; Wallet, S.M.; Trevino, J.G. Patient-Derived Xenograft Models for Pancreatic Adenocarcinoma Demonstrate Retention of Tumor Morphology through Incorporation of Murine Stromal Elements. Am. J. Pathol. 2015, 185, 1297–1303. [Google Scholar] [CrossRef]
- Karnoub, A.E.; Dash, A.B.; Vo, A.P.; Sullivan, A.; Brooks, M.W.; Bell, G.W.; Richardson, A.L.; Polyak, K.; Tubo, R.; Weinberg, R.A. Mesenchymal Stem Cells within Tumour Stroma Promote Breast Cancer Metastasis. Nature 2007, 449, 557–563. [Google Scholar] [CrossRef]
- Cho, J.; Lee, H.-J.; Hwang, S.J.; Min, H.-Y.; Kang, H.N.; Park, A.-Y.; Hyun, S.Y.; Sim, J.Y.; Lee, H.J.; Jang, H.-J.; et al. The Interplay between Slow-Cycling, Chemoresistant Cancer Cells and Fibroblasts Creates a Proinflammatory Niche for Tumor Progression. Cancer Res. 2020, 80, 2257–2272. [Google Scholar] [CrossRef]
- Han, J.; Yun, J.; Quan, M.; Kang, W.; Jung, J.-G.; Heo, W.; Li, S.; Lee, K.J.; Son, H.-Y.; Kim, J.H.; et al. Jak2 Regulates Paclitaxel Resistance in Triple Negative Breast Cancers. J. Mol. Med. 2021, 99, 1783–1795. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, A.; Neuwirth, I.; Herndler-Brandstetter, D. Modeling the Tumor Microenvironment and Cancer Immunotherapy in Next-Generation Humanized Mice. Cancers 2023, 15, 2989. https://doi.org/10.3390/cancers15112989
Chen A, Neuwirth I, Herndler-Brandstetter D. Modeling the Tumor Microenvironment and Cancer Immunotherapy in Next-Generation Humanized Mice. Cancers. 2023; 15(11):2989. https://doi.org/10.3390/cancers15112989
Chicago/Turabian StyleChen, Anna, Ines Neuwirth, and Dietmar Herndler-Brandstetter. 2023. "Modeling the Tumor Microenvironment and Cancer Immunotherapy in Next-Generation Humanized Mice" Cancers 15, no. 11: 2989. https://doi.org/10.3390/cancers15112989
APA StyleChen, A., Neuwirth, I., & Herndler-Brandstetter, D. (2023). Modeling the Tumor Microenvironment and Cancer Immunotherapy in Next-Generation Humanized Mice. Cancers, 15(11), 2989. https://doi.org/10.3390/cancers15112989







