Drug Repurposing in Oncology: A Systematic Review of Randomized Controlled Clinical Trials
Abstract
Simple Summary
Abstract
1. Introduction
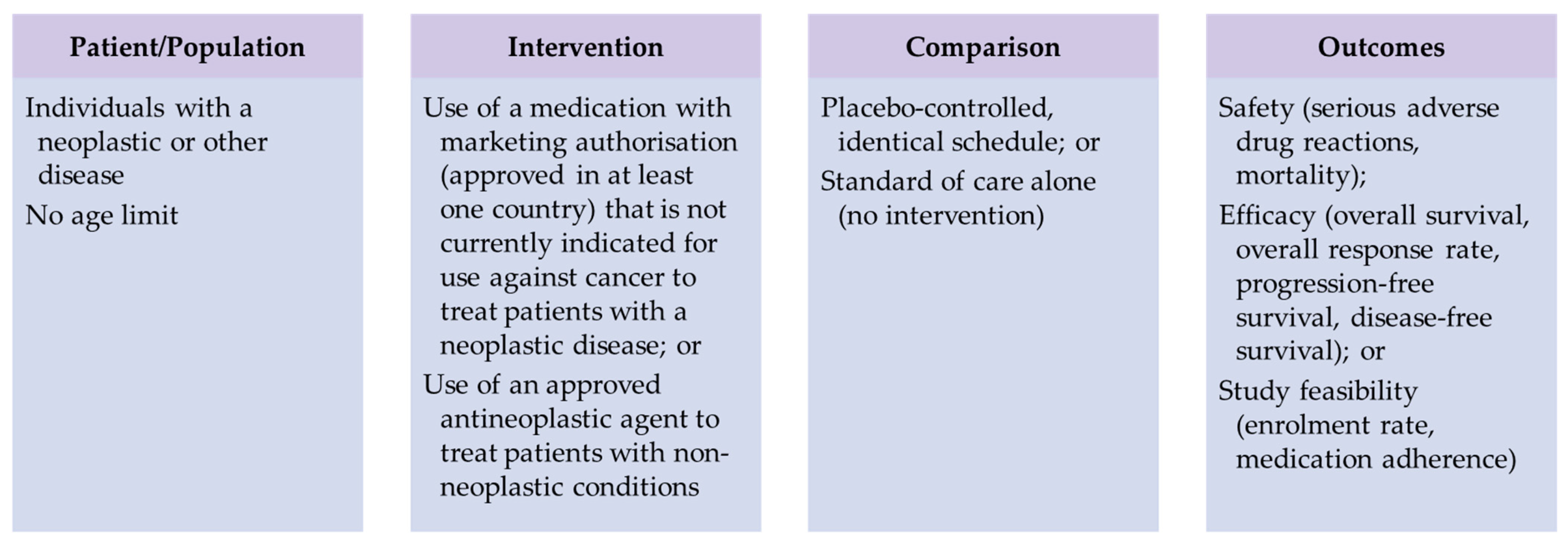
2. Materials and Methods
3. Results
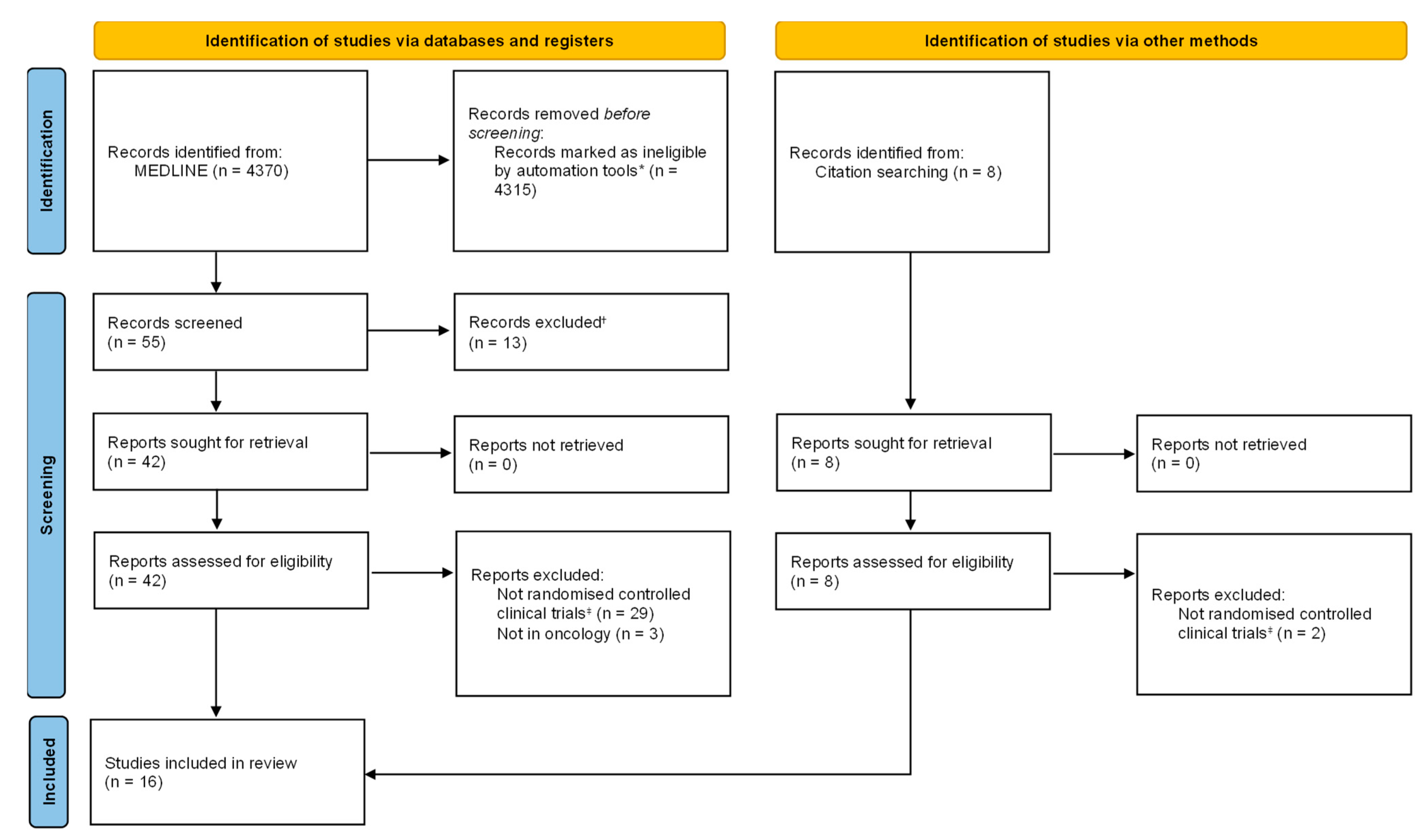
3.1. Literature Search Results
3.2. Excluded Articles
3.3. Drugs in Investigation for Repurposing and Use in Oncological Conditions
| Author (Year) | Country | Drug | Aim | Time Frame | Potential New Indication in Investigation | Main Findings | Author’s Conclusions | Limitations | Quality of Evidence (GRADE) |
|---|---|---|---|---|---|---|---|---|---|
| Drugs under investigation for use in oncological conditions | |||||||||
| Hegazy S.K. et al. (2022) [35] | Egypt | Mebendazole | Anti-tumor activity and safety | 12 months (mean) | Metastatic colorectal cancer (stage 4) | ORR improved 12 weeks after treatment but not significantly after 12 months; one-year OS did not significantly improve | Mebendazole was well tolerated and showed anti-tumor activity | Small sample size, high drop-out, molecular tumor characteristics not considered, lack of intention-to-treat analysis | Moderate ⨁⨁⨁◯ |
| Alghandour R. et al. (2021) [36] | Egypt | Metformin | Efficacy and safety | 22 months (mean) | Hormone-sensitive prostate cancer | The median CRPC-free survival was higher in the metformin group (p = 0.01). In patients with metastatic disease, there was no difference (p = 0.15) | Patients with high-risk localized disease, regional lymph node metastases, and those with metastatic low tumor volume disease seem to derive most of the benefit | Control group was standard of care and not placebo-controlled; it was a heterogenous population with heterogenous interventions (as standards of care) | Moderate ⨁⨁⨁◯ |
| Marrone K.A. et al. (2018) [37] | USA | Metformin | Efficacy and safety | 12 months | Advanced or metastatic NSCLC | There was a significant benefit in PFS with the use of metformin (p = 0.024), but OS was not significantly different | Metformin is a well-tolerated drug that, in addition to standard chemotherapy, can improve progression free survival | Due to the small sample size, the study was stopped because of changes in practice patterns for treatment, a lack of correlative analyses, and open-label | Moderate ⨁⨁⨁◯ |
| Knight J.M. et al. (2018) [38] | USA | Propranolol | Efficacy, safety, and feasibility | 100 days | Multiple myeloma | Enrollment rate: 16%; no serious ADRs were reported; MA: 94% | It is feasible to recruit and treat multiple myeloma patients with propranolol during HCT, with the greatest obstacle being other competing oncology trials | Small sample size, open-label | Moderate ⨁⨁⨁◯ |
| Taghizadeh Kermani A. et al. (2018) [39] | Iran | Enoxaparin | Efficacy and safety | 7 months | Oesophageal squamous cell carcinoma | Integration of enoxaparin into the chemoradiation protocol is safe and tolerable. Higher probability of neutropenia | The clinical and pathological response of squamous cell carcinoma to neoadjuvant chemoradiation was improved by the addition of enoxaparin (the difference was not significant) | Small sample size and no information about anti-Xa levels | Moderate ⨁⨁⨁◯ |
| Shaashua L. et al. (2017) [40] | Israel | Propranolol; etodolac | Efficacy and safety | 16 days (mean) | Primary operable breast cancer stages I–III | Decreased epithelial-to-mesenchymal transition, reduced activity of pro-metastatic/pro-inflammatory transcription factors, and decreased tumor-infiltrating monocytes while increasing tumor-infiltrating B cells | Perioperative inhibition of COX-2 and b-adrenergic signaling provides a safe and effective strategy for inhibiting multiple cellular and molecular pathways related to metastasis and disease recurrence in early-stage breast cancer | No information about long-term clinical outcomes | High ⨁⨁⨁⨁ |
| Kordes S. et al. (2015) [41] | Netherlands | Metformin | Efficacy and safety | 6 months | Pancreatic adenocarcinoma | OS at 6 months was higher in the placebo group (p = 0.41). Median OS was higher in the placebo group (hazard ratio 1.056 [95% CI 0.72–1.55]) | There is no advantage to the addition of metformin to erlotinib and gemcitabine in the treatment of advanced pancreatic cancer | No information on tumor biomarkers; high patient heterogeneity; and open-label | Moderate ⨁⨁⨁◯ |
| Antineoplastic agents under investigation for use in non-oncological conditions | |||||||||
| Aman J. et al. (2021) [47] | Netherlands | Imatinib | Efficacy and safety | 28 days | COVID-19 with hypoxic respiratory failure | There was no significant differences between the intervention and control groups regarding the time to discontinuation of supplemental oxygen and mechanical ventilation (HR 1.07, 95% CI 0.62–1.84; p = 0.82; adjusted for baseline characteristics) | Imatinib did not reduce the time to discontinuation of ventilation and supplemental oxygen for more than 48 consecutive hours in patients with COVID-19 requiring supplemental oxygen | Loss of follow-up (partly due to hospital relocations during the pandemics), imbalances in sex baseline clinical characteristics (comorbidities), and the treatment period of ten days were based on earlier observations and might need to be reconsidered | High ⨁⨁⨁⨁ |
| Author (Year) | Country | Drug | Aim | Time Frame | Potential New Indication in Investigation | Main Outcomes |
|---|---|---|---|---|---|---|
| Drugs under investigation for use in oncological conditions | ||||||
| Zeyen T. et al. (2022) [42] | Germany | Meclofenamate | Efficacy, safety, tolerability, and quality of life | 6 months | Progressive MGMT-methylated glioblastoma | OS, PFS, ADRs, and QoL |
| McCarthy C. et al. (2021) [43] | UK | Sodium valproate | Clinical activity, mechanism of action, and study feasibility | 6 months | High-risk oral epithelial dysplasia | Changes in lesion size, changes in histological grade, and loss of heterozygosity |
| Hüttner F.J. et al. (2020) [44] | Germany | Propranolol; etodolac | Safety, feasibility, and early parameters of efficacy | 24 months | Elective pancreatic head resection | Serious ADRs, post-operative mortality, pancreas-associated morbidity, MA, OS, DFS, and rates of local and distant recurrence |
| Polster S.P. et al. (2019) [45] | USA | Atorvastatin | Efficacy | 24 months | Cavernous angiomas | Change in QSM per year using intention-to-treat analysis, vascular permeability, andADRs |
| Jakola A.S. et al. (2018) [46] | Norway, Sweden | Disulfiram | Efficacy, safety, and health-related quality of life | 24 months | Recurrent glioblastoma | Six-month survival (primary endpoint), OS, PFS, safety, and health-related QoL |
| Antineoplastic agents under investigation for use in non-oncological conditions | ||||||
| Atmowihardjo L. et al. (2022) [48] | Netherlands | Imatinib mesylate | Efficacy, safety, and tolerability | 28 days | COVID-19 with acute distress respiratory syndrome | Change in Extravascular Lung Water Index between baseline (day 1) and day 4, SOFA score, 28-day mortality, ADRs |
| Butler T. et al. (2021) [49] | USA | Leuprolide | Efficacy | 52 weeks | Alzheimer’s disease | Change in cognition from baseline to post-treatment as measured by the ADAS-Cog |
| Emadi A. et al. (2020) [50] | USA | Imatinib | Efficacy, safety, tolerability, and pharmacokinetics | 60 days | COVID-19 | Proportion of patients with a two-point improvement at day 14 from baseline using an 8-category ordinal scale; all-cause mortality at day 28 and at day 60 |
3.4. Antineoplastics in Investigation for Repurposing and Use in Non-Oncological Conditions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ashburn, T.T.; Thor, K.B. Drug repositioning: Identifying and developing new uses for existing drugs. Nat. Rev. Drug Discov. 2004, 3, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Reichert, J.M. Trends in development and approval times for new therapeutics in the United States. Nat. Rev. Drug Discov. 2003, 2, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, J.; Henske, P.; Singh, A. Rebuilding big pharma’s business model. Vivo 2003, 21, 73–80. [Google Scholar]
- The Academy of Medical Sciences. Multimorbidity: A Priority for Global Health Research. 2018. Available online: https://acmedsci.ac.uk/file-download/82222577 (accessed on 10 January 2023).
- World Health Organization. Global Status Report on Noncommunicable Diseases 2014. Available online: https://apps.who.int/iris/handle/10665/148114 (accessed on 10 January 2023).
- World Health Organization. Noncommunicable Diseases. Available online: https://www.who.int/health-topics/noncommunicable-diseases#tab=tab_1 (accessed on 10 January 2023).
- World Health Organization. Global Action Plan for the Prevention and Control of Noncommunicable Diseases 2013–2020. Available online: https://www.who.int/publications/i/item/9789241506236 (accessed on 10 January 2023).
- World Health Organization. Implementation Roadmap 2023–2030 for the Global Action Plan for the Prevention and Control of NCDs 2013–2030. Available online: https://www.who.int/teams/noncommunicable-diseases/governance/roadmap (accessed on 10 January 2023).
- World Health Organization. Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 10 January 2023).
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Sleire, L.; Førde-Tislevoll, H.E.; Netland, I.A.; Leiss, L.; Skeie, B.S.; Enger, P.Ø. Drug repurposing in cancer. Pharmacol. Res. 2017, 124, 74–91. [Google Scholar] [CrossRef]
- Ghafouri-Fard, S.; Abak, A.; Tondro Anamag, F.; Shoorei, H.; Fattahi, F.; Javadinia, S.A.; Basiri, A.; Taheri, M. 5-Fluorouracil: A Narrative Review on the Role of Regulatory Mechanisms in Driving Resistance to This Chemotherapeutic Agent. Front. Oncol. 2021, 11, 658636. [Google Scholar] [CrossRef]
- Hua, Y.; Dai, X.; Xu, Y.; Xing, G.; Liu, H.; Lu, T.; Chen, Y.; Zhang, Y. Drug repositioning: Progress and challenges in drug discovery for various diseases. Eur. J. Med. Chem. 2022, 234, 114239. [Google Scholar] [CrossRef]
- Corsello, S.M.; Nagari, R.T.; Spangler, R.D.; Rossen, J.; Kocak, M.; Bryan, J.G.; Humeidi, R.; Peck, D.; Wu, X.; Tang, A.A.; et al. Discovering the anti-cancer potential of non-oncology drugs by systematic viability profiling. Nat. Cancer 2020, 1, 235–248. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 29, 372. [Google Scholar] [CrossRef]
- Miller, S.A.; Forrest, J.L. Enhancing your practice through evidence-based decision making: PICO, learning how to ask good questions. J. Evid.-Based Dent. Pract. 2001, 1, 136–141. [Google Scholar] [CrossRef]
- Granholm, A.; Alhazzani, W.; Møller, M.H. Use of the GRADE approach in systematic reviews and guidelines. Br. J. Anaesth. 2019, 123, 554–559. [Google Scholar] [CrossRef]
- Ryan, R.; Hill, S. How to GRADE the Quality of the Evidence. Cochrane Consumers and Communication Group. CCCG. Version 3.0 December 2016. Available online: http://cccrg.cochrane.org/author-resources (accessed on 1 January 2023).
- Patil, V.M.; Bhelekar, A.; Menon, N.; Bhattacharjee, A.; Simha, V.; Abhinav, R.; Abhyankar, A.; Sridhar, E.; Mahajan, A.; Puranik, A.D.; et al. Reverse swing-M, phase 1 study of repurposing mebendazole in recurrent high-grade glioma. Cancer Med. 2020, 9, 4676–4685. [Google Scholar] [CrossRef]
- Patil, V.M.; Menon, N.; Chatterjee, A.; Tonse, R.; Choudhari, A.; Mahajan, A.; Puranik, A.D.; Epari, S.; Jadhav, M.; Pathak, S.; et al. Mebendazole plus lomustine or temozolomide in patients with recurrent glioblastoma: A randomised open-label phase II trial. EClinicalMedicine 2022, 49, 101449. [Google Scholar] [CrossRef]
- Gallia, G.L.; Holdhoff, M.; Brem, H.; Joshi, A.D.; Hann, C.L.; Bai, R.Y.; Staedtke, V.; Blakeley, J.O.; Sengupta, S.; Jarrell, T.C.; et al. Mebendazole and temozolomide in patients with newly diagnosed high-grade gliomas: Results of a phase 1 clinical trial. Neurooncol. Adv. 2020, 3, vdaa154. [Google Scholar] [CrossRef]
- O’Rawe, M.; Wickremesekera, A.C.; Pandey, R.; Young, D.; Sim, D.; FitzJohn, T.; Burgess, C.; Kaye, A.H.; Tan, S.T. Treatment of glioblastoma with re-purposed renin-angiotensin system modulators: Results of a phase I clinical trial. J. Clin. Neurosci. 2022, 95, 48–54. [Google Scholar] [CrossRef]
- Huang, J.; Campian, J.L.; Gujar, A.D.; Tran, D.D.; Lockhart, A.C.; DeWees, T.A.; Tsien, C.I.; Kim, A.H. A phase I study to repurpose disulfiram in combination with temozolomide to treat newly diagnosed glioblastoma after chemoradiotherapy. J. Neurooncol. 2016, 128, 259–266. [Google Scholar] [CrossRef]
- Brown, J.R.; Walker, S.R.; Heppler, L.N.; Tyekucheva, S.; Nelson, E.A.; Klitgaard, J.; Nicolais, M.; Kroll, Y.; Xiang, M.; Yeh, J.E.; et al. Targeting constitutively active STAT3 in chronic lymphocytic leukemia: A clinical trial of the STAT3 inhibitor pyrimethamine with pharmacodynamic analyses. Am. J. Hematol. 2021, 96, E95–E98. [Google Scholar] [CrossRef]
- Rosenzweig, M.; Palmer, J.; Tsai, N.C.; Synold, T.; Wu, X.; Tao, S.; Hammond, S.N.; Buettner, R.; Duarte, L.; Htut, M.; et al. Repurposing leflunomide for relapsed/refractory multiple myeloma: A phase 1 study. Leuk. Lymphoma 2020, 61, 1669–1677. [Google Scholar] [CrossRef]
- Masuda, T.; Noda, M.; Kogawa, T.; Kitagawa, D.; Hayashi, N.; Jomori, T.; Nakanishi, Y.; Nakayama, K.I.; Ohno, S.; Mimori, K. Phase I dose-escalation trial to repurpose propagermanium, an oral CCL2 inhibitor, in patients with breast cancer. Cancer Sci. 2020, 111, 924–931. [Google Scholar] [CrossRef]
- Parikh, A.B.; Marrone, K.A.; Becker, D.J.; Brahmer, J.R.; Ettinger, D.S.; Levy, B.P. A pooled analysis of two phase II trials evaluating metformin plus platinum-based chemotherapy in advanced non-small cell lung cancer. Cancer Treat. Res. Commun. 2019, 20, 100150. [Google Scholar] [CrossRef]
- Schweizer, M.T.; Haugk, K.; McKiernan, J.S.; Gulati, R.; Cheng, H.H.; Maes, J.L.; Dumpit, R.F.; Nelson, P.S.; Montgomery, B.; McCune, J.S.; et al. A phase I study of niclosamide in combination with enzalutamide in men with castration-resistant prostate cancer. PLoS ONE 2018, 13, e0198389. [Google Scholar] [CrossRef] [PubMed]
- Antonarakis, E.S.; Heath, E.I.; Smith, D.C.; Rathkopf, D.; Blackford, A.L.; Danila, D.C.; King, S.; Frost, A.; Ajiboye, A.S.; Zhao, M.; et al. Repurposing itraconazole as a treatment for advanced prostate cancer: A noncomparative randomized phase II trial in men with metastatic castration-resistant prostate cancer. Oncologist 2013, 18, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.W.; Teraphongphom, N.; de Boer, E.; van den Berg, N.S.; Divi, V.; Kaplan, M.J.; Oberhelman, N.J.; Hong, S.S.; Capes, E.; Colevas, A.D.; et al. Safety of panitumumab-IRDye800CW and cetuximab-IRDye800CW for fluorescence-guided surgical navigation in head and neck cancers. Theranostics 2018, 8, 2488–2495. [Google Scholar] [CrossRef] [PubMed]
- Parikh, A.B.; Kozuch, P.; Rohs, N.; Becker, D.J.; Levy, B.P. Metformin as a repurposed therapy in advanced non-small cell lung cancer (NSCLC): Results of a phase II trial. Investig. New Drugs 2017, 35, 813–819. [Google Scholar] [CrossRef]
- Traore, F.; Togo, B.; Pasquier, E.; Dembélé, A.; André, N. Preliminary evaluation of children treated with metronomic chemotherapy and valproic acid in a low-income country: Metro-Mali-02. Indian J. Cancer 2013, 50, 250–253. [Google Scholar] [CrossRef]
- Todd, J.A.; Evangelou, M.; Cutler, A.J.; Pekalski, M.L.; Walker, N.M.; Stevens, H.E.; Porter, L.; Smyth, D.J.; Rainbow, D.B.; Ferreira, R.C.; et al. Regulatory T Cell Responses in Participants with Type 1 Diabetes after a Single Dose of Interleukin-2: A Non-Randomised, Open Label, Adaptive Dose-Finding Trial. PLoS Med. 2016, 13, e1002139. [Google Scholar] [CrossRef]
- Imamura, K.; Izumi, Y.; Banno, H.; Uozumi, R.; Morita, S.; Egawa, N.; Ayaki, T.; Nagai, M.; Nishiyama, K.; Watanabe, Y.; et al. Induced pluripotent stem cell-based Drug Repurposing for Amyotrophic lateral sclerosis Medicine (iDReAM) study: Protocol for a phase I dose escalation study of bosutinib for amyotrophic lateral sclerosis patients. BMJ Open 2019, 9, e033131. [Google Scholar] [CrossRef]
- Hegazy, S.K.; El-Azab, G.A.; Zakaria, F.; Mostafa, M.F.; El-Ghoneimy, R.A. Mebendazole; from an anti-parasitic drug to a promising candidate for drug repurposing in colorectal cancer. Life Sci. 2022, 299, 120536. [Google Scholar] [CrossRef]
- Alghandour, R.; Ebrahim, M.A.; Elshal, A.M.; Ghobrial, F.; Elzaafarany, M.; ELbaiomy, M.A. Repurposing metformin as anticancer drug: Randomized controlled trial in advanced prostate cancer (MANSMED). Urol. Oncol. 2021, 39, 831.e1–831.e10. [Google Scholar] [CrossRef]
- Marrone, K.A.; Zhou, X.; Forde, P.M.; Purtell, M.; Brahmer, J.R.; Hann, C.L.; Kelly, R.J.; Coleman, B.; Gabrielson, E.; Rosner, G.L.; et al. A Randomized Phase II Study of Metformin plus Paclitaxel/Carboplatin/Bevacizumab in Patients with Chemotherapy-Naïve Advanced or Metastatic Nonsquamous Non-Small Cell Lung Cancer. Oncologist 2018, 23, 859–865. [Google Scholar] [CrossRef]
- Knight, J.M.; Kerswill, S.A.; Hari, P.; Cole, S.W.; Logan, B.R.; D’Souza, A.; Shah, N.N.; Horowitz, M.M.; Stolley, M.R.; Sloan, E.K.; et al. Repurposing existing medications as cancer therapy: Design and feasibility of a randomized pilot investigating propranolol administration in patients receiving hematopoietic cell transplantation. BMC Cancer 2018, 18, 593. [Google Scholar] [CrossRef]
- Taghizadeh Kermani, A.; Hosseini, S.; Fanipakdel, A.; Joudi Mashhad, M.; Akhavan Rezayat, K.; Zardadi, M.; Gholami, A.; Javadinia, S.A.; Ferns, G.A.; Avan, A. A randomized clinical trial on the antitumoral effects of low molecular weight heparin in the treatment of esophageal cancer. J. Cell. Physiol. 2019, 234, 4191–4199. [Google Scholar] [CrossRef]
- Shaashua, L.; Shabat-Simon, M.; Haldar, R.; Matzner, P.; Zmora, O.; Shabtai, M.; Sharon, E.; Allweis, T.; Barshack, I.; Hayman, L.; et al. Perioperative COX-2 and β-Adrenergic Blockade Improves Metastatic Biomarkers in Breast Cancer Patients in a Phase-II Randomized Trial. Clin. Cancer Res. 2017, 23, 4651–4661. [Google Scholar] [CrossRef]
- Kordes, S.; Pollak, M.N.; Zwinderman, A.H.; Mathôt, R.A.; Weterman, M.J.; Beeker, A.; Punt, C.J.; Richel, D.J.; Wilmink, J.W. Metformin in patients with advanced pancreatic cancer: A double-blind, randomised, placebo-controlled phase 2 trial. Lancet Oncol. 2015, 16, 839–847. [Google Scholar] [CrossRef]
- Zeyen, T.; Potthoff, A.L.; Nemeth, R.; Heiland, D.H.; Burger, M.C.; Steinbach, J.P.; Hau, P.; Tabatabai, G.; Glas, M.; Schlegel, U.; et al. Phase I/II trial of meclofenamate in progressive MGMT-methylated glioblastoma under temozolomide second-line therapy-the MecMeth/NOA-24 trial. Trials 2022, 23, 57. [Google Scholar] [CrossRef]
- McCarthy, C.; Sacco, J.; Fedele, S.; Ho, M.; Porter, S.; Liloglou, T.; Greenhalf, B.; Robinson, M.; Young, B.; Cicconi, S.; et al. SAVER: Sodium valproate for the epigenetic reprogramming of high-risk oral epithelial dysplasia-a phase II randomised control trial study protocol. Trials 2021, 22, 428. [Google Scholar] [CrossRef]
- Hüttner, F.J.; Rooman, I.; Bouche, G.; Knebel, P.; Hüsing, J.; Mihaljevic, A.L.; Hackert, T.; Strobel, O.; Büchler, M.W.; Diener, M.K. Pancreatic resection with perioperative drug repurposing of propranolol and etodolac: Trial protocol of the phase-II randomised placebo controlled PROSPER trial. BMJ Open 2020, 10, e040406. [Google Scholar] [CrossRef]
- Polster, S.P.; Stadnik, A.; Akers, A.L.; Cao, Y.; Christoforidis, G.A.; Fam, M.D.; Flemming, K.D.; Girard, R.; Hobson, N.; Koenig, J.I.; et al. Atorvastatin Treatment of Cavernous Angiomas with Symptomatic Hemorrhage Exploratory Proof of Concept (AT CASH EPOC) Trial. Neurosurgery 2019, 85, 843–853. [Google Scholar] [CrossRef]
- Jakola, A.S.; Werlenius, K.; Mudaisi, M.; Hylin, S.; Kinhult, S.; Bartek, J., Jr.; Salvesen, Ø.; Carlsen, S.M.; Strandéus, M.; Lindskog, M.; et al. Disulfiram repurposing combined with nutritional copper supplement as add-on to chemotherapy in recurrent glioblastoma (DIRECT): Study protocol for a randomized controlled trial. F1000Research 2018, 7, 1797. [Google Scholar] [CrossRef]
- Aman, J.; Duijvelaar, E.; Botros, L.; Kianzad, A.; Schippers, J.R.; Smeele, P.J.; Azhang, S.; Bartelink, I.H.; Bayoumy, A.A.; Bet, P.M.; et al. Imatinib in patients with severe COVID-19: A randomised, double-blind, placebo-controlled, clinical trial. Lancet Respir. Med. 2021, 9, 957–968. [Google Scholar] [CrossRef]
- Atmowihardjo, L.; Schippers, J.R.; Bartelink, I.H.; Bet, P.M.; van Rein, N.; Purdy, K.; Cavalla, D.; Comberiati, V.; McElroy, A.; Snape, S.D.; et al. The INVENT COVID trial: A structured protocol for a randomized controlled trial investigating the efficacy and safety of intravenous imatinib mesylate (Impentri®) in subjects with acute respiratory distress syndrome induced by COVID-19. Trials 2022, 23, 158. [Google Scholar] [CrossRef] [PubMed]
- Butler, T.; Goldberg, J.D.; Galvin, J.E.; Maloney, T.; Ravdin, L.; Glodzik, L.; de Leon, M.J.; Hochman, T.; Bowen, R.L.; Atwood, C.S. Rationale, study design and implementation of the LUCINDA Trial: Leuprolide plus Cholinesterase Inhibition to reduce Neurologic Decline in Alzheimer’s. Contemp. Clin. Trials 2021, 107, 106488. [Google Scholar] [CrossRef] [PubMed]
- Emadi, A.; Chua, J.V.; Talwani, R.; Bentzen, S.M.; Baddley, J. Safety and Efficacy of Imatinib for Hospitalized Adults with COVID-19: A structured summary of a study protocol for a randomised controlled trial. Trials 2020, 21, 897. [Google Scholar] [CrossRef] [PubMed]
- Giovannucci, E.; Harlan, D.M.; Archer, M.C.; Bergenstal, R.M.; Gapstur, S.M.; Habel, L.A.; Pollak, M.; Regensteiner, J.G.; Yee, D. Diabetes and cancer: A consensus report. Diabetes Care 2010, 33, 1674–1685. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.M.; Donnelly, L.A.; Emslie-Smith, A.M.; Alessi, D.R.; Morris, A.D. Metformin and reduced risk of cancer in diabetic patients. BMJ 2005, 330, 1304–1305. [Google Scholar] [CrossRef]
- Jiralerspong, S.; Palla, S.L.; Giordano, S.H.; Meric-Bernstam, F.; Liedtke, C.; Barnett, C.M.; Hsu, L.; Hung, M.C.; Hortobagyi, G.N.; Gonzalez-Angulo, A.M. Metformin and pathologic complete responses to neoadjuvant chemotherapy in diabetic patients with breast cancer. J. Clin. Oncol. 2009, 27, 3297–3302. [Google Scholar] [CrossRef]
- Yao, K.; Zheng, H.; Li, T. Association Between Metformin Use and the Risk, Prognosis of Gynecologic Cancer. Front. Oncol. 2022, 12, 942380. [Google Scholar] [CrossRef]
- Barakat, H.E.; Hussein, R.R.S.; Elberry, A.A.; Zaki, M.A.; Elsherbiny Ramadan, M. Factors influencing the anticancer effects of metformin on breast cancer outcomes: A systematic review and meta-analysis. Expert Rev. Anticancer 2022, 22, 415–436. [Google Scholar] [CrossRef]
- Wang, Z.; Lai, S.T.; Xie, L.; Zhao, J.D.; Ma, N.Y.; Zhu, J.; Ren, Z.G.; Jiang, G.L. Metformin is associated with reduced risk of pancreatic cancer in patients with type 2 diabetes mellitus: A systematic review and meta-analysis. Diabetes Res. Clin. Pract. 2014, 106, 19–26. [Google Scholar] [CrossRef]
- Lin, J.J.; Gallagher, E.J.; Sigel, K.; Mhango, G.; Galsky, M.D.; Smith, C.B.; LeRoith, D.; Wisnivesky, J.P. Survival of patients with stage IV lung cancer with diabetes treated with metformin. Am. J. Respir. Crit. Care Med. 2015, 191, 448–454. [Google Scholar] [CrossRef]
- Wink, K.C.; Belderbos, J.S.; Dieleman, E.M.; Rossi, M.; Rasch, C.R.; Damhuis, R.A.; Houben, R.M.; Troost, E.G. Improved progression free survival for patients with diabetes and locally advanced non-small cell lung cancer (NSCLC) using metformin during concurrent chemoradiotherapy. Radiother. Oncol. 2016, 118, 453–459. [Google Scholar] [CrossRef]
- Hitron, A.; Adams, V.; Talbert, J.; Steinke, D. The influence of antidiabetic medications on the development and progression of prostate cancer. Cancer Epidemiol. 2012, 36, e243–e250. [Google Scholar] [CrossRef]
- Jafarzadeh, E.; Montazeri, V.; Aliebrahimi, S.; Sezavar, A.H.; Ghahremani, M.H.; Ostad, S.N. Combined regimens of cisplatin and metformin in cancer therapy: A systematic review and meta-analysis. Life Sci. 2022, 304, 120680. [Google Scholar] [CrossRef]
- Coyle, C.; Cafferty, F.H.; Vale, C.; Langley, R.E. Metformin as an adjuvant treatment for cancer: A systematic review and meta-analysis. Ann. Oncol. 2016, 27, 2184–2195. [Google Scholar] [CrossRef]
- Chevalier, B.; Pasquier, D.; Lartigau, E.F.; Chargari, C.; Schernberg, A.; Jannin, A.; Mirabel, X.; Vantyghem, M.C.; Escande, A. Metformin: (future) best friend of the radiation oncologist? Radiother. Oncol. 2020, 151, 95–105. [Google Scholar] [CrossRef]
- Jiao, Y.; Liu, D.; Sun, Y.; Chen, Z.; Liu, S. Survival Benefit of Metformin as an Adjuvant Treatment for Head and Neck Cancer: A Systematic Review and Meta-Analysis. Front. Pharm. 2022, 13, 850750. [Google Scholar] [CrossRef]
- World Health Organization Model List of Essential Medicines-22nd List, 2021; World Health Organization: Geneva, Switzerland, 2021; Available online: https://www.who.int/publications/i/item/WHO-MHP-HPS-EML-2021.02 (accessed on 10 January 2023).
- Cejuela, M.; Martin-Castillo, B.; Menendez, J.A.; Pernas, S. Metformin and Breast Cancer: Where Are We Now? Int. J. Mol. Sci. 2022, 23, 2705. [Google Scholar] [CrossRef]
- Soukas, A.A.; Hao, H.; Wu, L. Metformin as Anti-Aging Therapy: Is It for Everyone? Trends Endocrinol. Metab. 2019, 30, 745–755. [Google Scholar] [CrossRef]
- Triggle, C.R.; Mohammed, I.; Bshesh, K.; Marei, I.; Ye, K.; Ding, H.; MacDonald, R.; Hollenberg, M.D.; Hill, M.A. Metformin: Is it a drug for all reasons and diseases? Metabolism 2022, 133, 155223. [Google Scholar] [CrossRef]
- Brugmans, J.P.; Thienpont, D.C.; van Wijngaarden, I.; Vanparijs, O.F.; Schuermans, V.L.; Lauwers, H.L. Mebendazole in enterobiasis. Radiochemical and pilot clinical study in 1,278 subjects. JAMA 1971, 217, 313–316. [Google Scholar] [CrossRef]
- Mukhopadhyay, T.; Sasaki, J.; Ramesh, R.; Roth, J.A. Mebendazole elicits a potent antitumor effect on human cancer cell lines both in vitro and in vivo. Clin. Cancer Res. 2002, 8, 2963–2969. [Google Scholar] [PubMed]
- Sasaki, J.; Ramesh, R.; Chada, S.; Gomyo, Y.; Roth, J.A.; Mukhopadhyay, T. The anthelmintic drug mebendazole induces mitotic arrest and apoptosis by depolymerizing tubulin in non-small cell lung cancer cells. Mol. Cancer Ther. 2002, 1, 1201–1209. [Google Scholar] [PubMed]
- Pantziarka, P.; Bouche, G.; Meheus, L.; Sukhatme, V.; Sukhatme, V.P. Repurposing drugs in oncology (ReDO)-cimetidine as an anti-cancer agent. Ecancermedicalscience 2014, 8, 485. [Google Scholar] [CrossRef] [PubMed]
- Guerini, A.E.; Triggiani, L.; Maddalo, M.; Bonù, M.L.; Frassine, F.; Baiguini, A.; Alghisi, A.; Tomasini, D.; Borghetti, P.; Pasinetti, N.; et al. Mebendazole as a Candidate for Drug Repurposing in Oncology: An Extensive Review of Current Literature. Cancers 2019, 11, 1284. [Google Scholar] [CrossRef] [PubMed]
- Gales, L.; Forsea, L.; Mitrea, D.; Stefanica, I.; Stanculescu, I.; Mitrica, R.; Georgescu, M.; Trifanescu, O.; Anghel, R.; Serbanescu, L. Antidiabetics, Anthelmintics, Statins, and Beta-Blockers as Co-Adjuvant Drugs in Cancer Therapy. Medicina 2022, 58, 1239. [Google Scholar] [CrossRef]
- Nath, J.; Paul, R.; Ghosh, S.K.; Paul, J.; Singha, B.; Debnath, N. Drug repurposing and relabeling for cancer therapy: Emerging benzimidazole antihelminthics with potent anticancer effects. Life Sci. 2020, 258, 118189. [Google Scholar] [CrossRef]
- Larsen, A.R.; Bai, R.Y.; Chung, J.H.; Borodovsky, A.; Rudin, C.M.; Riggins, G.J.; Bunz, F. Repurposing the antihelmintic mebendazole as a hedgehog inhibitor. Mol. Cancer Ther. 2015, 14, 3–13. [Google Scholar] [CrossRef]
- Martarelli, D.; Pompei, P.; Baldi, C.; Mazzoni, G. Mebendazole inhibits growth of human adrenocortical carcinoma cell lines implanted in nude mice. Cancer Chemother. Pharmacol. 2008, 61, 809–817. [Google Scholar] [CrossRef]
- Dakshanamurthy, S.; Issa, N.T.; Assefnia, S.; Seshasaye, A.; Peters, O.J.; Madhavan, S.; Uren, A.; Brown, M.L.; Byers, S.W. Predicting new indications for approved drugs using a proteochemometric method. J. Med. Chem. 2012, 55, 6832–6848. [Google Scholar] [CrossRef]
- Blom, K.; Senkowski, W.; Jarvius, M.; Berglund, M.; Rubin, J.; Lenhammar, L.; Parrow, V.; Andersson, C.; Loskog, A.; Fryknäs, M.; et al. The anticancer effect of mebendazole may be due to M1 monocyte/macrophage activation via ERK1/2 and TLR8-dependent inflammasome activation. Immunopharmacol. Immunotoxicol. 2017, 39, 199–210. [Google Scholar] [CrossRef]
- Zhang, L.; Bochkur Dratver, M.; Yazal, T.; Dong, K.; Nguyen, A.; Yu, G.; Dao, A.; Bochkur Dratver, M.; Duhachek-Muggy, S.; Bhat, K.; et al. Mebendazole Potentiates Radiation Therapy in Triple-Negative Breast Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2019, 103, 195–207. [Google Scholar] [CrossRef]
- Bai, R.Y.; Staedtke, V.; Aprhys, C.M.; Gallia, G.L.; Riggins, G.J. Antiparasitic mebendazole shows survival benefit in 2 preclinical models of glioblastoma multiforme. Neuro-Oncology 2011, 13, 974–982. [Google Scholar] [CrossRef]
- Meco, D.; Attinà, G.; Mastrangelo, S.; Navarra, P.; Ruggiero, A. Emerging Perspectives on the Antiparasitic Mebendazole as a Repurposed Drug for the Treatment of Brain Cancers. Int. J. Mol. Sci. 2023, 24, 1334. [Google Scholar] [CrossRef]
- Doudican, N.A.; Byron, S.A.; Pollock, P.M.; Orlow, S.J. XIAP downregulation accompanies mebendazole growth inhibition in melanoma xenografts. Anti-Cancer Drugs 2013, 24, 181–188. [Google Scholar] [CrossRef]
- Simbulan-Rosenthal, C.M.; Dakshanamurthy, S.; Gaur, A.; Chen, Y.S.; Fang, H.B.; Abdussamad, M.; Zhou, H.; Zapas, J.; Calvert, V.; Petricoin, E.F.; et al. The repurposed anthelmintic mebendazole in combination with trametinib suppresses refractory NRASQ61K melanoma. Oncotarget 2017, 8, 12576–12595. [Google Scholar] [CrossRef]
- Walf-Vorderwülbecke, V.; Pearce, K.; Brooks, T.; Hubank, M.; van den Heuvel-Eibrink, M.M.; Zwaan, C.M.; Adams, S.; Edwards, D.; Bartram, J.; Samarasinghe, S.; et al. Targeting acute myeloid leukemia by drug-induced c-MYB degradation. Leukemia 2018, 32, 882–889. [Google Scholar] [CrossRef]
- Elayapillai, S.; Ramraj, S.; Benbrook, D.M.; Bieniasz, M.; Wang, L.; Pathuri, G.; Isingizwe, Z.R.; Kennedy, A.L.; Zhao, Y.D.; Lightfoot, S.; et al. Potential and mechanism of mebendazole for treatment and maintenance of ovarian cancer. Gynecol. Oncol. 2021, 160, 302–311. [Google Scholar] [CrossRef]
- Williamson, T.; Bai, R.Y.; Staedtke, V.; Huso, D.; Riggins, G.J. Mebendazole and a non-steroidal anti-inflammatory combine to reduce tumor initiation in a colon cancer preclinical model. Oncotarget 2016, 7, 68571–68584. [Google Scholar] [CrossRef]
- Dobrosotskaya, I.Y.; Hammer, G.D.; Schteingart, D.E.; Maturen, K.E.; Worden, F.P. Mebendazole monotherapy and long-term disease control in metastatic adrenocortical carcinoma. Endocr. Pract. 2011, 17, e59–e62. [Google Scholar] [CrossRef]
- Nygren, P.; Larsson, R. Drug repositioning from bench to bedside: Tumour remission by the antihelmintic drug mebendazole in refractory metastatic colon cancer. Acta Oncol. 2014, 53, 427–428. [Google Scholar] [CrossRef]
- Black, J.W.; Crowther, A.F.; Shanks, R.G.; Smith, L.H.; Dornhorst, A.C. A new adrenergic betareceptor antagonist. Lancet 1964, 1, 1080–1081. [Google Scholar] [CrossRef] [PubMed]
- Gillam, P.M.; Prichard, B.N. Use of propranolol in angina pectoris. Br. Med. J. 1965, 2, 337–339. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.; Modiri, O.; England, R.W.; Shawber, C.J.; Wu, J.K. Propranolol Therapy in Infantile Hemangioma: It Is Not Just About the Beta. Plast. Reconstr. Surg. 2021, 147, 875–885. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Gudipudi, R.; Nguyen, S.A.; Carroll, W.; Clemmens, C. Should Propranolol Remain the Gold Standard for Treatment of Infantile Hemangioma? A Systematic Review and Meta-Analysis of Propranolol Versus Atenolol. Ann. Otol Rhinol. Laryngol. 2023, 132, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Novoa, M.; Baselga, E.; Beltran, S.; Giraldo, L.; Shahbaz, A.; Pardo-Hernandez, H.; Arevalo-Rodriguez, I. Interventions for infantile haemangiomas of the skin. Cochrane Database Syst. Rev. 2018, 4, CD006545. [Google Scholar] [CrossRef] [PubMed]
- Park, P.G.; Merryman, J.; Orloff, M.; Schuller, H.M. Beta-adrenergic mitogenic signal transduction in peripheral lung adenocarcinoma: Implications for individuals with preexisting chronic lung disease. Cancer Res. 1995, 55, 3504–3508. [Google Scholar]
- Masur, K.; Niggemann, B.; Zanker, K.S.; Entschladen, F. Norepinephrine-induced migration of SW 480 colon carcinoma cells is inhibited by beta-blockers. Cancer Res. 2001, 61, 2866–2869. [Google Scholar]
- Sommers Smith, S.K.; Smith, D.M. Beta blockade induces apoptosis in cultured capillary endothelial cells. In Vitro Cell. Dev. Biol. Anim. 2002, 38, 298–304. [Google Scholar] [CrossRef]
- Ji, Y.; Chen, S.; Xiao, X.; Zheng, S.; Li, K. β-blockers: A novel class of antitumor agents. Onco Targets Ther. 2012, 5, 391–401. [Google Scholar] [CrossRef]
- Satilmis, H.; Verheye, E.; Vlummens, P.; Oudaert, I.; Vandewalle, N.; Fan, R.; Knight, J.M.; De Beule, N.; Ates, G.; Massie, A.; et al. Targeting the β2 -adrenergic receptor increases chemosensitivity in multiple myeloma by induction of apoptosis and modulating cancer cell metabolism. J. Pathol. 2023, 259, 69–80. [Google Scholar] [CrossRef]
- Grytli, H.H.; Fagerland, M.W.; Fosså, S.D.; Taskén, K.A.; Håheim, L.L. Use of β-blockers is associated with prostate cancer-specific survival in prostate cancer patients on androgen deprivation therapy. Prostate 2013, 73, 250–260. [Google Scholar] [CrossRef]
- Jansen, L.; Hoffmeister, M.; Arndt, V.; Chang-Claude, J.; Brenner, H. Stage-specific associations between beta blocker use and prognosis after colorectal cancer. Cancer 2014, 120, 1178–1186. [Google Scholar] [CrossRef]
- Schmidt, S.A.; Schmidt, M. Beta-blockers and improved survival from ovarian cancer: New miracle treatment or another case of immortal person-time bias? Cancer 2016, 22, 324–325. [Google Scholar] [CrossRef]
- Hwa, Y.L.; Shi, Q.; Kumar, S.K.; Lacy, M.Q.; Gertz, M.A.; Kapoor, P.; Buadi, F.K.; Leung, N.; Dingli, D.; Go, R.S.; et al. Beta-blockers improve survival outcomes in patients with multiple myeloma: A retrospective evaluation. Am. J. Hematol. 2017, 92, 50–55. [Google Scholar] [CrossRef]
- Kozanoglu, I.; Yandim, M.K.; Cincin, Z.B.; Ozdogu, H.; Cakmakoglu, B.; Baran, Y. New indication for therapeutic potential of an old well-known drug (propranolol) for multiple myeloma. J. Cancer Res. Clin. Oncol. 2013, 139, 327–335. [Google Scholar] [CrossRef]
- Knight, J.M.; Rizzo, J.D.; Hari, P.; Pasquini, M.C.; Giles, K.E.; D’Souza, A.; Logan, B.R.; Hamadani, M.; Chhabra, S.; Dhakal, B.; et al. Propranolol inhibits molecular risk markers in HCT recipients: A phase 2 randomized controlled biomarker trial. Blood Adv. 2020, 4, 467–476. [Google Scholar] [CrossRef]
- Del Toro, R.; Méndez-Ferrer, S. Autonomic regulation of hematopoiesis and cancer. Haematologica 2013, 98, 1663–1666. [Google Scholar] [CrossRef]
- Mendelson, A.; Frenette, P.S. Hematopoietic stem cell niche maintenance during homeostasis and regeneration. Nat. Med. 2014, 20, 833–846. [Google Scholar] [CrossRef]
- Benish, M.; Bartal, I.; Goldfarb, Y.; Levi, B.; Avraham, R.; Raz, A.; Ben-Eliyahu, S. Perioperative use of beta-blockers and COX-2 inhibitors may improve immune competence and reduce the risk of tumor metastasis. Ann. Surg Oncol. 2008, 15, 2042–2052. [Google Scholar] [CrossRef]
- Haldar, R.; Ricon-Becker, I.; Radin, A.; Gutman, M.; Cole, S.W.; Zmora, O.; Ben-Eliyahu, S. Perioperative COX2 and β-adrenergic blockade improves biomarkers of tumor metastasis, immunity, and inflammation in colorectal cancer: A randomized controlled trial. Cancer 2020, 126, 3991–4001. [Google Scholar] [CrossRef]
- Kern, M.A.; Haugg, A.M.; Koch, A.F.; Schilling, T.; Breuhahn, K.; Walczak, H.; Fleischer, B.; Trautwein, C.; Michalski, C.; Schulze-Bergkamen, H.; et al. Cyclooxygenase-2 inhibition induces apoptosis signaling via death receptors and mitochondria in hepatocellular carcinoma. Cancer Res. 2006, 66, 7059–7066. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.K.; Wang, H.; Peskar, B.M.; Levin, E.; Itani, R.M.; Sarfeh, I.J.; Tarnawski, A.S. Inhibition of angiogenesis by nonsteroidal anti-inflammatory drugs: Insight into mechanisms and implications for cancer growth and ulcer healing. Nat. Med. 1999, 5, 1418–1423. [Google Scholar] [CrossRef] [PubMed]
- Ménétrier-Caux, C.; Bain, C.; Favrot, M.C.; Duc, A.; Blay, J.Y. Renal cell carcinoma induces interleukin 10 and prostaglandin E2 production by monocytes. Br. J. Cancer 1999, 79, 119–130. [Google Scholar] [CrossRef]
- Regulska, K.; Regulski, M.; Karolak, B.; Murias, M.; Stanisz, B. Can cardiovascular drugs support cancer treatment? The rationale for drug repurposing. Drug Discov. Today 2019, 24, 1059–1065. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.N.; Mao, Z.X.; Wu, Y.; Liang, M.X.; Wang, D.D.; Chen, X.; Chang, P.A.; Zhang, W.; Tang, J.H. The anti-cancer properties of heparin and its derivatives: A review and prospect. Cell Adhes. Migr. 2020, 14, 118–128. [Google Scholar] [CrossRef]
- Kuderer, N.M.; Khorana, A.A.; Lyman, G.H.; Francis, C.W. A meta-analysis and systematic review of the efficacy and safety of anticoagulants as cancer treatment: Impact on survival and bleeding complications. Cancer 2007, 110, 1149–1161. [Google Scholar] [CrossRef]
- Stevenson, J.L.; Varki, A.; Borsig, L. Heparin attenuates metastasis mainly due to inhibition of P- and L-selectin, but non-anticoagulant heparins can have additional effects. Thromb. Res. 2007, 120, S107–S111, Erratum in: Thromb. Res. 2008, 123, 187–190. [Google Scholar] [CrossRef]
- Hejna, M.; Raderer, M.; Zielinski, C.C. Inhibition of metastases by anticoagulants. J. Natl. Cancer Inst. 1999, 91, 22–36. [Google Scholar] [CrossRef]
- Alyahya, R.; Sudha, T.; Racz, M.; Stain, S.C.; Mousa, S.A. Anti-metastasis efficacy and safety of non-anticoagulant heparin derivative versus low molecular weight heparin in surgical pancreatic cancer models. Int. J. Oncol. 2015, 46, 1225–1231. [Google Scholar] [CrossRef]
- Atallah, J.; Khachfe, H.H.; Berro, J.; Assi, H.I. The use of heparin and heparin-like molecules in cancer treatment: A review. Cancer Treat. Res. Commun. 2020, 24, 100192. [Google Scholar] [CrossRef]
- Malik, J.A.; Ahmed, S.; Momin, S.S.; Shaikh, S.; Alafnan, A.; Alanazi, J.; Said Almermesh, M.H.; Anwar, S. Drug Repurposing: A New Hope in Drug Discovery for Prostate Cancer. ACS Omega 2022, 8, 56–73. [Google Scholar] [CrossRef]
- Zhang, C.; Jin, H.; Wen, Y.F.; Yin, G. Efficacy of COVID-19 Treatments: A Bayesian Network Meta-Analysis of Randomized Controlled Trials. Front. Public Health 2021, 9, 729559. [Google Scholar] [CrossRef]
- Cheng, Q.; Chen, J.; Jia, Q.; Fang, Z.; Zhao, G. Efficacy and safety of current medications for treating severe and non-severe COVID-19 patients: An updated network meta-analysis of randomized placebo-controlled trials. Aging 2021, 13, 21866–21902. [Google Scholar] [CrossRef]
| Query | Search Algorithm | Number of Records ⴕ |
|---|---|---|
| #1 | (“Drug Repositioning”[MeSH Terms] OR “drug reposit*”[Title/Abstract] OR “drug repurpos*”[Title/Abstract] OR “drug resc*”[Title/Abstract] OR (“repurpos*”[Title/Abstract] AND “drug”[Title/Abstract]) OR (“reposit*”[Title/Abstract] AND “drug”[Title/Abstract]) OR “new indication”[Title/Abstract] OR “indication change”[Title/Abstract] OR “change indication”[Title/Abstract] OR “another indication”[Title/Abstract]) | 13,876 |
| #2 | (“antineoplastic agents”[MeSH Terms] OR “neoplasms”[MeSH Terms] OR “medical oncology”[MeSH Terms] OR “surgical oncology”[MeSH Terms] OR “carcinoma”[MeSH Terms] OR “hodgkin disease”[MeSH Terms] OR “leukemia, lymphocytic, chronic, b cell”[MeSH Terms] OR “lymphoma”[MeSH Terms] OR “myelodysplastic syndromes”[MeSH Terms] OR “tumo*”[Title/Abstract] OR “neoplas*”[Title/Abstract] OR “cancer”[Title/Abstract] OR “cancer*”[Title/Abstract] OR “malignan*”[Title/Abstract] OR “oncolog*”[Title/Abstract] OR “carcinom*”[Title/Abstract] OR “epitheliom*”[Title/Abstract] OR “Hodgkin”[Title/Abstract] OR “lymphom*”[Title/Abstract] OR “leukemia”[Title/Abstract] OR “Leucocythaemia”[Title/Abstract] OR “Leucocythemia”[Title/Abstract] OR “Leukocythemia”[Title/Abstract] OR “sarcoma”[Title/Abstract] OR “Reticulolymphosarcoma”[Title/Abstract] OR “Germinoblastoma”[Title/Abstract] OR “blastoma”[Title/Abstract] OR “myelodyspl*”[Title/Abstract] OR “Dysmyelopoietic”[Title/Abstract] OR “Anticancer”[Title/Abstract] OR “antineoplast*”[Title/Abstract] OR “antitumo*”[Title/Abstract] OR “chemotherap*”[Title/Abstract]) | 5,170,828 |
| #1 AND #2 | 4370 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ioakeim-Skoufa, I.; Tobajas-Ramos, N.; Menditto, E.; Aza-Pascual-Salcedo, M.; Gimeno-Miguel, A.; Orlando, V.; González-Rubio, F.; Fanlo-Villacampa, A.; Lasala-Aza, C.; Ostasz, E.; et al. Drug Repurposing in Oncology: A Systematic Review of Randomized Controlled Clinical Trials. Cancers 2023, 15, 2972. https://doi.org/10.3390/cancers15112972
Ioakeim-Skoufa I, Tobajas-Ramos N, Menditto E, Aza-Pascual-Salcedo M, Gimeno-Miguel A, Orlando V, González-Rubio F, Fanlo-Villacampa A, Lasala-Aza C, Ostasz E, et al. Drug Repurposing in Oncology: A Systematic Review of Randomized Controlled Clinical Trials. Cancers. 2023; 15(11):2972. https://doi.org/10.3390/cancers15112972
Chicago/Turabian StyleIoakeim-Skoufa, Ignatios, Natalia Tobajas-Ramos, Enrica Menditto, Mercedes Aza-Pascual-Salcedo, Antonio Gimeno-Miguel, Valentina Orlando, Francisca González-Rubio, Ana Fanlo-Villacampa, Carmen Lasala-Aza, Ewelina Ostasz, and et al. 2023. "Drug Repurposing in Oncology: A Systematic Review of Randomized Controlled Clinical Trials" Cancers 15, no. 11: 2972. https://doi.org/10.3390/cancers15112972
APA StyleIoakeim-Skoufa, I., Tobajas-Ramos, N., Menditto, E., Aza-Pascual-Salcedo, M., Gimeno-Miguel, A., Orlando, V., González-Rubio, F., Fanlo-Villacampa, A., Lasala-Aza, C., Ostasz, E., & Vicente-Romero, J. (2023). Drug Repurposing in Oncology: A Systematic Review of Randomized Controlled Clinical Trials. Cancers, 15(11), 2972. https://doi.org/10.3390/cancers15112972








