The Association between Statins and Liver Cancer Risk in Patients with Heart Failure: A Nationwide Population-Based Cohort Study
Abstract
Simple Summary
Abstract
1. Introduction
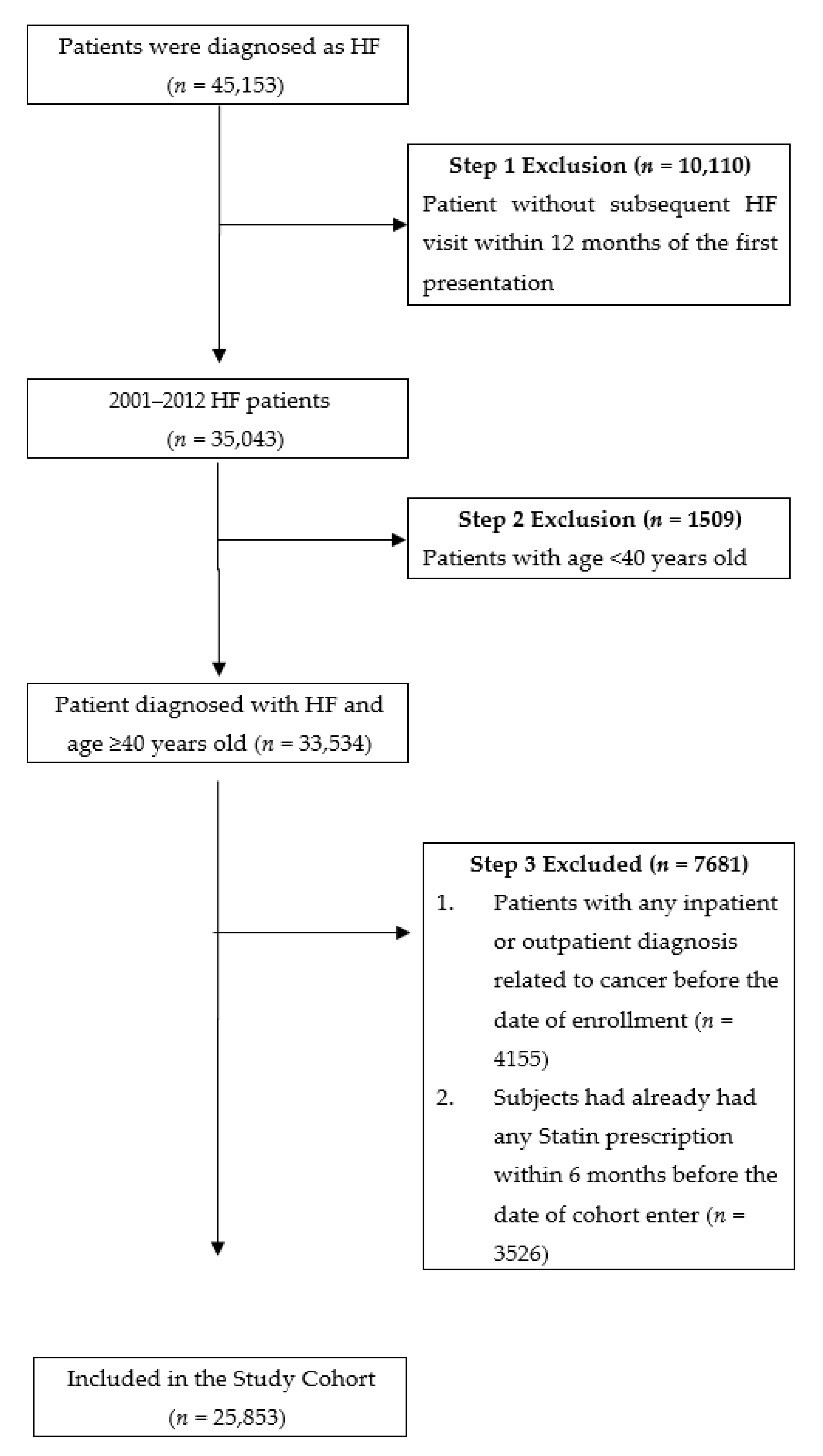
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roger, V.L. Epidemiology of heart failure. Circ. Res. 2013, 113, 646–659. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e895–e1032. [Google Scholar] [CrossRef]
- Jones, N.R.; Roalfe, A.K.; Adoki, I.; Hobbs, F.R.; Taylor, C.J. Survival of patients with chronic heart failure in the community: A systematic review and meta-analysis. Eur. J. Heart Fail 2019, 21, 1306–1325. [Google Scholar] [CrossRef] [PubMed]
- Hasin, T.; Gerber, Y.; McNallan, S.M.; Weston, S.A.; Kushwaha, S.S.; Nelson, T.J.; Cerhan, J.R.; Roger, V.L. Patients with heart failure have an increased risk of incident cancer. J. Am. Coll. Cardiol. 2013, 62, 881–886. [Google Scholar] [CrossRef] [PubMed]
- Banke, A.; Schou, M.; Videbaek, L.; Møller, J.E.; Torp-Pedersen, C.; Gustafsson, F.; Dahl, J.S.; Køber, L.; Hildebrandt, P.R.; Gislason, G.H.; et al. Incidence of cancer in patients with chronic heart failure: A long-term follow-up study. Eur. J. Heart Fail 2016, 18, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Hasin, T.; Gerber, Y.; Weston, S.A.; Jiang, R.; Killian, J.M.; Manemann, S.M.; Cerhan, J.R.; Roger, V.L. Heart Failure After Myocardial Infarction Is Associated With Increased Risk of Cancer. J. Am. Coll. Cardiol. 2016, 68, 265–271. [Google Scholar] [CrossRef]
- Bertero, E.; Canepa, M.; Maack, C.; Ameri, P. Linking Heart Failure to Cancer: Background Evidence and Research Perspectives. Circulation 2018, 138, 735–742. [Google Scholar] [CrossRef]
- de Boer, R.A.; Hulot, J.S.; Tocchetti, C.G.; Aboumsallem, J.P.; Ameri, P.; Anker, S.D.; Bauersachs, J.; Bertero, E.; Coats, A.J.; Čelutkienė, J.; et al. Common mechanistic pathways in cancer and heart failure. A scientific roadmap on behalf of the Translational Research Committee of the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur. J. Heart Fail 2020, 22, 2272–2289. [Google Scholar] [CrossRef]
- Joharatnam-Hogan, N.; Alexandre, L.; Yarmolinsky, J.; Lake, B.; Capps, N.; Martin, R.M.; Ring, A.; Cafferty, F.; E Langley, R. Statins as Potential Chemoprevention or Therapeutic Agents in Cancer: A Model for Evaluating Repurposed Drugs. Curr. Oncol. Rep. 2021, 23, 29. [Google Scholar] [CrossRef]
- Chan, K.K.W.; Oza, A.M.; Siu, L.L. The statins as anticancer agents. Clin. Cancer Res. 2003, 9, 10–19. [Google Scholar]
- Danesh, F.R.; Sadeghi, M.M.; Amro, N.; Philips, C.; Zeng, L.; Lin, S.; Sahai, A.; Kanwar, Y.S. 3-Hydroxy-3-methylglutaryl CoA reductase inhibitors prevent high glucose-induced proliferation of mesangial cells via modulation of Rho GTPase/ p21 signaling pathway: Implications for diabetic nephropathy. Proc. Natl. Acad. Sci. USA 2002, 99, 8301–8305. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Colio, L.M.; Muñoz-García, B.; Martín-Ventura, J.L.; Lorz, C.; Díaz, C.; Hernández, G.; Egido, J. 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors decrease Fas ligand expression and cytotoxicity in activated human T lymphocytes. Circulation 2003, 108, 1506–1513. [Google Scholar] [CrossRef] [PubMed]
- Juneja, M.; Kobelt, D.; Walther, W.; Voss, C.; Smith, J.; Specker, E.; Neuenschwander, M.; Gohlke, B.-O.; Dahlmann, M.; Radetzki, S.; et al. Statin and rottlerin small-molecule inhibitors restrict colon cancer progression and metastasis via MACC1. PLoS Biol. 2017, 15, e2000784. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.F.; Nordestgaard, B.G.; Bojesen, S.E. Statin use and reduced cancer-related mortality. N. Engl. J. Med. 2012, 367, 1792–1802. [Google Scholar] [CrossRef]
- Simon, T.G.; Duberg, A.-S.; Aleman, S.; Hagstrom, H.; Nguyen, L.H.; Khalili, H.; Chung, R.T.; Ludvigsson, J.F. Lipophilic Statins and Risk for Hepatocellular Carcinoma and Death in Patients with Chronic Viral Hepatitis: Results From a Nationwide Swedish Population. Ann. Intern. Med. 2019, 171, 318–327. [Google Scholar] [CrossRef]
- Jiang, W.; Hu, J.-W.; He, X.-R.; Jin, W.-L.; He, X.-Y. Statins: A repurposed drug to fight cancer. J. Exp. Clin. Cancer Res. 2021, 40, 241. [Google Scholar] [CrossRef]
- Shi, M.; Zheng, H.; Nie, B.; Gong, W.; Cui, X. Statin use and risk of liver cancer: An update meta-analysis. BMJ Open 2014, 4, e005399. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, W.; Wang, M.; Shi, J.; Jia, X.; Dang, S. A Meta-Analysis of Statin Use and Risk of Hepatocellular Carcinoma. Can. J. Gastroenterol. Hepatol. 2022, 2022, 5389044. [Google Scholar] [CrossRef]
- Villanueva, A. Hepatocellular Carcinoma. N. Engl. J. Med. 2019, 380, 1450–1462. [Google Scholar] [CrossRef]
- Ren, Q.-W.; Yu, S.-Y.; Teng, T.-H.K.; Li, X.; Cheung, K.-S.; Wu, M.-Z.; Li, H.-L.; Wong, P.-F.; Tse, H.-F.; Lam, C.S.P.; et al. Statin associated lower cancer risk and related mortality in patients with heart failure. Eur. Heart J. 2021, 42, 3049–3059. [Google Scholar] [CrossRef]
- Cheng, T.M. Reflections on the 20th anniversary of Taiwan’s single-payer National Health Insurance System. Health Aff. 2015, 34, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.-M.; Shin, D.W.; Yoo, T.G.; Cho, M.H.; Jang, W.; Lee, J.; Kim, S. Association between statin use and Alzheimer’s disease with dose response relationship. Sci. Rep. 2021, 11, 15280. [Google Scholar] [CrossRef]
- Singh, S.; Singh, P.P. Statins for prevention of hepatocellular cancer: One step closer? Hepatology 2014, 59, 724–726. [Google Scholar] [CrossRef] [PubMed]
- D’Agostino, R.B., Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat. Med. 1998, 17, 2265–2281. [Google Scholar] [CrossRef]
- Xing, Q.-Q.; Li, J.-M.; Dong, X.; Zeng, D.-Y.; Chen, Z.-J.; Lin, X.-Y.; Pan, J.-S. Socioeconomics and attributable etiology of primary liver cancer, 1990–2019. World J. Gastroenterol. 2022, 28, 2361–2382. [Google Scholar] [CrossRef]
- Schneeweiss, S. Sensitivity analysis and external adjustment for unmeasured confounders in epidemiologic database studies of therapeutics. Pharmacoepidemiol. Drug Saf. 2006, 15, 291–303. [Google Scholar] [CrossRef]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Kalogeropoulos, A.; Georgiopoulou, V.; Psaty, B.M.; Rodondi, N.; Smith, A.L.; Harrison, D.G.; Liu, Y.; Hoffmann, U.; Bauer, D.C.; Newman, A.B.; et al. Inflammatory markers and incident heart failure risk in older adults: The Health ABC (Health, Aging, and Body Composition) study. J. Am. Coll Cardiol. 2010, 55, 2129–2137. [Google Scholar] [CrossRef]
- Tsutamoto, T.; Hisanaga, T.; Wada, A.; Maeda, K.; Ohnishi, M.; Fukai, D.; Mabuchi, N.; Sawaki, M.; Kinoshita, M. Interleukin-6 spillover in the peripheral circulation increases with the severity of heart failure, and the high plasma level of interleukin-6 is an important prognostic predictor in patients with congestive heart failure. J. Am. Coll. Cardiol. 1998, 31, 391–398. [Google Scholar] [CrossRef]
- Meijers, W.C.; Maglione, M.; Bakker, S.J.L.; Oberhuber, R.; Kieneker, L.M.; de Jong, S.; Haubner, B.J.; Nagengast, W.B.; Lyon, A.R.; van der Vegt, B.; et al. Heart Failure Stimulates Tumor Growth by Circulating Factors. Circulation 2018, 138, 678–691. [Google Scholar] [CrossRef]
- Bertero, E.; Ameri, P.; Maack, C. Bidirectional Relationship Between Cancer and Heart Failure: Old and New Issues in Cardio-oncology. Card Fail Rev. 2019, 5, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Ausoni, S.; Azzarello, G. Development of Cancer in Patients With Heart Failure: How Systemic Inflammation Can Lay the Groundwork. Front. Cardiovasc. Med. 2020, 7, 598384. [Google Scholar] [CrossRef]
- Gbelcová, H.; Švéda, M.; Laubertová, L.; Varga, I.; Vítek, L.; Kolář, M.; Strnad, H.; Zelenka, J.; Böhmer, D.; Ruml, T. The effect of simvastatin on lipid droplets accumulation in human embryonic kidney cells and pancreatic cancer cells. Lipids Health Dis. 2013, 12, 126. [Google Scholar] [CrossRef] [PubMed]
- Pinchuk, T.V.; Fedulaev, Y.N.; Khairetdinova, G.A.; Denisova, N.N.; Chura, O.V.; Logunova, I.Y. Anti-inflammatory effects of simvastatin in patients with chronic heart failure. Bull. Exp. Biol. Med. 2014, 157, 552–554. [Google Scholar] [CrossRef] [PubMed]
- Cao, T.-T.; Chen, H.-H.; Dong, Z.; Xu, Y.-W.; Zhao, P.; Guo, W.; Wei, H.-C.; Zhang, C.; Lu, R. Stachydrine Protects Against Pressure Overload-Induced Cardiac Hypertrophy by Suppressing Autophagy. Cell Physiol. Biochem. 2017, 42, 103–114. [Google Scholar] [CrossRef]
- Sleijfer, S.; van der Gaast, A.; Planting, A.S.; Stoter, G.; Verweij, J. The potential of statins as part of anti-cancer treatment. Eur. J. Cancer 2005, 41, 516–522. [Google Scholar] [CrossRef]
- Takemoto, M.; Liao, J.K. Pleiotropic effects of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitors. Arterioscler Thromb Vasc Biol. 2001, 21, 1712–1719. [Google Scholar] [CrossRef]
- Freed-Pastor, W.A.; Mizuno, H.; Zhao, X.; Langerød, A.; Moon, S.-H.; Rodriguez-Barrueco, R.; Barsotti, A.; Chicas, A.; Li, W.; Polotskaia, A.; et al. Mutant p53 disrupts mammary tissue architecture via the mevalonate pathway. Cell 2012, 148, 244–258. [Google Scholar] [CrossRef]
- Wong, W.W.; Dimitroulakos, J.; Minden, M.D.; Penn, L.Z. HMG-CoA reductase inhibitors and the malignant cell: The statin family of drugs as triggers of tumor-specific apoptosis. Leukemia 2002, 16, 508–519. [Google Scholar] [CrossRef]
- Rao, S.; Porter, D.C.; Chen, X.; Herliczek, T.; Lowe, M.; Keyomarsi, K. Lovastatin-mediated G1 arrest is through inhibition of the proteasome, independent of hydroxymethyl glutaryl-CoA reductase. Proc. Natl. Acad. Sci. USA 1999, 96, 7797–7802. [Google Scholar] [CrossRef]
- Zhu, B.; Qu, S. The Relationship Between Diabetes Mellitus and Cancers and Its Underlying Mechanisms. Front. Endocrinol. 2022, 13, 800995. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-H.; Huang, C.-W.; Nguyen, P.A.; Lin, M.-C.; Yeh, C.-Y.; Islam, M.; Rahmanti, A.R.; Yang, H.-C. Chemopreventive Effects of Concomitant or Individual Use of Statins, Aspirin, Metformin, and Angiotensin Drugs: A Study Using Claims Data of 23 Million Individuals. Cancers 2022, 14, 1211. [Google Scholar] [CrossRef]
- Egom, E.E.A.; Hafeez, H. Biochemistry of Statins. Adv. Clin. Chem. 2016, 73, 127–168. [Google Scholar]
- Kapadia, S.B.; Chisari, F.V. Hepatitis C virus RNA replication is regulated by host geranylgeranylation and fatty acids. Proc. Natl. Acad. Sci. USA 2005, 102, 2561–2566. [Google Scholar] [CrossRef] [PubMed]
- Syed, G.H.; Amako, Y.; Siddiqui, A. Hepatitis C virus hijacks host lipid metabolism. Trends Endocrinol. Metab. 2010, 21, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Dorobantu, C.; Macovei, A.; Lazar, C.; Dwek, R.A.; Zitzmann, N.; Branza-Nichita, N. Cholesterol depletion of hepatoma cells impairs hepatitis B virus envelopment by altering the topology of the large envelope protein. J. Virol. 2011, 85, 13373–13383. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Xie, Y.; Yu, Z.; Xiao, H.; Jiang, G.; Zhou, X.; Yang, Y.; Li, X.; Zhao, M.; Li, L.; et al. The Mevalonate Pathway Is a Druggable Target for Vaccine Adjuvant Discovery. Cell 2018, 175, 1059–1073 e21. [Google Scholar] [CrossRef]
- Bader, T.; Korba, B. Simvastatin potentiates the anti-hepatitis B virus activity of FDA-approved nucleoside analogue inhibitors in vitro. Antivir. Res. 2010, 86, 241–245. [Google Scholar] [CrossRef]
- Zou, B.; Odden, M.C.; Nguyen, M.H. Statin Use and Reduced Hepatocellular Carcinoma Risk in Patients with Nonalcoholic Fatty Liver Disease. Clin. Gastroenterol. Hepatol. 2023, 21, 435–444.e6. [Google Scholar] [CrossRef]
- Zhong, G.-C.; Liu, Y.; Ye, Y.-Y.; Hao, F.-B.; Wang, K.; Gong, J.-P. Meta-analysis of studies using statins as a reducer for primary liver cancer risk. Sci. Rep. 2016, 6, 26256. [Google Scholar] [CrossRef]
- Nezasa, K.-I.; Higaki, K.; Matsumura, T.; Inazawa, K.; Hasegawa, H.; Nakano, M.; Koike, M. Liver-specific distribution of rosuvastatin in rats: Comparison with pravastatin and simvastatin. Drug Metab. Dispos. 2002, 30, 1158–1163. [Google Scholar] [CrossRef] [PubMed]
- Efthimiadis, A. Rosuvastatin and cardiovascular disease: Did the strongest statin hold the initial promises? Angiology 2008, 59 (Suppl 2), 62S–64S. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.H.; Davidson, M.H.; A Stein, E.; E Bays, H.; McKenney, J.M.; Miller, E.; A Cain, V.; Blasetto, J.W. Comparison of the efficacy and safety of rosuvastatin versus atorvastatin, simvastatin, and pravastatin across doses (STELLAR* Trial). Am. J. Cardiol. 2003, 92, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Paoletti, R.; Fahmy, M.; Mahla, G.; Mizan, J.; Southworth, H. Rosuvastatin demonstrates greater reduction of low-density lipoprotein cholesterol compared with pravastatin and simvastatin in hypercholesterolaemic patients: A randomized, double-blind study. J. Cardiovasc Risk 2001, 8, 383–390. [Google Scholar] [CrossRef]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; De Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 139, e1082–e1143. [Google Scholar]
- de Silva, R.; Nikitin, N.P.; Bhandari, S.; Nicholson, A.; Clark, A.L.; Cleland, J.G. Atherosclerotic renovascular disease in chronic heart failure: Should we intervene? Eur. Heart J. 2005, 26, 1596–1605. [Google Scholar] [CrossRef]
- Li, M.; Cima, M.J.; Milner, D.A., Jr. If It’s Not One Thing, It’s Another: An Inverse Relationship of Malignancy and Atherosclerotic Disease. PLoS ONE 2015, 10, e0126855. [Google Scholar] [CrossRef]
- Chen, W.; Zheng, R.; Zhang, S.; Zeng, H.; Zuo, T.; Xia, C.; Yang, Z.; He, J. Cancer incidence and mortality in China in 2013: An analysis based on urbanization level. Chin. J. Cancer Res. 2017, 29, 1–10. [Google Scholar] [CrossRef]
| Whole Cohort (n = 25,853) | Patients with Statin Use (≥28 Days; n = 7364) | Patients without Statin Use (<28 Days; n = 18,489) | p a | ||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Age, years (Mean ± SD) | 69.71 (13.18) | 65.86 (11.15) | 71.25 (12.58) | <0.001 | |||
| 40–54 | 3892 | 15.05 | 1428 | 19.39 | 2464 | 13.33 | <0.001 |
| 55–64 | 4545 | 17.58 | 1776 | 24.12 | 2769 | 14.98 | |
| 65–74 | 7665 | 29.65 | 2505 | 34.02 | 5160 | 27.91 | |
| ≥75 | 9751 | 37.72 | 1655 | 22.47 | 8096 | 43.79 | |
| Gender | |||||||
| Female | 13,409 | 51.87 | 4126 | 56.03 | 9283 | 50.21 | <0.001 |
| Male | 12,444 | 48.13 | 3238 | 43.97 | 9206 | 49.79 | |
| CCI + | |||||||
| 0 | 5562 | 21.51 | 1825 | 24.78 | 3737 | 20.21 | <0.001 |
| 1 | 6499 | 25.14 | 1997 | 27.12 | 4502 | 24.35 | |
| 2 | 5411 | 20.93 | 1523 | 20.68 | 3888 | 21.03 | |
| ≥3 | 8381 | 32.42 | 2019 | 27.42 | 6362 | 34.41 | |
| Diabetes | |||||||
| No | 17,126 | 66.24 | 4461 | 60.58 | 12,665 | 68.50 | <0.001 |
| Yes | 8727 | 33.76 | 2903 | 39.42 | 5824 | 31.50 | |
| Hypertension | |||||||
| No | 6878 | 26.60 | 1725 | 23.42 | 5153 | 27.87 | <0.001 |
| Yes | 18,975 | 73.40 | 5639 | 76.58 | 13,336 | 72.13 | |
| Dyslipidemia | |||||||
| No | 17,358 | 67.14 | 4057 | 55.09 | 13,301 | 71.94 | <0.001 |
| Yes | 8495 | 32.86 | 3307 | 44.91 | 5188 | 28.06 | |
| Non-statin lipid-lowering drugs | |||||||
| <28 days | 22,909 | 88.61 | 5427 | 73.70 | 17,482 | 94.55 | <0.001 |
| 28–365 days | 2027 | 7.84 | 1274 | 17.30 | 753 | 4.07 | |
| >365 days | 917 | 3.55 | 663 | 9.00 | 254 | 1.37 | |
| Metformin | |||||||
| <28 days | 20,061 | 77.60 | 4502 | 61.14 | 15,559 | 84.15 | <0.001 |
| 28–365 days | 2203 | 8.52 | 823 | 11.18 | 1380 | 7.46 | |
| >365 days | 3589 | 13.88 | 2039 | 27.69 | 1550 | 8.38 | |
| RAA | |||||||
| <28 days | 7257 | 28.07 | 957 | 13.00 | 6300 | 34.07 | <0.001 |
| 28–365 days | 6906 | 26.71 | 1521 | 20.65 | 5385 | 29.13 | |
| >365 days | 11,690 | 45.22 | 4886 | 66.35 | 6804 | 36.80 | |
| Aspirin | |||||||
| <28 days | 10,706 | 41.41 | 1701 | 23.10 | 9005 | 48.70 | <0.001 |
| 28–365 days | 6737 | 26.06 | 1873 | 25.43 | 4864 | 26.31 | |
| >365 days | 8410 | 32.53 | 3790 | 51.47 | 4620 | 24.99 | |
| Level of Urbanization | |||||||
| Urban | 16,521 | 63.90 | 4943 | 67.12 | 11,578 | 62.62 | <0.001 |
| Suburban | 5935 | 22.96 | 1542 | 20.94 | 4393 | 23.76 | |
| Rural | 3397 | 13.14 | 879 | 11.94 | 2518 | 13.62 | |
| Monthly income (TWD) | |||||||
| 0 | 3275 | 12.67 | 791 | 10.74 | 2484 | 13.44 | <0.001 |
| 1–21,000 | 6379 | 24.67 | 1567 | 21.28 | 4812 | 26.03 | |
| 21,000–33,300 | 10,337 | 39.98 | 2888 | 39.22 | 7449 | 40.29 | |
| ≥33,301 | 5862 | 22.67 | 2118 | 28.76 | 3744 | 20.25 | |
| Hepatitis B/C | |||||||
| No | 23,090 | 89.31 | 6710 | 91.12 | 16,380 | 88.59 | <0.001 |
| Yes | 2763 | 10.69 | 654 | 8.88 | 2109 | 11.41 | |
| Whole Cohort (n = 25,853) | ||
|---|---|---|
| n | % | |
| Total cohort | ||
| Never used | 17,973 | 69.52 |
| ≤28 days of statin use | 516 | 2.00 |
| >28 days of statin use | 7364 | 28.48 |
| Ever statin users | ||
| Single statin users | 4385 | 55.65 |
| Sequential multiple statins users | 3495 | 44.35 |
| Statin type ever prescribed a | ||
| Simvastatin | 2210 | 28.05 |
| Lovastatin | 1200 | 15.23 |
| Atorvastatin | 4264 | 54.11 |
| Fluvastatin | 1197 | 15.19 |
| Pravastatin | 1017 | 12.91 |
| Rosuvastatin | 2224 | 28.22 |
| All Group (n = 25,853) | Patients without Statin Use (Total Follow-Up 85,867.4 Person–Years) | Patients with Statin Use (Total Follow-Up 51,080.5 Person–Years) | Adjusted HR † (95% CI) | ||||
|---|---|---|---|---|---|---|---|
| No. of Patients with Liver Cancer | Incidence Rate (per 105 Person–Years) (95% CI) | No. of Patients with Liver Cancer | Incidence Rate (per 105 Person–Years) (95% CI) | ||||
| Whole cohort | |||||||
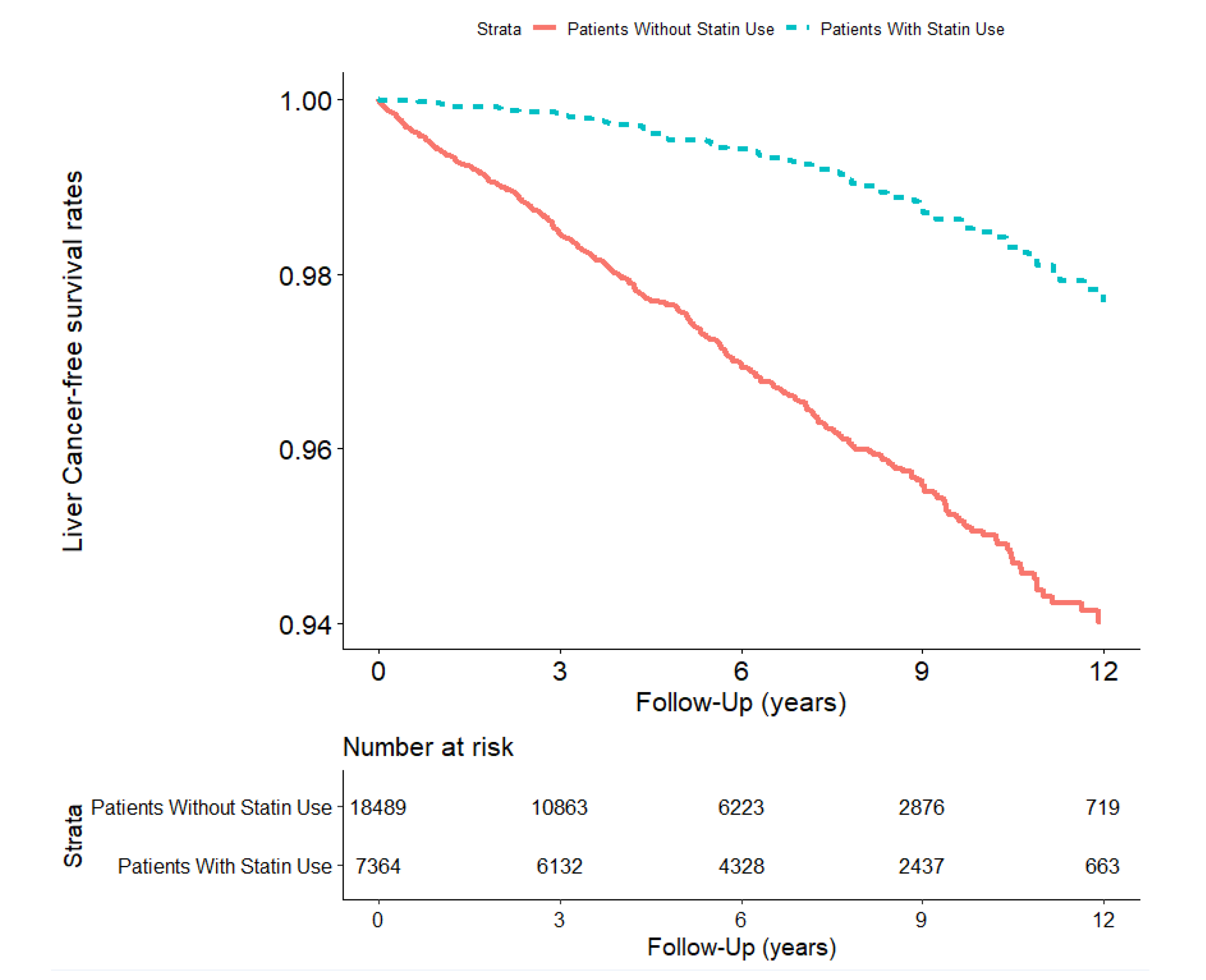
| Liver Cancer | 441 | 513.6 | (465.6, 561.5) | 71 | 139.0 | (106.7, 171.3) | 0.26(0.20, 0.33) *** |
| Age, 40–64 a | |||||||
| Liver Cancer | 153 | 537.0 | (451.9, 622.1) | 30 | 126.9 | (81.5, 172.3) | 0.23(0.15, 0.34) *** |
| Age, 65–74 b | |||||||
| Liver Cancer | 160 | 590.7 | (499.2, 682.2) | 31 | 171.6 | (111.2, 232.0) | 0.30(0.20, 0.44) *** |
| Age, ≥75 c | |||||||
| Liver Cancer | 128 | 422.6 | (349.4, 495.8) | 10 | 106.6 | (40.5, 172.7) | 0.25(0.13, 0.48) *** |
| Female d | |||||||
| Liver Cancer | 202 | 447.2 | (385.5, 508.8) | 33 | 110.0 | (72.5, 147.5) | 0.25(0.17, 0.36) *** |
| Male e | |||||||
| Liver Cancer | 239 | 587.3 | (512.9, 661.8) | 38 | 180.3 | (123.0, 237.6) | 0.27(0.19, 0.38) *** |
| Variable | No. of Patients | No. of Person-Years | No. of Patients with Liver Cancer | Incidence Rate (per 105 Person–Years) (95% CI) | Adjusted HR (95% CI) | p-Value for Trend | |
|---|---|---|---|---|---|---|---|
| Total statin use | |||||||
| Non-user (<28 cDDDs) | 18,489 | 85,867.4 | 441 | 513.6 | (465.6, 561.5) | 1.00 | <0.001 |
| User (≥28 cDDDs) | 7364 | 51,080.5 | 71 | 139.0 | (106.7, 171.3) | 0.26(0.20, 0.33) *** | |
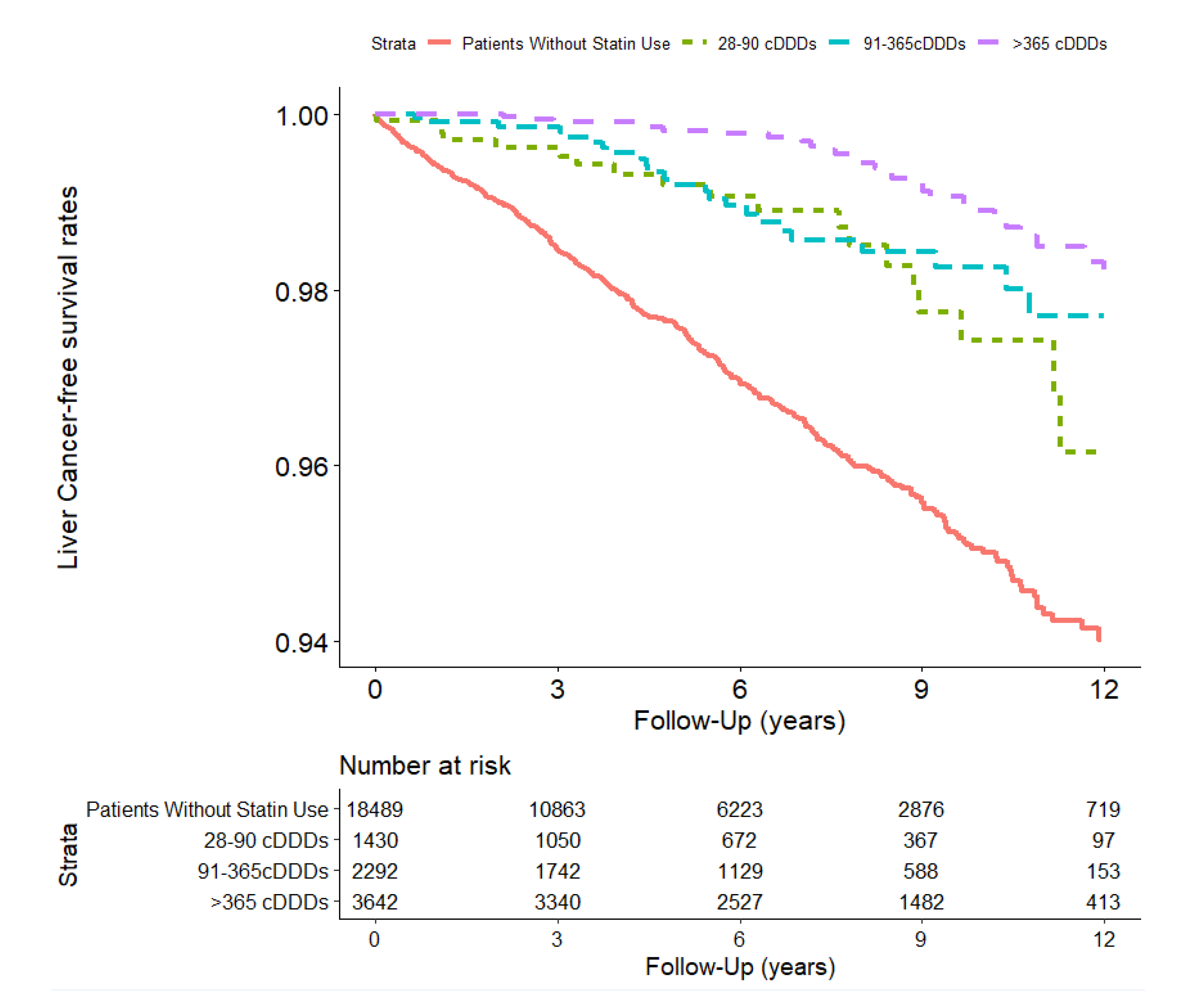
| 28–90 cDDDs | 1430 | 8530.1 | 19 | 222.7 | (122.6, 322.9) | 0.42(0.27, 0.67) *** | |
| 91–365 cDDDs | 2292 | 14,038.9 | 24 | 171.0 | (102.6, 239.3) | 0.32(0.21, 0.48) *** | |
| >365 cDDDs | 3642 | 28,511.5 | 28 | 98.2 | (61.8, 134.6) | 0.18(0.12, 0.26) *** | |
| Lipophilia statin use † | |||||||
| Non-user (<28 cDDDs) | 19,558 | 91,882.1 | 448 | 487.6 | (442.4, 532.7) | 1.00 | <0.001 |
| User (≥28 cDDDs) | 6295 | 45,065.8 | 64 | 142.0 | (107.2, 176.8) | 0.34(0.26, 0.44) *** | |
| 28–90 cDDDs | 1376 | 8485.9 | 19 | 223.9 | (123.2, 324.6) | 0.49(0.31, 0.78) ** | |
| 91–365 cDDDs | 2150 | 14,083.9 | 20 | 142.0 | (79.8, 204.2) | 0.33(0.21, 0.52) *** | |
| >365 cDDDs | 2769 | 22,495.9 | 25 | 111.1 | (67.6, 154.7) | 0.27(0.18, 0.41) *** | |
| Hydrophilia statin use † | |||||||
| Non-user (<28 cDDDs) | 18,489 | 85,867.4 | 488 | 568.3 | (517.9, 618.7) | 1.00 | <0.001 |
| User (≥28 cDDDs) | 2986 | 21,378.3 | 24 | 112.3 | (67.3, 157.2) | 0.42(0.28, 0.64) *** | |
| 28–90 cDDDs | 779 | 5168.5 | 7 | 135.4 | (35.1, 235.8) | 0.47(0.22, 1.00) | |
| 91–365 cDDDs | 1086 | 7493.3 | 9 | 120.1 | (41.6, 198.6) | 0.46(0.24, 0.90) * | |
| >365 cDDDs | 1121 | 8716.5 | 8 | 91.8 | (28.2, 155.4) | 0.35(0.17, 0.71) ** | |
| Individual statin use (≥28 cDDDs) ‡ | |||||||
| Simvastatin | 2210 | 17,280.4 | 22 | 127.3 | (74.1, 180.5) | 0.51(0.33, 0.79) ** | |
| Lovastatin | 1200 | 10,016.9 | 18 | 179.7 | (96.7, 262.7) | 0.70(0.43, 1.13) | |
| Atorvastatin | 4264 | 30,556.4 | 39 | 127.6 | (87.6, 167.7) | 0.39(0.28, 0.55) *** | |
| Fluvastatin | 1197 | 9276.5 | 13 | 140.1 | (64.0, 216.3) | 0.63(0.36, 1.10) | |
| Pravastatin | 1017 | 7827.8 | 13 | 166.1 | (75.8, 256.4) | 0.73(0.41, 1.27) | |
| Rosuvastatin | 2224 | 15,714.8 | 14 | 89.1 | (42.4, 135.8) | 0.34(0.20, 0.58) *** | |
| Statin Use | p for Trend | ||||
|---|---|---|---|---|---|
| <28 cDDDs | 28–90 cDDDs | 91–365 cDDDs | >365 cDDDs | ||
| Adjusted HR (95% CI) | Adjusted HR (95% CI) | Adjusted HR (95% CI) | Adjusted HR (95% CI) | ||
| Main model † | 1.00 | 0.42 (0.27, 0.67) *** | 0.32 (0.21, 0.48) *** | 0.18 (0.12, 0.26) *** | <0.001 |
| Additional covariates ‡ | |||||
| Main model + Non-statin | 1.00 | 0.43 (0.27, 0.69) *** | 0.34 (0.22, 0.51) *** | 0.19 (0.13, 0.29) *** | <0.001 |
| Main model + Metformin | 1.00 | 0.41 (0.26, 0.65) *** | 0.31 (0.20, 0.47) *** | 0.18 (0.12, 0.27) *** | <0.001 |
| Main model + RAA | 1.00 | 0.44 (0.28, 0.69) *** | 0.37 (0.24, 0.56) *** | 0.23 (0.15, 0.34) *** | <0.001 |
| Main model + Aspirin | 1.00 | 0.45 (0.29, 0.72) *** | 0.37 (0.24, 0.56) *** | 0.22 (0.15, 0.33) *** | <0.001 |
| Subgroup effects | |||||
| Age, years | |||||
| 40–64 | 1.00 | 0.21 (0.08, 0.57) ** | 0.23 (0.11, 0.47) *** | 0.23 (0.14, 0.38) *** | <0.001 |
| 65–74 | 1.00 | 0.49 (0.25, 0.97) ** | 0.46 (0.26, 0.81) *** | 0.15 (0.08, 0.30) *** | <0.001 |
| ≥75 | 1.00 | 0.72 (0.32, 1.63) | 0.24 (0.08, 0.75) * | 0.05 (0.01, 0.37) ** | <0.001 |
| Sex | |||||
| Female | 1.00 | 0.48 (0.26, 0.89) * | 0.24 (0.12, 0.48) *** | 0.17 (0.10, 0.30) *** | <0.001 |
| Male | 1.00 | 0.36 (0.18, 0.73) ** | 0.39 (0.23, 0.66) *** | 0.18 (0.11, 0.31) *** | <0.001 |
| CCI + | |||||
| 0 | 1.00 | 0.30 (0.07, 1.23) | 0.41 (0.16, 1.02) | 0.32 (0.15, 0.65) ** | <0.001 |
| 1 | 1.00 | 0.14 (0.04, 0.58) ** | 0.22 (0.09, 0.54) *** | 0.13 (0.06, 0.29) *** | <0.001 |
| 2 | 1.00 | 0.46 (0.19, 1.13) | 0.35 (0.16, 0.76) ** | 0.13 (0.05, 0.32) *** | <0.001 |
| ≥3 | 1.00 | 0.63 (0.33, 1.21) | 0.30 (0.14, 0.63) ** | 0.17 (0.08, 0.36) *** | <0.001 |
| Diabetes | |||||
| No | 1.00 | 0.53 (0.31, 0.90) * | 0.37 (0.22, 0.63) *** | 0.17 (0.10, 0.30) *** | <0.001 |
| Yes | 1.00 | 0.25 (0.10, 0.62) ** | 0.23 (0.12, 0.46) *** | 0.16 (0.09, 0.29) *** | <0.001 |
| Dyslipidemia | |||||
| No | 1.00 | 0.35 (0.19, 0.66) ** | 0.28 (0.16, 0.50) *** | 0.18 (0.11, 0.30) *** | <0.001 |
| Yes | 1.00 | 0.54 (0.27, 1.06) | 0.36 (0.20, 0.66) *** | 0.17 (0.10, 0.31) *** | <0.001 |
| Hypertension | |||||
| No | 1.00 | 0.42 (0.17, 1.04) | 0.39 (0.17, 0.89) * | 0.31 (0.16, 0.62) *** | <0.001 |
| Yes | 1.00 | 0.42 (0.25, 0.72) ** | 0.30 (0.18, 0.48) *** | 0.14 (0.09, 0.23) *** | <0.001 |
| Hepatitis B/C | |||||
| No | 1.00 | 0.55 (0.29, 1.04) | 0.42 (0.24, 0.74) ** | 0.20 (0.11, 0.35) *** | <0.001 |
| Yes | 1.00 | 0.38 (0.19, 0.73) ** | 0.29 (0.16, 0.53) *** | 0.28 (0.17, 0.48) *** | <0.001 |
| Non-Statin | |||||
| <28 days | 1.00 | 0.39 (0.23, 0.66) *** | 0.33 (0.21, 0.53) *** | 0.17 (0.11, 0.27) *** | <0.001 |
| 28–365 days | 1.00 | 0.86 (0.32, 2.33) | 0.46 (0.17, 1.23) | 0.28 (0.12, 0.66) ** | 0.002 |
| >365 days | 1.00 | 0.22 (0.03, 1.97) | 0.29 (0.07, 1.33) | 0.123 | |
| Metformin | |||||
| <28 days | 1.00 | 0.50 (0.30, 0.84) ** | 0.37 (0.23, 0.62) *** | 0.15 (0.08, 0.27) *** | <0.001 |
| 28–365 days | 1.00 | 0.22 (0.05, 0.90) * | 0.19 (0.06, 0.63) ** | 0.16 (0.05, 0.51) ** | <0.001 |
| >365 days | 1.00 | 0.27 (0.07, 1.11) | 0.27 (0.11, 0.67) ** | 0.22 (0.12, 0.41) *** | <0.001 |
| RAA | |||||
| <28 days | 1.00 | 0.68 (0.33, 1.39) | 0.53 (0.25, 1.13) | 0.17 (0.06, 0.55) ** | <0.001 |
| 28–365 days | 1.00 | 0.27 (0.10, 0.73) ** | 0.26 (0.10, 0.63) ** | 0.27 (0.12, 0.61) ** | <0.001 |
| >365 days | 1.00 | 0.45 (0.21, 0.97) * | 0.38 (0.21, 0.69) ** | 0.23 (0.14, 0.38) *** | <0.001 |
| Aspirin | |||||
| <28 days | 1.00 | 0.47 (0.22, 0.99) * | 0.32 (0.14, 0.71) ** | 0.20 (0.09, 0.46) *** | <0.001 |
| 28–365 days | 1.00 | 0.52 (0.25, 1.06) | 0.42 (0.21, 0.84) * | 0.24 (0.11, 0.53) *** | <0.001 |
| >365 days | 1.00 | 0.36 (0.13, 0.99) * | 0.38 (0.19, 0.76) ** | 0.23 (0.13, 0.40) *** | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, M.-C.; Chen, C.-C.; Lu, M.-Y.; Lin, K.-J.; Chiu, C.-C.; Yang, T.-Y.; Fang, Y.-A.; Jian, W.; Chen, M.-Y.; Hsu, M.-H.; et al. The Association between Statins and Liver Cancer Risk in Patients with Heart Failure: A Nationwide Population-Based Cohort Study. Cancers 2023, 15, 2959. https://doi.org/10.3390/cancers15112959
Lu M-C, Chen C-C, Lu M-Y, Lin K-J, Chiu C-C, Yang T-Y, Fang Y-A, Jian W, Chen M-Y, Hsu M-H, et al. The Association between Statins and Liver Cancer Risk in Patients with Heart Failure: A Nationwide Population-Based Cohort Study. Cancers. 2023; 15(11):2959. https://doi.org/10.3390/cancers15112959
Chicago/Turabian StyleLu, Meng-Chuan, Chun-Chao Chen, Meng-Ying Lu, Kuan-Jie Lin, Chun-Chih Chiu, Tsung-Yeh Yang, Yu-Ann Fang, William Jian, Ming-Yao Chen, Min-Huei Hsu, and et al. 2023. "The Association between Statins and Liver Cancer Risk in Patients with Heart Failure: A Nationwide Population-Based Cohort Study" Cancers 15, no. 11: 2959. https://doi.org/10.3390/cancers15112959
APA StyleLu, M.-C., Chen, C.-C., Lu, M.-Y., Lin, K.-J., Chiu, C.-C., Yang, T.-Y., Fang, Y.-A., Jian, W., Chen, M.-Y., Hsu, M.-H., Lai, Y.-H., Yang, T.-L., Hao, W.-R., & Liu, J.-C. (2023). The Association between Statins and Liver Cancer Risk in Patients with Heart Failure: A Nationwide Population-Based Cohort Study. Cancers, 15(11), 2959. https://doi.org/10.3390/cancers15112959









