Predictive Biomarkers of Pathological Response to Neoadjuvant Chemoradiotherapy for Locally Advanced Soft Tissue Sarcomas
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Characteristics of the Patient
2.2. Pathological Response
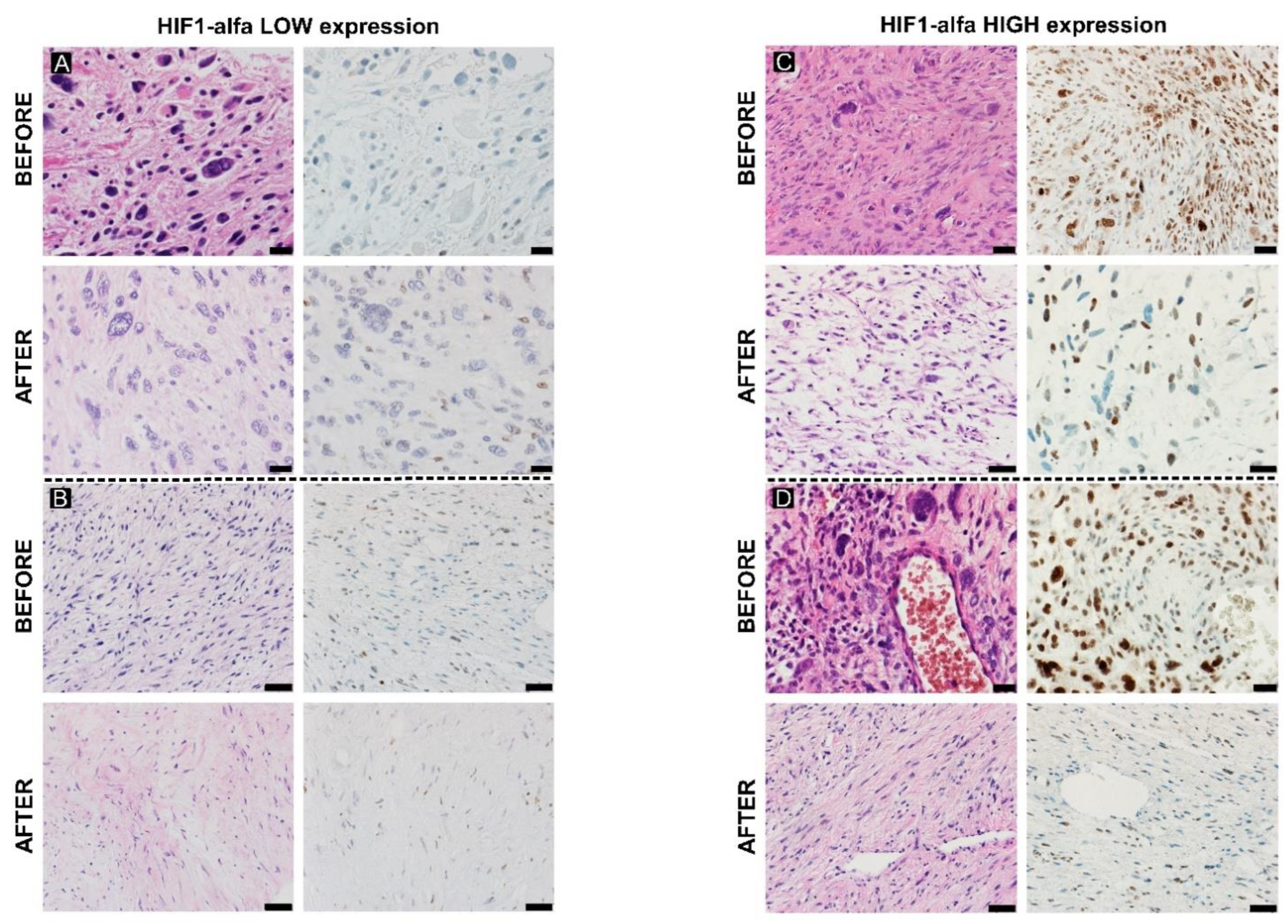
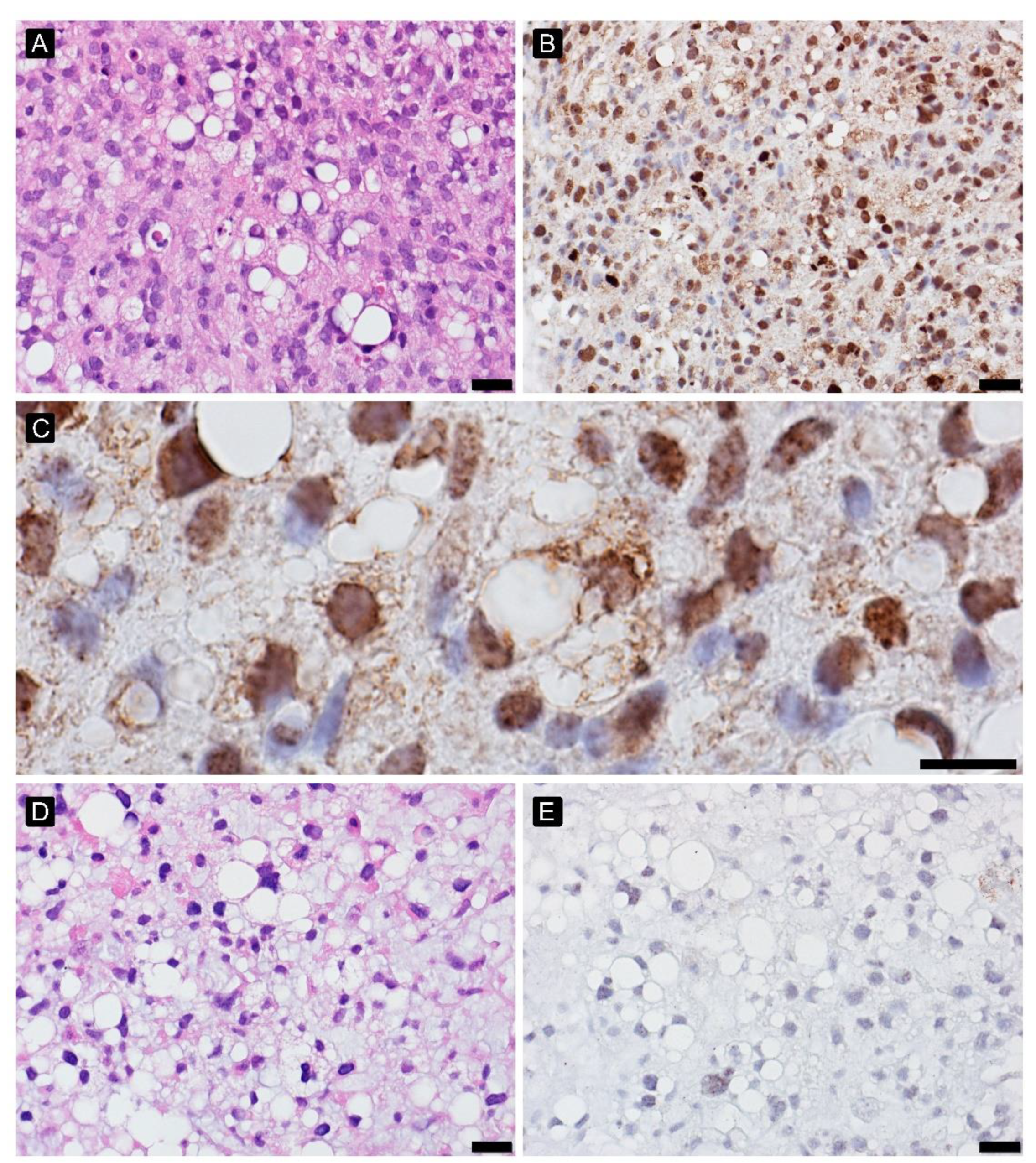
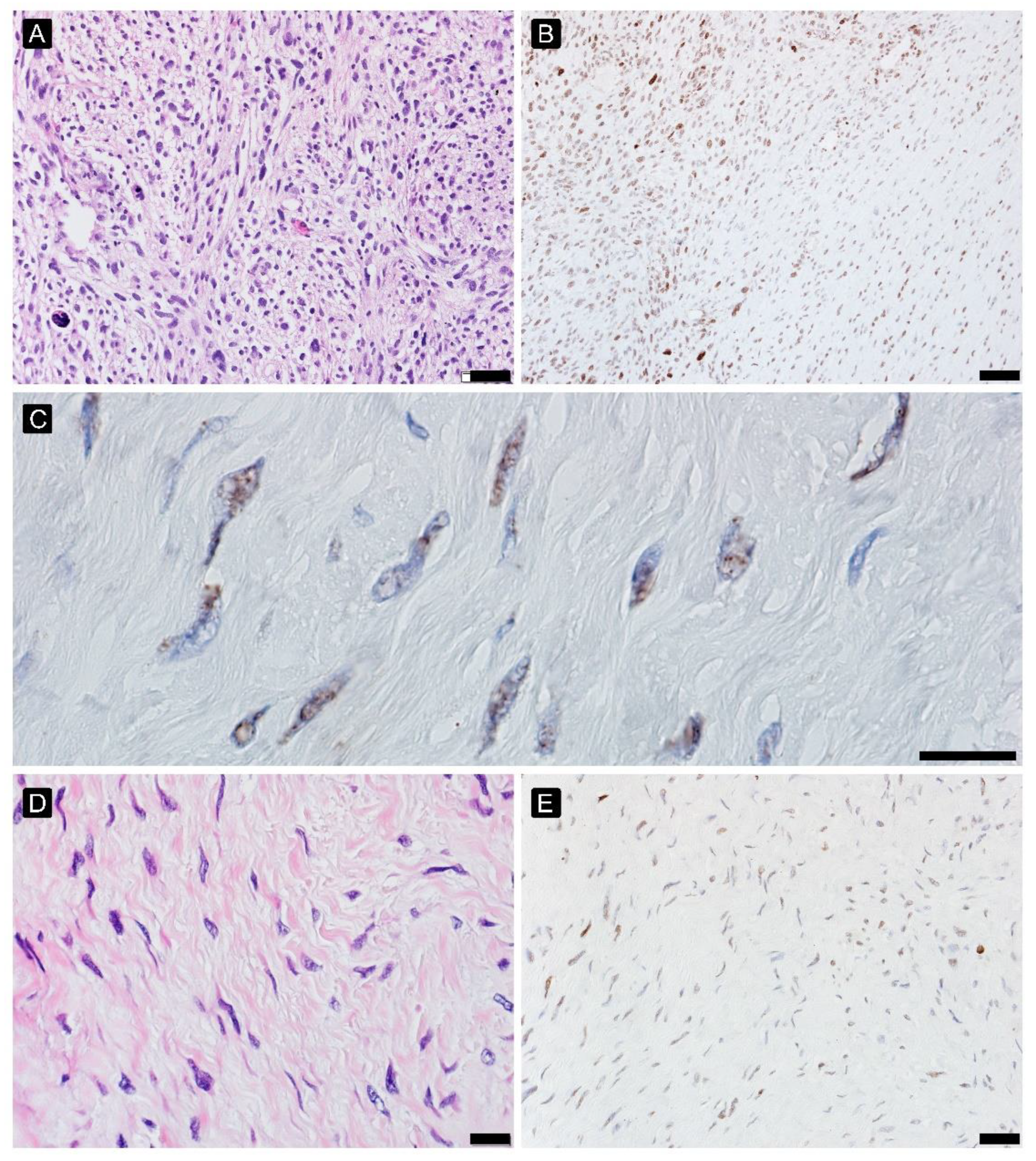
2.3. Hypoxia Response
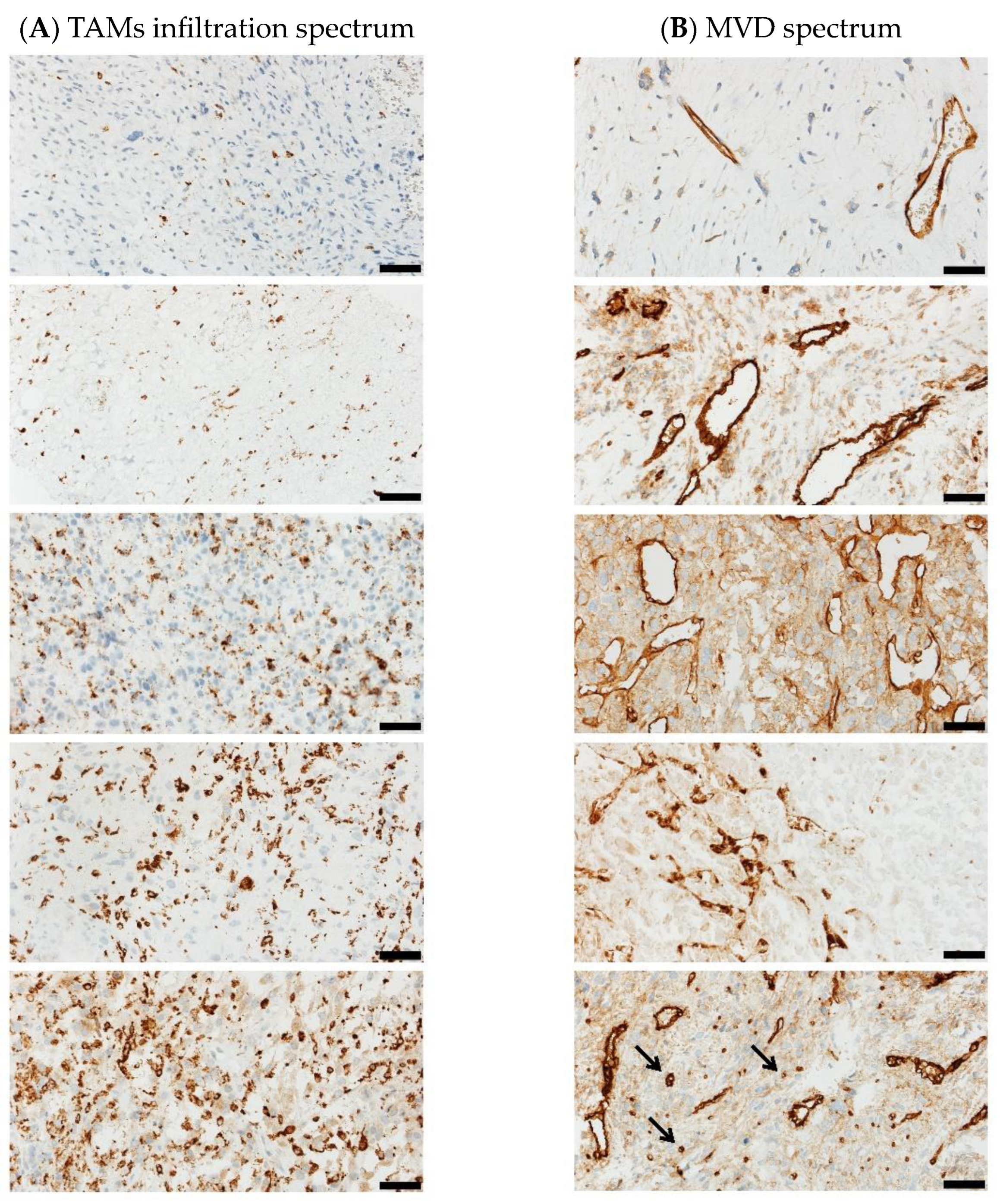
2.4. Immune Infiltration
2.5. Microvessel Density Analysis
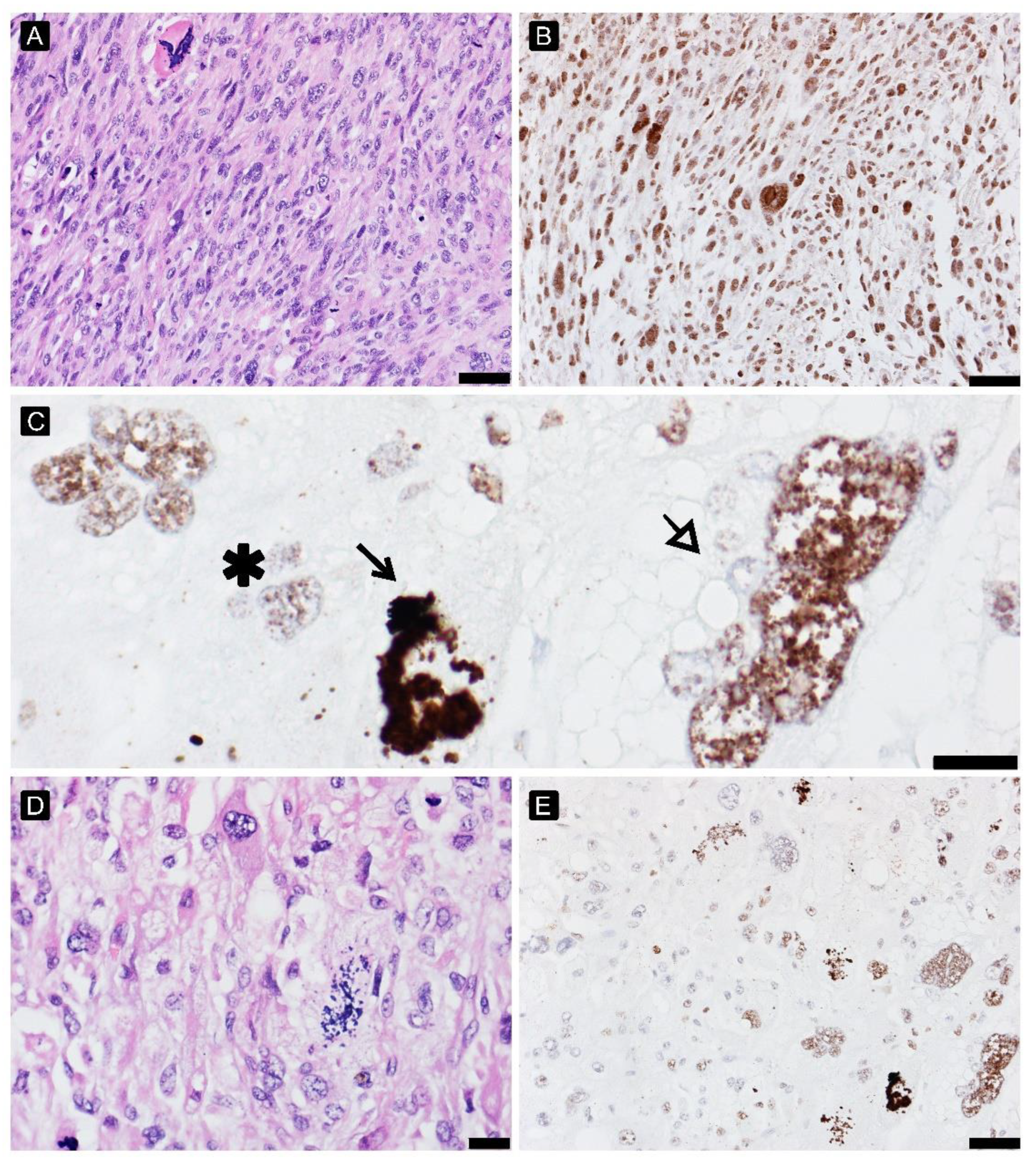
2.6. DNA Damage Analysis
2.7. Statistical Analysis
3. Results
3.1. Characteristics of the Patient
3.2. Pathological Response
3.3. Hypoxia Response
3.4. Immune Infiltration
3.5. Microvessel Density Analysis
3.6. DNA Damage Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gronchi, A.; Miah, A.B.; Dei Tos, A.P.; Abecassis, N.; Bajpai, J.; Bauer, S.; Biagini, R.; Bielack, S.; Blay, J.Y.; Bolle, S.; et al. Soft tissue and visceral sarcomas: ESMO-EURACAN-GENTURIS Clinical Practice Guidelines for diagnosis, treatment and follow-up(☆). Ann. Oncol. 2021, 32, 1348–1365. [Google Scholar] [CrossRef]
- Rutkowski, P.; Koseła-Paterczyk, H.; Kozak, K.; Ługowska, I.; Fijuth, J.; Jeziorski, A.; Ryś, J.; Spałek, M.; Borkowska, A.; Wągrodzki, M.; et al. Postępowanie diagnostyczno-terapeutyczne u chorych na mięsaki tkanek miękkich u dorosłych—Zalecenia ekspertów. Onkologia w Praktyce Klinicznej Edukacja. 2022. Available online: https://journals.viamedica.pl/onkologia_w_praktyce_klin_edu/article/view/91853 (accessed on 27 March 2023).
- Chan, C.H.; Wong, P. Molecular Predictors of Radiotherapy Response in Sarcoma. Curr. Treat. Options Oncol. 2016, 17, 2. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Yuan, Z.; Ahmed, K.; Welsh, E.A.; Fulp, W.J.; Gonzalez, R.J.; Mullinax, J.E.; Letson, D.; Bui, M.; Harrison, L.B.; et al. Genomic identification of sarcoma radiosensitivity and the clinical implications for radiation dose personalization. Transl. Oncol. 2021, 14, 101165. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Zeng, Q.; Li, Y.; Zhang, X.; Suto, M.J.; Xu, B.; Yi, N. Predicting radiotherapy response for patients with soft tissue sarcoma by developing a molecular signature. Oncol. Rep. 2017, 38, 2814–2824. [Google Scholar] [CrossRef] [PubMed]
- Shah, D.; Borys, D.; Martinez, S.R.; Li, C.-S.; Tamurian, R.M.; Bold, R.J.; Monjazeb, A.; Canter, R.J. Complete Pathologic Response to Neoadjuvant Radiotherapy is Predictive of Oncological Outcome in Patients with Soft Tissue Sarcoma. Anticancer Res. 2012, 32, 3911–3915. [Google Scholar] [PubMed]
- Taylor, B.S.; Barretina, J.; Maki, R.G.; Antonescu, C.R.; Singer, S.; Ladanyi, M. Advances in sarcoma genomics and new therapeutic targets. Nat. Rev. Cancer 2011, 11, 541–557. [Google Scholar] [CrossRef] [PubMed]
- Sbaraglia, M.; Dei Tos, A.P. The pathology of soft tissue sarcomas. Radiol. Med. 2019, 124, 266–281. [Google Scholar] [CrossRef]
- Zhang, R.S.; Liu, J.; Deng, Y.T.; Wu, X.; Jiang, Y. The real-world clinical outcomes and treatment patterns of patients with unresectable locally advanced or metastatic soft tissue sarcoma treated with anlotinib in the post-ALTER0203 trial era. Cancer Med. 2022, 11, 2271–2283. [Google Scholar] [CrossRef]
- Fiedorowicz, M.; Bartnik, E.; Sobczuk, P.; Teterycz, P.; Czarnecka, A.M. Molecular biology of sarcoma. Oncol. Clin. Pract. 2018, 14, 307–330. [Google Scholar] [CrossRef]
- Ballinger, M.L.; Goode, D.L.; Ray-Coquard, I.; James, P.A.; Mitchell, G.; Niedermayr, E.; Puri, A.; Schiffman, J.D.; Dite, G.S.; Cipponi, A.; et al. Monogenic and polygenic determinants of sarcoma risk: An international genetic study. Lancet Oncol. 2016, 17, 1261–1271. [Google Scholar] [CrossRef]
- Nacev, B.A.; Sanchez-Vega, F.; Smith, S.A.; Antonescu, C.R.; Rosenbaum, E.; Shi, H.; Tang, C.; Socci, N.D.; Rana, S.; Gularte-Mérida, R.; et al. Clinical sequencing of soft tissue and bone sarcomas delineates diverse genomic landscapes and potential therapeutic targets. Nat. Commun. 2022, 13, 3405. [Google Scholar] [CrossRef]
- Czarnecka, A.M.; Synoradzki, K.; Firlej, W.; Bartnik, E.; Sobczuk, P.; Fiedorowicz, M.; Grieb, P.; Rutkowski, P. Molecular Biology of Osteosarcoma. Cancers 2020, 12, 2130. [Google Scholar] [CrossRef] [PubMed]
- Marino-Enriquez, A.; Bovee, J.V. Molecular Pathogenesis and Diagnostic, Prognostic and Predictive Molecular Markers in Sarcoma. Surg. Pathol. Clin. 2016, 9, 457–473. [Google Scholar] [CrossRef] [PubMed]
- Bui, N.Q.; Przybyl, J.; Trabucco, S.E.; Frampton, G.; Hastie, T.; van de Rijn, M.; Ganjoo, K.N. A clinico-genomic analysis of soft tissue sarcoma patients reveals CDKN2A deletion as a biomarker for poor prognosis. Clin. Sarcoma Res. 2019, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Spalek, M.; Kosela Paterczyk, H.M.; Borkowska, A.; Wagrodzki, M.; Cieszanowski, A.; Castaneda-Wysocka, P.; Switaj, T.; Dudzisz-Sledz, M.E.; Czarnecka, A.M.; Dabrowska-Szewczyk, E.; et al. Preoperative hypofractionated radiotherapy (RT) combined with chemotherapy in primary marginally resectable high grade soft tissue sarcomas (STS) of extremities or trunk wall: Interim analysis of prospective phase II clinical trial. Ann. Oncol. 2018, 29, viii585–viii586. [Google Scholar] [CrossRef]
- Kane, J.M., 3rd; Magliocco, A.; Zhang, Q.; Wang, D.; Klimowicz, A.; Harris, J.; Simko, J.; DeLaney, T.; Kraybill, W.; Kirsch, D.G. Correlation of High-Risk Soft Tissue Sarcoma Biomarker Expression Patterns with Outcome following Neoadjuvant Chemoradiation. Sarcoma 2018, 2018, 8310950. [Google Scholar] [CrossRef]
- Tang, Z.; Zeng, Q.; Li, Y.; Zhang, X.; Ma, J.; Suto, M.J.; Xu, B.; Yi, N. Development of a radiosensitivity gene signature for patients with soft tissue sarcoma. Oncotarget 2017, 8, 27428–27439. [Google Scholar] [CrossRef]
- Spałek, M.J.; Koseła-Paterczyk, H.; Borkowska, A.; Wągrodzki, M.; Szumera-Ciećkiewicz, A.; Czarnecka, A.M.; Castaneda-Wysocka, P.; Kalinowska, I.; Poleszczuk, J.; Dąbrowska-Szewczyk, E.; et al. Combined Preoperative Hypofractionated Radiotherapy With Doxorubicin-Ifosfamide Chemotherapy in Marginally Resectable Soft Tissue Sarcomas: Results of a Phase 2 Clinical Trial. Int. J. Radiat. Oncol. Biol. Phys. 2021, 110, 1053–1063. [Google Scholar] [CrossRef]
- Spalek, M.; Koseła-Paterczyk, H.; Borkowska, A.; Wągrodzki, M.; Szumera-Ciećkiewicz, A.; Cieszanowski, A.; Castaneda-Wysocka, P.; Świtaj, T.; Dudzisz-Śledź, M.; Czarnecka, A.; et al. 5 × 5 Gy with chemotherapy in borderline resectable soft tissue sarcomas: Early results of a trial. Radiother. Oncol. 2019, 133, S31–S32. [Google Scholar] [CrossRef]
- Wardelmann, E.; Haas, R.L.; Bovée, J.V.M.G.; Terrier, P.; Lazar, A.; Messiou, C.; LePechoux, C.; Hartmann, W.; Collin, F.; Fisher, C.; et al. Evaluation of response after neoadjuvant treatment in soft tissue sarcomas; the European Organization for Research and Treatment of Cancer–Soft Tissue and Bone Sarcoma Group (EORTC–STBSG) recommendations for pathological examination and reporting. Eur. J. Cancer 2016, 53, 84–95. [Google Scholar] [CrossRef]
- Godbole, G.B.; Modi, D.N.; Puri, C.P. Regulation of homeobox A10 expression in the primate endometrium by progesterone and embryonic stimuli. Reproduction 2007, 134, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Gaber, G.; El Achy, S.; Khedr, G.A.; Parimi, V.; Helenowksi, I.; Donnelly, E.D.; Strauss, J.B.; Woloschak, G.; Wei, J.-J.; Small, W.; et al. Impact of p53, HIF1a, Ki-67, CA-9, and GLUT1 Expression on Treatment Outcomes in Locally Advanced Cervical Cancer Patients Treated With Definitive Chemoradiation Therapy. Am. J. Clin. Oncol. 2021, 44, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Weidner, N. Current pathologic methods for measuring intratumoral microvessel density within breast carcinoma and other solid tumors. Breast Cancer Res. Treat. 1995, 36, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Weidner, N.; Semple, J.P.; Welch, W.R.; Folkman, J. Tumor angiogenesis and metastasis--correlation in invasive breast carcinoma. N. Engl. J. Med. 1991, 324, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Qiu, Q.; Li, Z.; Sachdeva, M.; Min, H.; Cardona, D.M.; DeLaney, T.F.; Han, T.; Ma, Y.; Luo, L.; et al. HIF-1 Alpha Regulates the Response of Primary Sarcomas to Radiation Therapy through a Cell Autonomous Mechanism. Radiat. Res. 2015, 183, 594–609. [Google Scholar] [CrossRef]
- Yehia, L.; Boulos, F.; Jabbour, M.; Mahfoud, Z.; Fakhruddin, N.; El-Sabban, M. Expression of HIF-1α and Markers of Angiogenesis Are Not Significantly Different in Triple Negative Breast Cancer Compared to Other Breast Cancer Molecular Subtypes: Implications for Future Therapy. PLoS ONE 2015, 10, e0129356. [Google Scholar] [CrossRef]
- Ellingsen, C.; Andersen, L.M.; Galappathi, K.; Rofstad, E.K. Hypoxia biomarkers in squamous cell carcinoma of the uterine cervix. BMC Cancer 2015, 15, 805. [Google Scholar] [CrossRef]
- Kim, Y.H.; Yoo, K.C.; Cui, Y.H.; Uddin, N.; Lim, E.J.; Kim, M.J.; Nam, S.Y.; Kim, I.G.; Suh, Y.; Lee, S.J. Radiation promotes malignant progression of glioma cells through HIF-1alpha stabilization. Cancer Lett. 2014, 354, 132–141. [Google Scholar] [CrossRef]
- Lee, H.J.; Yoon, C.; Park, D.J.; Kim, Y.J.; Schmidt, B.; Lee, Y.J.; Tap, W.D.; Eisinger-Mathason, T.S.; Choy, E.; Kirsch, D.G.; et al. Inhibition of vascular endothelial growth factor A and hypoxia-inducible factor 1α maximizes the effects of radiation in sarcoma mouse models through destruction of tumor vasculature. Int. J. Radiat. Oncol. Biol. Phys. 2015, 91, 621–630. [Google Scholar] [CrossRef]
- Shintani, K.; Matsumine, A.; Kusuzaki, K.; Matsubara, T.; Satonaka, H.; Wakabayashi, T.; Hoki, Y.; Uchida, A. Expression of hypoxia-inducible factor (HIF)-1α as a biomarker of outcome in soft-tissue sarcomas. Virchows Archiv 2006, 449, 673–681. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, W.; Li, S.; Tu, C. Prognosis value of Hypoxia-inducible factor-1α expression in patients with bone and soft tissue sarcoma: A meta-analysis. Springerplus 2016, 5, 1370. [Google Scholar] [CrossRef] [PubMed]
- Najbauer, J.; Fukushima, S.; Endo, M.; Matsumoto, Y.; Fukushi, J.-I.; Matsunobu, T.; Kawaguchi, K.-I.; Setsu, N.; Iida, K.; Yokoyama, N.; et al. Hypoxia-inducible factor 1 alpha is a poor prognostic factor and potential therapeutic target in malignant peripheral nerve sheath tumor. PLoS ONE 2017, 12, e0178064. [Google Scholar] [CrossRef]
- Nie, C.; Lv, H.; Bie, L.; Hou, H.; Chen, X. Hypoxia-inducible factor 1-alpha expression correlates with response to neoadjuvant chemotherapy in women with breast cancer. Medicine 2018, 97, e13551. [Google Scholar] [CrossRef] [PubMed]
- Aebersold, D.M.; Burri, P.; Beer, K.T.; Laissue, J.; Djonov, V.; Greiner, R.H.; Semenza, G.L. Expression of hypoxia-inducible factor-1alpha: A novel predictive and prognostic parameter in the radiotherapy of oropharyngeal cancer. Cancer Res. 2001, 61, 2911–2916. [Google Scholar]
- Koukourakis, M.I.; Giatromanolaki, A.; Sivridis, E.; Simopoulos, C.; Turley, H.; Talks, K.; Gatter, K.C.; Harris, A.L. Hypoxia-inducible factor (HIF1A and HIF2A), angiogenesis, and chemoradiotherapy outcome of squamous cell head-and-neck cancer. Int. J. Radiat. Oncol. Biol. Phys. 2002, 53, 1192–1202. [Google Scholar] [CrossRef]
- Koukourakis, M.I.; Giatromanolaki, A.; Skarlatos, J.; Corti, L.; Blandamura, S.; Piazza, M.; Gatter, K.C.; Harris, A.L. Hypoxia inducible factor (HIF-1a and HIF-2a) expression in early esophageal cancer and response to photodynamic therapy and radiotherapy. Cancer Res. 2001, 61, 1830–1832. [Google Scholar]
- Hui, E.P.; Chan, A.T.; Pezzella, F.; Turley, H.; To, K.F.; Poon, T.C.; Zee, B.; Mo, F.; Teo, P.M.; Huang, D.P.; et al. Coexpression of hypoxia-inducible factors 1alpha and 2alpha, carbonic anhydrase IX, and vascular endothelial growth factor in nasopharyngeal carcinoma and relationship to survival. Clin. Cancer Res. 2002, 8, 2595–2604. [Google Scholar]
- Bachtiary, B.; Schindl, M.; Potter, R.; Dreier, B.; Knocke, T.H.; Hainfellner, J.A.; Horvat, R.; Birner, P. Overexpression of Hypoxia-inducible Factor 1α Indicates Diminished Response to Radiotherapy and Unfavorable Prognosis in Patients Receiving Radical Radiotherapy for Cervical Cancer1. Clin. Cancer Res. 2003, 9, 2234–2240. [Google Scholar]
- Xia, Y.; Jiang, L.; Zhong, T. The role of HIF-1alpha in chemo-/radioresistant tumors. Onco Targets Ther. 2018, 11, 3003–3011. [Google Scholar] [CrossRef]
- Tsagozis, P.; Augsten, M.; Zhang, Y.; Li, T.; Hesla, A.; Bergh, J.; Haglund, F.; Tobin, N.P.; Ehnman, M. An immunosuppressive macrophage profile attenuates the prognostic impact of CD20-positive B cells in human soft tissue sarcoma. Cancer Immunol. Immunother. 2019, 68, 927–936. [Google Scholar] [CrossRef]
- Kather, J.N.; Hörner, C.; Weis, C.-A.; Aung, T.; Vokuhl, C.; Weiss, C.; Scheer, M.; Marx, A.; Simon-Keller, K. CD163+ immune cell infiltrates and presence of CD54+ microvessels are prognostic markers for patients with embryonal rhabdomyosarcoma. Sci. Rep. 2019, 9, 9211. [Google Scholar] [CrossRef]
- Pervaiz, N.; Colterjohn, N.; Farrokhyar, F.; Tozer, R.; Figueredo, A.; Ghert, M. A systematic meta-analysis of randomized controlled trials of adjuvant chemotherapy for localized resectable soft-tissue sarcoma. Cancer 2008, 113, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Dumars, C.; Ngyuen, J.M.; Gaultier, A.; Lanel, R.; Corradini, N.; Gouin, F.; Heymann, D.; Heymann, M.F. Dysregulation of macrophage polarization is associated with the metastatic process in osteosarcoma. Oncotarget 2016, 7, 78343–78354. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Yang, Y.; Fang, M.; Li, X.; Yuan, X.; Yuan, J. Co-evolution of tumor-associated macrophages and tumor neo-vessels during cervical cancer invasion. Oncol. Lett. 2016, 12, 2625–2631. [Google Scholar] [CrossRef] [PubMed]
- Raj, S.K.; Kooshki, M.; Winters, M.; Russell, G.B.; Miller, L.D.; Laurini, J.A.; Pierre, T.; Savage, P.D. Prognostic implications of tumor associated macrophages (TAMs) in soft tissue sarcoma. J. Clin. Oncol. 2019, 37, e22548. [Google Scholar] [CrossRef]
- Fujiwara, T.; Fukushi, J.; Yamamoto, S.; Matsumoto, Y.; Setsu, N.; Oda, Y.; Yamada, H.; Okada, S.; Watari, K.; Ono, M.; et al. Macrophage infiltration predicts a poor prognosis for human ewing sarcoma. Am. J. Pathol. 2011, 179, 1157–1170. [Google Scholar] [CrossRef]
- Ganjoo, K.N.; Witten, D.; Patel, M.; Espinosa, I.; La, T.; Tibshirani, R.; van de Rijn, M.; Jacobs, C.; West, R.B. The prognostic value of tumor-associated macrophages in leiomyosarcoma: A single institution study. Am. J. Clin. Oncol. 2011, 34, 82–86. [Google Scholar] [CrossRef]
- Avdalyan, A.; Bobrov, I.; Klimachev, V.; Lazarev, A. Prognostic Value of Microvessel Density in Tumor and Peritumoral Area as Evaluated by CD31 Protein Expression and Argyrophilic Nucleolar Organizer Region Count in Endothelial Cells in Uterine Leiomyosarcoma. Sarcoma 2012, 2012, 594512. [Google Scholar] [CrossRef] [PubMed]
- Marioni, G.; Franz, L.; Ottaviano, G.; Contro, G.; Tealdo, G.; Carli, A.; Frigo, A.C.; Nicolai, P.; Alessandrini, L. Prognostic Significance of CD105- and CD31-Assessed Microvessel Density in Paired Biopsies and Surgical Samples of Laryngeal Carcinoma. Cancers 2020, 12, 2059. [Google Scholar] [CrossRef]
- Yudoh, K.; Kanamori, M.; Ohmori, K.; Yasuda, T.; Aoki, M.; Kimura, T. Concentration of vascular endothelial growth factor in the tumour tissue as a prognostic factor of soft tissue sarcomas. Br. J. Cancer 2001, 84, 1610–1615. [Google Scholar] [CrossRef]
- Guo, C.-R.; Han, R.; Xue, F.; Xu, L.; Ren, W.-G.; Li, M.; Feng, Z.; Hu, B.-C.; Peng, Z.-M. Expression and clinical significance of CD31, CD34, and CD105 in pulmonary ground glass nodules with different vascular manifestations on CT. Front. Oncol. 2022, 12, 956451. [Google Scholar] [CrossRef] [PubMed]
- Venkataramani, V.; Küffer, S.; Cheung, K.C.P.; Jiang, X.; Trümper, L.; Wulf, G.G.; Ströbel, P. CD31 Expression Determines Redox Status and Chemoresistance in Human Angiosarcomas. Clin. Cancer Res. 2018, 24, 460–473. [Google Scholar] [CrossRef]
- Nepomnyashchaya, E.M.; Ulianova, E.P.; Sagakyants, A.B.; Novikova, I.A.; Vashchenko, L.N.; Ausheva, T.V.; Shulgina, O.G.; Dashkova, I.R.; Vladimirova, L.Y.; Kit, O.I. Factors of angiogenesis (VEGF and CD34) in primary and recurrent soft tissue sarcomas. J. Clin. Oncol. 2020, 38, e23545. [Google Scholar] [CrossRef]
- Kubo, T.; Shimose, S.; Fujimori, J.; Arihiro, K.; Ochi, M. Diversity of angiogenesis among malignant bone tumors. Mol. Clin. Oncol. 2013, 1, 131–136. [Google Scholar] [CrossRef]
- Ollauri-Ibanez, C.; Lopez-Novoa, J.M.; Pericacho, M. Endoglin-based biological therapy in the treatment of angiogenesis-dependent pathologies. Expert Opin. Biol. Ther. 2017, 17, 1053–1063. [Google Scholar] [CrossRef]
- Radzikowska, J.; Krzeski, A.; Czarnecka, A.M.; Klepacka, T.; Rychlowska-Pruszynska, M.; Raciborska, A.; Dembowska-Baginska, B.; Pronicki, M.; Kukwa, A.; Sierdzinski, J.; et al. Endoglin Expression and Microvessel Density as Prognostic Factors in Pediatric Rhabdomyosarcoma. J. Clin. Med. 2021, 10, 512. [Google Scholar] [CrossRef] [PubMed]
- Puerto-Camacho, P.; Diaz-Martin, J.; Olmedo-Pelayo, J.; Bolado-Carrancio, A.; Salguero-Aranda, C.; Jordan-Perez, C.; Esteban-Medina, M.; Alamo-Alvarez, I.; Delgado-Bellido, D.; Lobo-Selma, L.; et al. Endoglin and MMP14 Contribute to Ewing Sarcoma Spreading by Modulation of Cell-Matrix Interactions. Int. J. Mol. Sci. 2022, 23, 8657. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.L.; Ravi, V.; Brohl, A.S.; Chawla, S.; Ganjoo, K.N.; Italiano, A.; Attia, S.; Burgess, M.A.; Thornton, K.; Cranmer, L.D.; et al. Efficacy and Safety of TRC105 Plus Pazopanib vs Pazopanib Alone for Treatment of Patients With Advanced Angiosarcoma: A Randomized Clinical Trial. JAMA Oncol. 2022, 8, 740–747. [Google Scholar] [CrossRef]
- Rahmanian, N.; Shokrzadeh, M.; Eskandani, M. Recent advances in γH2AX biomarker-based genotoxicity assays: A marker of DNA damage and repair. DNA Repair. 2021, 108, 103243. [Google Scholar] [CrossRef]
- Palla, V.-V.; Karaolanis, G.; Katafigiotis, I.; Anastasiou, I.; Patapis, P.; Dimitroulis, D.; Perrea, D. gamma-H2AX: Can it be established as a classical cancer prognostic factor? Tumor Biol. 2017, 39. [Google Scholar] [CrossRef]
- Sak, A.; Stuschke, M. Use of gammaH2AX and other biomarkers of double-strand breaks during radiotherapy. Semin. Radiat. Oncol. 2010, 20, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.M.; Moon, Y.J.; Park, S.H.; Park, H.J.; Wang, S.I.; Park, H.S.; Lee, H.; Kwon, K.S.; Moon, W.S.; Lee, D.G.; et al. Individual and Combined Expression of DNA Damage Response Molecules PARP1, γH2AX, BRCA1, and BRCA2 Predict Shorter Survival of Soft Tissue Sarcoma Patients. PLoS ONE 2016, 11, e0163193. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Value, n (%) |
|---|---|
| Age at diagnosis | |
| Median | 58 |
| Range | 32–75 |
| Gender | |
| Female | 8 (42.11%) |
| Male | 11 (57.9%) |
| Tumor pathology | |
| Undifferentiated pleomorphic sarcoma (UPS) | 11 (57.9%) |
| Myxofibrosarcoma (MFS) | 4 (21.05%) |
| Leiomyosarcoma (LMS) | 2 (10.53%) |
| Pleomorphic liposarcoma (PLPS) | 1 (5.26%) |
| Malignant peripheral nerve sheath tumor (MPNST) | 1 (5.26%) |
| Tumor site | |
| Trunk wall | 2 (10.53%) |
| Arm/shoulder | 1 (5.26%) |
| Thigh/buttock | 2 (10.53%) |
| Calf | 14 (73.68%) |
| Grade | |
| G2 | 8 (42.11%) |
| G3 | 11 (57.9%) |
| Largest tumor dimension | |
| 5–10 cm | 3 (15.79%) |
| >10–15 cm | 8 (42.11%) |
| >15–20 cm | 7 (36.84%) |
| >20–25 cm | 1 (5.26%) |
| >30 cm | 1 (5.26%) |
| Given doxorubicin-ifosfamide chemotherapy | |
| 1 cycle | 19 (100%) |
| 2 cycles | 17 (89.47%) |
| 3 cycles | 17 (89.47%) |
| Completed radiotherapy | |
| Yes | 18 (94.74%) |
| No | 1 (5.26%) |
| Before Treatment | After Treatment | |
|---|---|---|
| HIF-1α | 176 (range 90–240) | 122 (range 60–180) |
| TAM | 1–4 (21.05%); 2–4 (21.05%); 3–4 (21.05%); 4–2 (10.53%); 5–5 (26.32%) | |
| IMVD | 1–7 (36.84%); 2–3 (15.79%); 3–4 (21.05%); 4–2 (10.53%); 5–3 (15.79%) | |
| γH2AFX | 216 (range 140–300) | |
| Necrosis | 67% (range 0–100%) | |
| Response score | A—1 (5.26%); B—2 (10.53%); C—1 (5.26%); D—11 (57.89%); E—4 (21.05%) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szumera-Ciećkiewicz, A.; Bobak, K.; Spałek, M.J.; Sokół, K.; Wągrodzki, M.; Owczarek, D.; Kawecka, M.; Puton, B.; Koseła-Paterczyk, H.; Rutkowski, P.; et al. Predictive Biomarkers of Pathological Response to Neoadjuvant Chemoradiotherapy for Locally Advanced Soft Tissue Sarcomas. Cancers 2023, 15, 2960. https://doi.org/10.3390/cancers15112960
Szumera-Ciećkiewicz A, Bobak K, Spałek MJ, Sokół K, Wągrodzki M, Owczarek D, Kawecka M, Puton B, Koseła-Paterczyk H, Rutkowski P, et al. Predictive Biomarkers of Pathological Response to Neoadjuvant Chemoradiotherapy for Locally Advanced Soft Tissue Sarcomas. Cancers. 2023; 15(11):2960. https://doi.org/10.3390/cancers15112960
Chicago/Turabian StyleSzumera-Ciećkiewicz, Anna, Klaudia Bobak, Mateusz J. Spałek, Kamil Sokół, Michał Wągrodzki, Daria Owczarek, Monika Kawecka, Beata Puton, Hanna Koseła-Paterczyk, Piotr Rutkowski, and et al. 2023. "Predictive Biomarkers of Pathological Response to Neoadjuvant Chemoradiotherapy for Locally Advanced Soft Tissue Sarcomas" Cancers 15, no. 11: 2960. https://doi.org/10.3390/cancers15112960
APA StyleSzumera-Ciećkiewicz, A., Bobak, K., Spałek, M. J., Sokół, K., Wągrodzki, M., Owczarek, D., Kawecka, M., Puton, B., Koseła-Paterczyk, H., Rutkowski, P., & Czarnecka, A. M. (2023). Predictive Biomarkers of Pathological Response to Neoadjuvant Chemoradiotherapy for Locally Advanced Soft Tissue Sarcomas. Cancers, 15(11), 2960. https://doi.org/10.3390/cancers15112960









