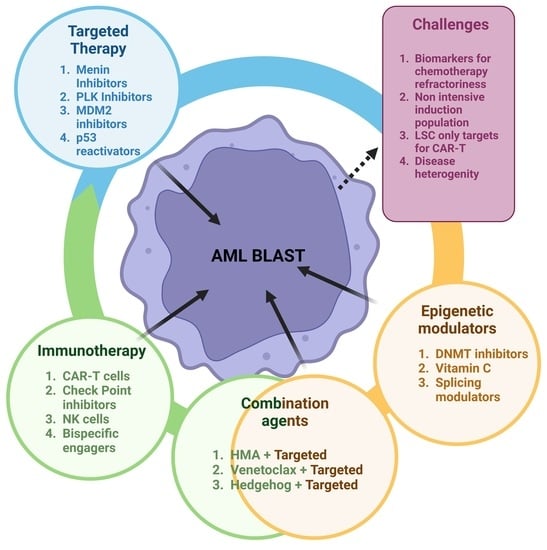
Novel Investigational Agents and Pathways That May Influence the Future Management of Acute Myeloid Leukemia
Abstract
Simple Summary
Abstract
1. Introduction
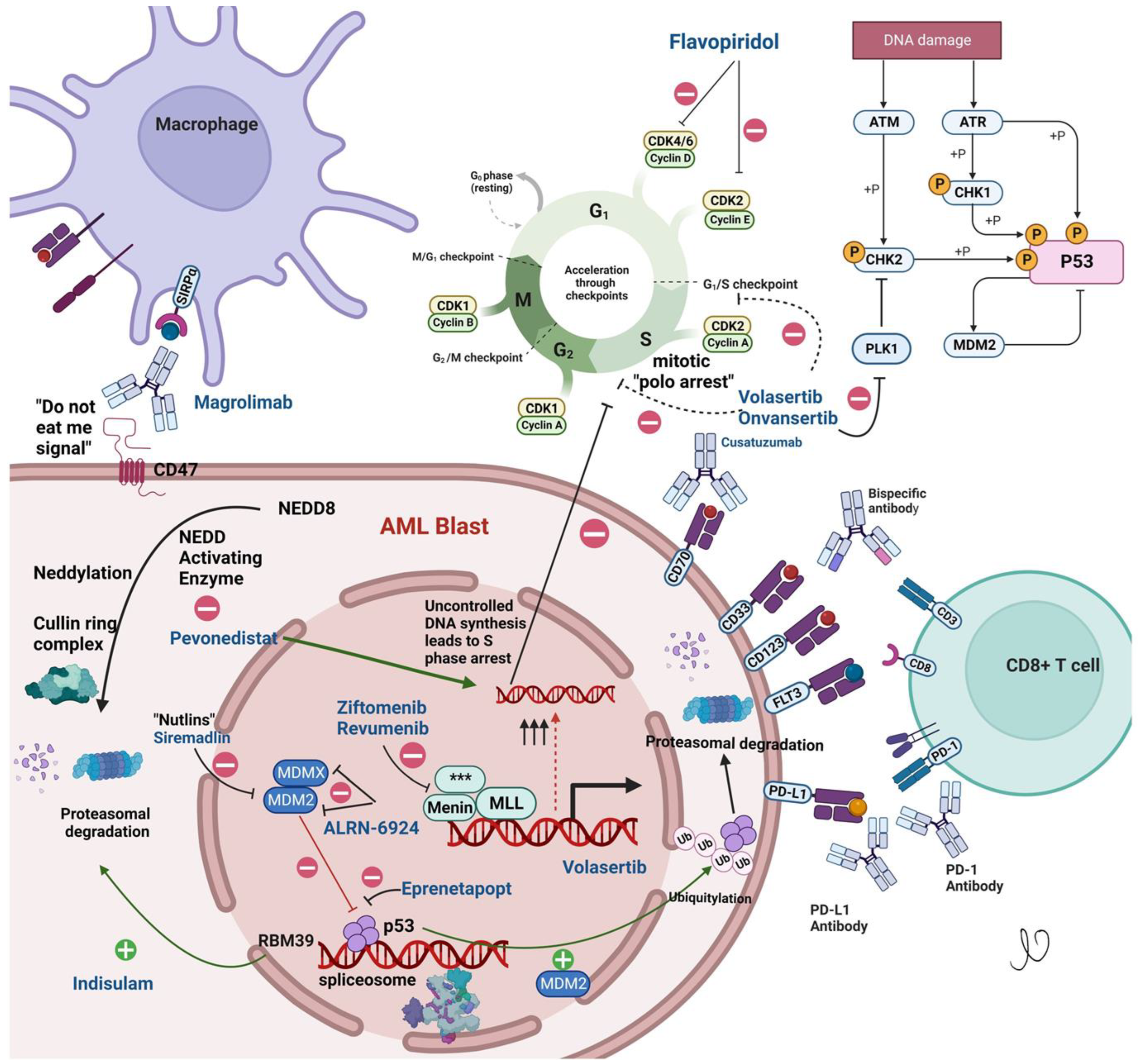
2. Menin Inhibitors
3. Anti-CD47 Antibody
4. Polo-Like Kinase Inhibitors
5. NAE Inhibitors
6. The Mouse Double Minute 2 (MDM2) Pathway
7. P53 Reactivators
8. Cell Cycle Inhibitors
Cyclin-Dependent Kinase (CDK) Inhibitors
9. Immunotherapies
9.1. Antibodies
9.2. CD33-Antibody Drug Conjugate
9.3. CD33xCD3 Bispecific T Cell Engager (BiTE) Antibody AMG330
9.4. CD123
9.5. CD70
10. Splicing Modulators
11. Immunotherapy
Checkpoint Inhibitors
12. Cell Therapy
13. Vitamin C
14. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- DiNardo, C.D.; Jonas, B.A.; Pullarkat, V.; Thirman, M.J.; Garcia, J.S.; Wei, A.H.; Konopleva, M.; Döhner, H.; Letai, A.; Fenaux, P.; et al. Azacitidine and Venetoclax in Previously Untreated Acute Myeloid Leukemia. N. Engl. J. Med. 2020, 383, 617–629. [Google Scholar] [CrossRef] [PubMed]
- Stelmach, P.; Trumpp, A. Leukemic stem cells and therapy resistance in acute myeloid leukemia. Haematologica 2023, 108, 353–366. [Google Scholar] [CrossRef] [PubMed]
- Merino, A.; Maakaron, J.; Bachanova, V. Advances in NK cell therapy for hematologic malignancies: NK source, persistence and tumor targeting. Blood Rev. 2023, 101073, in press. [Google Scholar] [CrossRef]
- Wang, J.; Tomlinson, B.; Lazarus, H.M. Update on Small Molecule Targeted Therapies for Acute Myeloid Leukemia. Curr. Treat. Options Oncol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.X.; Yan, J.; Keeshan, K.; Tubbs, A.T.; Wang, H.; Silva, A.; Brown, E.J.; Hess, J.L.; Pear, W.S.; Hua, X. The tumor suppressor menin regulates hematopoiesis and myeloid transformation by influencing Hox gene expression. Proc. Natl. Acad. Sci. USA 2006, 103, 1018–1023. [Google Scholar] [CrossRef] [PubMed]
- Caslini, C.; Yang, Z.; El-Osta, M.; Milne, T.A.; Slany, R.K.; Hess, J.L. Interaction of MLL amino terminal sequences with menin is required for transformation. Cancer Res. 2007, 67, 7275–7283. [Google Scholar] [CrossRef]
- Kühn, M.W.M.; Song, E.; Feng, Z.; Sinha, A.; Chen, C.-W.; Deshpande, A.J.; Cusan, M.; Farnoud, N.; Mupo, A.; Grove, C.; et al. Targeting Chromatin Regulators Inhibits Leukemogenic Gene Expression in NPM1 Mutant Leukemia. Cancer Discov. 2016, 6, 1166–1181. [Google Scholar] [CrossRef]
- Borkin, D.; He, S.; Miao, H.; Kempinska, K.; Pollock, J.; Chase, J.; Purohit, T.; Malik, B.; Zhao, T.; Wang, J.; et al. Pharmacologic inhibition of the Menin-MLL interaction blocks progression of MLL leukemia in vivo. Cancer Cell 2015, 27, 589–602. [Google Scholar] [CrossRef]
- Issa, G.C.; Aldoss, I.; DiPersio, J.F.; Cuglievan, B.; Stone, R.M.; Arellano, M.L.; Thirman, M.J.; Patel, M.R.; Dickens, D.; Shenoy, S.; et al. The Menin Inhibitor SNDX-5613 (revumenib) Leads to Durable Responses in Patients (Pts) with KMT2A-Rearranged or NPM1 Mutant AML: Updated Results of a Phase (Ph) 1 Study. Blood 2022, 140, 150–152. [Google Scholar] [CrossRef]
- Erba, H.P.; Fathi, A.T.; Issa, G.C.; Altman, J.K.; Montesinos, P.; Patnaik, M.M.; Foran, J.M.; De Botton, S.; Baer, M.R.; Schiller, G.J.; et al. Update on a Phase 1/2 First-in-Human Study of the Menin-KMT2A (MLL) Inhibitor Ziftomenib (KO-539) in Patients with Relapsed or Refractory Acute Myeloid Leukemia. Blood 2022, 140, 153–156. [Google Scholar] [CrossRef]
- Fiskus, W.; Boettcher, S.; Daver, N.; Mill, C.P.; Sasaki, K.; Birdwell, C.E.; Davis, J.A.; Takahashi, K.; Kadia, T.M.; DiNardo, C.D.; et al. Effective Menin inhibitor-based combinations against AML with MLL rearrangement or NPM1 mutation (NPM1c). Blood Cancer J. 2022, 12, 5. [Google Scholar] [CrossRef] [PubMed]
- Dafflon, C.; Craig, V.J.; Méreau, H.; Gräsel, J.; Schacher Engstler, B.; Hoffman, G.; Nigsch, F.; Gaulis, S.; Barys, L.; Ito, M.; et al. Complementary activities of DOT1L and Menin inhibitors in MLL-rearranged leukemia. Leukemia 2017, 31, 1269–1277. [Google Scholar] [CrossRef] [PubMed]
- Miao, H.; Kim, E.; Chen, D.; Purohit, T.; Kempinska, K.; Ropa, J.; Klossowski, S.; Trotman, W.; Danet-Desnoyers, G.; Cierpicki, T.; et al. Combinatorial treatment with menin and FLT3 inhibitors induces complete remission in AML models with activating FLT3 mutations. Blood 2020, 136, 2958–2963. [Google Scholar] [CrossRef] [PubMed]
- Blazar, B.R.; Lindberg, F.P.; Ingulli, E.; Panoskaltsis-Mortari, A.; Oldenborg, P.A.; Iizuka, K.; Yokoyama, W.M.; Taylor, P.A. CD47 (integrin-associated protein) engagement of dendritic cell and macrophage counterreceptors is required to prevent the clearance of donor lymphohematopoietic cells. J. Exp. Med. 2001, 194, 541–549. [Google Scholar] [CrossRef]
- Jaiswal, S.; Jamieson, C.H.M.; Pang, W.W.; Park, C.Y.; Chao, M.P.; Majeti, R.; Traver, D.; van Rooijen, N.; Weissman, I.L. CD47 Is Upregulated on Circulating Hematopoietic Stem Cells and Leukemia Cells to Avoid Phagocytosis. Cell 2009, 138, 271–285. [Google Scholar] [CrossRef]
- Majeti, R.; Chao, M.P.; Alizadeh, A.A.; Pang, W.W.; Jaiswal, S.; Gibbs, K.D.; van Rooijen, N.; Weissman, I.L. CD47 Is an Adverse Prognostic Factor and Therapeutic Antibody Target on Human Acute Myeloid Leukemia Stem Cells. Cell 2009, 138, 286–299. [Google Scholar] [CrossRef]
- Liu, J.; Wang, L.; Zhao, F.; Tseng, S.; Narayanan, C.; Shura, L.; Willingham, S.; Howard, M.; Prohaska, S.; Volkmer, J.; et al. Pre-Clinical Development of a Humanized Anti-CD47 Antibody with Anti-Cancer Therapeutic Potential. PLoS ONE 2015, 10, e0137345. [Google Scholar] [CrossRef]
- Vyas, P.; Knapper, S.; Kelly, R.; Salim, R.; Lubowiecki, M.; Royston, D.; Johnson, H.; Roberts, C.; Chen, J.; Agoram, B. Initial Phase 1 Results of the First-in-Class Anti-CD47 Antibody Hu5F9-G4 in Relapsed/Refractory Acute Myeloid Leukemia Patients. European Hematology Association EHA Library Abstract PF232. 2018. Available online: https://library.ehaweb.org/eha/2018/stockholm/214718/paresh.vyas.initial.phase.1.results.of.the.first-in-class.anti-cd47.antibody.html (accessed on 18 April 2023).
- Brierley, C.K.; Staves, J.; Roberts, C.; Johnson, H.; Vyas, P.; Goodnough, L.T.; Murphy, M.F. The effects of monoclonal anti-CD47 on RBCs, compatibility testing, and transfusion requirements in refractory acute myeloid leukemia. Transfusion 2019, 59, 2248–2254. [Google Scholar] [CrossRef]
- Chen, J.Y.; McKenna, K.M.; Choi, T.S.; Duan, J.; Brown, L.; Stewart, J.J.; Sompalli, K.; Vyas, P.; Schrier, S.; Majeti, R.; et al. RBC-Specific CD47 Pruning Confers Protection and Underlies the Transient Anemia in Patients Treated with Anti-CD47 Antibody 5F9. Blood 2018, 132, 2327. [Google Scholar] [CrossRef]
- Zeidan, A.M.; DeAngelo, D.J.; Palmer, J.; Seet, C.S.; Tallman, M.S.; Wei, X.; Raymon, H.; Sriraman, P.; Kopytek, S.; Bewersdorf, J.P.; et al. Phase 1 study of anti-CD47 monoclonal antibody CC-90002 in patients with relapsed/refractory acute myeloid leukemia and high-risk myelodysplastic syndromes. Ann. Hematol. 2022, 101, 557–569. [Google Scholar] [CrossRef]
- Boasman, K.; Bridle, C.; Simmonds, M.; Rinaldi, C. Role of pro-phagocytic calreticulin and anti-phagocytic CD47 in MDS and MPN models treated with azacytidine or ruxolitinib. In Haematologica; Ferrata Storti Foundation: Pavia, Italy, 2017; p. 763. [Google Scholar]
- Daver, N.G.; Vyas, P.; Kambhampati, S.; Al Malki, M.M.; Larson, R.A.; Asch, A.S.; Mannis, G.N.; Chai-Ho, W.; Tanaka, T.N.; Bradley, T.J.; et al. Tolerability and efficacy of the first-in-class anti-CD47 antibody magrolimab combined with azacitidine in frontline TP53m AML patients: Phase 1b results. J. Clin. Oncol. 2022, 40, 7020. [Google Scholar] [CrossRef]
- Reinhardt, H.C.; Yaffe, M.B. Phospho-Ser/Thr-binding domains: Navigating the cell cycle and DNA damage response. Nat. Rev. Mol. Cell Biol. 2013, 14, 563–580. [Google Scholar] [CrossRef] [PubMed]
- Barr, F.A.; Silljé, H.H.W.; Nigg, E.A. Polo-like kinases and the orchestration of cell division. Nat. Rev. Mol. Cell Biol. 2004, 5, 429–441. [Google Scholar] [CrossRef]
- Kumar, S.; Kim, J. PLK-1 Targeted Inhibitors and Their Potential against Tumorigenesis. Biomed Res. Int. 2015, 2015, 705745. [Google Scholar] [CrossRef]
- Renner, A.G.; Dos Santos, C.; Recher, C.; Bailly, C.; Créancier, L.; Kruczynski, A.; Payrastre, B.; Manenti, S. Polo-like kinase 1 is overexpressed in acute myeloid leukemia and its inhibition preferentially targets the proliferation of leukemic cells. Blood 2009, 114, 659–662. [Google Scholar] [CrossRef] [PubMed]
- Döhner, H.; Symeonidis, A.; Deeren, D.; Demeter, J.; Sanz, M.A.; Anagnostopoulos, A.; Esteve, J.; Fiedler, W.; Porkka, K.; Kim, H.J.; et al. Adjunctive Volasertib in Patients With Acute Myeloid Leukemia not Eligible for Standard Induction Therapy: A Randomized, Phase 3 Trial. Hemasphere 2021, 5, e617. [Google Scholar] [CrossRef]
- Zeidan, A.M.; Ridinger, M.; Lin, T.L.; Becker, P.S.; Schiller, G.J.; Patel, P.A.; Spira, A.I.; Tsai, M.L.; Samuëlsz, E.; Silberman, S.L.; et al. A Phase Ib Study of Onvansertib, a Novel Oral PLK1 Inhibitor, in Combination Therapy for Patients with Relapsed or Refractory Acute Myeloid Leukemia. Clin. Cancer Res. 2020, 26, 6132–6140. [Google Scholar] [CrossRef]
- Tao, Y.F.; Li, Z.H.; Du, W.W.; Xu, L.X.; Ren, J.L.; Li, X.L.; Fang, F.; Xie, Y.; Li, M.; Qian, G.H.; et al. Inhibiting PLK1 induces autophagy of acute myeloid leukemia cells via mammalian target of rapamycin pathway dephosphorylation. Oncol. Rep. 2017, 37, 1419–1429. [Google Scholar] [CrossRef]
- Sheth, A.S.; Chan, K.-K.; Nalepa, G.; Clapp, D.W.; Sierra Potchanant, E. Targeting PLK1 As a Novel Strategy for Acute Myeloid Leukemias with Fanconi Anemia Pathway Mutations. Blood 2022, 140, 6233–6234. [Google Scholar] [CrossRef]
- Moison, C.; Lavallée, V.-P.; Thiollier, C.; Lehnertz, B.; Boivin, I.; Mayotte, N.; Gareau, Y.; Fréchette, M.; Blouin-Chagnon, V.; Corneau, S.; et al. Complex karyotype AML displays G2/M signature and hypersensitivity to PLK1 inhibition. Blood Adv. 2019, 3, 552–563. [Google Scholar] [CrossRef]
- Xia, Y.; An, J.; Li, J.; Gu, W.; Zhang, Y.; Zhao, S.; Zhao, C.; Xu, Y.; Li, B.; Zhong, Z.; et al. Transferrin-guided intelligent nanovesicles augment the targetability and potency of clinical PLK1 inhibitor to acute myeloid leukemia. Bioact. Mater. 2023, 21, 499–510. [Google Scholar] [CrossRef]
- Soucy, T.A.; Smith, P.G.; Milhollen, M.A.; Berger, A.J.; Gavin, J.M.; Adhikari, S.; Brownell, J.E.; Burke, K.E.; Cardin, D.P.; Critchley, S.; et al. An inhibitor of NEDD8-activating enzyme as a new approach to treat cancer. Nature 2009, 458, 732–736. [Google Scholar] [CrossRef]
- Swords, R.T.; Kelly, K.R.; Smith, P.G.; Garnsey, J.J.; Mahalingam, D.; Medina, E.; Oberheu, K.; Padmanabhan, S.; O’Dwyer, M.; Nawrocki, S.T.; et al. Inhibition of NEDD8-activating enzyme: A novel approach for the treatment of acute myeloid leukemia. Blood 2010, 115, 3796–3800. [Google Scholar] [CrossRef] [PubMed]
- Swords, R.T.; Coutre, S.; Maris, M.B.; Zeidner, J.F.; Foran, J.M.; Cruz, J.; Erba, H.P.; Berdeja, J.G.; Tam, W.; Vardhanabhuti, S.; et al. Pevonedistat, a first-in-class NEDD8-activating enzyme inhibitor, combined with azacitidine in patients with AML. Blood 2018, 131, 1415–1424. [Google Scholar] [CrossRef] [PubMed]
- Adès, L.; Girshova, L.; Doronin, V.A.; Díez-Campelo, M.; Valcárcel, D.; Kambhampati, S.; Viniou, N.-A.; Woszczyk, D.; De Paz Arias, R.; Symeonidis, A.; et al. Pevonedistat plus azacitidine vs azacitidine alone in higher-risk MDS/chronic myelomonocytic leukemia or low-blast-percentage AML. Blood Adv. 2022, 6, 5132–5145. [Google Scholar] [CrossRef] [PubMed]
- Cojocari, D.; Smith, B.N.; Purkal, J.J.; Arrate, M.P.; Huska, J.D.; Xiao, Y.; Gorska, A.; Hogdal, L.J.; Ramsey, H.E.; Boghaert, E.R.; et al. Pevonedistat and azacitidine upregulate NOXA (PMAIP1) to increase sensitivity to venetoclax in preclinical models of acute myeloid leukemia. Haematologica 2022, 107, 825–835. [Google Scholar] [CrossRef] [PubMed]
- Guru Murthy, G.S.; Kaufmann, S.; Saliba, A.; Szabo, A.; Michaelis, L.; Abedin, S.; Runaas, L.; Carlson, K.; Maldonado-Schmidt, S.; Hinman, A.; et al. P540: Pevonedistat, Azacitidine and Venetoclax for Patients with Relapsed/Refractory Acute Myeloid Leukemia—A Phase I Study. HemaSphere 2022, 6, 439–440. [Google Scholar] [CrossRef]
- Short, N.J.; Montalban-Bravo, G.; Alvarado, Y.; Konopleva, M.; Jabbour, E.J.; Garcia-Manero, G.; Yilmaz, M.; Jain, N.; Borthakur, G.; DiNardo, C.D.; et al. Azacitidine, Venetoclax and Pevonedistat As Frontline Therapy for Patients with Secondary Acute Myeloid Leukemia Who Are Unfit for Intensive Chemotherapy: Results from a Phase I/II Study. Blood 2021, 138, 2349. [Google Scholar] [CrossRef]
- Wu, X.; Bayle, J.H.; Olson, D.; Levine, A.J. The p53-mdm-2 autoregulatory feedback loop. Genes. Dev. 1993, 7, 1126–1132. [Google Scholar] [CrossRef]
- Faderl, S.; Kantarjian, H.M.; Estey, E.; Manshouri, T.; Chan, C.-Y.; Rahman Elsaied, A.; Kornblau, S.M.; Cortes, J.; Thomas, D.A.; Pierce, S.; et al. The prognostic significance of p16INK4a/p14ARF locus deletion and MDM-2 protein expression in adult acute myelogenous leukemia. Cancer 2000, 89, 1976–1982. [Google Scholar] [CrossRef]
- Vassilev, L.T.; Vu, B.T.; Graves, B.; Carvajal, D.; Podlaski, F.; Filipovic, Z.; Kong, N.; Kammlott, U.; Lukacs, C.; Klein, C.; et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science 2004, 303, 844–848. [Google Scholar] [CrossRef]
- Andreeff, M.; Kelly, K.R.; Yee, K.; Assouline, S.; Strair, R.; Popplewell, L.; Bowen, D.; Martinelli, G.; Drummond, M.W.; Vyas, P.; et al. Results of the Phase I Trial of RG7112, a Small-Molecule MDM2 Antagonist in Leukemia. Clin. Cancer Res. 2016, 22, 868–876. [Google Scholar] [CrossRef] [PubMed]
- Konopleva, M.Y.; Röllig, C.; Cavenagh, J.; Deeren, D.; Girshova, L.; Krauter, J.; Martinelli, G.; Montesinos, P.; Schäfer, J.A.; Ottmann, O.; et al. Idasanutlin plus cytarabine in relapsed or refractory acute myeloid leukemia: Results of the MIRROS trial. Blood Adv. 2022, 6, 4147–4156. [Google Scholar] [CrossRef] [PubMed]
- Uy, G.L.; Assouline, S.; Young, A.M.; Blotner, S.; Higgins, B.; Chen, L.C.; Yee, K. Phase 1 study of the MDM2 antagonist RO6839921 in patients with acute myeloid leukemia. Investig. New Drugs 2020, 38, 1430–1441. [Google Scholar] [CrossRef] [PubMed]
- Stein, E.M.; DeAngelo, D.J.; Chromik, J.; Chatterjee, M.; Bauer, S.; Lin, C.C.; Suarez, C.; de Vos, F.; Steeghs, N.; Cassier, P.A.; et al. Results from a First-in-Human Phase I Study of Siremadlin (HDM201) in Patients with Advanced Wild-Type TP53 Solid Tumors and Acute Leukemia. Clin. Cancer Res. 2022, 28, 870–881. [Google Scholar] [CrossRef]
- Sallman, D.A.; Borate, U.; Cull, E.H.; Donnellan, W.B.; Komrokji, R.S.; Steidl, U.G.; Corvez, M.M.; Payton, M.; Annis, D.A.; Pinchasik, D.; et al. Phase 1/1b Study of the Stapled Peptide ALRN-6924, a Dual Inhibitor of MDMX and MDM2, As Monotherapy or in Combination with Cytarabine for the Treatment of Relapsed/Refractory AML and Advanced MDS with TP53 Wild-Type. Blood 2018, 132, 4066. [Google Scholar] [CrossRef]
- Mayo, M.; Chutake, Y.; Karnik, R.; McDonald, A.; Cho, P.S.; Filiatrault, J.; Chen, D.; Dixit, V.; Proctor, W.; Breitkopf, S.; et al. Development of KT-253, a Highly Potent and Selective Heterobifunctional MDM2 Degrader for the Treatment of Acute Myeloid Leukemia. Blood 2022, 140, 6239–6240. [Google Scholar] [CrossRef]
- Arber, D.A.; Orazi, A.; Hasserjian, R.P.; Borowitz, M.J.; Calvo, K.R.; Kvasnicka, H.M.; Wang, S.A.; Bagg, A.; Barbui, T.; Branford, S.; et al. International Consensus Classification of Myeloid Neoplasms and Acute Leukemias: Integrating morphologic, clinical, and genomic data. Blood 2022, 140, 1200–1228. [Google Scholar] [CrossRef]
- Cluzeau, T.; Sebert, M.; Rahmé, R.; Cuzzubbo, S.; Lehmann-Che, J.; Madelaine, I.; Peterlin, P.; Bève, B.; Attalah, H.; Chermat, F.; et al. Eprenetapopt Plus Azacitidine in TP53-Mutated Myelodysplastic Syndromes and Acute Myeloid Leukemia: A Phase II Study by the Groupe Francophone des Myélodysplasies (GFM). J. Clin. Oncol. 2021, 39, 1575–1583. [Google Scholar] [CrossRef]
- Lambert, J.M.; Gorzov, P.; Veprintsev, D.B.; Söderqvist, M.; Segerbäck, D.; Bergman, J.; Fersht, A.R.; Hainaut, P.; Wiman, K.G.; Bykov, V.J. PRIMA-1 reactivates mutant p53 by covalent binding to the core domain. Cancer Cell 2009, 15, 376–388. [Google Scholar] [CrossRef]
- Maslah, N.; Salomao, N.; Drevon, L.; Verger, E.; Partouche, N.; Ly, P.; Aubin, P.; Naoui, N.; Schlageter, M.H.; Bally, C.; et al. Synergistic effects of PRIMA-1(Met) (APR-246) and 5-azacitidine in TP53-mutated myelodysplastic syndromes and acute myeloid leukemia. Haematologica 2020, 105, 1539–1551. [Google Scholar] [CrossRef] [PubMed]
- Sallman, D.A.; Komrokji, R.S.; DeZern, A.E.; Sebert, M.; Garcia-Manero, G.; Rahmé, R.; Steensma, D.P.; Lehmann che, J.; Roboz, G.J.; Madelaine, I.; et al. Long Term Follow-up and Combined Phase 2 Results of Eprenetapopt (APR-246) and Azacitidine (AZA) in Patients with TP53 mutant Myelodysplastic Syndromes (MDS) and Oligoblastic Acute Myeloid Leukemia (AML). Blood 2021, 138, 246. [Google Scholar] [CrossRef]
- Mishra, A.; Tamari, R.; DeZern, A.E.; Byrne, M.T.; Gooptu, M.; Chen, Y.B.; Deeg, H.J.; Sallman, D.; Gallacher, P.; Wennborg, A.; et al. Eprenetapopt Plus Azacitidine After Allogeneic Hematopoietic Stem-Cell Transplantation for TP53-Mutant Acute Myeloid Leukemia and Myelodysplastic Syndromes. J. Clin. Oncol. 2022, 40, 3985–3993. [Google Scholar] [CrossRef] [PubMed]
- Aprea Therapeutics Announces Results of Primary Endpoint from Phase 3 Trial of Eprenetapopt in TP53 Mutant Myelodysplastic Syndromes (MDS). 2020. Available online: https://www.globenewswire.com/news-release/2020/12/28/2150874/0/en/Aprea-Therapeutics-Announces-Results-of-Primary-Endpoint-from-Phase-3-Trial-of-Eprenetapopt-in-TP53-Mutant-Myelodysplastic-Syndromes-MDS.html (accessed on 18 April 2023).
- Zeidner, J.F.; Karp, J.E. Clinical activity of alvocidib (flavopiridol) in acute myeloid leukemia. Leuk. Res. 2015, 39, 1312–1318. [Google Scholar] [CrossRef]
- Zeidner, J.F.; Foster, M.C.; Blackford, A.L.; Litzow, M.R.; Morris, L.E.; Strickland, S.A.; Lancet, J.E.; Bose, P.; Levy, M.Y.; Tibes, R.; et al. Randomized multicenter phase II study of flavopiridol (alvocidib), cytarabine, and mitoxantrone (FLAM) versus cytarabine/daunorubicin (7+3) in newly diagnosed acute myeloid leukemia. Haematologica 2015, 100, 1172–1179. [Google Scholar] [CrossRef]
- Zeidner, J.F.; Lee, D.J.; Frattini, M.; Fine, G.D.; Costas, J.; Kolibaba, K.; Anthony, S.P.; Bearss, D.; Smith, B.D. Phase I Study of Alvocidib Followed by 7+3 (Cytarabine + Daunorubicin) in Newly Diagnosed Acute Myeloid Leukemia. Clin. Cancer Res. 2021, 27, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.S.; Adés, L.; Fathi, A.T.; Kreuzer, K.A.; O’Meara, M.M.; Liang, S.-Y.; Ravandi, F. CASCADE: A phase 3, randomized, double-blind study of vadastuximab talirine (33A) versus placebo in combination with azacitidine or decitabine in the treatment of older patients with newly diagnosed acute myeloid leukemia (AML). J. Clin. Oncol. 2017, 35, TPS7066. [Google Scholar] [CrossRef]
- Ravandi, F.; Walter, R.B.; Subklewe, M.; Buecklein, V.; Jongen-Lavrencic, M.; Paschka, P.; Ossenkoppele, G.J.; Kantarjian, H.M.; Hindoyan, A.; Agarwal, S.K.; et al. Updated results from phase I dose-escalation study of AMG 330, a bispecific T-cell engager molecule, in patients with relapsed/refractory acute myeloid leukemia (R/R AML). J. Clin. Oncol. 2020, 38, 7508. [Google Scholar] [CrossRef]
- Subklewe, M.; Stein, A.; Walter, R.B.; Bhatia, R.; Wei, A.H.; Ritchie, D.; Bücklein, V.; Vachhani, P.; Dai, T.; Hindoyan, A.; et al. Preliminary Results from a Phase 1 First-in-Human Study of AMG 673, a Novel Half-Life Extended (HLE) Anti-CD33/CD3 BiTE® (Bispecific T-Cell Engager) in Patients with Relapsed/Refractory (R/R) Acute Myeloid Leukemia (AML). Blood 2019, 134, 833. [Google Scholar] [CrossRef]
- Westervelt, P.; Cortes, J.E.; Altman, J.K.; Long, M.; Oehler, V.G.; Gojo, I.; Guenot, J.; Chun, P.; Roboz, G.J. Phase 1 First-in-Human Trial of AMV564, a Bivalent Bispecific (2:2) CD33/CD3 T-Cell Engager, in Patients with Relapsed/Refractory Acute Myeloid Leukemia (AML). Blood 2019, 134, 834. [Google Scholar] [CrossRef]
- Abedin, S.; Guru Murthy, G.S.; Szabo, A.; Hamadani, M.; Michaelis, L.C.; Carlson, K.-S.; Runaas, L.; Gauger, K.; Desai, A.G.; Chen, M.M.; et al. Lintuzumab-Ac225 with Combination with Intensive Chemotherapy Yields High Response Rate and MRD Negativity in R/R AML with Adverse Features. Blood 2022, 140, 157–158. [Google Scholar] [CrossRef]
- Uy, G.L.; Aldoss, I.; Foster, M.C.; Sayre, P.H.; Wieduwilt, M.J.; Advani, A.S.; Godwin, J.E.; Arellano, M.L.; Sweet, K.L.; Emadi, A.; et al. Flotetuzumab as salvage immunotherapy for refractory acute myeloid leukemia. Blood 2021, 137, 751–762. [Google Scholar] [CrossRef]
- Vadakekolathu, J.; Lai, C.; Reeder, S.; Church, S.E.; Hood, T.; Lourdusamy, A.; Rettig, M.P.; Aldoss, I.; Advani, A.S.; Godwin, J.; et al. TP53 abnormalities correlate with immune infiltration and associate with response to flotetuzumab immunotherapy in AML. Blood Adv. 2020, 4, 5011–5024. [Google Scholar] [CrossRef] [PubMed]
- Ravandi, F.; Bashey, A.; Stock, W.; Foran, J.M.; Mawad, R.; Egan, D.; Blum, W.; Yang, A.; Pastore, A.; Johnson, C.; et al. Complete Responses in Relapsed/Refractory Acute Myeloid Leukemia (AML) Patients on a Weekly Dosing Schedule of Vibecotamab (XmAb14045), a CD123 x CD3 T Cell-Engaging Bispecific Antibody; Initial Results of a Phase 1 Study. Blood 2020, 136, 4–5. [Google Scholar] [CrossRef]
- Watts, J.; Maris, M.; Lin, T.L.; Patel, P.; Madanat, Y.F.; Cogle, C.R.; Borthakur, G.; Huebner, D.; Khaskhely, N.; Bonham, L.; et al. Updated Results from a Phase 1 Study of APVO436, a Novel Bispecific Anti-CD123 x Anti-CD3 Adaptir™ Molecule, in Relapsed/Refractory Acute Myeloid Leukemia and Myelodysplastic Syndrome. Blood 2022, 140, 6204–6205. [Google Scholar] [CrossRef]
- Aptevo Therapeutics Inc. 100% Clinical Benefit Rate Achieved in Phase 1b Trial Evaluating APVO436 in Combination with Venetoclax and Azacitidine for Venetoclax Treatment Naïve Patients with Acute Myeloid Leukemia (AML). 2022. Available online: https://www.accesswire.com/731275/100-Clinical-Benefit-Rate-Achieved-in-Phase-1b-Trial-Evaluating-APVO436-in-Combination-with-Venetoclax-and-Azacitidine-for-Venetoclax-Treatment-Nave-Patients-with-Acute-Myeloid-Leukemia-AML (accessed on 18 April 2023).
- Riether, C.; Pabst, T.; Höpner, S.; Bacher, U.; Hinterbrandner, M.; Banz, Y.; Müller, R.; Manz, M.G.; Gharib, W.H.; Francisco, D.; et al. Targeting CD70 with cusatuzumab eliminates acute myeloid leukemia stem cells in patients treated with hypomethylating agents. Nat. Med. 2020, 26, 1459–1467. [Google Scholar] [CrossRef]
- Brauchle, B.; Goldstein, R.L.; Karbowski, C.M.; Henn, A.; Li, C.-M.; Bücklein, V.L.; Krupka, C.; Boyle, M.C.; Koppikar, P.; Haubner, S.; et al. Characterization of a Novel FLT3 BiTE Molecule for the Treatment of Acute Myeloid Leukemia. Mol. Cancer Ther. 2020, 19, 1875–1888. [Google Scholar] [CrossRef]
- Adamia, S.; Haibe-Kains, B.; Pilarski, P.M.; Bar-Natan, M.; Pevzner, S.; Avet-Loiseau, H.; Lode, L.; Verselis, S.; Fox, E.A.; Burke, J.; et al. A genome-wide aberrant RNA splicing in patients with acute myeloid leukemia identifies novel potential disease markers and therapeutic targets. Clin. Cancer Res. 2014, 20, 1135–1145. [Google Scholar] [CrossRef]
- Steensma, D.P.; Wermke, M.; Klimek, V.M.; Greenberg, P.L.; Font, P.; Komrokji, R.S.; Yang, J.; Brunner, A.M.; Carraway, H.E.; Ades, L.; et al. Phase I First-in-Human Dose Escalation Study of the oral SF3B1 modulator H3B-8800 in myeloid neoplasms. Leukemia 2021, 35, 3542–3550. [Google Scholar] [CrossRef]
- Han, T.; Goralski, M.; Gaskill, N.; Capota, E.; Kim, J.; Ting, T.C.; Xie, Y.; Williams, N.S.; Nijhawan, D. Anticancer sulfonamides target splicing by inducing RBM39 degradation via recruitment to DCAF15. Science 2017, 356, eaal3755. [Google Scholar] [CrossRef]
- Assi, R.; Kantarjian, H.M.; Kadia, T.M.; Pemmaraju, N.; Jabbour, E.; Jain, N.; Daver, N.; Estrov, Z.; Uehara, T.; Owa, T.; et al. Final results of a phase 2, open-label study of indisulam, idarubicin, and cytarabine in patients with relapsed or refractory acute myeloid leukemia and high-risk myelodysplastic syndrome. Cancer 2018, 124, 2758–2765. [Google Scholar] [CrossRef] [PubMed]
- Hsiehchen, D.; Goralski, M.; Kim, J.; Xie, Y.; Nijhawan, D. Biomarkers for RBM39 degradation in acute myeloid leukemia. Leukemia 2020, 34, 1924–1928. [Google Scholar] [CrossRef] [PubMed]
- Fong, J.Y.; Pignata, L.; Goy, P.-A.; Kawabata, K.C.; Lee, S.C.-W.; Koh, C.M.; Musiani, D.; Massignani, E.; Kotini, A.G.; Penson, A.; et al. Therapeutic Targeting of RNA Splicing Catalysis through Inhibition of Protein Arginine Methylation. Cancer Cell 2019, 36, 194–209.e9. [Google Scholar] [CrossRef]
- Watts, J.M.; Bradley, T.J.; Thomassen, A.; Brunner, A.M.; Minden, M.D.; Papadantonakis, N.; Abedin, S.; Baines, A.J.; Barbash, O.; Gorman, S.; et al. A Phase I/II Study to Investigate the Safety and Clinical Activity of the Protein Arginine Methyltransferase 5 Inhibitor GSK3326595 in Subjects with Myelodysplastic Syndrome and Acute Myeloid Leukemia. Blood 2019, 134, 2656. [Google Scholar] [CrossRef]
- Wang, E.; Pineda, J.M.B.; Kim, W.J.; Chen, S.; Bourcier, J.; Stahl, M.; Hogg, S.J.; Bewersdorf, J.P.; Han, C.; Singer, M.E.; et al. Modulation of RNA splicing enhances response to BCL2 inhibition in leukemia. Cancer Cell 2023, 41, 164–180.e8. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.; Basu, S.; Garcia-Manero, G.; Hourigan, C.S.; Oetjen, K.A.; Cortes, J.E.; Ravandi, F.; Jabbour, E.J.; Al-Hamal, Z.; Konopleva, M.; et al. The distribution of T-cell subsets and the expression of immune checkpoint receptors and ligands in patients with newly diagnosed and relapsed acute myeloid leukemia. Cancer 2019, 125, 1470–1481. [Google Scholar] [CrossRef] [PubMed]
- Sallman, D.A.; McLemore, A.F.; Aldrich, A.L.; Komrokji, R.S.; McGraw, K.L.; Dhawan, A.; Geyer, S.; Hou, H.-A.; Eksioglu, E.A.; Sullivan, A.; et al. TP53 mutations in myelodysplastic syndromes and secondary AML confer an immunosuppressive phenotype. Blood 2020, 136, 2812–2823. [Google Scholar] [CrossRef]
- Ørskov, A.D.; Treppendahl, M.B.; Skovbo, A.; Holm, M.S.; Friis, L.S.; Hokland, M.; Grønbæk, K. Hypomethylation and up-regulation of PD-1 in T cells by azacytidine in MDS/AML patients: A rationale for combined targeting of PD-1 and DNA methylation. Oncotarget 2015, 6, 9612. [Google Scholar] [CrossRef]
- Zeidan, A.M.; Boss, I.; Beach, C.L.; Copeland, W.B.; Thompson, E.; Fox, B.A.; Hasle, V.E.; Hellmann, A.; Taussig, D.C.; Tormo, M.; et al. A randomized phase 2 trial of azacitidine with or without durvalumab as first-line therapy for older patients with AML. Blood Adv. 2022, 6, 2219–2229. [Google Scholar] [CrossRef]
- Daver, N.; Garcia-Manero, G.; Basu, S.; Boddu, P.C.; Alfayez, M.; Cortes, J.E.; Konopleva, M.; Ravandi-Kashani, F.; Jabbour, E.; Kadia, T.; et al. Efficacy, Safety, and Biomarkers of Response to Azacitidine and Nivolumab in Relapsed/Refractory Acute Myeloid Leukemia: A Nonrandomized, Open-Label, Phase II Study. Cancer Discov. 2019, 9, 370–383. [Google Scholar] [CrossRef]
- Daver, N.G.; Garcia-Manero, G.; Konopleva, M.Y.; Alfayez, M.; Pemmaraju, N.; Kadia, T.M.; DiNardo, C.D.; Cortes, J.E.; Ravandi, F.; Abbas, H.; et al. Azacitidine (AZA) with Nivolumab (Nivo), and AZA with Nivo + Ipilimumab (Ipi) in Relapsed/Refractory Acute Myeloid Leukemia: A Non-Randomized, Prospective, Phase 2 Study. Blood 2019, 134, 830. [Google Scholar] [CrossRef]
- Brunner, A.M.; Esteve, J.; Porkka, K.; Knapper, S.; Traer, E.; Scholl, S.; Garcia-Manero, G.; Vey, N.; Wermke, M.; Janssen, J.; et al. Efficacy and Safety of Sabatolimab (MBG453) in Combination with Hypomethylating Agents (HMAs) in Patients (Pts) with Very High/High-Risk Myelodysplastic Syndrome (vHR/HR-MDS) and Acute Myeloid Leukemia (AML): Final Analysis from a Phase Ib Study. Blood 2021, 138, 244. [Google Scholar] [CrossRef]
- Maude, S.L.; Laetsch, T.W.; Buechner, J.; Rives, S.; Boyer, M.; Bittencourt, H.; Bader, P.; Verneris, M.R.; Stefanski, H.E.; Myers, G.D.; et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N. Engl. J. Med. 2018, 378, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Locke, F.L.; Miklos, D.B.; Jacobson, C.A.; Perales, M.A.; Kersten, M.J.; Oluwole, O.O.; Ghobadi, A.; Rapoport, A.P.; McGuirk, J.; Pagel, J.M.; et al. Axicabtagene Ciloleucel as Second-Line Therapy for Large B-Cell Lymphoma. N. Engl. J. Med. 2022, 386, 640–654. [Google Scholar] [CrossRef]
- Vishwasrao, P.; Li, G.; Boucher, J.C.; Smith, D.L.; Hui, S.K. Emerging CAR T Cell Strategies for the Treatment of AML. Cancers 2022, 14, 1241. [Google Scholar] [CrossRef] [PubMed]
- Budde, L.; Song, J.Y.; Kim, Y.; Blanchard, S.; Wagner, J.; Stein, A.S.; Weng, L.; Del Real, M.; Hernandez, R.; Marcucci, E.; et al. Remissions of Acute Myeloid Leukemia and Blastic Plasmacytoid Dendritic Cell Neoplasm Following Treatment with CD123-Specific CAR T Cells: A First-in-Human Clinical Trial. Blood 2017, 130, 811. [Google Scholar] [CrossRef]
- Liu, F.; Cao, Y.; Pinz, K.; Ma, Y.; Wada, M.; Chen, K.; Ma, G.; Shen, J.; Tse, C.O.; Su, Y.; et al. First-in-Human CLL1-CD33 Compound CAR T Cell Therapy Induces Complete Remission in Patients with Refractory Acute Myeloid Leukemia: Update on Phase 1 Clinical Trial. Blood 2018, 132, 901. [Google Scholar] [CrossRef]
- Zhang, H.; Bu, C.; Peng, Z.; luo, m.; Li, C. The efficacy and safety of anti-CLL1 based CAR-T cells in children with relapsed or refractory acute myeloid leukemia: A multicenter interim analysis. J. Clin. Oncol. 2021, 39, 10000. [Google Scholar] [CrossRef]
- Cui, Q.; Qian, C.; Xu, N.; Kang, L.; Dai, H.; Cui, W.; Song, B.; Yin, J.; Li, Z.; Zhu, X.; et al. CD38-directed CAR-T cell therapy: A novel immunotherapy strategy for relapsed acute myeloid leukemia after allogeneic hematopoietic stem cell transplantation. J. Hematol. Oncol. 2021, 14, 82. [Google Scholar] [CrossRef]
- Daver, N. A bispecific approach to improving CAR T cells in AML. Blood 2020, 135, 703–704. [Google Scholar] [CrossRef]
- Mussai, F.; De Santo, C.; Abu-Dayyeh, I.; Booth, S.; Quek, L.; McEwen-Smith, R.M.; Qureshi, A.; Dazzi, F.; Vyas, P.; Cerundolo, V. Acute myeloid leukemia creates an arginase-dependent immunosuppressive microenvironment. Blood 2013, 122, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Kaur, G.; Sankin, A.I.; Chen, F.; Guan, F.; Zang, X. Immune checkpoint blockade and CAR-T cell therapy in hematologic malignancies. J. Hematol. Oncol. 2019, 12, 59. [Google Scholar] [CrossRef] [PubMed]
- Perna, F.; Berman, S.H.; Soni, R.K.; Mansilla-Soto, J.; Eyquem, J.; Hamieh, M.; Hendrickson, R.C.; Brennan, C.W.; Sadelain, M. Integrating Proteomics and Transcriptomics for Systematic Combinatorial Chimeric Antigen Receptor Therapy of AML. Cancer Cell 2017, 32, 506–519.e5. [Google Scholar] [CrossRef]
- Gu, X.; He, D.; Li, C.; Wang, H.; Yang, G. Development of Inducible CD19-CAR T Cells with a Tet-On System for Controlled Activity and Enhanced Clinical Safety. Int. J. Mol. Sci. 2018, 19, 3455. [Google Scholar] [CrossRef]
- Ruggeri, L.; Capanni, M.; Urbani, E.; Perruccio, K.; Shlomchik, W.D.; Tosti, A.; Posati, S.; Rogaia, D.; Frassoni, F.; Aversa, F.; et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science 2002, 295, 2097–2100. [Google Scholar] [CrossRef] [PubMed]
- Paust, S.; Blish, C.A.; Reeves, R.K. Redefining Memory: Building the Case for Adaptive NK Cells. J. Virol. 2017, 91, e00169-17. [Google Scholar] [CrossRef] [PubMed]
- Foltz, J.A.; Berrien-Elliott, M.M.; Neal, C.; Foster, M.; McClain, E.; Schappe, T.; Desai, S.; Becker-Hapak, M.; Cashen, A.F.; Fehniger, T.A. Cytokine-Induced Memory-like (ML) NK Cells Persist for >2 Months Following Adoptive Transfer into Leukemia Patients with a MHC-Compatible Hematopoietic Cell Transplant (HCT). Blood 2019, 134, 1954. [Google Scholar] [CrossRef]
- Gleason, M.K.; Verneris, M.R.; Todhunter, D.A.; Zhang, B.; McCullar, V.; Zhou, S.X.; Panoskaltsis-Mortari, A.; Weiner, L.M.; Vallera, D.A.; Miller, J.S. Bispecific and trispecific killer cell engagers directly activate human NK cells through CD16 signaling and induce cytotoxicity and cytokine production. Mol. Cancer Ther. 2012, 11, 2674–2684. [Google Scholar] [CrossRef] [PubMed]
- Vallera, D.A.; Felices, M.; McElmurry, R.; McCullar, V.; Zhou, X.; Schmohl, J.U.; Zhang, B.; Lenvik, A.J.; Panoskaltsis-Mortari, A.; Verneris, M.R.; et al. IL15 Trispecific Killer Engagers (TriKE) Make Natural Killer Cells Specific to CD33+ Targets While Also Inducing Persistence, In Vivo Expansion, and Enhanced Function. Clin. Cancer Res. 2016, 22, 3440–3450. [Google Scholar] [CrossRef]
- Gillberg, L.; Ørskov, A.D.; Nasif, A.; Ohtani, H.; Madaj, Z.; Hansen, J.W.; Rapin, N.; Mogensen, J.B.; Liu, M.; Dufva, I.H.; et al. Oral vitamin C supplementation to patients with myeloid cancer on azacitidine treatment: Normalization of plasma vitamin C induces epigenetic changes. Clin. Epigenetics 2019, 11, 143. [Google Scholar] [CrossRef]
- Agathocleous, M.; Meacham, C.E.; Burgess, R.J.; Piskounova, E.; Zhao, Z.; Crane, G.M.; Cowin, B.L.; Bruner, E.; Murphy, M.M.; Chen, W.; et al. Ascorbate regulates haematopoietic stem cell function and leukaemogenesis. Nature 2017, 549, 476–481. [Google Scholar] [CrossRef]
- Steensma, D.P.; Bejar, R.; Jaiswal, S.; Lindsley, R.C.; Sekeres, M.A.; Hasserjian, R.P.; Ebert, B.L. Clonal hematopoiesis of indeterminate potential and its distinction from myelodysplastic syndromes. Blood 2015, 126, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zhu, H.; Huang, J.; Zhu, Y.; Hong, M.; Zhu, H.; Zhang, J.; Li, S.; Yang, L.; Lian, Y.; et al. The synergy of Vitamin C with decitabine activates TET2 in leukemic cells and significantly improves overall survival in elderly patients with acute myeloid leukemia. Leuk. Res. 2018, 66, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Premnath, N.; Chung, S.S.; Weinberg, O.K.; Ikpefan, R.; Pandey, M.; Kaur, G.; Geethakumari, P.R.; Afrough, A.; Awan, F.T.; Anderson, L.D., Jr.; et al. Clinical and molecular characteristics associated with Vitamin C deficiency in myeloid malignancies; real world data from a prospective cohort. Leuk. Res. 2023, 125, 107001. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Lasho, T.L.; Fernandez, J.; Finke, C.; Amundson, M.; LaPlant, B.; Mangaonkar, A.A.; Witzig, T.E.; Patnaik, M.M. Phase II trial assessing safety and preliminary efficacy of high-dose intravenous ascorbic acid in patients with TET2-mutant clonal cytopenias of undetermined significance. J. Clin. Oncol. 2022, 40, TPS7076. [Google Scholar] [CrossRef]
- Khoury, J.D.; Solary, E.; Abla, O.; Akkari, Y.; Alaggio, R.; Apperley, J.F.; Bejar, R.; Berti, E.; Busque, L.; Chan, J.K.C.; et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Myeloid and Histiocytic/Dendritic Neoplasms. Leukemia 2022, 36, 1703–1719. [Google Scholar] [CrossRef]
- Meisel, M.; Hinterleitner, R.; Pacis, A.; Chen, L.; Earley, Z.M.; Mayassi, T.; Pierre, J.F.; Ernest, J.D.; Galipeau, H.J.; Thuille, N.; et al. Microbial signals drive pre-leukaemic myeloproliferation in a Tet2-deficient host. Nature 2018, 557, 580–584. [Google Scholar] [CrossRef]
- Zhang, D.; Gao, X.; Li, H.; Borger, D.K.; Wei, Q.; Yang, E.; Xu, C.; Pinho, S.; Frenette, P.S. The microbiota regulates hematopoietic stem cell fate decisions by controlling iron availability in bone marrow. Cell Stem Cell 2022, 29, 232–247.e7. [Google Scholar] [CrossRef]
- Unger, J.M.; Xiao, H.; LeBlanc, M.; Hershman, D.L.; Blanke, C.D. Cancer Clinical Trial Participation at the 1-Year Anniversary of the Outbreak of the COVID-19 Pandemic. JAMA Netw. Open 2021, 4, e2118433. [Google Scholar] [CrossRef]
| NCT Number | Study Name | Study Design and Status | Drugs Tested | Drug Class | Primary Outcome |
|---|---|---|---|---|---|
| NCT02013648 | Randomized Phase III Study of Intensive Chemotherapy with or Without Dasatinib | Randomized, open label Active not recruiting | Dasatinib | Tyrosine Kinase inhibitor |
|
| NCT04102020 | Randomized, Double-Blind, 2-Arm, Multicenter, Phase 3 Study of Venetoclax and Oral Azacitidine Versus Oral Azacitidine as Maintenance Therapy for Patients with Acute Myeloid Leukemia in First Remission After Conventional Chemotherapy (VIALE-M) | Randomized, double blind Active and recruiting | Venetoclax Azacitidine CC-486 | BCL2 inhibitor |
|
| NCT04716114 | A Phase 3, Open-Label, Multicenter, Randomized Study of SKLB1028 Versus Salvage Chemotherapy in Patients with FLT3-mutated Acute Myeloid Leukemia Refractory to or Relapsed After First-line Treatment (ALIVE) | Randomized, open label Active and recruiting | SKLB1028 | Multikinase inhibitor of EGFR, FLT3 and ABL |
|
| NCT05586074 | HEC73543 Versus Salvage Chemotherapy in Relapsed or Refractory FLT3-ITD Acute Myeloid Leukemia: a Multicenter, Open-label, Randomized Phase 3 Trial | Randomized, Open label Active not yet recruiting | Clifutinib | FLT3 inhibitor |
|
| NCT04161885 | A Randomized, Open Label Phase 3 Study Evaluating Safety and Efficacy of Venetoclax in Combination with Azacitidine After Allogeneic Stem Cell Transplantation in Subjects With Acute Myeloid Leukemia (AML) (VIALE-T) | Randomized, Open label Active, recruiting | Venetoclax Azacitidine | BCL2 inhibitor |
|
| NCT04628026 | A Randomized, Placebo-Controlled Phase III Study of Induction and Consolidation Chemotherapy with Venetoclax in Adult Patients with Newly Diagnosed Acute Myeloid Leukemia or Myelodysplastic Syndrome with Excess Blasts-2 | Randomized, double blind Active, recruiting | Venetoclax | BCL2 inhibitor |
|
| NCT04571645 | A Randomized, Double-blind, Placebo-controlled Study to Evaluate the Efficacy and Safety of Dociparstat Sodium in Combination with Standard Chemotherapy for the Treatment of Newly Diagnosed Acute Myeloid Leukemia | Randomized, double blind Active, not recruiting | Dociparastat | CXCR4/CXCL12 inhibitor |
|
| NCT02997202 | A Multi-center, Randomized, Double-blind, Placebo-controlled Phase III Trial of the FLT3 Inhibitor Gilteritinib Administered as Maintenance Therapy Following Allogeneic Transplant for Patients with FLT3/ITD AML | Randomized, double blind Active, not recruiting | Gilteritinib | FLT3 inhibitor |
|
| NCT05429632 | Randomized, Double-blind, Placebo-controlled, Multi-center Phase III Study to Evaluate the Efficacy and Safety of Mocravimod as Adjunctive and Maintenance Treatment in Adult AML Patients Undergoing Allogeneic HCT | Randomized, double blind Active, recruiting | Macrovimod | Sphingosine-1-phosphate receptor modulator |
|
| NCT03258931 | Phase III Randomized Study of Crenolanib Versus Midostaurin Administered Following Induction Chemotherapy and Consolidation Therapy in Newly Diagnosed Subjects With FLT3 Mutated Acute Myeloid Leukemia | Randomized, open label Active, recruiting | Crenolanib | FLT3 inhibitor |
|
| NCT04229979 | A Randomized, Open-Label Study of the Efficacy and Safety of Galinpepimut-S (GPS) Maintenance Monotherapy Compared to Investigator’s Choice of Best Available Therapy in Subjects with Acute Myeloid Leukemia Who Have Achieved Complete Remission After Second-Line Salvage Therapy | Randomized, Open label Active, recruiting | Galinpepimut-S | WT-1 peptide vaccine |
|
| NCT05079230 | A Phase 3, Randomized, Double-Blind, Placebo-Controlled Study Evaluating the Safety and Efficacy of Magrolimab Versus Placebo in Combination with Venetoclax and Azacitidine in Newly Diagnosed, Previously Untreated Patients with Acute Myeloid Leukemia Who Are Ineligible for Intensive Chemotherapy | Randomized, Double blind Active, recruiting | Magrolimab | Anti-CD47 |
|
| NCT03616470 | A Phase III Randomized, Double-Blind Trial to Evaluate the Efficacy of Uproleselan Administered with Chemotherapy Versus Chemotherapy Alone in Patients With Relapsed/Refractory Acute Myeloid Leukemia | Randomized, Double blind Active, not recruiting | Uproleselan | E-selectin antagonist |
|
| NCT02668653 | A Phase 3, Double-Blind, Placebo-controlled Study of Quizartinib Administered in Combination with Induction and Consolidation Chemotherapy, and Administered as Continuation Therapy in Subjects 18 to 75 Years Old with Newly Diagnosed FLT3-ITD (+) Acute Myeloid Leukemia (QuANTUM First) | Randomized, Double blind Active, not recruiting | Quizartinib | FLT3 inhibitor |
|
| NCT03250338 | Phase III Randomized, Double-blind, Placebo-controlled Study Investigating the Efficacy of the Addition of Crenolanib to Salvage Chemotherapy Versus Salvage Chemotherapy Alone in Subjects ≤ 75 Years of Age with Relapsed/Refractory FLT3 Mutated Acute Myeloid Leukemia | Randomized, double blind Active, recruiting | Crenolanib | FLT3 inhibitor |
|
| NCT04161885 | A Randomized, Open Label Phase 3 Study Evaluating Safety and Efficacy of Venetoclax in Combination with Azacitidine After Allogeneic Stem Cell Transplantation in Subjects With Acute Myeloid Leukemia (AML) (VIALE-T) | Randomized, open label Active, recruiting | Venetoclax Azacitidine | BCL2 inhibitor |
|
| NCT05020665 | A Phase 3, Randomized, Double-blind, Placebo-controlled Study to Assess the Efficacy and Safety of Entospletinib in Combination With Intensive Induction and Consolidation Chemotherapy in Adults With Newly Diagnosed Nucleophosmin 1-mutated Acute Myeloid Leukemia | Randomized, double blind Active, not recruiting | Entospletinib | spleen tyrosine kinase (SYK) inhibitor |
|
| NCT04778397 | A Phase 3, Randomized, Open-Label Study Evaluating the Safety and Efficacy of Magrolimab in Combination With Azacitidine Versus Physician’s Choice of Venetoclax in Combination With Azacitidine or Intensive Chemotherapy in Previously Untreated Patients With TP53 Mutant Acute Myeloid Leukemia ENHANCE-2 | Randomized, Open label Active, recruiting | Magrolimab | Anti-CD47 |
|
| NCT02665065 | A Multicenter, Pivotal Phase 3 Study of Iomab-B Prior to Allogeneic Hematopoietic Cell Transplant Versus Conventional Care in Older Subjects with Active, Relapsed or Refractory Acute Myeloid Leukemia (AML) (SIERRA) | Randomized, Open label Active, not recruiting | Iomab-B | Anti-CD45-131I apamistamab |
|
| NCT03926624 | Phase 3 Randomized Trial of DFP-10917 vs. Non-Intensive Reinduction (LoDAC, Azacitidine, Decitabine, Venetoclax Combination Regimens) or Intensive Reinduction (High & Intermediate Dose Cytarabine Regimens) for Acute Myelogenous Leukemia Patients in Second, Third, or Fourth Salvage | Randomized, Open label Active, recruiting | DFP-10917 | deoxycytidine nucleoside analogue |
|
| NCT03268954 | A Phase 3, Randomized, Controlled, Open-label, Clinical Study of Pevonedistat Plus Azacitidine Versus Single-Agent Azacitidine as First-Line Treatment for Patients with Higher-Risk Myelodysplastic Syndromes, Chronic Myelomonocytic Leukemia, or Low-Blast Acute Myelogenous Leukemia | Randomized, Open label Active, not recruiting | Pevonedistat | NAE inhibitor |
|
| NCT03092674 | A Randomized Phase II/III Trial of “Novel Therapeutics” Versus Azacitidine in Newly Diagnosed Patients With Acute Myeloid Leukemia (AML) or High-Risk Myelodysplastic Syndrome (MDS), Age 60 or Older LEAP: Less-Intense AML Platform Trial | Randomized, Open label Active, not recruiting | Nivolumab | PD-1 inhibitor |
|
| NCT03257241 | A PALG Prospective Multicenter Clinical Trial to Compare the Efficacy of Two Standard Induction Therapies (DA-90 vs. DAC) and Two Standard Salvage Regimens (FLAG-IDA vs. CLAG-M) in AML Patients ≤ 60 Years Old | Randomized, Open label Active, recruiting | DNR 90 DAC CLAG-M FLAG- IDA | Anthracycline |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Premnath, N.; Madanat, Y.F. Novel Investigational Agents and Pathways That May Influence the Future Management of Acute Myeloid Leukemia. Cancers 2023, 15, 2958. https://doi.org/10.3390/cancers15112958
Premnath N, Madanat YF. Novel Investigational Agents and Pathways That May Influence the Future Management of Acute Myeloid Leukemia. Cancers. 2023; 15(11):2958. https://doi.org/10.3390/cancers15112958
Chicago/Turabian StylePremnath, Naveen, and Yazan F. Madanat. 2023. "Novel Investigational Agents and Pathways That May Influence the Future Management of Acute Myeloid Leukemia" Cancers 15, no. 11: 2958. https://doi.org/10.3390/cancers15112958
APA StylePremnath, N., & Madanat, Y. F. (2023). Novel Investigational Agents and Pathways That May Influence the Future Management of Acute Myeloid Leukemia. Cancers, 15(11), 2958. https://doi.org/10.3390/cancers15112958