Screening of Breast Cancer from Sweat Samples Analyzed by 2-Dimensional Gas Chromatography-Mass Spectrometry: A Preliminary Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects & Study Design
2.2. Sweat Sample Collection
2.3. Analytical Devices
2.4. Data Analysis
2.4.1. Extraction and Pre-Treatment of the GC × GC − MS Raw Data
2.4.2. Statistical Analysis
3. Results
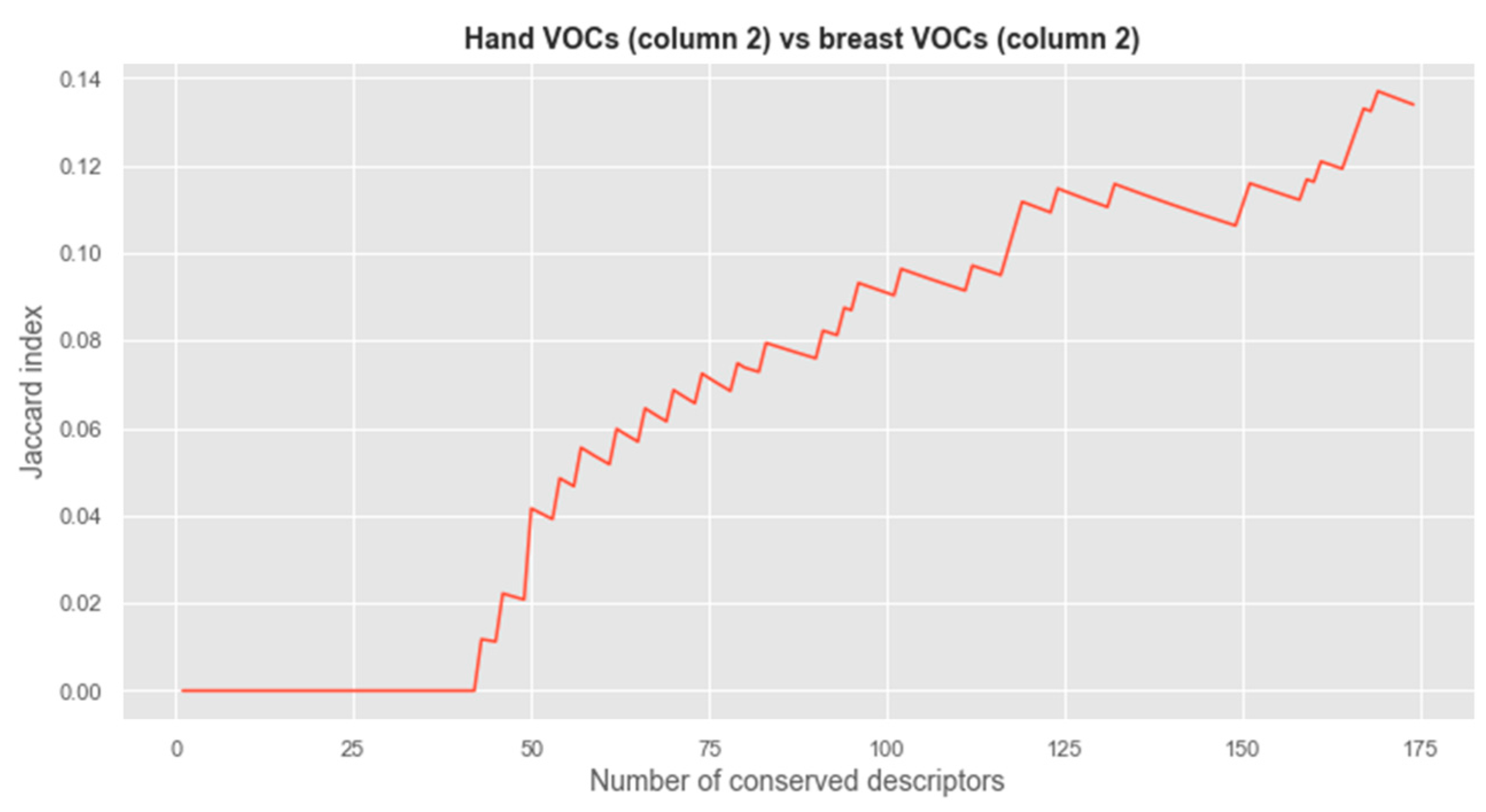
3.1. Vector Analysis of Chemical Compounds of Sweat Volatomic Pattern from GC × GC − MS Analysis
3.2. Multivariate Statistical Analysis of Sweat Volatomics Profile
3.2.1. A 2-Dimensional Representation of the Datasets
3.2.2. TPOT
3.2.3. Grid Search
3.2.4. Shapley Values
3.2.5. Probe Variable Method
4. Discussion
4.1. Pipeline for Identification of BC-Related VOCs
4.2. VOCs Matrices
4.3. Confounding Parameters
4.4. Instrumental Parameters
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Cancer. 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 1 February 2023).
- Glasziou, P.; Houssami, N. The evidence base for breast cancer screening. Prev. Med. 2011, 53, 100–102. [Google Scholar] [CrossRef] [PubMed]
- Dobi, Á.; Kelemen, G.; Kaizer, L.; Weiczner, R.; Thurzó, L.; Kahán, Z. Breast cancer under 40 years of age: Increasing number and worse prognosis. Pathol. Oncol. Res. 2011, 17, 425–428. [Google Scholar] [CrossRef] [PubMed]
- Leemans, M.; Bauër, P.; Cuzuel, V.; Audureau, E.; Fromantin, I. Volatile Organic Compounds Analysis as a Potential Novel Screening Tool for Breast Cancer: A Systematic Review. Biomark. Insights 2022, 17, 117727192211007. [Google Scholar] [CrossRef]
- Berenguer, C.V.; Pereira, F.; Pereira, J.A.M.; Câmara, J.S. Volatilomics: An Emerging and Promising Avenue for the Detection of Potential Prostate Cancer Biomarkers. Cancers 2022, 14, 3982. [Google Scholar] [CrossRef] [PubMed]
- Bauër, P.; Leemans, M.; Audureau, E.; Gilbert, C.; Armal, C.; Fromantin, I. Remote Medical Scent Detection of Cancer and Infectious Diseases with Dogs and Rats: A Systematic Review. Integr. Cancer Ther. 2022, 21, 153473542211405. [Google Scholar] [CrossRef]
- Janfaza, S.; Khorsand, B.; Nikkhah, M.; Zahiri, J. Digging deeper into volatile organic compounds associated with cancer. Biol. Methods Protoc. 2019, 4, bpz014. [Google Scholar] [CrossRef] [PubMed]
- Buljubasic, F.; Buchbauer, G. The scent of human diseases: A review on specific volatile organic compounds as diagnostic biomarkers. Flavour Fragr. J. 2015, 30, 5–25. [Google Scholar] [CrossRef]
- Janssens, E.; Van Meerbeeck, J.P.; Lamote, K. Volatile organic compounds in human matrices as lung cancer biomarkers: A systematic review. Crit. Rev. Oncol. Hematol. 2020, 153, 103037. [Google Scholar] [CrossRef]
- Phillips, M.; Cataneo, R.N.; Chaturvedi, A.; Kaplan, P.D.; Libardoni, M.; Mundada, M.; Patel, U.; Zhang, X. Detection of an Extended Human Volatome with Comprehensive Two-Dimensional Gas Chromatography Time-of-Flight Mass Spectrometry. PLoS ONE 2013, 8, e75274. [Google Scholar] [CrossRef]
- Cuzuel, V.; Portas, E.; Cognon, G.; Rivals, I.; Heulard, F.; Thiébaut, D. Sampling method development and optimization in view of human hand odor analysis by thermal desorption coupled with gas chromatography and mass spectrometry. Anal. Bioanal. Chem. 2017, 409, 5113–5124. [Google Scholar] [CrossRef]
- Cuzuel, V.; Sizun, A.; Cognon, G.; Rivals, I.; Heulard, F.; Thiébaut, D.; Vial, J. Human odor and forensics. Optimization of a comprehensive two-dimensional gas chromatography method based on orthogonality: How not to choose between criteria. J. Chromatogr. A 2018, 1536, 58–66. [Google Scholar] [CrossRef]
- Cuzuel, V.; Leconte, R.; Cognon, G.; Thiebaut, D.; Vial, J.; Sauleau, C.; Rivals, I. Human odor and forensics: Towards Bayesian suspect identification using GC × GC − MS characterization of hand odor. J. Chromatogr. B 2018, 1092, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Manduchi, E.; Romano, J.D.; Moore, J.H. The promise of automated machine learning for the genetic analysis of complex traits. Hum. Genet. 2022, 141, 1529–1544. [Google Scholar] [CrossRef] [PubMed]
- Chawla, N.V.; Bowyer, K.W.; Hall, L.O.; Kegelmeyer, W.P. SMOTE: Synthetic Minority Over-sampling Technique. J. Artif. Intell. Res. 2011, 16, 321–357. [Google Scholar] [CrossRef]
- London, A.J. Artificial Intelligence and Black-Box Medical Decisions: Accuracy versus Explainability. Hastings Cent. Rep. 2019, 49, 15–21. [Google Scholar] [CrossRef]
- Lundberg, S.M.; Lee, S.I. A unified approach to interpreting model predictions. Adv. Neural Inf. Process. Syst. 2017, 30, 4766–4775. [Google Scholar]
- Montagna, W. The Structure and Function of Skin, 3rd ed. 1974. Available online: https://www.elsevier.com/books/the-structure-and-function-of-skin/montagna/978-0-12-505263-4 (accessed on 1 February 2023).
- Nicolaides, N. Skin Lipids: Their Biochemical Uniqueness: Unlike internal organs, the skin biosynthesizes and excretes unusual fat soluble substances. Science 1974, 186, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.-J.; Oh, C.-H. 2nd Dimensional GC-MS analysis of sweat volatile organic compounds prepared by solid phase micro-extraction. Technol. Health Care 2014, 22, 481–488. [Google Scholar] [CrossRef]
- Johnson, K.S.; Conant, E.F.; Soo, M.S. Molecular Subtypes of Breast Cancer: A Review for Breast Radiologists. J. Breast Imaging 2021, 3, 12–24. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, L.; Qiu, Z.; Lv, Y.; Chen, G.; Li, E. Early diagnosis of breast cancer from exhaled breath by gas chromatography-mass spectrometry (GC/MS) analysis: A prospective cohort study. J. Clin. Lab. Anal. 2020, 34, e23526. [Google Scholar] [CrossRef]
- Barash, O.; Zhang, W.; Halpern, J.M.; Hua, Q.-L.; Pan, Y.-Y.; Kayal, H.; Khoury, K.; Liu, H.; Davies, M.P.A.; Haick, H. Differentiation between genetic mutations of breast cancer by breath volatolomics. Oncotarget 2015, 6, 44864–44876. [Google Scholar] [CrossRef] [PubMed]
- Thuleau, A.; Gilbert, C.; Bauër, P.; Alran, S.; Fourchotte, V.; Guillot, E.; Vincent-Salomon, A.; Kerihuel, J.-C.; Dugay, J.; Semetey, V.; et al. A New Transcutaneous Method for Breast Cancer Detection with Dogs. Oncology 2018, 96, 110–123. [Google Scholar] [CrossRef] [PubMed]
- Wackermannová, M.; Pinc, L.; Jebavý, L. Olfactory Sensitivity in Mammalian Species. Physiol. Res. 2016, 65, 369–390. [Google Scholar] [CrossRef] [PubMed]
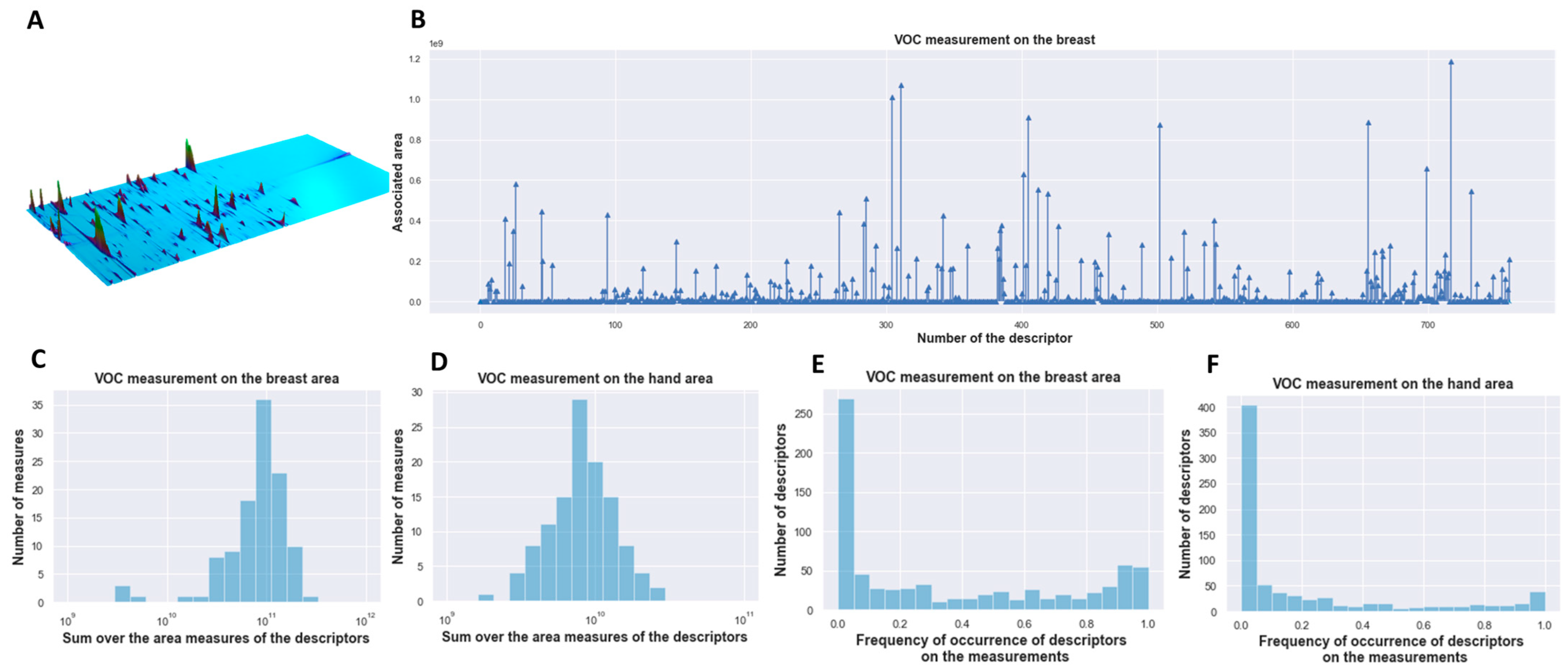
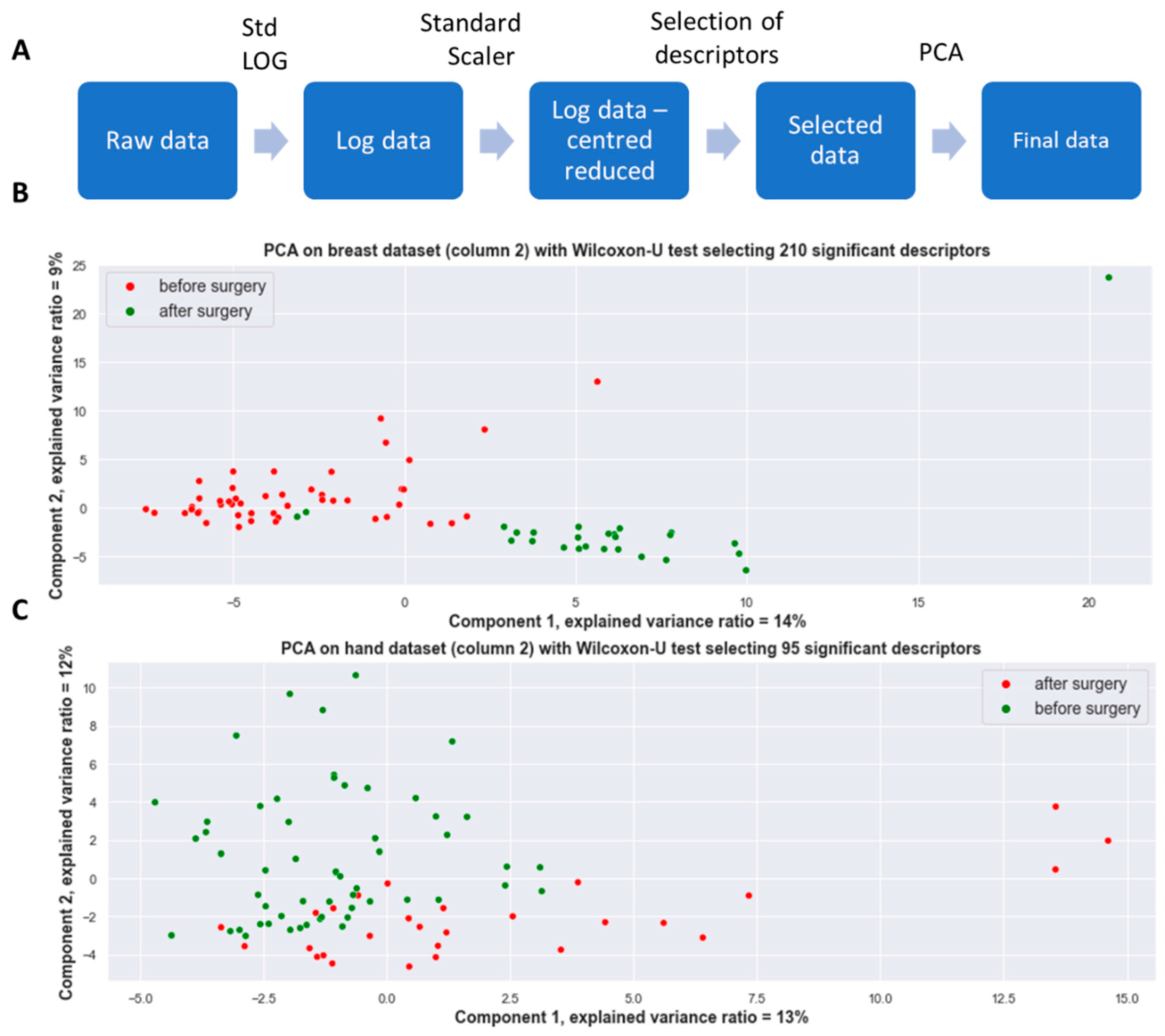
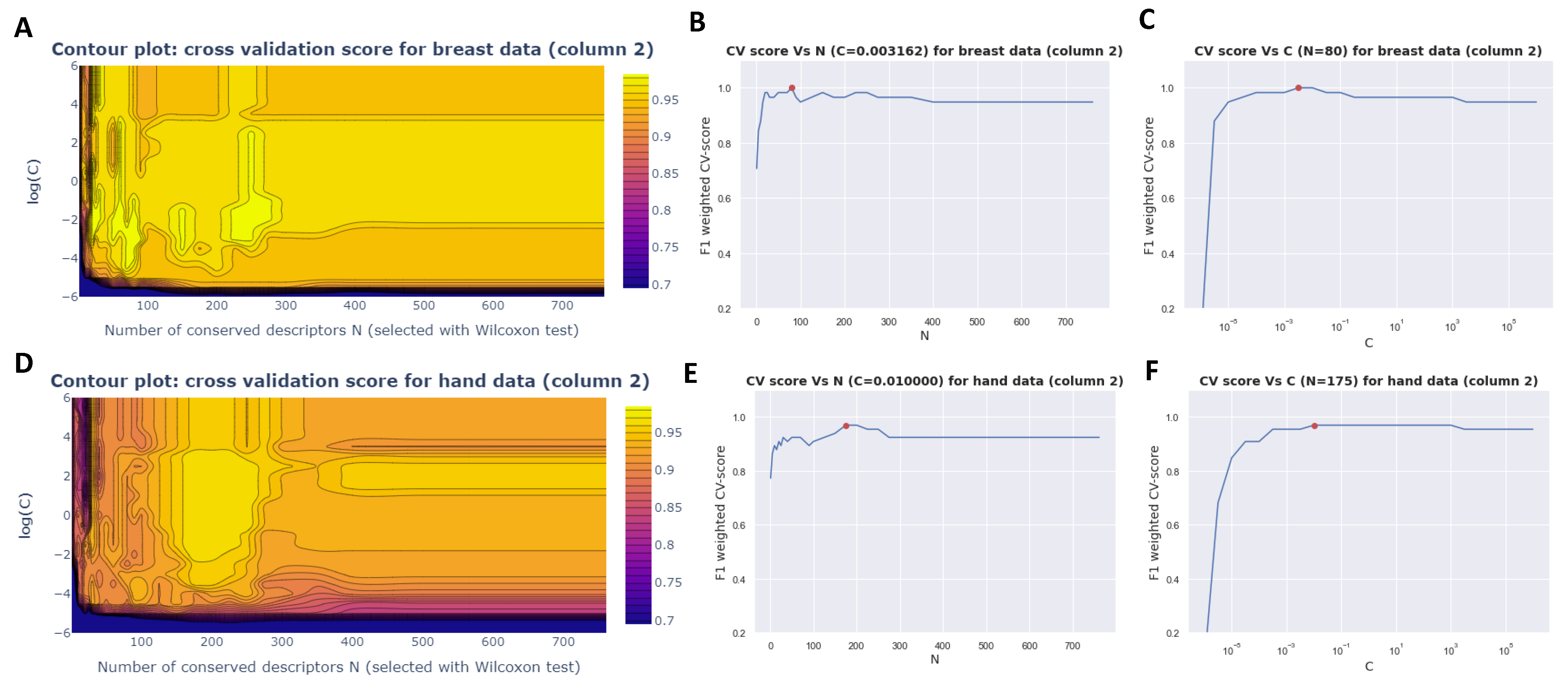
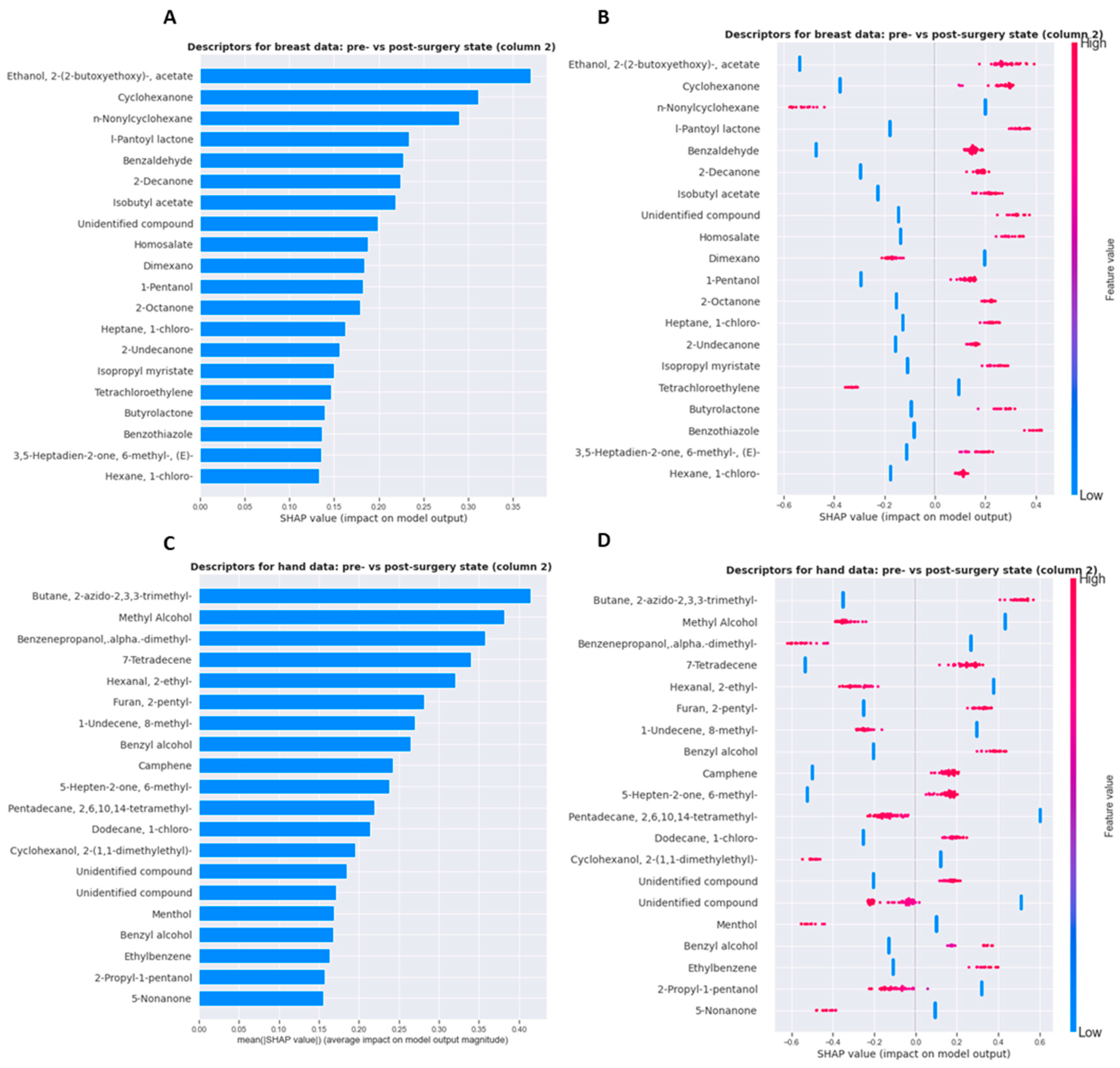

| Sweat Samples before Surgery | Sweat Samples after Surgery | |||
|---|---|---|---|---|
| Body part sweat collection | Hands | Breast | Hands | Breast |
| Subjects | 21 | 20 | 13 | 12 |
| Number of samples | 74 | 70 | 43 | 41 |
| Age (range, median) | mean 51, range (36–76) | mean 51, range (36–76) | mean 52, range (41–76) | mean 51, range (36–71) |
| 1. | Application of LOG chemical standardization. |
| 2. | Principal component analysis: visualization of clusters of samples based on their similarity. |
| 3. | Split the data into two sets: a learning/validation set and a test set and rebalance the classes/labels on the learning set employing SMOTE. |
| 4. | Train models with TPOT. |
| 5. | Evaluation of the performance of the selected models by computing their F1-weighted score on the test set |
| 6. | Interpret the model using SHAP |
| 7. | Determine the relevance of VOCs using the probe variable method |
| Pre-vs. Post-Surgery Status on Breast VOCs (GC Column 2) | Pre-vs. Post-Surgery Status on Hand VOCs (GC Column 2) | |
|---|---|---|
| F1-score | 0.93 | 0.85 |
| Sensitivity | 1.0 | 0.96 |
| Specificity | 0.82 | 0.64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leemans, M.; Cuzuel, V.; Bauër, P.; Baba Aissa, H.; Cournelle, G.; Baelde, A.; Thuleau, A.; Cognon, G.; Pouget, N.; Guillot, E.; et al. Screening of Breast Cancer from Sweat Samples Analyzed by 2-Dimensional Gas Chromatography-Mass Spectrometry: A Preliminary Study. Cancers 2023, 15, 2939. https://doi.org/10.3390/cancers15112939
Leemans M, Cuzuel V, Bauër P, Baba Aissa H, Cournelle G, Baelde A, Thuleau A, Cognon G, Pouget N, Guillot E, et al. Screening of Breast Cancer from Sweat Samples Analyzed by 2-Dimensional Gas Chromatography-Mass Spectrometry: A Preliminary Study. Cancers. 2023; 15(11):2939. https://doi.org/10.3390/cancers15112939
Chicago/Turabian StyleLeemans, Michelle, Vincent Cuzuel, Pierre Bauër, Hind Baba Aissa, Gabriel Cournelle, Aurélien Baelde, Aurélie Thuleau, Guillaume Cognon, Nicolas Pouget, Eugénie Guillot, and et al. 2023. "Screening of Breast Cancer from Sweat Samples Analyzed by 2-Dimensional Gas Chromatography-Mass Spectrometry: A Preliminary Study" Cancers 15, no. 11: 2939. https://doi.org/10.3390/cancers15112939
APA StyleLeemans, M., Cuzuel, V., Bauër, P., Baba Aissa, H., Cournelle, G., Baelde, A., Thuleau, A., Cognon, G., Pouget, N., Guillot, E., Fromantin, I., & Audureau, E. (2023). Screening of Breast Cancer from Sweat Samples Analyzed by 2-Dimensional Gas Chromatography-Mass Spectrometry: A Preliminary Study. Cancers, 15(11), 2939. https://doi.org/10.3390/cancers15112939





