Hope and Hype around Immunotherapy in Triple-Negative Breast Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Main Studies with Immunotherapy
2.1. Advanced Triple-Negative Breast Cancer
2.1.1. Immune-Checkpoint Inhibitors in Monotherapy
| Study Name | Phase | Line of Therapy and Population | TNBC (=N) | Treatment | Findings |
|---|---|---|---|---|---|
| KEYNOTE-012 [16] | Ib | mTNBC All lines (first in 15.6%) | 32 | Pembrolizumab | ORR: 18.5% mPFS: 1.9 mo OS: 11.2 mo |
| JAVELIN [23] | I | mTNBC All lines (first or second line in 50% of patients) | 58 | Avelumab | ORR (TNBC cohort): 5.2% ORR (PD-L1 positive): 22.2% ORR (PD-L1 negative): 2.6% |
| KEYNOTE-086 (cohort A) [17] | II | mTNBC Second or later lines (second in 31%) | 170 | Pembrolizumab | ORR (ITT): 5.3% ORR (PD-L1 positive): 5.7% ORR (PD-L1 negative): 4.7% |
| KEYNOTE-086 (cohort B) [18] | II | mTNBC First line | 84 | Pembrolizumab | ORR: 21.4% mPFS: 2.1 mom OS: 18 mo |
| TAPUR [22] | II | mBC with HTMB Second or later lines (≥3 in 93%) | 28 | Pembrolizumab | ORR: 21% DCR: 37% mPFS: 10.6 weeks mOS: 30.6 weeks |
| ENCORE 602/TRIO025 [24] | II | mTNBC Second or later lines (second in 69%) | 41 | Atezolizumab | ORR: 2.0% mPFS: 1.51 mo mOS: 12.4 mo |
| TONIC [25] | II | mTNBC First to fourth lines (first in 24%) | 67 | Nivolumab with (1) no induction or (2) irradiation or (3) cyclophosphamide or (4) cisplatin or (5) doxorubicin | ORR (overall cohort): 20% ORR (cisplatin cohor): 23% ORR (doxorubicin cohort): 35% ORR (TNBC cohort): 5% |
| SAFIR02-BREAST IMMUNO [26] | II | mBC First or second lines (second in 52%) | 82 | Durvalumab vs. chemotherapy | HR for OS (ITT): 0.84, 95 Cl: 0.54–1.29; p = 0.423. HR for OS (TNBC cohort): 0.54, 95% CI 0.30–0.97, p = 0.0377 HR for OS (TNBC PD-L1 positive) 0.37, 95% CI 0.12–1.13 |
| KEYNOTE-119 [19] | III | mTNBC Second or third lines (second in 60%) | 622 | Pembrolizumab vs. chemotherapy | mOS (PD-L1 CPS > 1): 10.7 vs. 10.2 mo (HR 0.86, 95%CI 0.69–1.06) mOS (PD-L1 CPS > 10): 12.7 vs. 11.6 mo (HR 0.78, 95%CI 0.57–1.06) OS (ITT): 9.9 vs. 10.8 mo (HR 0.97, 95%CI 0.82–1.15) PFS (ITT): 2.1 vs. 3.3 mo (HR 1.60, 95%CI 1.33–1.92) |
2.1.2. Immune-Checkpoint Inhibitors in Combination with Chemotherapy
| Study Name | IMpassion130 [28,29,30] | IMpassion131 [31] | KEYNOTE-355 [34,35,36] | |
|---|---|---|---|---|
| Population | mTNBC (=902) | mTNBC (=651) | mTNBC (=847) | |
| Random | 1:1 | 2:1 | 2:1 | |
| ICI | Atezolizumab | Atezolizumab | Pembrolizumab | |
| Chemotherapy | Nab-paclitaxel | Paclitaxel | Nab-paclitaxel Paclitaxel Carboplatin and gemcitabine | |
| Primary endpoint | PFS and OS in ITT and PD-L1 positive (hierarchical) | PFS in PD-L1 positive and ITT (hierarchical) | PFS and OS in PD-L1 CPS score ≥10, ≥1, and ITT (hierarchical) | |
| PD-L1 definition | IC > 1 | IC > 1 | CPS > 1 and CPS > 10 | |
| Assay | SP142 | SP142 | 22C3 | |
| Findings | PFS | ITT: 7.2 vs. 5.5 mo (HR 0.80, 95% CI 0.69–0.92, p = 0.002) PD-L1: 7.5 vs. 5.3 mo (HR 0.63, 95% CI 0.50–0.80) | ITT: 5.7 vs. 5.6 mo (HR 0.86, 95% CI 0.70–1.05) PD-L1: 6.0 vs. 5.7 mo (HR 0.82, 95% CI 0.60–1.12; p = 0.20) | ITT: 7.5 mos vs. 5.6 (HR 0.82, 95%CI 0.70–0.98) PD-L1 CPS ≥ 10: 9.7 vs. 5.6 (HR 0.66, 95%CI 0.50–0.88) PD-L1 CPS ≥ 1: 7.5 vs. 5.6 (HR 0.75, 95%CI 0.62–0.91) |
| OS | ITT: 21.0 vs. 18.7 mo (HR 0.87, 95% CI 0.75–1.02, p = 0.08) PD-L1: 25.4 vs. 17.9 mo (HR 0.67, 95%CI 0.53–0.86) | ITT: 19.2 vs. 22.8 mo (HR 1.12, 95% CI 0.88–1.43) PD-L1: 22.1 vs. 28.3 mo (HR 1.11, 95% CI 0.76–1.64) | ITT: 17.2 mos vs. 15.5 (HR 0.89, 95%CI 0.76–1.05) PD-L1 CPS ≥ 10: 23.0 vs. 16.1 (HR 0.73, 95%CI 0.55–0.95, p = 0.0093) PD-L1 CPS ≥ 1: 17.6 vs. 16.0 (HR 0.86, 95%CI 0.72–1.04, p = 9.0563) | |
2.2. Early Triple-Negative Breast Cancer
| ICI | Study Name | Phase | Population | Treatment | Findings | pCR Improvement | EFS Benefit | |
|---|---|---|---|---|---|---|---|---|
| pCR | EFS | |||||||
| Pembrolizumab | I-SPY2 [47] | II | Stage II/III HER2-negative (=250) | A: paclitaxel + pembrolizumab → AC B: paclitaxel → AC | HER2-negative: 44 vs. 17% TNBC: 60 vs. 22% | Numerically higher but not powered for statistical significance | Yes | Not reported |
| KEYNOTE-522 [39,40,41] | III | T1c N1-2 or T2-4 N0-2 TNBC (=1174) | A: carboplatin and paclitaxel + pembrolizumab → AC/EC → surgery → pembrolizumab B: carboplatin and paclitaxel + placebo → AC/EC → surgery → placebo | ITT: 63% vs. 55.6% PD-L1 positive: 54.9 vs. 68.9% PD-L1 negative: 30.3 vs. 45.4% | 3-year EFS: 84.5 vs. 76.8% (HR 0.63, 95% CI 0.48–0.88, p = 0.0003) | Yes | Yes | |
| Atezolizumab | NeoTRIPaPDL1 [44] | III | Early high-risk or locally advanced TNBC (=280) | A: carboplatin and nab-paclitaxel + atezolizumab → surgery → AC/EC B: carboplatin and nab-paclitaxel → surgery → AC/EC | ITT: 48.6 vs. 44.4% PD-L1 positive: 51.9 vs. 48% | Not reported | Not significant | Not reported |
| IMpassion031 [43] | III | cT2-4 cN0-3 TNBC (=333) | A: Atezolizumab → nabpaclitaxel → AC B: placebo → nabpaclitaxel → AC | ITT: 57.6 vs. 41.1% PD-L1 positive: 68.8% vs. 49.3% | Not reported | Yes | Not reported | |
| Durvalumab | GeparNUEVO [46] | II | T1b-T4a-d TNBC (=174) | A: durvalumab + nabpaclitaxel → AC B: placebo + nabpaclitaxel → AC | ITT: 53.4 vs. 44.2% PD-L1 positive: 58 vs. 50.7% window-cohort: 61.0 vs. 41.4% | 3-year iDFS: 84.9 vs. 76.9% 3-year OS: 95.1 vs. 83.1% | Not significant | Yes |
3. Novel Strategies and Future Directions
3.1. Dual Immunotherapy
3.2. PARPi
3.3. Angiogenesis Inhibitors
3.4. ADCs
3.5. Current and Future Directions
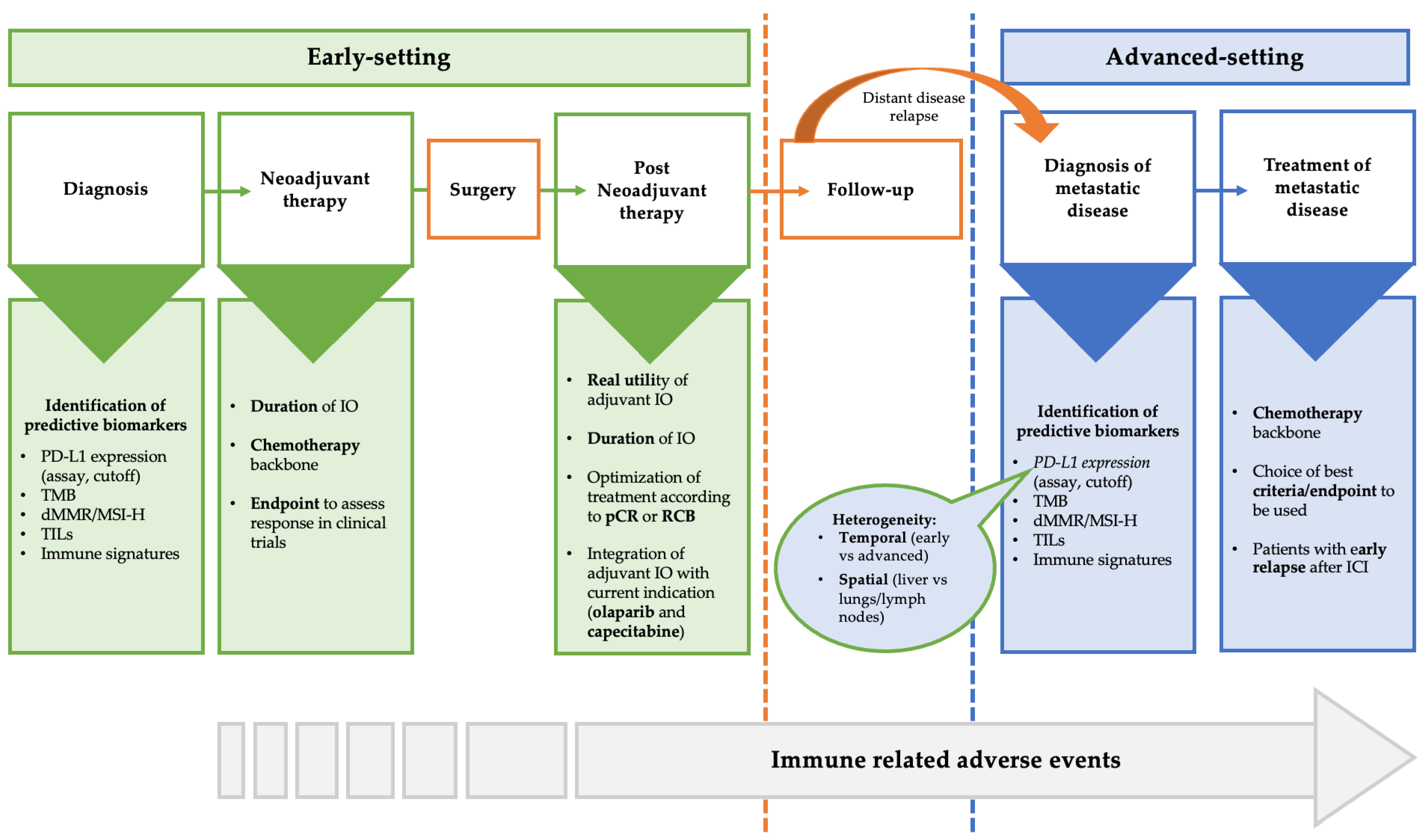
4. Open Question about the Use of Immunotherapy in Triple-Negative Breast Cancer
4.1. PD-L1 as Valid Biomarkers
4.2. Other Predictive Biomarkers
4.3. Backbone Chemotherapy
4.4. Optimal Duration of Immunotherapy
4.5. Patients without pCR after Neoadjuvant Immunotherapy
4.6. Patients with Early Relapse after (Neo)Adjuvant Immunotherapy
4.7. Endpoints and Criteria to Assess Response to Immunotherapy in Clinical Trials
4.8. Immune-Related Adverse Event (irAEs)
4.9. Financial Toxicity
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- SEER Cancer Statistics Review, 1975–2016. Available online: https://seer.cancer.gov/csr/1975_2016/index.html (accessed on 3 March 2023).
- Dent, R.; Trudeau, M.; Pritchard, K.I.; Hanna, W.M.; Kahn, H.K.; Sawka, C.A.; Lickley, L.A.; Rawlinson, E.; Sun, P.; Narod, S.A. Triple-negative breast cancer: Clinical features and patterns of recurrence. Clin. Cancer Res. 2007, 13, 4429–4434. [Google Scholar] [CrossRef]
- Li, C.H.; Karantza, V.; Aktan, G.; Lala, M. Current treatment landscape for patients with locally recurrent inoperable or metastatic triple-negative breast cancer: A systematic literature review. Breast Cancer Res. 2019, 21, 143. [Google Scholar] [CrossRef] [PubMed]
- Anders, C.; Carey, L.A. Understanding and treating triple-negative breast cancer. Oncology 2008, 22, 1233–1239; discussion 1239–1240, 1243. [Google Scholar] [PubMed]
- Agostinetto, E.; Eiger, D.; Punie, K.; de Azambuja, E. Emerging Therapeutics for Patients with Triple-Negative Breast Cancer. Curr. Oncol. Rep. 2021, 23, 57. [Google Scholar] [CrossRef] [PubMed]
- Bianchini, G.; Balko, J.M.; Mayer, I.A.; Sanders, M.E.; Gianni, L. Triple-negative breast cancer: Challenges and opportunities of a heterogeneous disease. Nat. Rev. Clin. Oncol. 2016, 13, 674–690. [Google Scholar] [CrossRef]
- Denkert, C.; Liedtke, C.; Tutt, A.; von Minckwitz, G. Molecular alterations in triple-negative breast cancer-the road to new treatment strategies. Lancet 2017, 389, 2430–2442. [Google Scholar] [CrossRef]
- Lehmann, B.D.; Bauer, J.A.; Chen, X.; Sanders, M.E.; Chakravarthy, A.B.; Shyr, Y.; Pietenpol, J.A. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J. Clin. Investig. 2011, 121, 2750–2767. [Google Scholar] [CrossRef]
- Burstein, M.D.; Tsimelzon, A.; Poage, G.M.; Covington, K.R.; Contreras, A.; Fuqua, S.A.W.; Savage, M.I.; Osborne, C.K.; Hilsenbeck, S.G.; Chang, J.C.; et al. Comprehensive genomic analysis identifies novel subtypes and targets of triple-negative breast cancer. Clin. Cancer Res. 2015, 21, 1688–1698. [Google Scholar] [CrossRef]
- Bareche, Y.; Venet, D.; Ignatiadis, M.; Aftimos, P.; Piccart, M.; Rothe, F.; Sotiriou, C. Unravelling triple-negative breast cancer molecular heterogeneity using an integrative multiomic analysis. Ann. Oncol. 2018, 29, 895–902. [Google Scholar] [CrossRef]
- Debien, V.; De Caluwé, A.; Wang, X.; Piccart-Gebhart, M.; Tuohy, V.K.; Romano, E.; Buisseret, L. Immunotherapy in breast cancer: An overview of current strategies and perspectives. NPJ Breast Cancer 2023, 9, 7. [Google Scholar] [CrossRef]
- Agostinetto, E.; Losurdo, A.; Nader-Marta, G.; Santoro, A.; Punie, K.; Barroso, R.; Popovic, L.; Solinas, C.; Kok, M.; de Azambuja, E.; et al. Progress and pitfalls in the use of immunotherapy for patients with triple negative breast cancer. Expert Opin. Investig. Drugs 2022, 31, 567–591. [Google Scholar] [CrossRef]
- Jacob, S.L.; Huppert, L.A.; Rugo, H.S. Role of Immunotherapy in Breast Cancer. JCO Oncol. Pract. 2023, 19, 167–179. [Google Scholar] [CrossRef]
- Tarantino, P.; Corti, C.; Schmid, P.; Cortes, J.; Mittendorf, E.A.; Rugo, H.; Tolaney, S.M.; Bianchini, G.; Andrè, F.; Curigliano, G. Immunotherapy for early triple negative breast cancer: Research agenda for the next decade. NPJ Breast Cancer 2022, 8, 23. [Google Scholar] [CrossRef]
- Li, L.; Zhang, F.; Liu, Z.; Fan, Z. Immunotherapy for Triple-Negative Breast Cancer: Combination Strategies to Improve Outcome. Cancers 2023, 15, 321. [Google Scholar] [CrossRef]
- Nanda, R.; Chow, L.Q.M.; Dees, E.C.; Berger, R.; Gupta, S.; Geva, R.; Pusztai, L.; Pathiraja, K.; Aktan, G.; Cheng, J.D.; et al. Pembrolizumab in Patients with Advanced Triple-Negative Breast Cancer: Phase Ib KEYNOTE-012 Study. J. Clin. Oncol. 2016, 34, 2460–2467. [Google Scholar] [CrossRef]
- Adams, S.; Schmid, P.; Rugo, H.S.; Winer, E.P.; Loirat, D.; Awada, A.; Cescon, D.W.; Iwata, H.; Campone, M.; Nanda, R.; et al. Pembrolizumab monotherapy for previously treated metastatic triple-negative breast cancer: Cohort A of the phase II KEYNOTE-086 study. Ann. Oncol. 2019, 30, 397–404. [Google Scholar] [CrossRef]
- Adams, S.; Loi, S.; Toppmeyer, D.; Cescon, D.W.; De Laurentiis, M.; Nanda, R.; Winer, E.P.; Mukai, H.; Tamura, K.; Armstrong, A.; et al. Pembrolizumab monotherapy for previously untreated, PD-L1-positive, metastatic triple-negative breast cancer: Cohort B of the phase II KEYNOTE-086 study. Ann. Oncol. 2019, 30, 405–411. [Google Scholar] [CrossRef]
- Winer, E.P.; Lipatov, O.; Im, S.-A.; Goncalves, A.; Muñoz-Couselo, E.; Lee, K.S.; Schmid, P.; Tamura, K.; Testa, L.; Witzel, I.; et al. Pembrolizumab versus investigator-choice chemotherapy for metastatic triple-negative breast cancer (KEYNOTE-119): A randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 499–511. [Google Scholar] [CrossRef]
- Maio, M.; Ascierto, P.A.; Manzyuk, L.; Motola-Kuba, D.; Penel, N.; Cassier, P.A.; Bariani, G.M.; De Jesus Acosta, A.; Doi, T.; Longo, F.; et al. Pembrolizumab in microsatellite instability high or mismatch repair deficient cancers: Updated analysis from the phase II KEYNOTE-158 study. Ann. Oncol. 2022, 33, 929–938. [Google Scholar] [CrossRef]
- Marabelle, A.; Le, D.T.; Ascierto, P.A.; Di Giacomo, A.M.; De Jesus-Acosta, A.; Delord, J.-P.; Geva, R.; Gottfried, M.; Penel, N.; Hansen, A.R.; et al. Efficacy of Pembrolizumab in Patients With Noncolorectal High Microsatellite Instability/Mismatch Repair-Deficient Cancer: Results From the Phase II KEYNOTE-158 Study. J. Clin. Oncol. 2020, 38, 1–10. [Google Scholar] [CrossRef]
- Alva, A.S.; Mangat, P.K.; Garrett-Mayer, E.; Halabi, S.; Hansra, D.; Calfa, C.J.; Khalil, M.F.; Ahn, E.R.; Cannon, T.L.; Crilley, P.; et al. Pembrolizumab in Patients with Metastatic Breast Cancer With High Tumor Mutational Burden: Results From the Targeted Agent and Profiling Utilization Registry (TAPUR) Study. J. Clin. Oncol. 2021, 39, 2443–2451. [Google Scholar] [CrossRef]
- Dirix, L.Y.; Takacs, I.; Jerusalem, G.; Nikolinakos, P.; Arkenau, H.-T.; Forero-Torres, A.; Boccia, R.; Lippman, M.E.; Somer, R.; Smakal, M.; et al. Avelumab, an anti-PD-L1 antibody, in patients with locally advanced or metastatic breast cancer: A phase 1b JAVELIN Solid Tumor study. Breast Cancer Res. Treat. 2018, 167, 671–686. [Google Scholar] [CrossRef] [PubMed]
- O’Shaughnessy, J.; Moroose, R.; Babu, S.; Baramidze, K.; Chan, D.; Leitner, S.; Nemsadze, G.; Ordentlich, P.; Quaranto, C.; Meyers, M.; et al. Results of ENCORE 602 (TRIO025), a phase II, randomized, placebo-controlled, double-blinded, multicenter study of atezolizumab with or without entinostat in patients with advanced triple-negative breast cancer (aTNBC). J. Clin. Oncol. 2020, 38, 1014. [Google Scholar] [CrossRef]
- Voorwerk, L.; Slagter, M.; Horlings, H.M.; Sikorska, K.; van de Vijver, K.K.; de Maaker, M.; Nederlof, I.; Kluin, R.J.C.; Warren, S.; Ong, S.; et al. Immune induction strategies in metastatic triple-negative breast cancer to enhance the sensitivity to PD-1 blockade: The TONIC trial. Nat. Med. 2019, 25, 920–928. [Google Scholar] [CrossRef] [PubMed]
- Bachelot, T.; Filleron, T.; Bieche, I.; Arnedos, M.; Campone, M.; Dalenc, F.; Coussy, F.; Sablin, M.-P.; Debled, M.; Lefeuvre-Plesse, C.; et al. Durvalumab compared to maintenance chemotherapy in metastatic breast cancer: The randomized phase II SAFIR02-BREAST IMMUNO trial. Nat. Med. 2021, 27, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Emens, L.A.; Middleton, G. The interplay of immunotherapy and chemotherapy: Harnessing potential synergies. Cancer Immunol. Res. 2015, 3, 436–443. [Google Scholar] [CrossRef]
- Schmid, P.; Adams, S.; Rugo, H.S.; Schneeweiss, A.; Barrios, C.H.; Iwata, H.; Diéras, V.; Hegg, R.; Im, S.-A.; Shaw Wright, G.; et al. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N. Engl. J. Med. 2018, 379, 2108–2121. [Google Scholar] [CrossRef]
- Emens, L.A.; Adams, S.; Barrios, C.H.; Diéras, V.; Iwata, H.; Loi, S.; Rugo, H.S.; Schneeweiss, A.; Winer, E.P.; Patel, S.; et al. First-line atezolizumab plus nab-paclitaxel for unresectable, locally advanced, or metastatic triple-negative breast cancer: IMpassion130 final overall survival analysis. Ann. Oncol. 2021, 32, 983–993, Erratum in Ann. Oncol. 2021, 32, 1650. [Google Scholar] [CrossRef]
- Schmid, P.; Rugo, H.S.; Adams, S.; Schneeweiss, A.; Barrios, C.H.; Iwata, H.; Diéras, V.; Henschel, V.; Molinero, L.; Chui, S.Y.; et al. Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): Updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2020, 21, 44–59. [Google Scholar] [CrossRef]
- Miles, D.; Gligorov, J.; André, F.; Cameron, D.; Schneeweiss, A.; Barrios, C.; Xu, B.; Wardley, A.; Kaen, D.; Andrade, L.; et al. Primary results from IMpassion131, a double-blind, placebo-controlled, randomised phase III trial of first-line paclitaxel with or without atezolizumab for unresectable locally advanced/metastatic triple-negative breast cancer. Ann. Oncol. 2021, 32, 994–1004. [Google Scholar] [CrossRef]
- Franzoi, M.A.; de Azambuja, E. Atezolizumab in metastatic triple-negative breast cancer: IMpassion130 and 131 trials—How to explain different results? ESMO Open 2020, 5, e001112. [Google Scholar] [CrossRef]
- Cortes, J.; Andre, F.; Goncalves, A.; Kummel, S.; Martin, M.; Schmid, P.; Schuetz, F.; Swain, S.; Easton, V.; Pollex, E.; et al. IMpassion132 Phase III trial: Atezolizumab and chemotherapy in early relapsing metastatic triple-negative breast cancer. Future Oncol. 2019, 15, 1951–1961. [Google Scholar] [CrossRef]
- Cortes, J.; Rugo, H.S.; Cescon, D.W.; Im, S.-A.; Yusof, M.M.; Gallardo, C.; Lipatov, O.; Barrios, C.H.; Perez-Garcia, J.; Iwata, H.; et al. Pembrolizumab plus Chemotherapy in Advanced Triple-Negative Breast Cancer. N. Engl. J. Med. 2022, 387, 217–226. [Google Scholar] [CrossRef]
- Cortes, J.; Cescon, D.W.; Rugo, H.S.; Nowecki, Z.; Im, S.-A.; Yusof, M.M.; Gallardo, C.; Lipatov, O.; Barrios, C.H.; Holgado, E.; et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): A randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet 2020, 396, 1817–1828. [Google Scholar] [CrossRef]
- Cortés, J.; Cescon, D.W.; Rugo, H.S. Final results of KEYNOTE-355: Randomized, double-blind, phase 3 study of pembrolizumab + chemotherapy vs. placebo + chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer. In Proceedings of the 2021 San Antonio Breast Cancer Symposium, San Antonio, TX, USA, 7–10 December. Abstract GS1-02.
- Szekely, B.; Bossuyt, V.; Li, X.; Wali, V.B.; Patwardhan, G.A.; Frederick, C.; Silber, A.; Park, T.; Harigopal, M.; Pelekanou, V.; et al. Immunological differences between primary and metastatic breast cancer. Ann. Oncol. 2018, 29, 2232–2239. [Google Scholar] [CrossRef]
- Cortazar, P.; Zhang, L.; Untch, M.; Mehta, K.; Costantino, J.P.; Wolmark, N.; Bonnefoi, H.; Cameron, D.; Gianni, L.; Valagussa, P.; et al. Pathological complete response and long-term clinical benefit in breast cancer: The CTNeoBC pooled analysis. Lancet 2019, 384, 164–172, Erratum in: Lancet 2019, 393, 986. [Google Scholar] [CrossRef]
- Schmid, P.; Cortes, J.; Pusztai, L.; McArthur, H.; Kümmel, S.; Bergh, J.; Denkert, C.; Park, Y.H.; Hui, R.; Harbeck, N.; et al. Pembrolizumab for Early Triple-Negative Breast Cancer. N. Engl. J. Med. 2020, 382, 810–821. [Google Scholar] [CrossRef]
- Schmid, P.; Cortes, J.; Dent, R.; Pusztai, L.; McArthur, H.; Kümmel, S.; Bergh, J.; Denkert, C.; Park, Y.H.; Hui, R.; et al. VP7-2021: KEYNOTE-522: Phase III study of neoadjuvant pembrolizumab + chemotherapy vs. placebo + chemotherapy, followed by adjuvant pembrolizumab vs. placebo for early-stage TNBC. Ann. Oncol. 2021, 32, 1198–1200. [Google Scholar] [CrossRef]
- Schmid, P.; Cortes, J.; Dent, R.; Pusztai, L.; McArthur, H.; Kümmel, S.; Bergh, J.; Denkert, C.; Park, Y.H.; Hui, R.; et al. Event-free Survival with Pembrolizumab in Early Triple-Negative Breast Cancer. N. Engl. J. Med. 2022, 386, 556–567. [Google Scholar] [CrossRef]
- FDA USF& DA. FDA Approves Pembrolizumab for High-Risk Early-Stage Triple-Negative Breast Cancer. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-pembrolizumab-high-risk-early-stage-triple-negative-breast-cancer (accessed on 21 May 2023).
- Mittendorf, E.A.; Zhang, H.; Barrios, C.H.; Saji, S.; Jung, K.H.; Hegg, R.; Koehler, A.; Sohn, J.; Iwata, H.; Telli, M.L.; et al. Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031): A randomised, double-blind, phase 3 trial. Lancet 2020, 396, 1090–1100. [Google Scholar] [CrossRef]
- Gianni, L.; Huang, C.S.; Egle, D.; Bermejo, B.; Zamagni, C.; Thill, M.; Anton, A.; Zambelli, S.; Bianchini, G.; Russo, S.; et al. Pathologic complete response (pCR) to neoadjuvant treatment with or without atezolizumab in triple-negative, early high-risk and locally advanced breast cancer: NeoTRIP Michelangelo randomized study. Ann. Oncol. 2022, 33, 534–543. [Google Scholar] [CrossRef] [PubMed]
- Bianchini, G.; Huang, C.-S.; Egle, D.; Bermejo, B.; Zamagni, C.; Thill, M.; Anton, A.; Zambelli, S.; Russo, S.; Ciruelos, E.M.; et al. LBA13 Tumour infiltrating lymphocytes (TILs), PD-L1 expression and their dynamics in the NeoTRIPaPDL1 trial. Ann. Oncol. 2020, 31, S1145–S1146. [Google Scholar] [CrossRef]
- Loibl, S.; Untch, M.; Burchardi, N.; Huober, J.; Sinn, B.V.; Blohmer, J.-U.; Grischke, E.-M.; Furlanetto, J.; Tesch, H.; Hanusch, C.; et al. A randomised phase II study investigating durvalumab in addition to an anthracycline taxane-based neoadjuvant therapy in early triple-negative breast cancer: Clinical results and biomarker analysis of GeparNuevo study. Ann. Oncol. 2019, 30, 1279–1288, Erratum in: Ann Oncol. 2022, 33, 743–744. [Google Scholar] [CrossRef] [PubMed]
- Nanda, R.; Liu, M.C.; Yau, C.; Shatsky, R.; Pusztai, L.; Wallace, A.; Chien, A.J.; Forero-Torres, A.; Ellis, E.; Han, H.; et al. Effect of Pembrolizumab Plus Neoadjuvant Chemotherapy on Pathologic Complete Response in Women with Early-Stage Breast Cancer: An Analysis of the Ongoing Phase 2 Adaptively Randomized I-SPY2 Trial. JAMA Oncol. 2020, 6, 676–684. [Google Scholar] [CrossRef]
- Saji, S.; McArthur, H.L.; Ignatiadis, M.; Bailey, A.; El-Abed, S.; Brandao, M.; Metzger, O.; Lai, C.; Guillaume, S.; Fumagalli, D.; et al. ALEXANDRA/IMpassion030: A phase 3 study of standard adjuvant chemotherapy with or without atezolizumab in patients with early-stage triple-negative breast cancer. J. Clin. Oncol. 2021, 39 (Suppl. S15), TPS597. [Google Scholar] [CrossRef]
- Barroso-Sousa, R.; Li, T.; Reddy, S.; Emens, L.A.; Overmoyer, B.; Lange, P.; Dilullo, M.K.; Attaya, V.; Kimmel, J.; Winer, E.P.; et al. Abstract GS2-10 Nimbus: A phase 2 trial of nivolumab plus ipilimumab for patients with hypermutated her2-negative metastatic breast cancer (MBC). Cancer Res. 2022, 82 (Suppl. S4), GS2-10. [Google Scholar] [CrossRef]
- Adams, S.; Othus, M.; Patel, S.P.; Miller, K.D.; Chugh, R.; Schuetze, S.M.; Chamberlin, M.D.; Haley, B.J.; Storniolo, A.M.V.; Reddy, M.P.; et al. A Multicenter Phase II Trial of Ipilimumab and Nivolumab in Unresectable or Metastatic Metaplastic Breast Cancer: Cohort 36 of Dual Anti-CTLA-4 and Anti-PD-1 Blockade in Rare Tumors (DART, SWOG S1609). Clin. Cancer Res. 2022, 28, 271–278. [Google Scholar] [CrossRef]
- Kok, M.; Nederlof, I.; Isaeva, O.I. LBA13 Nivolumab and ipilimumab in early-stage triple negative breast cancer (TNBC) with tumor-infiltrating lymphocytes (TILs): First results from the BELLINI trial. Ann. Oncol. 2022, 33 (Suppl. 7), S1382. [Google Scholar] [CrossRef]
- De La Motte Rouge, T.; Frenel, J.-S.; Borcoman, E.; Isambert, N.; Emile, G.; Augereau, P.; Cropet, C.; Legrand, F.; Gonçalves, A. 274P metronomic oral vinorelbine (MOV) combined with tremelimumab (T) + durvalumab (D): Efficacy and safety results of the advanced breast cancer (ABC) patients (pts) cohort of the MOVIE study. Ann. Oncol. 2020, 31 (Suppl. S4), S348–S395. [Google Scholar] [CrossRef]
- Tutt, A.N.J.; Garber, J.E.; Kaufman, B.; Viale, G.; Fumagalli, D.; Rastogi, P.; Gelber, R.D.; de Azambuja, E.; Fielding, A.; Balmaña, J.; et al. Adjuvant Olaparib for Patients with BRCA1- or BRCA2-Mutated Breast Cancer. N. Engl. J. Med. 2021, 384, 2394–2405. [Google Scholar] [CrossRef]
- Robson, M.E.; Tung, N.; Conte, P.; Im, S.-A.; Senkus, E.; Xu, B.; Masuda, N.; Delaloge, S.; Li, W.; Armstrong, A.; et al. OlympiAD final overall survival and tolerability results: Olaparib versus chemotherapy treatment of physician’s choice in patients with a germline BRCA mutation and HER2-negative metastatic breast cancer. Ann. Oncol. 2019, 30, 558–566. [Google Scholar] [CrossRef]
- Litton, J.K.; Hurvitz, S.A.; Mina, L.A.; Rugo, H.S.; Lee, K.-H.; Gonçalves, A.; Diab, S.; Woodward, N.; Goodwin, A.; Yerushalmi, R.; et al. Talazoparib versus chemotherapy in patients with germline BRCA1/2-mutated HER2-negative advanced breast cancer: Final overall survival results from the EMBRACA trial. Ann. Oncol. 2020, 31, 1526–1535. [Google Scholar] [CrossRef]
- Domchek, S.M.; Postel-Vinay, S.; Im, S.-A.; Park, Y.H.; Delord, J.-P.; Italiano, A.; Alexandre, J.; You, B.; Bastian, S.; Krebs, M.G.; et al. Olaparib and durvalumab in patients with germline BRCA-mutated metastatic breast cancer (MEDIOLA): An open-label, multicentre, phase 1/2, basket study. Lancet Oncol. 2020, 21, 1155–1164. [Google Scholar] [CrossRef]
- Vinayak, S.; Tolaney, S.M.; Schwartzberg, L.; Mita, M.; McCann, G.; Tan, A.R.; Wahner-Hendrickson, A.E.; Forero, A.; Anders, C.; Wulf, G.M.; et al. Open-label Clinical Trial of Niraparib Combined with Pembrolizumab for Treatment of Advanced or Metastatic Triple-Negative Breast Cancer. JAMA Oncol. 2019, 5, 1132–1140. [Google Scholar] [CrossRef]
- Sammons, S.L.; Tan, T.J.; Im, Y.H.; She, L.; Mundy, K.; Bigelow, R.; Traina, T.A.; Anders, C.; Renzulli, E.; Kim, S.-B.; et al. Dora: A Phase II, Multicenter, International Study of Olaparib with or without Durvalumab as a Chemotherapy-Free Maintenance Strategy in Platinum-Pretreated Advanced Triple-Negative Breast Cancer (TNBC). In Proceedings of the San Antonio Breast Cancer Symposium 2022, San Antonio, TX, USA, 6–10 December 2022. [Google Scholar]
- de Aguiar, R.B.; de Moraes, J.Z. Exploring the Immunological Mechanisms Underlying the Anti-vascular Endothelial Growth Factor Activity in Tumors. Front. Immunol. 2019, 10, 1023. [Google Scholar] [CrossRef]
- Chen, L.; Jiang, Y.-Z.; Wu, S.; Wu, J.; Di, G.; Liu, G.; Yu, K.; Fan, L.; Li, J.; Hou, Y.; et al. Famitinib with Camrelizumab and Nab-Paclitaxel for Advanced Immunomodulatory Triple-Negative Breast Cancer (FUTURE-C-Plus): An Open-Label, Single-Arm, Phase II Trial. Cancer Res. 2022, 28, 2807–2817. [Google Scholar] [CrossRef]
- Ozaki, Y.; Tsurutani, J.; Mukohara, T.; Iwasa, T.; Takahashi, M.; Tanabe, Y.; Kawabata, H.; Masuda, N.; Futamura, M.; Minami, H.; et al. Safety and efficacy of nivolumab plus bevacizumab, paclitaxel for HER2-negative metastatic breast cancer: Primary results and biomarker data from a phase 2 trial (WJOG9917B). Eur. J. Cancer 2022, 171, 193–202. [Google Scholar] [CrossRef]
- Goldenberg, D.M.; Cardillo, T.M.; Govindan, S.V.; Rossi, E.A.; Sharkey, R.M. Trop-2 is a novel target for solid cancer therapy with sacituzumab govitecan (IMMU-132), an antibody-drug conjugate (ADC). Oncotarget 2015, 6, 22496–22512, Erratum in: Oncotarget 2020, 11, 942. [Google Scholar] [CrossRef]
- Bardia, A.; Tolaney, S.M.; Punie, K.; Loirat, D.; Oliveira, M.; Kalinsky, K.; Zelnak, A.; Aftimos, P.; Dalenc, F.; Sardesai, S.; et al. Biomarker analyses in the phase III ASCENT study of sacituzumab govitecan versus chemotherapy in patients with metastatic triple-negative breast cancer. Ann. Oncol. 2021, 32, 1148–1156. [Google Scholar] [CrossRef]
- Modi, S.; Jacot, W.; Yamashita, T.; Sohn, J.; Vidal, M.; Tokunaga, E.; Tsurutani, J.; Ueno, N.T.; Prat, A.; Chae, Y.S.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Low Advanced Breast Cancer. N. Engl. J. Med. 2022, 387, 9–20. [Google Scholar] [CrossRef]
- Saini, K.S.; Punie, K.; Twelves, C.; Bortini, S.; de Azambuja, E.; Anderson, S.; Criscitiello, C.; Awada, A.; Loi, S. Antibody-drug conjugates, immune-checkpoint inhibitors, and their combination in breast cancer therapeutics. Expert Opin. Biol. Ther. 2021, 21, 945–962. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Humeau, J.; Buqué, A.; Zitvogel, L.; Kroemer, G. Immunostimulation with chemotherapy in the era of immune checkpoint inhibitors. Nat. Rev. Clin. Oncol. 2020, 17, 725–741. [Google Scholar] [CrossRef] [PubMed]
- Molinelli, C.; Jacobs, F.; Marchiò, C.; Pitto, F.; Cosso, M.; Spinaci, S.; de Azambuja, E.; Schettini, F.; Agostinetto, E.; Lambertini, M. HER2-Low Breast Cancer: Where Are We? Breast Care 2022, 17, 533–545. [Google Scholar] [CrossRef] [PubMed]
- Schmid, P.; Wysocki, P.; Ma, C.; Park, Y.H.; Fernandes, R.; Lord, S.; Baird, R.D.; Prady, C.; Jung, K.H.; Asselah, J.; et al. Abstract PD11-09: PD11-09 Datopotamab deruxtecan (Dato-DXd) + durvalumab (D) as first-line (1L) treatment for unresectable locally advanced/metastatic triple-negative breast cancer (a/mTNBC): Updated results from BEGONIA, a phase 1b/2 study. Cancer Res. 2023, 83 (Suppl. S5), PD11-09. [Google Scholar] [CrossRef]
- Schmid, P.; Wysocki, P.; Park, Y.H.; Jassem, J.; Jung, K.H.; Lord, S.; Huisden, R.; Stewart, R.; Vuković, P.; Nunes, A.T.; et al. Abstract PD11-08: PD11-08 Trastuzumab deruxtecan (T-DXd) + durvalumab (D) as first-line (1L) treatment for unresectable locally advanced/metastatic hormone receptor-negative (HR−), HER2-low breast cancer: Updated results from BEGONIA, a phase 1b/2 study. Cancer Res. 2023, 83 (Suppl. S5), PD11-08. [Google Scholar] [CrossRef]
- Chan, A.S.H.; Jonas, A.B.; Qiu, X.; Ottoson, N.R.; Walsh, R.M.; Gorden, K.B.; Harrison, B.; Maimonis, P.J.; Leonardo, S.M.; Ertelt, K.E.; et al. Imprime PGG-Mediated Anti-Cancer Immune Activation Requires Immune Complex Formation. PLoS ONE 2016, 11, e0165909. [Google Scholar] [CrossRef]
- Buisseret, L.; Loirat, D.; Aftimos, P.G.; Punie, K.; Maurer, C.; Debien, V.; Goncalves, A.; Ghiringhelli, F.; Taylor, D.; Clatot, F.; et al. LBA17 Primary endpoint results of SYNERGY, a randomized phase II trial, first-line chemo-immunotherapy trial of durvalumab, paclitaxel, and carboplatin with or without the anti-CD73 antibody oleclumab in patients with advanced or metastatic triple-negative breast cancer (TNBC). Ann. Oncol. 2022, 33 (Suppl. S7), S1385. [Google Scholar] [CrossRef]
- Hosseini, M.; Seyedpour, S.; Khodaei, B.; Loghman, A.H.; Seyedpour, N.; Yazdi, M.H.; Rezaei, N. Cancer Vaccines for Triple-Negative Breast Cancer: A Systematic Review. Vaccines 2023, 11, 146. [Google Scholar] [CrossRef]
- Rozenblit, M.; Huang, R.; Danziger, N.; Hegde, P.; Alexander, B.; Ramkissoon, S.; Blenman, K.; Ross, J.S.; Rimm, D.L.; Pusztai, L. Comparison of PD-L1 protein expression between primary tumors and metastatic lesions in triple negative breast cancers. J. Immunother. Cancer 2020, 8, e001558. [Google Scholar] [CrossRef]
- Rugo, H.S.; Loi, S.; Adams, S.; Schmid, P.; Schneeweiss, A.; Barrios, C.H.; Iwata, H.; Dieras, V.C.; Winer, E.P.; Kockx, M.; et al. Performance of PD-L1 immunohistochemistry (IHC) assays in unresectable locally advanced or metastatic triple-negative breast cancer (mTNBC): Post-hoc analysis of IMpassion130. Ann. Oncol. 2019, 30, v858–v859. [Google Scholar] [CrossRef]
- Zambelli, A.; Sgarra, R.; De Sanctis, R.; Agostinetto, E.; Santoro, A.; Manfioletti, G. Heterogeneity of triple-negative breast cancer: Understanding the Daedalian labyrinth and how it could reveal new drug targets. Expert Opin. Ther. Targets 2022, 26, 557–573. [Google Scholar] [CrossRef] [PubMed]
- Loi, S.; Salgado, R.; Adams, S.; Pruneri, G.; Francis, P.A.; Lacroix-Triki, M.; Joensuu, H.; Dieci, M.V.; Badve, S.; Demaria, S.; et al. Tumor infiltrating lymphocyte stratification of prognostic staging of early-stage triple negative breast cancer. NPJ Breast Cancer 2022, 8, 3. [Google Scholar] [CrossRef] [PubMed]
- Emens, L.A.; Molinero, L.; Loi, S.; Rugo, H.S.; Schneeweiss, A.; Diéras, V.; Iwata, H.; Barrios, C.H.; Nechaeva, M.; Nguyen-Duc, A.; et al. Atezolizumab and nab-Paclitaxel in Advanced Triple-Negative Breast Cancer: Biomarker Evaluation of the IMpassion130 Study. J. Natl. Cancer Inst. 2021, 113, 1005–1016. [Google Scholar] [CrossRef] [PubMed]
- Loi, S.; Drubay, D.; Adams, S.; Pruneri, G.; Francis, P.A.; Lacroix-Triki, M.; Joensuu, H.; Dieci, M.V.; Badve, S.; Demaria, S.; et al. Tumor-Infiltrating Lymphocytes and Prognosis: A Pooled Individual Patient Analysis of Early-Stage Triple-Negative Breast Cancers. J. Clin. Oncol. 2019, 37, 559–569. [Google Scholar] [CrossRef] [PubMed]
- Barroso-Sousa, R.; Jain, E.; Cohen, O.; Kim, D.; Buendia-Buendia, J.; Winer, E.; Lin, N.; Tolaney, S.M.; Wagle, N. Prevalence and mutational determinants of high tumor mutation burden in breast cancer. Ann. Oncol. 2020, 31, 387–394. [Google Scholar] [CrossRef]
- Iwase, T.; Blenman, K.R.M.; Li, X.; Reisenbichler, E.; Seitz, R.; Hout, D.; Nielsen, T.J.; Schweitzer, B.L.; Bailey, D.B.; Shen, Y.; et al. A Novel Immunomodulatory 27-Gene Signature to Predict Response to Neoadjuvant Immunochemotherapy for Primary Triple-Negative Breast Cancer. Cancers 2021, 13, 4839. [Google Scholar] [CrossRef]
- Yee, D.; Shatsky, R.; Yau, C.; Wolf, D.; Nanda, R.; Van ’t Veer, L.; Berry, D.A.; DeMichele, A.; Esserman, L.; I-SPY2 Consortium. Improved pathologic complete response rates for triple-negative breast cancer in the ISPY2 Trial. 2022 ASCO Annual Meeting. J. Clin. Oncol. 2022, 40, 591. [Google Scholar] [CrossRef]
- Mittempergher, L.; Kuilman, M.M.; Barcaru, A.; Nota, B.; Delahaye, L.J.M.J.; Audeh, M.W.; Wolf, D.M.; Yau, C.; Brown Swigart, L.; Hirst, G.L.; et al. The ImPrint immune signature to identify patients with high-risk early breast cancer who may benefit from PD1 checkpoint inhibition in I-SPY2. J. Clin. Oncol. 2022, 40, 514. [Google Scholar] [CrossRef]
- Masuda, N.; Lee, S.-J.; Ohtani, S.; Im, Y.-H.; Lee, E.-S.; Yokota, I.; Kuroi, K.; Im, S.-A.; Park, B.-W.; Kim, S.-B.; et al. Adjuvant Capecitabine for Breast Cancer after Preoperative Chemotherapy. N. Engl. J. Med. 2017, 376, 2147–2159. [Google Scholar] [CrossRef]
- Agostinetto, E.; Gligorov, J.; Piccart, M. Systemic therapy for early-stage breast cancer: Learning from the past to build the future. Nat. Rev. Clin. Oncol. 2022, 19, 763–774. [Google Scholar] [CrossRef]
- Berruti, A.; Amoroso, V.; Gallo, F.; Bertaglia, V.; Simoncini, E.; Pedersini, R.; Ferrari, L.; Bottini, A.; Bruzzi, P.; Sormani, M.P. Pathologic complete response as a potential surrogate for the clinical outcome in patients with breast cancer after neoadjuvant therapy: A meta-regression of 29 randomized prospective studies. J. Clin. Oncol. 2014, 32, 3883–3891. [Google Scholar] [CrossRef]
- Gumusay, O.; Callan, J.; Rugo, H.S. Immunotherapy toxicity: Identification and management. Breast Cancer Res. Treat. 2022, 192, 1–17. [Google Scholar] [CrossRef]
- Duma, N.; Lambertini, M. It Is Time to Talk About Fertility and Immunotherapy. Oncologist 2020, 25, 277–278. [Google Scholar] [CrossRef]
- Dent, R.A.; Cortés, J.; Pusztai, L. 135MO HRQoL with neoadjuvant pembrolizumab + chemotherapy vs placebo + chemotherapy, followed by adjuvant pembrolizumab vs placebo for early-stage TNBC: Results from KEYNOTE-522. Ann. Oncol. 2022, 33 (Suppl. S7), S55–S84. [Google Scholar] [CrossRef]
- Agostinetto, E.; Eiger, D.; Lambertini, M.; Ceppi, M.; Bruzzone, M.; Pondé, N.; Plummer, C.; Awada, A.H.; Santoro, A.; Piccart-Gebhart, M.; et al. Cardiotoxicity of immune checkpoint inhibitors: A systematic review and meta-analysis of randomised clinical trials. Eur. J. Cancer 2021, 148, 76–91. [Google Scholar] [CrossRef]

| Combinations of ICIs with | Study Name | Phase | Status | Population | Treatment | Findings |
|---|---|---|---|---|---|---|
| Dual immunotherapy | MOVIE (NCT03518606) | Ib/II | Active, not rectuiting | Pretreated MBC | Durvalumab + Tremelimumab + metronomic vinorelbine | ORR 20.7% |
| NIMBUS (NCT03789110) | II | Active, not rectuiting | Hypermutated HER2 negative mBC | Nivolumab + Ipilimumab | ORR (TMB ≥ 9 mut/Mb): 16.7% ORR (TMB ≥ 14 mut/Mb): 60% | |
| DART (NCT02834013) | II | Active, not rectuiting | Advanced MpBC | Nivolumab + Ipilimumab | ORR 18% | |
| BELLINI (NCT03815890) | II | Recruiting | Stage I-III TNBC | 4 weeks of neoadjuvant Nivolumab +/− Ipilimumab low dose | PR: 23% Immune activation: 58% | |
| PARPi | MEDIOLA (NCT02734004) | Ib/II | Active, not rectuiting | BRCA mutated HER2-negative MBC | Durvalumab + Olaparib | DCR at week 12: 80% DCR at week 28: 50% ORR: 63.3% |
| Topacio/KEYNOTE-162 (NCT02657889) | I/II | Completed | Pretreated mTNBC | Pembrolizumab + Niraparib | ORR (ITT): 21% ORR (PD-L1 positive): 32% ORR (PD-L1 negative): 8% ORR (gBRCAmut): 47% | |
| DORA (NCT03167619) | II | Completed | Platinum-treated mTNBC | Durvalumab + Olaparib vs. Olaparib | mPFS: 6.1 vs. 4.0 mo mPFS (gBRCA mut vs. wt): 8.2 vs. 2.9 mo | |
| KEYLINK (NCT04191135) | II | Active, not rectuiting | mTNBC after induction with first-line CT + Pembrolizumab | Pembrolizumab + Olaparib vs. Pembrolizumab + CT | Not reported | |
| NCT03594396 | II | Active, not rectuiting | Preoperative treatment before NACT for StageII/III early TNBC | Durvalumab + Olaparib | pCR: 75% pCR (gBRCA): 84.6% | |
| Anti-angiogenic drugs | FUTURE-C-PLUS (NCT04129996) | II | Active, not rectuiting | First-line treatment in mTNBC | Camrelizumab + nab-paclitaxel and famitinib | ORR: 81.3% mPFS: 13.6 mo mDOR: 14.9 mo |
| NEWBEAT (WJOG9917B) | II | Active, not rectuiting | First-line treatment in HER2 negative mBC | Nivolumab + Bevacizumab and paclitaxel | ORR: 70% DCR: 98% mPFS: 14 mo mOS: 32.2 | |
| ADCs | ASCENT-04(NCT05382286) | III | Recruiting | Previously untreated PD-L1-positive mTNBC | Pembrolizumab + SG vs. pembrolizumab + CT | Not reported |
| ASPRIA (NCT04434040) | II | Recruiting | Early-stage TNBC with residual disease after NACT | Atezolizumab + SG | Not reported | |
| NCT04468061 | II | Recruiting | PD-L1-negative mTNBC | Pembrolizumab + SG vs. SG | Not reported | |
| BEGONIA (NCT03742102) | Ib/II | Active, not rectuiting | First-line treatment in mTNBC | Durvalumab + Dato-DXd | ORR: 74% | |
| BEGONIA (NCT03742102) | Ib/II | Active, not rectuiting | First-line treatment in mTNBC | Durvalumab + T-DXd | ORR: 57% | |
| Other approaches | IMPRIME-1(NCT02981303) | II | Completed | Pretreated mTNBC | Pembrolizumab + Imprime-PGG | ORR: 15.9% DCR: 54.5% mOS: 16.4 vs. 9 mo |
| SYNERGY | Ib/II | Active, not rectuiting | First-line treatment for mTNBC | Durvalumab + Oleclumab + CT vs. Durvalumab + CT | CBR: 43 vs. 44% mPFS: 6 vs. 7.7 mo |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jacobs, F.; Agostinetto, E.; Miggiano, C.; De Sanctis, R.; Zambelli, A.; Santoro, A. Hope and Hype around Immunotherapy in Triple-Negative Breast Cancer. Cancers 2023, 15, 2933. https://doi.org/10.3390/cancers15112933
Jacobs F, Agostinetto E, Miggiano C, De Sanctis R, Zambelli A, Santoro A. Hope and Hype around Immunotherapy in Triple-Negative Breast Cancer. Cancers. 2023; 15(11):2933. https://doi.org/10.3390/cancers15112933
Chicago/Turabian StyleJacobs, Flavia, Elisa Agostinetto, Chiara Miggiano, Rita De Sanctis, Alberto Zambelli, and Armando Santoro. 2023. "Hope and Hype around Immunotherapy in Triple-Negative Breast Cancer" Cancers 15, no. 11: 2933. https://doi.org/10.3390/cancers15112933
APA StyleJacobs, F., Agostinetto, E., Miggiano, C., De Sanctis, R., Zambelli, A., & Santoro, A. (2023). Hope and Hype around Immunotherapy in Triple-Negative Breast Cancer. Cancers, 15(11), 2933. https://doi.org/10.3390/cancers15112933








