Radiotherapy in Combination with Systemic Therapy for Multiple Myeloma—A Critical Toxicity Evaluation in the Modern Treatment Era
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinical Data
2.2. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alaggio, R.; Amador, C.; Anagnostopoulos, I.; Attygalle, A.D.; Araujo, I.B.d.O.; Berti, E.; Bhagat, G.; Borges, A.M.; Boyer, D.; Calaminici, M.; et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Lymphoid Neoplasms. Leukemia 2022, 36, 1720–1748. [Google Scholar] [CrossRef] [PubMed]
- Cowan, A.J.; Allen, C.; Barac, A.; Basaleem, H.; Bensenor, I.; Curado, M.P.; Foreman, K.; Gupta, R.; Harvey, J.; Hosgood, H.D.; et al. Global Burden of Multiple Myeloma: A Systematic Analysis for the Global Burden of Disease Study 2016. JAMA Oncol. 2018, 4, 1221–1227. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Dong, X.; Li, H.; Cao, M.; Sun, D.; He, S.; Yang, F.; Yan, X.; Zhang, S.; Li, N.; et al. Cancer statistics in China and United States, 2022: Profiles, trends, and determinants. Chin. Med. J. 2022, 135, 584–590. [Google Scholar] [CrossRef] [PubMed]
- Erdmann, F.; Spix, C.; Katalinic, A.; Christ, M.; Folkerts, J.; Hansmann, J.; Kranzhöfer, K.; Kunz, B.; Manegold, K.; Penzkofer, A.; et al. Krebs in Deutschland für 2017/2018. 2021. Available online: https://www.krebsdaten.de/Krebs/DE/Content/Publikationen/Krebs_in_Deutschland/kid_2021/krebs_in_deutschland_2021.pdf? (accessed on 23 April 2023).
- Kyle, R.A.; Gertz, M.A.; Witzig, T.E.; Lust, J.A.; Lacy, M.Q.; Dispenzieri, A.; Fonseca, R.; Rajkumar, S.V.; Offord, J.R.; Larson, D.R.; et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin. Proc. 2003, 78, 21–33. [Google Scholar] [CrossRef]
- Balducci, M.; Chiesa, S.; Manfrida, S.; Rossi, E.; Za, T.; Frascino, V.; de Bari, B.; Hohaus, S.; Cellini, F.; Mantini, G.; et al. Impact of radiotherapy on pain relief and recalcification in plasma cell neoplasms: Longterm experience. Strah-Lentherapie Onkol. 2011, 187, 114–119. [Google Scholar] [CrossRef]
- Lecouvet, F.; Richard, F.; Vande Berg, B.; Malghem, J.; Maldague, B.; Jamart, J.; Ferrant, A.; Michaux, J.L. Longterm effects of localized spinal radiation therapy on vertebral fractures and focal lesions appearance in patients with multiple myeloma. Br. J. Haematol. 1997, 96, 743–745. [Google Scholar] [CrossRef]
- Oertel, M.; Elsayad, K.; Kroeger, K.J.; Haverkamp, U.; Rudack, C.; Lenz, G.; Eich, H.T. Impact of radiation dose on local control and survival in extramedullary head and neck plasmacytoma. Radiat. Oncol. 2019, 14, 63. [Google Scholar] [CrossRef]
- Elsayad, K.; Oertel, M.; König, L.; Hüske, S.; Le Ray, E.; Meheissen, M.A.M.; Elsaid, A.A.; Elfaham, E.; Debus, J.; Kirova, Y.; et al. Maximizing the Clinical Benefit of Radiotherapy in Solitary Plasmacytoma: An International Multi-center Analysis. Cancers 2020, 12, 676. [Google Scholar] [CrossRef]
- Hosny, M.; Verkleij, C.P.M.; van der Schans, J.; Frerichs, K.A.; Mutis, T.; Zweegman, S.; van de Donk, N.W.C.J. Current State of the Art and Prospects of T Cell-Redirecting Bispecific Antibodies in Multiple Myeloma. J. Clin. Med. 2021, 10, 4593. [Google Scholar] [CrossRef]
- Tan, D.; Lee, J.H.; Chen, W.; Shimizu, K.; Hou, J.; Suzuki, K.; Nawarawong, W.; Huang, S.-Y.; Sang Chim, C.; Kim, K.; et al. Recent advances in the management of multiple myeloma: Clinical impact based on resource-stratification. Consensus statement of the Asian Myeloma Network at the 16th international myeloma workshop. Leuk. Lymphoma 2018, 59, 2305–2317. [Google Scholar] [CrossRef]
- Berges, O.; Decaudin, D.; Servois, V.; Kirova, Y.M. Concurrent radiation therapy and bortezomib in myeloma patient. Radiother. Oncol. 2008, 86, 290–292. [Google Scholar] [CrossRef]
- Mohiuddin, M.M.; Harmon, D.C.; Delaney, T.F. Severe acute enteritis in a multiple myeloma patient receiving bortezomib and spinal radiotherapy: Case report. J. Chemother. 2005, 17, 343–346. [Google Scholar] [CrossRef] [PubMed]
- Sauer, S.; Erdmann, K.; Jensen, A.D.; Wennmann, M.; Pavel, P.; Jordan, K.; Schmitt, A.; Kriegsmann, M.; Wuchter, P.; Goldschmidt, H.; et al. Local Radiation Therapy Before and During Induction Delays Stem Cell Mobilization and Collection in Multiple Myeloma Patients. Transplant. Cell. Ther. 2021, 27, 876.e1–876.e11. [Google Scholar] [CrossRef] [PubMed]
- Oertel, M.; Elsayad, K.; Engenhart-Cabillic, R.; Reinartz, G.; Baues, C.; Schmidberger, H.; Vordermark, D.; Marnitz, S.; Lukas, P.; Ruebe, C.; et al. Radiation treatment of hemato-oncological patients in times of the COVID-19 pandemic: Expert recommendations from the radiation oncology panels of the German Hodgkin Study Group and the German Lymphoma Alliance. Strahlenther. Onkol. 2020, 196, 1096–1102. [Google Scholar] [CrossRef] [PubMed]
- Yahalom, J.; Dabaja, B.S.; Ricardi, U.; Ng, A.; Mikhaeel, N.G.; Vogelius, I.R.; Illidge, T.; Qi, S.; Wirth, A.; Specht, L. ILROG emergency guidelines for radiation therapy of hematological malignancies during the COVID-19 pandemic. Blood 2020, 135, 1829–1832. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.M.; Chouake, R.J.; Sanfilippo, N.J.; Rapp, T.B.; Cook, P.; Formenti, S.C.; Mazumder, A.; Silverman, J.S. Feasibility and efficacy of local radiotherapy with concurrent novel agents in patients with multiple myeloma. Clin. Lymphoma Myeloma Leuk. 2014, 14, 480–484. [Google Scholar] [CrossRef]
- Resende Salgado, L.; Wang, S.; Adler, A.; Chang, S.; Ru, M.; Moshier, E.; Dharmarajan, K.; Jay Cho, H.; Bakst, R. The Safety Profile of Concurrent Therapy for Multiple Myeloma in the Modern Era. Adv. Radiat. Oncol. 2019, 4, 112–117. [Google Scholar] [CrossRef]
- Guerini, A.E.; Tucci, A.; Alongi, F.; Mataj, E.; Belotti, A.; Borghetti, P.; Triggiani, L.; Pegurri, L.; Pedretti, S.; Bonù, M.; et al. RR Myelo POINT: A Retrospective Single-Center Study Assessing the Role of Radiotherapy in the Management of Multiple Myeloma and Possible Interactions with Concurrent Systemic Treatment. Cancers 2022, 14, 2273. [Google Scholar] [CrossRef]
- Nehlsen, A.D.; Sindhu, K.K.; Moshier, E.; Richter, J.; Richard, S.; Chari, A.; Sanchez, L.; Parekh, S.; Cho, H.J.; Jagannath, S.; et al. The Safety and Efficacy of Radiation Therapy with Concurrent Dexamethasone, Cyclophosphamide, Etoposide, and Cisplatin-Based Systemic Therapy for Multiple Myeloma. Clin. Lymphoma Myeloma Leuk. 2022, 22, 192–197. [Google Scholar] [CrossRef]
- National Institutes of Health National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE), Version 5.0. 2017. Available online: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf (accessed on 23 April 2023).
- Palumbo, A.; Avet-Loiseau, H.; Oliva, S.; Lokhorst, H.M.; Goldschmidt, H.; Rosinol, L.; Richardson, P.; Caltagirone, S.; Lahuerta, J.J.; Facon, T.; et al. Revised International Staging System for Multiple Myeloma: A Report From International Myeloma Working Group. J. Clin. Oncol. 2015, 33, 2863–2869. [Google Scholar] [CrossRef]
- Oken, M.M.; Creech, R.H.; Tormey, D.C.; Horton, J.; Davis, T.E.; McFadden, E.T.; Carbone, P.P. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am. J. Clin. Oncol. 1982, 5, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Attal, M.; Lauwers-Cances, V.; Hulin, C.; LeLeu, X.; Caillot, D.; Escoffre, M.; Arnulf, B.; Macro, M.; Belhadj, K.; Garderet, L.; et al. Lenalidomide, Bortezomib, and Dexamethasone with Transplantation for Myeloma. N. Engl. J. Med. 2017, 376, 1311–1320. [Google Scholar] [CrossRef] [PubMed]
- Benboubker, L.; Dimopoulos, M.A.; Dispenzieri, A.; Catalano, J.; Belch, A.R.; Cavo, M.; Pinto, A.; Weisel, K.; Ludwig, H.; Bahlis, N.; et al. Lenalidomide and dexamethasone in transplant-ineligible patients with myeloma. N. Engl. J. Med. 2014, 371, 906–917. [Google Scholar] [CrossRef] [PubMed]
- Jackson, G.H.; Davies, F.E.; Pawlyn, C.; Cairns, D.A.; Striha, A.; Collett, C.; Hockaday, A.; Jones, J.R.; Kishore, B.; Garg, M.; et al. Lenalidomide maintenance versus observation for patients with newly diagnosed multiple myeloma (Myeloma XI): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2019, 20, 57–73. [Google Scholar] [CrossRef]
- Durie, B.G.M.; Hoering, A.; Abidi, M.H.; Rajkumar, S.V.; Epstein, J.; Kahanic, S.P.; Thakuri, M.; Reu, F.; Reynolds, C.M.; Sexton, R.; et al. Bortezomib with lenalidomide and dexamethasone versus lenalidomide and dexamethasone alone in patients with newly diagnosed myeloma without intent for immediate autologous stem-cell transplant (SWOG S0777): A randomised, openlabel, phase 3 trial. Lancet 2017, 389, 519–527. [Google Scholar] [CrossRef]
- Facon, T.; Kumar, S.; Plesner, T.; Orlowski, R.Z.; Moreau, P.; Bahlis, N.; Basu, S.; Nahi, H.; Hulin, C.; Quach, H.; et al. Daratumumab plus Lenalidomide and Dexamethasone for Untreated Myeloma. N. Engl. J. Med. 2019, 380, 2104–2115. [Google Scholar] [CrossRef]
- Miguel, J.S.; Weisel, K.; Moreau, P.; Lacy, M.; Song, K.; Delforge, M.; Karlin, L.; Goldschmidt, H.; Banos, A.; Oriol, A.; et al. Pomalidomide plus low-dose dexamethasone versus high-dose dexamethasone alone for patients with re-lapsed and refractory multiple myeloma (MM-003): A randomised, open-label, phase 3 trial. Lancet Oncol. 2013, 14, 1055–1066. [Google Scholar] [CrossRef]
- Mateos, M.-V.; Sonneveld, P.; Hungria, V.; Nooka, A.K.; Estell, J.A.; Barreto, W.; Corradini, P.; Min, C.-K.; Medvedova, E.; Weisel, K.; et al. Daratumumab, Bortezomib, and Dexamethasone Versus Bortezomib and Dexamethasone in Patients with Previously Treated Multiple Myeloma: Three-year Follow-up of CASTOR. Clin. Lymphoma Myeloma Leuk. 2020, 20, 509–518. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Palumbo, A.; Corradini, P.; Cavo, M.; Delforge, M.; Di Raimondo, F.; Weisel, K.C.; Oriol, A.; Hansson, M.; Vacca, A.; et al. Safety and efficacy of pomalidomide plus low-dose dexamethasone in STRATUS (MM-010): A phase 3b study in refractory multiple myeloma. Blood 2016, 128, 497–503. [Google Scholar] [CrossRef]
- Tsang, R.W.; Campbell, B.A.; Goda, J.S.; Kelsey, C.R.; Kirova, Y.M.; Parikh, R.R.; Ng, A.K.; Ricardi, U.; Suh, C.-O.; Mauch, P.M.; et al. Radiation Therapy for Solitary Plasmacytoma and Multiple Myeloma: Guidelines from the International Lymphoma Radiation Oncology Group. Int. J. Radiat. Oncol. Biol. Phys. 2018, 101, 794–808. [Google Scholar] [CrossRef]
- Rudzianskiene, M.; Inciura, A.; Gerbutavicius, R.; Rudzianskas, V.; Macas, A.; Simoliuniene, R.; Dambrauskiene, R.; Kiavialaitis, G.E.; Juozaityte, E. Einzelne Fraktion vs. multiple Fraktionen in der palliativen Strahlentherapie des multiplen Myeloms: Eine prospektive randomisierte Studie. Strahlenther. Onkol. 2017, 193, 742–749. [Google Scholar] [CrossRef] [PubMed]
- Stölting, T.; Knauerhase, H.; Klautke, G.; Kundt, G.; Fietkau, R. Total and single doses influence the effectiveness of radiotherapy in palliative treatment of plasmacytoma. Strahlenther. Onkol. 2008, 184, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Rades, D.; Hoskin, P.J.; Stalpers, L.J.A.; Schulte, R.; Poortmans, P.; Veninga, T.; Dahm-Daphi, J.; Obralic, N.; Wild-fang, I.; Bahrehmand, R.; et al. Short-course radiotherapy is not optimal for spinal cord compression due to mye-loma. Int. J. Radiat. Oncol. Biol. Phys. 2006, 64, 1452–1457. [Google Scholar] [CrossRef] [PubMed]
- Matuschek, C.; Ochtrop, T.A.; Bölke, E.; Ganswindt, U.; Fenk, R.; Gripp, S.; Kröpil, P.; Gerber, P.A.; Kammers, K.; Hamilton, J.; et al. Effects of Radiotherapy in the treatment of multiple myeloma: A retrospective analysis of a Single Institution. Radiat. Oncol. 2015, 10, 71. [Google Scholar] [CrossRef] [PubMed]
- Price, J.G.; Niedzwiecki, D.; Oyekunle, T.; Arcasoy, M.O.; Champ, C.E.; Kelsey, C.R.; Salama, J.K.; Moravan, M.J. Effective Pain Control with Very Low Dose Palliative Radiation Therapy for Patients with Multiple Myeloma with Uncomplicated Osseous Lesions. Adv. Radiat. Oncol. 2021, 6, 100729. [Google Scholar] [CrossRef] [PubMed]
- Ballas, L.K.; Luo, C.; Millstein, J.; Bakst, R.L.; Dandapani, S.V.; Khan, M.K.; Patel, C.G.; Paydar, I.; Plastaras, J.P.; Ng, A.K. Phase II Multi-Institutional Study of Low-Dose (2 Gy x 2) Palliative Radiotherapy for Symptomatic Bone Metastases from Multiple Myeloma. Int. J. Radiat. Oncol.*Biol.*Phys. 2021, 111, e310–e311. [Google Scholar] [CrossRef]
- Mose, S.; Pfitzner, D.; Rahn, A.; Nierhoff, C.; Schiemann, M.; Böttcher, H.D. Wertigkeit der Radiotherapie in der Behandlung des multiplen Myeloms. Strahlenther. Onkol. Organ Der Dtsch. Rontgenges. 2000, 176, 506–512. [Google Scholar] [CrossRef]
- Martin, T.; Usmani, S.Z.; Berdeja, J.G.; Agha, M.; Cohen, A.D.; Hari, P.; Avigan, D.; Deol, A.; Htut, M.; Lesokhin, A.; et al. Ciltacabtagene Autoleucel, an Anti-B-cell Maturation Antigen Chimeric Antigen Receptor T-Cell Therapy, for Relapsed/Refractory Multiple Myeloma: CARTITUDE-1 2-Year Follow-Up. J. Clin. Oncol. 2023, 41, 1265–1274. [Google Scholar] [CrossRef]
- Moreau, P.; Garfall, A.L.; van de Donk, N.W.C.J.; Nahi, H.; San-Miguel, J.F.; Oriol, A.; Nooka, A.K.; Martin, T.; Rosinol, L.; Chari, A.; et al. Teclistamab in Relapsed or Refractory Multiple Myeloma. N. Engl. J. Med. 2022, 387, 495–505. [Google Scholar] [CrossRef]
- Moreau, P.; Facon, T.; Attal, M.; Hulin, C.; Michallet, M.; Maloisel, F.; Sotto, J.-J.; Guilhot, F.; Marit, G.; Doyen, C.; et al. Comparison of 200 mg/m(2) melphalan and 8 Gy total body irradiation plus 140 mg/m(2) melphalan as conditioning regimens for peripheral blood stem cell transplantation in patients with newly diagnosed multiple myeloma: Final analysis of the Intergroupe Francophone du Myélome 9502 randomized trial. Blood 2002, 99, 731–735. [Google Scholar] [CrossRef]
- Einsele, H.; Bamberg, M.; Budach, W.; Schmidberger, H.; Hess, C.F.; Wörmann, B.; Meisner, C.; Straka, C.; Hebart, H.; Trümper, L.; et al. A new conditioning regimen involving total marrow irradiation, busulfan and cyclophosphamide followed by autologous PBSCT in patients with advanced multiple myeloma. Bone Marrow Transplant. 2003, 32, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Cailleteau, A.; Maingon, P.; Choquet, S.; Bourdais, R.; Antoni, D.; Lioure, B.; Hulin, C.; Batard, S.; Llagostera, C.; Guimas, V.; et al. Phase 1 Study of the Combination of Escalated Total Marrow Irradiation Using Helical Tomotherapy and Fixed High-Dose Melphalan (140 mg/m2) Followed by Autologous Stem Cell Transplantation at First Relapse in Multiple Myeloma. Int. J. Radiat. Oncol. Biol. Phys. 2023, 115, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Guerini, A.E.; Filippi, A.R.; Tucci, A.; Simontacchi, G.; Re, A.; Guaineri, A.; Morelli, V.; Borghetti, P.; Triggiani, L.; Pegurri, L.; et al. ‘Le Roi est mort, vive le Roi’: New Roles of Radiotherapy in the Treatment of Lymphomas in Combination with Immunotherapy. Clin. Lymphoma Myeloma Leuk. 2022, 22, e135–e148. [Google Scholar] [CrossRef] [PubMed]
| Patient Characteristics | n (% or Range) |
|---|---|
| Number of Patients | 82 |
| Sex | |
| Male | 50 (61) |
| Female | 32 (39) |
| Median age at diagnosis, y | 58.5 (39–85) |
| Median time to RT, m | 1 (0–86) |
| R-ISS at initial diagnosis | |
| I | 17 (20.7) |
| II | 44 (53.7) |
| III | 5 (6.1) |
| Not recorded | 16 (19.5) |
| ECOG Performance Status | |
| 0 | 14 (17.1) |
| 1 | 27 (32.9) |
| 2 | 7 (8.5) |
| 3 | 5 (6.1) |
| 4 | 0 |
| Not recorded | 29 (35.4) |
| Localization | |
| Spine | 80 (97.6) |
| Pelvis | 58 (70.7) |
| Extremities | 31 (37.8) |
| Skull | 32 (39.0) |
| Rib cage | 35 (42.7) |
| Shoulders | 17 (20.7) |
| Treatment Characteristics | n (%) |
|---|---|
| Prior surgery | |
| Yes | 36 (43.9) |
| No | 46 (56.1) |
| Type of surgery | |
| Biopsy | 17 (47.2) |
| Osteosynthesis | 18 (50.0) |
| Laminectomy | 7 (19.4) |
| Resection | 5 (13.9) |
| Anti-myeloma agents applied within 30 days prior to 90 days after RT * | |
| PI | 56 (68.3) |
| IMiD | 32 (39.0) |
| Glucocorticoid | 72 (87.8) |
| Alkylating Agent | 35 (42.7) |
| Topoisomerase Inhibitor | 16 (19.5) |
| Others | 4 (4.9) |
| Anti-myeloma agents within concurrent systemic therapy ** | |
| PI | 39 (47.6) |
| IMiD | 16 (19.5) |
| Glucocorticoid | 53 (64.6) |
| Alkylating Agent | 12 (14.6) |
| Topoisomerase Inhibitor | 2 (2.4) |
| Others | 1 (1.2) |
| Time range of systemic therapy *** | |
| Up to 30 d before | 39 (47.6) |
| 30 d before | 10 (12.2) |
| 14 d before | 22 (26.8) |
| 7 d before | 31 (37.8) |
| <7 d before | 36 (43.9) |
| During | 53 (64.6) |
| Up to 90 d after | 75 (91.5) |
| 14 d after | 61 (74.4) |
| 30 d after | 67 (81.7) |
| 90 d after | 60 (73.2) |
| Stem Cell Mobilization | 13 (15.9) |
| Auto-SCT | 8 (9.8) |
| Osteoprotection | 68 (82.9) |
| Radiotherapy Characteristics | n (% of PTV or Range) |
|---|---|
| PTV | |
| Number | 134 |
| Per patient | 1 (1–4) |
| Irradiated bones per patient | 5 (1–14) |
| Dose-category | |
| <20 Gy | 1 (0.7) |
| 20-40 Gy | 75 (56.0) |
| >40 Gy | 51 (38.1) |
| Not recorded | 7 (5.2) |
| Fractions | 20 (4–33) |
| Dose per Fraction | 2 Gy (1.8–3 Gy) |
| Technique | |
| 2D | 8 (6.0) |
| 3D-CRT | 54 (40.3) |
| IMRT, Sliding-Window-technique | 41 (30.6) |
| IMRT, VMAT | 18 (13.4) |
| Tomotherapy | 3 (2.2) |
| Not recorded | 10 (7.5) |
| Localization * | |
| Spine | 95 (70.9) |
| Pelvis | 34 (25.4) |
| Extremities | 3 (2.2) |
| Skull | 2 (1.5) |
| Ribcage | 6 (4.5) |
| Shoulder | 3 (2.2) |
| Non-Hematological Toxicity and Grade | Total (n = 82) | RT (n = 29) | RT/ST (n = 53) | p |
|---|---|---|---|---|
| Any Non-Hematological Toxicity * | ||||
| Any grade | 54 (65.9) | 19 (65.5) | 35 (66) | 1.000 |
| Low-grade | 50 (61.0) | 19 (65.5) | 31 (58.5) | 0.638 |
| High-grade | 14 (17.1) | 6 (20.7) | 8 (15.1) | 0.550 |
| Fatigue | 0.735 | |||
| Low-grade | 18 (22.0) | 6 (20.7) | 12 (22.6) | |
| High-grade | 1 (1.2) | 0 | 1 (1.9) | |
| Skin | 0.718 | |||
| Low-grade | 27 (32.9) | 9 (31.0) | 18 (34.0) | |
| High-grade | 1 (1.2) | 0 | 1 (1.9) | |
| Gastrointestinal | 0.251 | |||
| Low-grade | 11 (13.4) | 2 (6.9) | 9 (17.0) | |
| High-grade | 3 (3.7) | 2 (6.9) | 1 (1.9) | |
| Mucosa | 0.814 | |||
| Low-grade | 11 (13.4) | 4 (13.8) | 7 (13.2) | |
| High-grade | 4 (4.9) | 2 (6.9) | 2 (3.8) | |
| Nausea | 1.000 | |||
| Low-grade | 10 (12.2) | 3 (10.3) | 7 (13.2) | |
| High-grade | 0 | 0 | 0 | |
| Vomiting | 1.000 | |||
| Low-grade | 2 (2.4) | 1 (3.4) | 1 (1.9) | |
| High-grade | 0 | 0 | 0 | |
| Pulmonary | 0.524 | |||
| Low-grade | 2 (2.4) | 1 (3.4) | 1 (1.9) | |
| High-grade | 2 (2.4) | 0 | 2 (3.8) | |
| Neurologic | 0.233 | |||
| Low-grade | 4 (4.9) | 0 | 4 (7.5) | |
| High-grade | 1 (1.2) | 0 | 1 (1.9) | |
| Musculoskeletal | 1.000 | |||
| Low-grade | 1 (1.2) | 0 | 1 (1.9) | |
| High-grade | 0 | 0 | 0 | |
| Other | 0.819 | |||
| Low-grade | 6 (7.3) | 2 (6.9) | 4 (7.5) | |
| High-grade | 4 (4.9) | 2 (6.9) | 2 (3.8) |
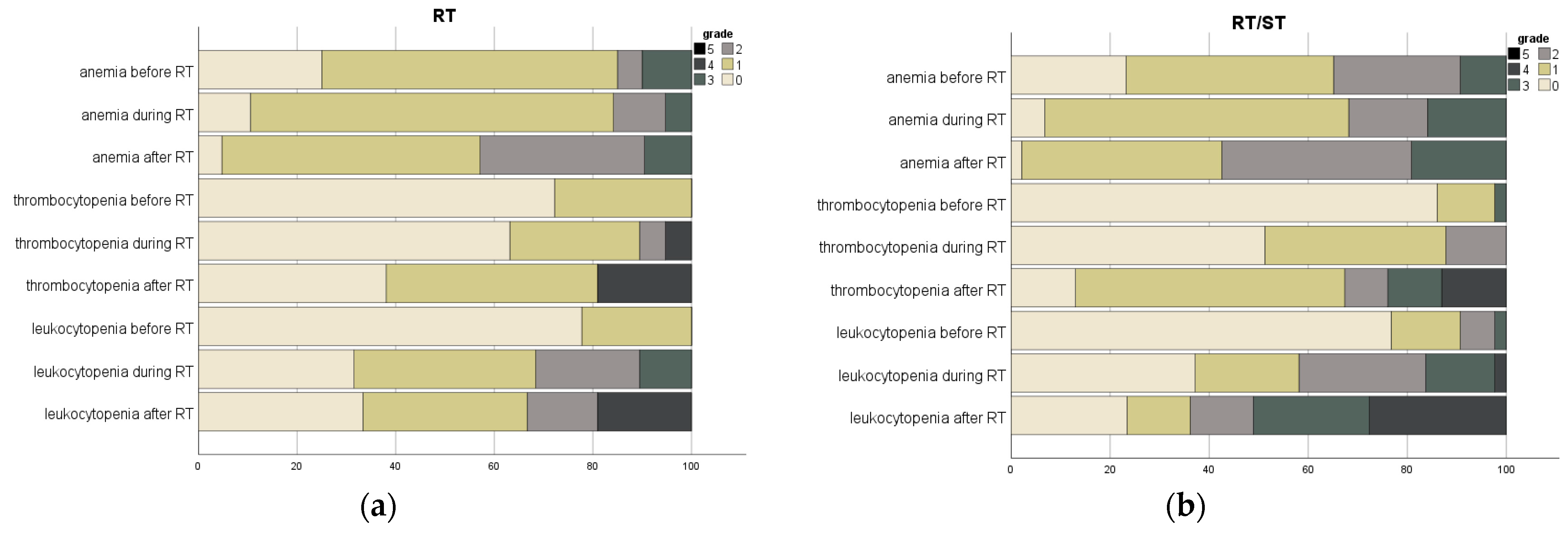
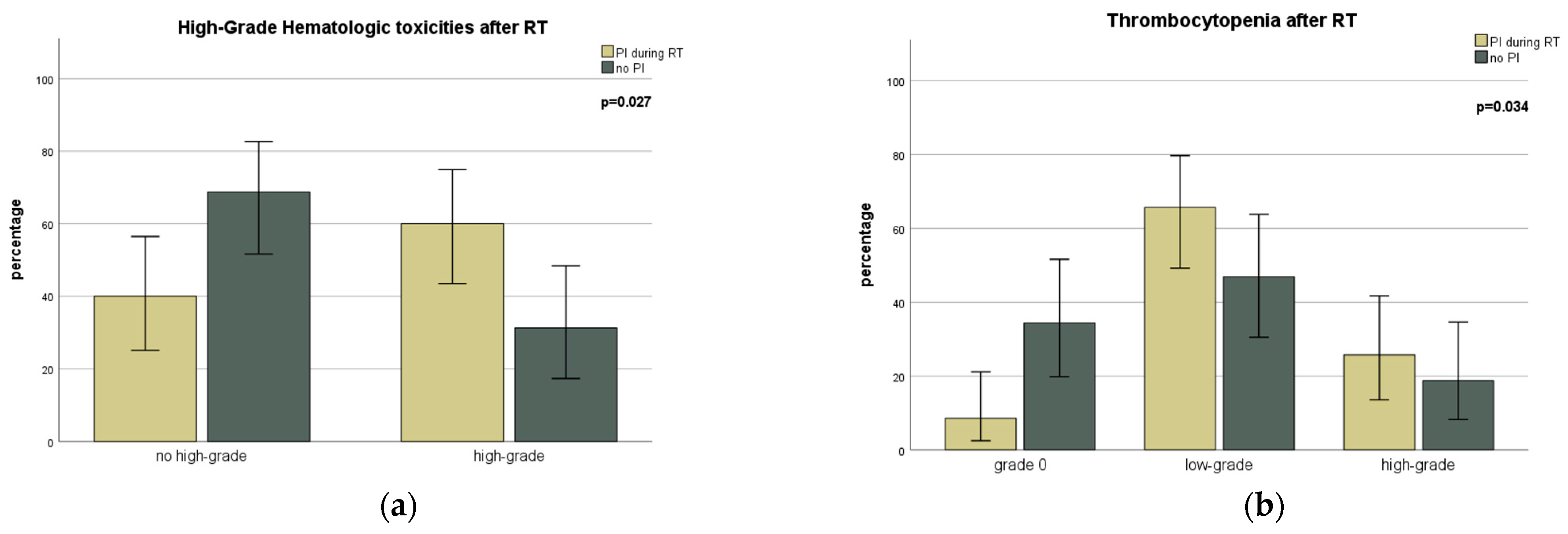
| Hematological Toxicity | Time | Grade | Total | RT | RT/ST | p |
|---|---|---|---|---|---|---|
| Before RT | No Toxicity | 15 (18.3) | 5 (17.2) | 10 (18.9) | 0.982 | |
| Low-Grade | 42 (51.2) | 13 (44.8) | 29 (54.7) | |||
| High-Grade | 6 (7.3) | 2 (6.9) | 4 (7.5) | |||
| Not recorded | 19 (23.2) | 9 (31.0) | 10 (18.9) | |||
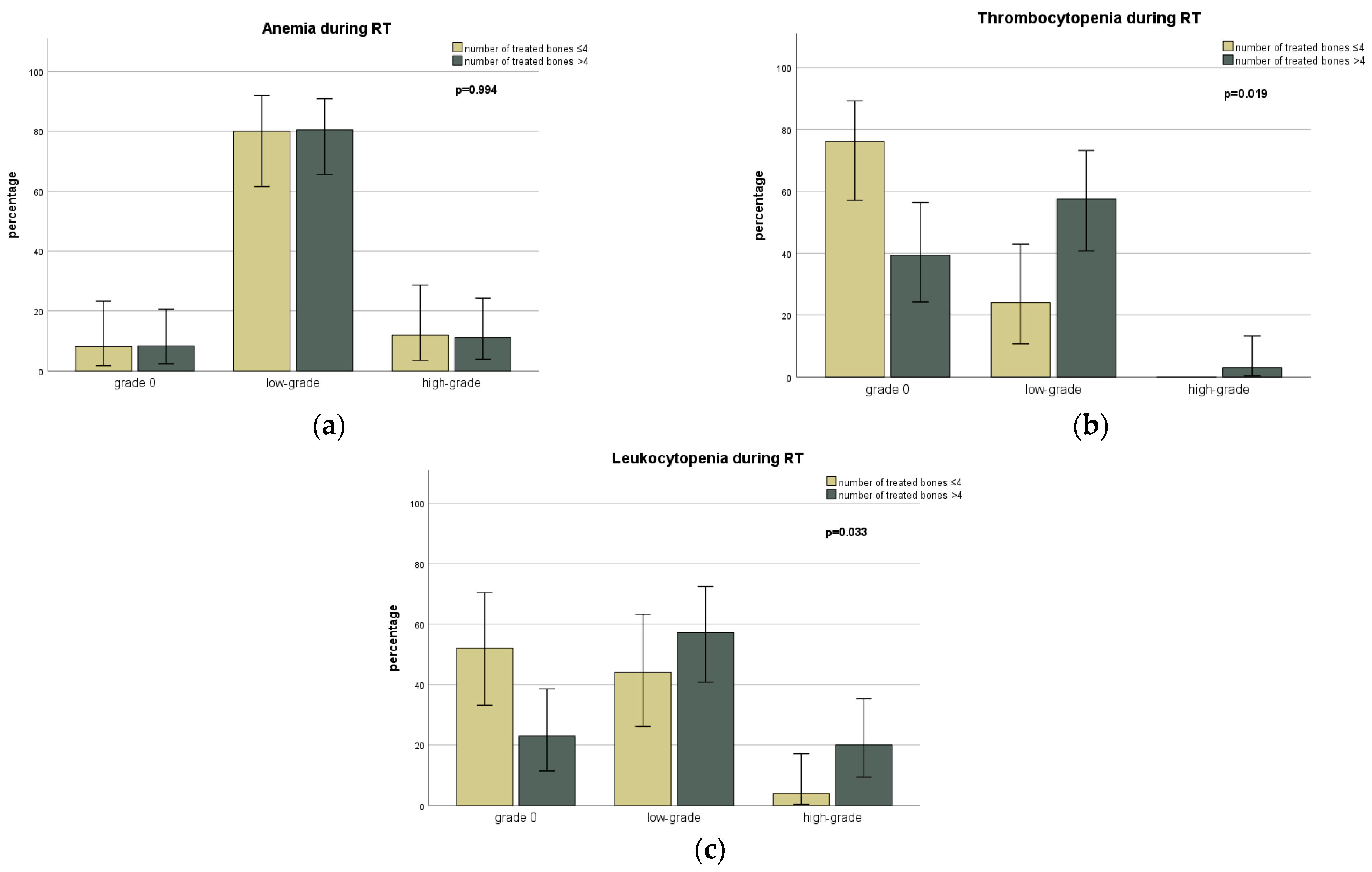
| Anemia | During RT | No Toxicity | 5 (6.1) | 2 (6.9) | 3 (5.7) | 0.474 |
| Low-Grade | 50 (61.0) | 16 (55.2) | 34 (64.2) | |||
| High-Grade | 8 (9.8) | 1 (3.4) | 7 (13.2) | |||
| Not recorded | 19 (23.2) | 10 (34.5) | 9 (17.0) | |||
| After RT | No Toxicity | 2 (2.4) | 1 (3.4) | 1 (1.9) | 0.532 | |
| Low-Grade | 55 (67.1) | 18 (62.1) | 37 (69.8) | |||
| High-Grade | 11 (13.4) | 2 (6.9) | 9 (17.0) | |||
| Not recorded | 14 (17.1) | 8 (27.6) | 6 (11.3) | |||
| Before RT | No Toxicity | 50 (61.0) | 13 (44.8) | 37 (69.8) | 0.255 | |
| Low-Grade | 10 (12.2) | 5 (17.2) | 5 (9.4) | |||
| High-Grade | 1 (1.2) | 0 | 1 (1.9) | |||
| Not recorded | 21 (25.6) | 11 (37.9) | 10 (18.9) | |||
| Thrombocytopenia | During RT | No Toxicity | 33 (40.2) | 12 (41.4) | 21 (39.6) | 0.184 |
| Low-Grade | 26 (31.7) | 6 (20.7) | 20 (37.7) | |||
| High-Grade | 1 (1.2) | 1 (3.4) | 0 | |||
| Not recorded | 22 (26.8) | 10 (34.5) | 12 (22.6) | |||
| After RT | No Toxicity | 14 (17.1) | 8 (27.6) | 6 (11.3) | 0.063 | |
| Low-Grade | 38 (46.3) | 9 (31.0) | 29 (54.7) | |||
| High-Grade | 15 (18.3) | 4 (13.8) | 11 (20.8) | |||
| Not recorded | 15 (18.3) | 8 (27.6) | 7 (13.2) | |||
| Before RT | No Toxicity | 47 (57.3) | 14 (48.3) | 33 (62.3) | 0.806 | |
| Low-Grade | 13 (15.9) | 4 (13.8) | 9 (17.0) | |||
| High-Grade | 1 (1.2) | 0 | 1 (1.9) | |||
| Not recorded | 21 (25.6) | 11 (37.9) | 10 (18.9) | |||
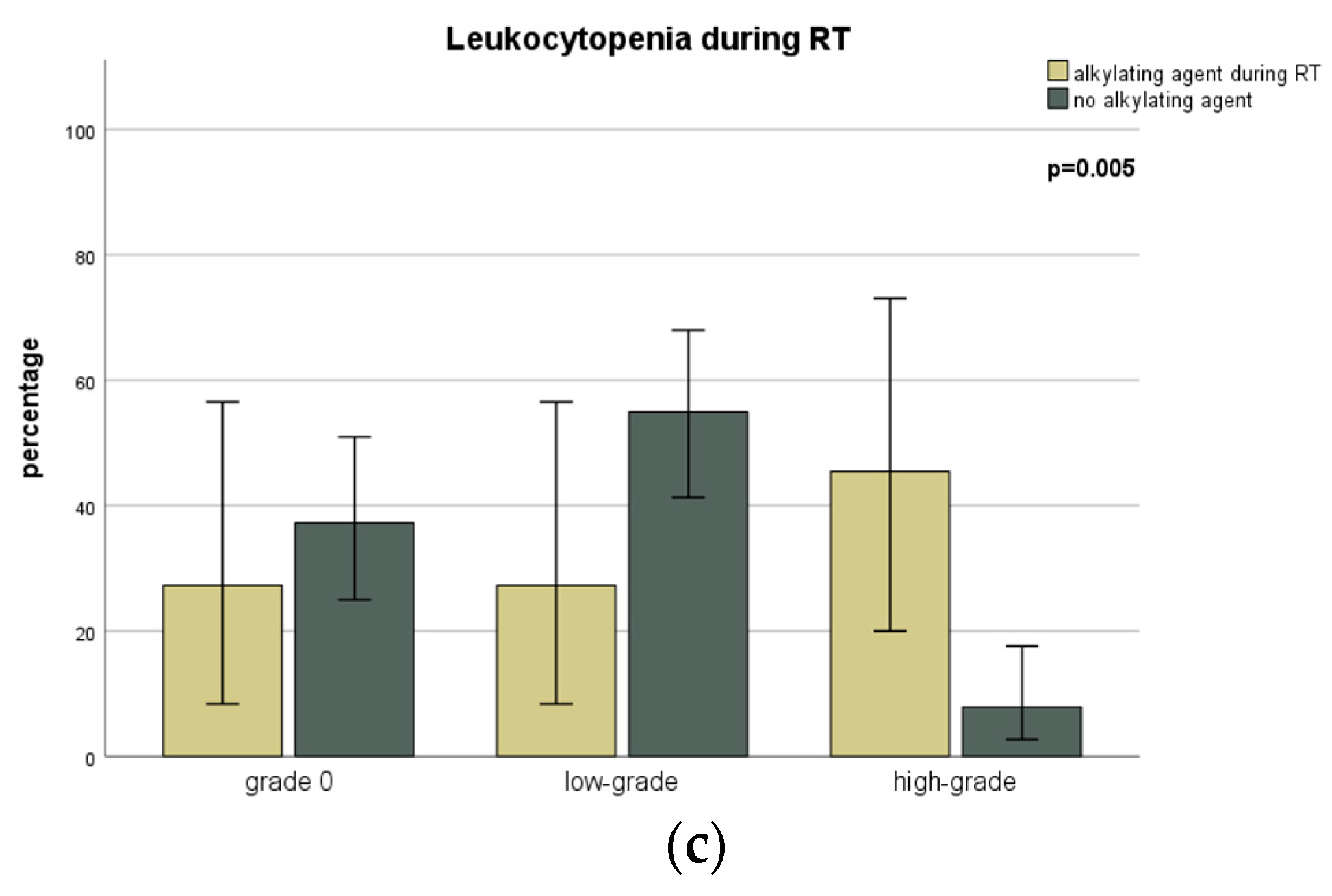
| Leukocytopenia | During RT | No Toxicity | 22 (26.8) | 6 (20.7) | 16 (30.2) | 0.684 |
| Low-Grade | 31 (37.8) | 11 (37.9) | 20 (37.7) | |||
| High-Grade | 9 (11.0) | 2 (6.9) | 7 (13.2) | |||
| Not recorded | 20 (24.4) | 10 (34.5) | 10 (18.9) | |||
| After RT | No Toxicity | 18 (22.0) | 7 (24.1) | 11 (20.8) | 0.042 | |
| Low-Grade | 22 (26.8) | 10 (34.5) | 12 (22.6) | |||
| High-Grade | 28 (34.1) | 4 (13.8) | 24 (45.3) | |||
| Not recorded | 14 (17.1) | 8 (27.6) | 6 (11.3) |
| Study | n | Median FU (m) | Dose (Gy) | IMRT * (%) | Novel Agent (%) | Non-Hematological Toxicity (Any grade) | Hematological Toxicity (Grade III or IV) |
|---|---|---|---|---|---|---|---|
| Shin et al., 2014 [17] | 39 | 6 | 8–37.5 (Mean 26.8) | n.a. | 43.6% | 23.8–40% ** | n.a. |
| Salgado et al., 2019 [18] | 130 | 14 | 2–40 (Median 20) | 15.4% | 70% | 45.9% | n.a. |
| Guerini et al., 2022 [19] | 312 | n.a. | 8–30 | 3.5% | 48.7% | 41% | n.a. |
| Nehlsen et al., 2022 [20] | 55 | 59.8 | 8–32.5 (Median 20) | n.a. | 40% | 14.6% | 35% |
| Present study | 82 | 46.5 | 8–59 (Median 40) | 46.3% | 85.4% | 65.9% | 45.1% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oertel, M.; Schlusemann, T.; Shumilov, E.; Reinartz, G.; Bremer, A.; Rehn, S.; Lenz, G.; Khandanpour, C.; Eich, H.T. Radiotherapy in Combination with Systemic Therapy for Multiple Myeloma—A Critical Toxicity Evaluation in the Modern Treatment Era. Cancers 2023, 15, 2909. https://doi.org/10.3390/cancers15112909
Oertel M, Schlusemann T, Shumilov E, Reinartz G, Bremer A, Rehn S, Lenz G, Khandanpour C, Eich HT. Radiotherapy in Combination with Systemic Therapy for Multiple Myeloma—A Critical Toxicity Evaluation in the Modern Treatment Era. Cancers. 2023; 15(11):2909. https://doi.org/10.3390/cancers15112909
Chicago/Turabian StyleOertel, Michael, Tom Schlusemann, Evgenii Shumilov, Gabriele Reinartz, Anne Bremer, Stephan Rehn, Georg Lenz, Cyrus Khandanpour, and Hans Theodor Eich. 2023. "Radiotherapy in Combination with Systemic Therapy for Multiple Myeloma—A Critical Toxicity Evaluation in the Modern Treatment Era" Cancers 15, no. 11: 2909. https://doi.org/10.3390/cancers15112909
APA StyleOertel, M., Schlusemann, T., Shumilov, E., Reinartz, G., Bremer, A., Rehn, S., Lenz, G., Khandanpour, C., & Eich, H. T. (2023). Radiotherapy in Combination with Systemic Therapy for Multiple Myeloma—A Critical Toxicity Evaluation in the Modern Treatment Era. Cancers, 15(11), 2909. https://doi.org/10.3390/cancers15112909








