Role of AI and Radiomic Markers in Early Diagnosis of Renal Cancer and Clinical Outcome Prediction: A Brief Review
Abstract
Simple Summary
Abstract
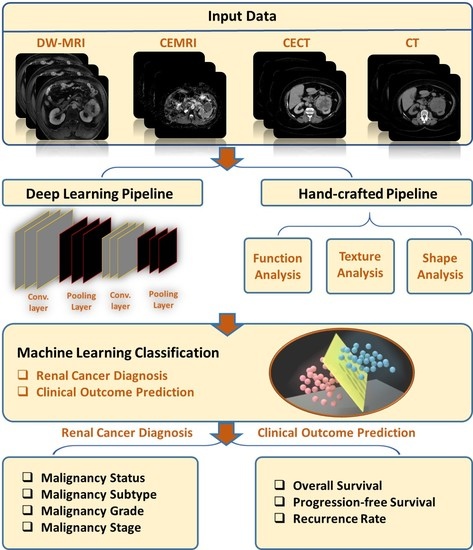
1. Introduction
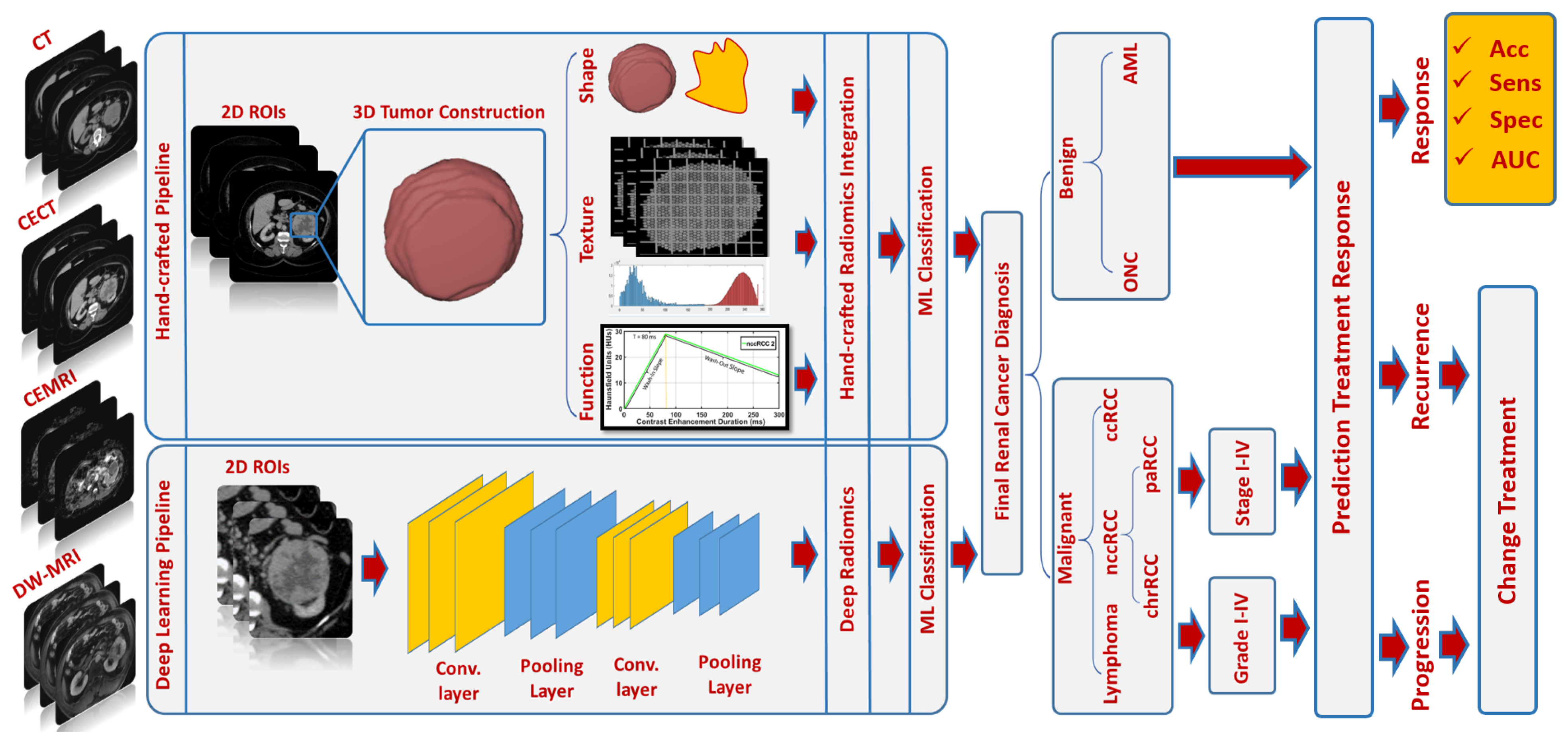
2. AI-Based Diagnostic Studies
2.1. Computed Tomography (CT) Studies
2.2. Magnetic Resonance Imaging (MRI) Studies
3. AI-Based Prediction of Clinical Outcome/Treatment Response Studies
4. Discussion and Future Directions
- Suggested Diagnostic Radiomic Markers:
- In terms of differentiating malignant from benign renal tumors, CT studies have demonstrated a slightly higher diagnostic accuracy [52,54,55,63,68,83] when compared with the results obtained by MRI studies [29,31,92,93]. This can be partially attributed to the superior resolution provided by CT in comparison with MRI. In both imaging modalities, first-order texture markers, including entropy, mean, MPP, skewness, and kurtosis, were reported to be sufficient for the intended purpose.
- For subtyping and grading, both CT [68,71,73,74,76,79,80,88,89,90,91] and MRI [22,41,95,96] studies exhibited adequate diagnostic performance, suggesting that second-order texture markers, particularly those derived from the GLCM and GLRLM, should be combined with first-order texture markers. A limited number of studies have relied on morphological or functional markers, which, if integrated, could significantly enhance diagnostic performance [68]. In this context, both imaging modalities can be utilized for subtyping and grading purposes. However, MRIs are preferable in cases involving pediatric patients or pregnant women [97] to prevent exposure to ionizing radiation. For staging, a few CT studies demonstrated promising diagnostic performance [39], while MRI studies did not investigate radiological staging.
- Suggested Diagnostic Radiomic Techniques: Generally, handcrafted radiomic techniques were more commonly investigated in both CT [14,27,28,34,35,37,54,62,68,74,82,86] and MRI [22,23,29,30,32,93,94] studies, as opposed to deep learning radiomic techniques, which were less frequently utilized in CT [66,81,83,88,95] and MRI [92] studies. Handcrafted techniques have proven efficient, as evidenced by high diagnostic accuracy, sensitivity, and specificity, as well as being well-understood (i.e., explainable AI), making them desirable and dependable.
- Suggested Diagnostic Classifiers: The RF, SVM, and ANN classifiers were the most frequently utilized AI-based classification models in CT studies [14,27,28,35,36,38,39,52,53,54,55,58,59,67,68,70,74,76,78,79,80,85,86,89,90], while the RF classifier was predominantly selected in MRI studies [29,32,33,41,93,95,96]. These classifiers have provided impressive diagnostic results and have been widely accepted by researchers in the field due to their ability to handle nonlinear and multiclass classification problems.
- Suggested Imaging Modalities/Phases: Contrast-enhanced phases 2 and 3 (corticomedullary/, arterial phase, and nephrographic/portal venous phase) were reported to be the most informative phases for extracting radiomic markers in both CT [35,36,38,58,62,64,68,70,71,72,73,74,75,76,80,81,87,89,90,91] and MRI [41] studies. Meanwhile, texture analysis of ADCs on DW-MRI was the most commonly employed technique to extract radiomic markers in MRI studies [29,30,31,32,94].
- Suggested Prediction Radiomic Markers: In terms of treatment response prediction, entropy, mean, skewness, kurtosis, STD, and median have been identified by most CT studies [46,50] as potential radiomic markers for predicting OS and PFS. On the other hand, histogram measures of ADC maps extracted from DW-MR images, specifically changes in mean ADC, ADC energy, and ADC kurtosis, were the most promising predictors of clinical outcome in MRI studies [43,44,51]. To the best of our knowledge, no studies have employed AI, ML, or DL for the purpose of predicting treatment response; rather, they have relied on statistical analyses to identify significant markers correlated with clinical outcome/treatment response. A limited number of studies have depended on morphological or functional markers, which, if integrated, could significantly enhance clinical outcome/treatment response prediction [43,46,48,49,51].
- Future Directions: While renal cancer diagnosis is a well-established research area, with numerous CT and MRI studies having developed radiomic and AI-based CAD systems for determining malignancy status, subtyping, grading, and staging, some investigations still suffer from low sensitivity or specificity [36,38,40,53,58,60,66,78,82,87,89,93,96]. Consequently, integrating radiomic markers extracted from multiple imaging modalities, such as CT and MRI, may improve diagnostic performance. Furthermore, as radiological-based analysis may not be sufficient for predicting clinical outcome/treatment responses, incorporating histopathological image analysis that captures characteristics such as cell color, shape, size, and staining could enhance prediction capabilities. Identifying robust AI models may reduce subjectivity by pinpointing optimal markers for treatment response prediction purposes. It is worth noting that a new trend in predicting treatment response using radiogenomics has recently emerged in a few studies and requires further investigation [98,99,100,101,102,103].
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| RC | Renal Cancer |
| RCC | Renal Cell Carcinoma |
| ccRCC | Clear-Cell RCC |
| nccRCC | Non-Clear-Cell RCC |
| paRCC | Papillary RCC |
| ChrRCC | Chromophobe RCC |
| AMLwvf | Angiomyolipoma without visible fat |
| ONC | Oncocytoma |
| CECT | Contrast-Enhanced Computed Tomography |
| CEMRI | Contrast-Enhanced Magnetic Resonance Imaging |
| DW-MRI | Diffusion-Weighted MRI |
| ADC | Apparent Diffusion Coefficient |
| AI | Artificial Intelligence |
| ML | Machine Learning |
| DL | Deep Learning |
| CAD | Computer-Aided Diagnosis |
| CAP | Computer-Aided Prediction |
| ROI | Region of Interest |
| AUC | Area Under the Curve |
| OS | Overall Survival |
| PFS | Progression-Free Survival |
| ANNs | Artificial Neural Networks |
| LR | Logistic Regression |
| RF | Random Forests |
| SVM | Support Vector Machine |
| CNN | Convolutional Neural Network |
| ROC | Receiver Operating Characteristics |
| MPP | Mean of Positive Pixels |
| SW-MRI | Susceptibility-Weighted MRI |
| GLCM | Gray-Level Co-occurrence Matrix |
| GLRLM | Gray-Level Run-Length Matrix |
| GLSZM | Gray-Level Size-Zone Matrix |
| NGTDM | Neighboring Gray-Tone Difference Matrix |
| GLDZM | Gray-Level Distance Zone Matrix |
| NGLDM | Neighboring Gray-Level Dependence Matrix |
| mRCC | Metastatic RCC |
| KM | Kaplan–Meier |
| PET | Positron Emission Tomography |
| SUV | Standard Uptake value |
| SABR | Stereotactic Ablative Body Radiotherapy |
| IRE | Initial Rate of Enhancement |
| MaxE | Maximum Enhancement |
| IRW | Initial Rate of Washout |
| iAUCAC60 | Initial Area Under Contrast Agent Concentration Curve for 60 s postinjection |
| HUs | Hounsfield Units |
| VEGFR | Vascular Endothelial Growth Factor Receptor |
| LassoCV | Least Absolute Shrinkage and Selection Operator Cross-Validation |
| KPS | Karnofsky Performance Status |
References
- ASCO. Kidney Cancer. Available online: https://www.cancer.net/cancer-types/kidney-cancer/statistics/ (accessed on 15 July 2022).
- American Cancer Society. Key Statistics About Kidney Cancer. Available online: https://www.cancer.org/cancer/kidney-cancer/about/key-statistics.html (accessed on 1 July 2022).
- National Cancer Institute. Cancer Prevalence and Cost of Care Projections. Available online: https://costprojections.cancer.gov/graph.php (accessed on 3 January 2018).
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2015. CA A Cancer J. Clin. 2015, 65, 5–29. [Google Scholar] [CrossRef]
- Chen, W.; Zheng, R.; Baade, P.D.; Zhang, S.; Zeng, H.; Bray, F.; Jemal, A.; Yu, X.Q.; He, J. Cancer statistics in China, 2015. CA Cancer J. Clin. 2016, 66, 115–132. [Google Scholar] [CrossRef]
- Moch, H.; Cubilla, A.L.; Humphrey, P.A.; Reuter, V.E.; Ulbright, T.M. The 2016 WHO classification of tumours of the urinary system and male genital organs—Part A: Renal, penile, and testicular tumours. Eur. Urol. 2016, 70, 93–105. [Google Scholar] [CrossRef]
- Delahunt, B.; Bethwaite, P.B.; Nacey, J.N. Outcome prediction for renal cell carcinoma: Evaluation of prognostic factors for tumours divided according to histological subtype. Pathology 2007, 39, 459–465. [Google Scholar] [CrossRef]
- Cheville, J.C.; Lohse, C.M.; Zincke, H.; Weaver, A.L.; Blute, M.L. Comparisons of outcome and prognostic features among histologic subtypes of renal cell carcinoma. Am. J. Surg. Pathol. 2003, 27, 612–624. [Google Scholar] [CrossRef]
- Rendon, R.A. Active surveillance as the preferred management option for small renal masses. Can. Urol. Assoc. J. 2010, 4, 136. [Google Scholar] [CrossRef]
- Mues, A.C.; Landman, J. Small renal masses: Current concepts regarding the natural history and reflections on the American Urological Association guidelines. Curr. Opin. Urol. 2010, 20, 105–110. [Google Scholar] [CrossRef]
- Heuer, R.; Gill, I.S.; Guazzoni, G.; Kirkali, Z.; Marberger, M.; Richie, J.P.; de la Rosette, J.J. A critical analysis of the actual role of minimally invasive surgery and active surveillance for kidney cancer. Eur. Urol. 2010, 57, 223–232. [Google Scholar] [CrossRef]
- Xipell, J. The incidence of benign renal nodules (a clinicopathologic study). J. Urol. 1971, 106, 503–506. [Google Scholar] [CrossRef]
- Gill, I.S.; Aron, M.; Gervais, D.A.; Jewett, M.A. Small renal mass. N. Engl. J. Med. 2010, 362, 624–634. [Google Scholar] [CrossRef]
- Hodgdon, T.; McInnes, M.D.; Schieda, N.; Flood, T.A.; Lamb, L.; Thornhill, R.E. Can quantitative CT texture analysis be used to differentiate fat-poor renal angiomyolipoma from renal cell carcinoma on unenhanced CT images? Radiology 2015, 276, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Mindrup, S.R.; Pierre, J.S.; Dahmoush, L.; Konety, B.R. The prevalence of renal cell carcinoma diagnosed at autopsy. BJU Int. 2005, 95, 31–33. [Google Scholar] [CrossRef]
- American Cancer Society. Test for Kidney Cancer. Available online: https://www.cancer.org/cancer/kidney-cancer/detection-diagnosis-staging/how-diagnosed.html (accessed on 10 April 2022).
- Lim, R.S.; Flood, T.A.; McInnes, M.D.; Lavallee, L.T.; Schieda, N. Renal angiomyolipoma without visible fat: Can we make the diagnosis using CT and MRI? Eur. Radiol. 2018, 28, 542–553. [Google Scholar] [CrossRef]
- Chandarana, H.; Rosenkrantz, A.B.; Mussi, T.C.; Kim, S.; Ahmad, A.A.; Raj, S.D.; McMenamy, J.; Melamed, J.; Babb, J.S.; Kiefer, B.; et al. Histogram analysis of whole-lesion enhancement in differentiating clear cell from papillary subtype of renal cell cancer. Radiology 2012, 265, 790–798. [Google Scholar] [CrossRef]
- Zhou, X.; Yan, F.; Luo, Y.; Peng, Y.-L.; Parajuly, S.S.; Wen, X.R.; Cai, D.-M.; Li, Y.-Z. Characterization and diagnostic confidence of contrast-enhanced ultrasound for solid renal tumors. Ultrasound Med. Biol. 2011, 37, 845–853. [Google Scholar] [CrossRef] [PubMed]
- Dyer, R.; DiSantis, D.J.; McClennan, B.L. Simplified imaging approach for evaluation of the solid renal mass in adults. Radiology 2008, 247, 331–343. [Google Scholar] [CrossRef]
- Zhang, J.; Lefkowitz, R.A.; Ishill, N.M.; Wang, L.; Moskowitz, C.S.; Russo, P.; Eisenberg, H.; Hricak, H. Solid renal cortical tumors: Differentiation with CT. Radiology 2007, 244, 494–504. [Google Scholar] [CrossRef]
- Goyal, A.; Razik, A.; Kandasamy, D.; Seth, A.; Das, P.; Ganeshan, B.; Sharma, R. Role of MR texture analysis in histological subtyping and grading of renal cell carcinoma: A preliminary study. Abdom. Radiol. 2019, 44, 3336–3349. [Google Scholar] [CrossRef]
- Razik, A.; Goyal, A.; Sharma, R.; Kandasamy, D.; Seth, A.; Das, P.; Ganeshan, B. MR texture analysis in differentiating renal cell carcinoma from lipid-poor angiomyolipoma and oncocytoma. Br. J. Radiol. 2020, 93, 20200569. [Google Scholar] [CrossRef]
- Lubner, M.G.; Smith, A.D.; Sandrasegaran, K.; Sahani, D.V.; Pickhardt, P.J. CT texture analysis: Definitions, applications, biologic correlates, and challenges. Radiographics 2017, 37, 1483–1503. [Google Scholar] [CrossRef] [PubMed]
- Gillies, R.J.; Kinahan, P.E.; Hricak, H. Radiomics: Images are more than pictures, they are data. Radiology 2016, 278, 563–577. [Google Scholar] [CrossRef] [PubMed]
- Scapicchio, C.; Gabelloni, M.; Barucci, A.; Cioni, D.; Saba, L.; Neri, E. A deep look into radiomics. La Radiol. Medica 2021, 126, 1296–1311. [Google Scholar] [CrossRef]
- Yang, R.; Wu, J.; Sun, L.; Lai, S.; Xu, Y.; Liu, X.; Ma, Y.; Zhen, X. Radiomics of small renal masses on multiphasic CT: Accuracy of machine learning–based classification models for the differentiation of renal cell carcinoma and angiomyolipoma without visible fat. Eur. Radiol. 2020, 30, 1254–1263. [Google Scholar] [CrossRef]
- You, M.W.; Kim, N.; Choi, H. The value of quantitative CT texture analysis in differentiation of angiomyolipoma without visible fat from clear cell renal cell carcinoma on four-phase contrast-enhanced CT images. Clin. Radiol. 2019, 74, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Zhu, Q.; Liu, H.; Chang, L.; Duan, S.; Dou, W.; Li, S.; Ye, J. Differentiating Benign from Malignant Renal Tumors Using T2-and Diffusion-Weighted Images: A Comparison of Deep Learning and Radiomics Models Versus Assessment from Radiologists. J. Magn. Reson. Imaging 2022, 55, 1251–1259. [Google Scholar] [CrossRef]
- van Oostenbrugge, T.J.; Spenkelink, I.M.; Bokacheva, L.; Rusinek, H.; van Amerongen, M.J.; Langenhuijsen, J.F.; Mulders, P.F.; Fütterer, J.J. Kidney tumor diffusion-weighted magnetic resonance imaging derived ADC histogram parameters combined with patient characteristics and tumor volume to discriminate oncocytoma from renal cell carcinoma. Eur. J. Radiol. 2021, 145, 110013. [Google Scholar] [CrossRef]
- Li, A.; Xing, W.; Li, H.; Hu, Y.; Hu, D.; Li, Z.; Kamel, I.R. Subtype differentiation of small (≤4 cm) solid renal mass using volumetric histogram analysis of DWI at 3-T MRI. Am. J. Roentgenol. 2018, 211, 614–623. [Google Scholar] [CrossRef]
- Matsumoto, S.; Arita, Y.; Yoshida, S.; Fukushima, H.; Kimura, K.; Yamada, I.; Tanaka, H.; Yagi, F.; Yokoyama, M.; Matsuoka, Y.; et al. Utility of radiomics features of diffusion-weighted magnetic resonance imaging for differentiation of fat-poor angiomyolipoma from clear cell renal cell carcinoma: Model development and external validation. Abdom. Radiol. 2022, 47, 2178–2186. [Google Scholar] [CrossRef] [PubMed]
- Hoang, U.N.; Mojdeh Mirmomen, S.; Meirelles, O.; Yao, J.; Merino, M.; Metwalli, A.; Marston Linehan, W.; Malayeri, A.A. Assessment of multiphasic contrast-enhanced MR textures in differentiating small renal mass subtypes. Abdom. Radiol. 2018, 43, 3400–3409. [Google Scholar] [CrossRef]
- Deng, Y.; Soule, E.; Samuel, A.; Shah, S.; Cui, E.; Asare-Sawiri, M.; Sundaram, C.; Lall, C.; Sandrasegaran, K. CT texture analysis in the differentiation of major renal cell carcinoma subtypes and correlation with Fuhrman grade. Eur. Radiol. 2019, 29, 6922–6929. [Google Scholar] [CrossRef]
- Zhang, G.M.Y.; Shi, B.; Xue, H.D.; Ganeshan, B.; Sun, H.; Jin, Z.Y. Can quantitative CT texture analysis be used to differentiate subtypes of renal cell carcinoma? Clin. Radiol. 2019, 74, 287–294. [Google Scholar] [CrossRef]
- Uhlig, J.; Biggemann, L.; Nietert, M.M.; Beißbarth, T.; Lotz, J.; Kim, H.S.; Trojan, L.; Uhlig, A. Discriminating malignant and benign clinical T1 renal masses on computed tomography: A pragmatic radiomics and machine learning approach. Medicine 2020, 99, e19725. [Google Scholar] [CrossRef]
- Feng, Z.; Shen, Q.; Li, Y.; Hu, Z. CT texture analysis: A potential tool for predicting the Fuhrman grade of clear-cell renal carcinoma. Cancer Imaging 2019, 19, 6. [Google Scholar] [CrossRef]
- Shu, J.; Tang, Y.; Cui, J.; Yang, R.; Meng, X.; Cai, Z.; Zhang, J.; Xu, W.; Wen, D.; Yin, H. Clear cell renal cell carcinoma: CT-based radiomics features for the prediction of Fuhrman grade. Eur. J. Radiol. 2018, 109, 8–12. [Google Scholar] [CrossRef]
- Demirjian, N.L.; Varghese, B.A.; Cen, S.Y.; Hwang, D.H.; Aron, M.; Siddiqui, I.; Fields, B.K.; Lei, X.; Yap, F.Y.; Rivas, M.; et al. CT-based radiomics stratification of tumor grade and TNM stage of clear cell renal cell carcinoma. Eur. Radiol. 2022, 32, 2552–2563. [Google Scholar] [CrossRef]
- Sun, J.; Pan, L.; Zha, T.; Xing, W.; Chen, J.; Duan, S. The role of MRI texture analysis based on susceptibility-weighted imaging in predicting Fuhrman grade of clear cell renal cell carcinoma. Acta Radiol. 2021, 62, 1104–1111. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Y.; Zhang, Y.; Chen, Y.X.; Huang, Z.Q.; Xia, X.Y.; Yan, Y.X.; Xu, M.P.; Chen, W.; Wang, X.l.; Chen, Q.L. MRI-Based Grading of Clear Cell Renal Cell Carcinoma Using a Machine Learning Classifier. Front. Oncol. 2021, 11, 708655. [Google Scholar] [CrossRef] [PubMed]
- Goh, V.; Ganeshan, B.; Nathan, P.; Juttla, J.K.; Vinayan, A.; Miles, K.A. Assessment of response to tyrosine kinase inhibitors in metastatic renal cell cancer: CT texture as a predictive biomarker. Radiology 2011, 261, 165–171. [Google Scholar] [CrossRef]
- Bharwani, N.; Miquel, M.; Powles, T.; Dilks, P.; Shawyer, A.; Sahdev, A.; Wilson, P.; Chowdhury, S.; Berney, D.; Rockall, A. Diffusion-weighted and multiphase contrast-enhanced MRI as surrogate markers of response to neoadjuvant sunitinib in metastatic renal cell carcinoma. Br. J. Cancer 2014, 110, 616–624. [Google Scholar] [CrossRef]
- Antunes, J.; Viswanath, S.; Rusu, M.; Valls, L.; Hoimes, C.; Avril, N.; Madabhushi, A. Radiomics analysis on FLT-PET/MRI for characterization of early treatment response in renal cell carcinoma: A proof-of-concept study. Transl. Oncol. 2016, 9, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Boos, J.; Revah, G.; Brook, O.R.; Rangaswamy, B.; Bhatt, R.S.; Brook, A.; Raptopoulos, V. CT intensity distribution curve (histogram) analysis of patients undergoing antiangiogenic therapy for metastatic renal cell carcinoma. Am. J. Roentgenol. 2017, 209, W85–W92. [Google Scholar] [CrossRef] [PubMed]
- Haider, M.A.; Vosough, A.; Khalvati, F.; Kiss, A.; Ganeshan, B.; Bjarnason, G.A. CT texture analysis: A potential tool for prediction of survival in patients with metastatic clear cell carcinoma treated with sunitinib. Cancer Imaging 2017, 17, 4. [Google Scholar] [CrossRef] [PubMed]
- Mains, J.R.; Donskov, F.; Pedersen, E.M.; Madsen, H.H.T.; Thygesen, J.; Thorup, K.; Rasmussen, F. Use of patient outcome endpoints to identify the best functional CT imaging parameters in metastatic renal cell carcinoma patients. Br. J. Radiol. 2017, 91, 20160795. [Google Scholar] [CrossRef] [PubMed]
- Khodabakhshi, Z.; Amini, M.; Mostafaei, S.; Haddadi Avval, A.; Nazari, M.; Oveisi, M.; Shiri, I.; Zaidi, H. Overall survival prediction in renal cell carcinoma patients using computed tomography radiomic and clinical information. J. Digit. Imaging 2021, 34, 1086–1098. [Google Scholar] [CrossRef]
- Zhang, H.; Yin, F.; Chen, M.; Yang, L.; Qi, A.; Cui, W.; Yang, S.; Wen, G. Development and Validation of a CT-Based Radiomics Nomogram for Predicting Postoperative Progression-Free Survival in Stage I–III Renal Cell Carcinoma. Front. Oncol. 2022, 11, 5373. [Google Scholar] [CrossRef]
- Lubner, M.G.; Stabo, N.; Abel, E.J.; Del Rio, A.M.; Pickhardt, P.J. CT textural analysis of large primary renal cell carcinomas: Pretreatment tumor heterogeneity correlates with histologic findings and clinical outcomes. Am. J. Roentgenol. 2016, 207, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, H.M.; Parameswaran, B.K.; Finnegan, M.E.; Roettger, D.; Lau, E.; Kron, T.; Shaw, M.; Chander, S.; Siva, S. Diffusion weighted and dynamic contrast enhanced MRI as an imaging biomarker for stereotactic ablative body radiotherapy (SABR) of primary renal cell carcinoma. PLoS ONE 2018, 13, e0202387. [Google Scholar] [CrossRef]
- Cui, E.M.; Lin, F.; Li, Q.; Li, R.G.; Chen, X.M.; Liu, Z.S.; Long, W.S. Differentiation of renal angiomyolipoma without visible fat from renal cell carcinoma by machine learning based on whole-tumor computed tomography texture features. Acta Radiol. 2019, 60, 1543–1552. [Google Scholar] [CrossRef]
- Lee, H.S.; Hong, H.; Jung, D.C.; Park, S.; Kim, J. Differentiation of fat-poor angiomyolipoma from clear cell renal cell carcinoma in contrast-enhanced MDCT images using quantitative feature classification. Med. Phys. 2017, 44, 3604–3614. [Google Scholar] [CrossRef]
- Feng, Z.; Rong, P.; Cao, P.; Zhou, Q.; Zhu, W.; Yan, Z.; Liu, Q.; Wang, W. Machine learning-based quantitative texture analysis of CT images of small renal masses: Differentiation of angiomyolipoma without visible fat from renal cell carcinoma. Eur. Radiol. 2018, 28, 1625–1633. [Google Scholar] [CrossRef]
- Yan, L.; Liu, Z.; Wang, G.; Huang, Y.; Liu, Y.; Yu, Y.; Liang, C. Angiomyolipoma with minimal fat: Differentiation from clear cell renal cell carcinoma and papillary renal cell carcinoma by texture analysis on CT images. Acad. Radiol. 2015, 22, 1115–1121. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Cao, F.; Xu, X.; Ma, W. Can whole-tumor radiomics-based CT analysis better differentiate fat-poor angiomyolipoma from clear cell renal cell caricinoma: Compared with conventional CT analysis? Abdom. Radiol. 2020, 45, 2500–2507. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Yu, D.; Ni, T.; Zhao, T.; Jin, Y.; Dong, E. Quantitative analysis of multiphase contrast-enhanced CT images: A pilot study of preoperative prediction of Fat-Poor angiomyolipoma and renal cell carcinoma. Am. J. Roentgenol. 2020, 214, 370–382. [Google Scholar] [CrossRef]
- Nassiri, N.; Maas, M.; Cacciamani, G.; Varghese, B.; Hwang, D.; Lei, X.; Aron, M.; Desai, M.; Oberai, A.A.; Cen, S.Y.; et al. A Radiomic-based Machine Learning Algorithm to Reliably Differentiate Benign Renal Masses from Renal Cell Carcinoma. Eur. Urol. Focus 2021, 8, 988–994. [Google Scholar] [CrossRef]
- Yap, F.Y.; Varghese, B.A.; Cen, S.Y.; Hwang, D.H.; Lei, X.; Desai, B.; Lau, C.; Yang, L.L.; Fullenkamp, A.J.; Hajian, S.; et al. Shape and texture-based radiomics signature on CT effectively discriminates benign from malignant renal masses. Eur. Radiol. 2021, 31, 1011–1021. [Google Scholar] [CrossRef] [PubMed]
- Coy, H.; Hsieh, K.; Wu, W.; Nagarajan, M.B.; Young, J.R.; Douek, M.L.; Brown, M.S.; Scalzo, F.; Raman, S.S. Deep learning and radiomics: The utility of Google TensorFlow™ Inception in classifying clear cell renal cell carcinoma and oncocytoma on multiphasic CT. Abdom. Radiol. 2019, 44, 2009–2020. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.Y.; Lubner, M.G.; Nystrom, J.T.; Swietlik, J.F.; Abel, E.J.; Havighurst, T.C.; Silverman, S.G.; McGahan, J.P.; Pickhardt, P.J. Utility of CT texture analysis in differentiating low-attenuation renal cell carcinoma from cysts: A bi-institutional retrospective study. Am. J. Roentgenol. 2019, 213, 1259–1266. [Google Scholar] [CrossRef]
- Tanaka, T.; Huang, Y.; Marukawa, Y.; Tsuboi, Y.; Masaoka, Y.; Kojima, K.; Iguchi, T.; Hiraki, T.; Gobara, H.; Yanai, H.; et al. Differentiation of small (≤4 cm) renal masses on multiphase contrast-enhanced CT by deep learning. Am. J. Roentgenol. 2020, 214, 605–612. [Google Scholar] [CrossRef]
- Li, Y.; Huang, X.; Xia, Y.; Long, L. Value of radiomics in differential diagnosis of chromophobe renal cell carcinoma and renal oncocytoma. Abdom. Radiol. 2020, 45, 3193–3201. [Google Scholar] [CrossRef]
- Li, X.; Ma, Q.; Tao, C.; Liu, J.; Nie, P.; Dong, C. A CT-based radiomics nomogram for differentiation of small masses (<4 cm) of renal oncocytoma from clear cell renal cell carcinoma. Abdom. Radiol. 2021, 46, 5240–5249. [Google Scholar]
- Li, X.; Ma, Q.; Nie, P.; Zheng, Y.; Dong, C.; Xu, W. A CT-based radiomics nomogram for differentiation of renal oncocytoma and chromophobe renal cell carcinoma with a central scar-matched study. Br. J. Radiol. 2022, 95, 20210534. [Google Scholar] [CrossRef] [PubMed]
- Zabihollahy, F.; Schieda, N.; Krishna, S.; Ukwatta, E. Automated classification of solid renal masses on contrast-enhanced computed tomography images using convolutional neural network with decision fusion. Eur. Radiol. 2020, 30, 5183–5190. [Google Scholar] [CrossRef]
- Yu, H.; Scalera, J.; Khalid, M.; Touret, A.S.; Bloch, N.; Li, B.; Qureshi, M.M.; Soto, J.A.; Anderson, S.W. Texture analysis as a radiomic marker for differentiating renal tumors. Abdom. Radiol. 2017, 42, 2470–2478. [Google Scholar] [CrossRef]
- Shehata, M.; Alksas, A.; Abouelkheir, R.T.; Elmahdy, A.; Shaffie, A.; Soliman, A.; Ghazal, M.; Abu Khalifeh, H.; Salim, R.; Abdel Razek, A.A.K.; et al. A comprehensive computer-assisted diagnosis system for early assessment of renal cancer tumors. Sensors 2021, 21, 4928. [Google Scholar] [CrossRef]
- Varghese, B.A.; Chen, F.; Hwang, D.H.; Cen, S.Y.; Desai, B.; Gill, I.S.; Duddalwar, V.A. Differentiation of predominantly solid enhancing lipid-poor renal cell masses by use of contrast-enhanced CT: Evaluating the role of texture in tumor subtyping. Am. J. Roentgenol. 2018, 211, W288–W296. [Google Scholar] [CrossRef]
- Uhlig, J.; Leha, A.; Delonge, L.M.; Haack, A.M.; Shuch, B.; Kim, H.S.; Bremmer, F.; Trojan, L.; Lotz, J.; Uhlig, A. Radiomic features and machine learning for the discrimination of renal tumor histological subtypes: A pragmatic study using clinical-routine computed tomography. Cancers 2020, 12, 3010. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Yin, F.; Yu, Y.; Zhang, H.; Wen, G. CT-based multi-phase Radiomic models for differentiating clear cell renal cell carcinoma. Cancer Imaging 2021, 21, 42. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Xing, Z.; Jiang, Z.; Chen, J.; Pan, L.; Qiu, J.; Xing, W. CT-based radiomic model predicts high grade of clear cell renal cell carcinoma. Eur. J. Radiol. 2018, 103, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Yin, R.H.; Yang, Y.C.; Tang, X.Q.; Shi, H.F.; Duan, S.F.; Pan, C.J. Enhanced computed tomography radiomics-based machine-learning methods for predicting the Fuhrman grades of renal clear cell carcinoma. J. X-ray Sci. Technol. 2021, 29, 1149–1160. [Google Scholar] [CrossRef]
- Bektas, C.T.; Kocak, B.; Yardimci, A.H.; Turkcanoglu, M.H.; Yucetas, U.; Koca, S.B.; Erdim, C.; Kilickesmez, O. Clear cell renal cell carcinoma: Machine learning-based quantitative computed tomography texture analysis for prediction of fuhrman nuclear grade. Eur. Radiol. 2019, 29, 1153–1163. [Google Scholar] [CrossRef]
- Lin, F.; Cui, E.M.; Lei, Y.; Luo, L.P. CT-based machine learning model to predict the Fuhrman nuclear grade of clear cell renal cell carcinoma. Abdom. Radiol. 2019, 44, 2528–2534. [Google Scholar] [CrossRef] [PubMed]
- Haji-Momenian, S.; Lin, Z.; Patel, B.; Law, N.; Michalak, A.; Nayak, A.; Earls, J.; Loew, M. Texture analysis and machine learning algorithms accurately predict histologic grade in small (<4 cm) clear cell renal cell carcinomas: A pilot study. Abdom. Radiol. 2020, 45, 789–798. [Google Scholar]
- Lai, S.; Sun, L.; Wu, J.; Wei, R.; Luo, S.; Ding, W.; Liu, X.; Yang, R.; Zhen, X. Multiphase contrast-enhanced CT-based machine learning models to predict the Fuhrman nuclear grade of clear cell renal cell carcinoma. Cancer Manag. Res. 2021, 13, 999. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Wei, R.; Lu, S.; Lai, S.; Wu, J.; Wu, Z.; Pang, X.; Wei, X.; Jiang, X.; Zhen, X.; et al. Fuhrman nuclear grade prediction of clear cell renal cell carcinoma: Influence of volume of interest delineation strategies on machine learning-based dynamic enhanced CT radiomics analysis. Eur. Radiol. 2022, 32, 2340–2350. [Google Scholar] [CrossRef]
- Yi, X.; Xiao, Q.; Zeng, F.; Yin, H.; Li, Z.; Qian, C.; Wang, C.; Lei, G.; Xu, Q.; Li, C.; et al. Computed tomography radiomics for predicting pathological grade of renal cell carcinoma. Front. Oncol. 2021, 10, 570396. [Google Scholar] [CrossRef]
- He, X.; Wei, Y.; Zhang, H.; Zhang, T.; Yuan, F.; Huang, Z.; Han, F.; Song, B. Grading of clear cell renal cell carcinomas by using machine learning based on artificial neural networks and radiomic signatures extracted from multidetector computed tomography images. Acad. Radiol. 2020, 27, 157–168. [Google Scholar] [CrossRef]
- Xu, L.; Yang, C.; Zhang, F.; Cheng, X.; Wei, Y.; Fan, S.; Liu, M.; He, X.; Deng, J.; Xie, T.; et al. Deep Learning Using CT Images to Grade Clear Cell Renal Cell Carcinoma: Development and Validation of a Prediction Model. Cancers 2022, 14, 2574. [Google Scholar] [CrossRef]
- Deng, Y.; Soule, E.; Cui, E.; Samuel, A.; Shah, S.; Lall, C.; Sundaram, C.; Sandrasegaran, K. Usefulness of CT texture analysis in differentiating benign and malignant renal tumours. Clin. Radiol. 2020, 75, 108–115. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, Z.; Chen, Y.C.; Zhao, Z.Y.; Yin, X.D.; Jiang, H.B. A deep learning-based radiomics model for differentiating benign and malignant renal tumors. Transl. Oncol. 2019, 12, 292–300. [Google Scholar] [CrossRef]
- Nie, P.; Yang, G.; Wang, Z.; Yan, L.; Miao, W.; Hao, D.; Wu, J.; Zhao, Y.; Gong, A.; Cui, J.; et al. A CT-based radiomics nomogram for differentiation of renal angiomyolipoma without visible fat from homogeneous clear cell renal cell carcinoma. Eur. Radiol. 2020, 30, 1274–1284. [Google Scholar] [CrossRef]
- Lee, H.; Hong, H.; Kim, J.; Jung, D.C. Deep feature classification of angiomyolipoma without visible fat and renal cell carcinoma in abdominal contrast-enhanced CT images with texture image patches and hand-crafted feature concatenation. Med. Phys. 2018, 45, 1550–1561. [Google Scholar] [CrossRef] [PubMed]
- Kunapuli, G.; Varghese, B.A.; Ganapathy, P.; Desai, B.; Cen, S.; Aron, M.; Gill, I.; Duddalwar, V. A decision-support tool for renal mass classification. J. Digit. Imaging 2018, 31, 929–939. [Google Scholar] [CrossRef]
- Ma, Y.; Xu, X.; Pang, P.; Wen, Y. A CT-Based Tumoral and Mini-Peritumoral Radiomics Approach: Differentiate Fat-Poor Angiomyolipoma from Clear Cell Renal Cell Carcinoma. Cancer Manag. Res. 2021, 13, 1417. [Google Scholar] [CrossRef] [PubMed]
- Uhm, K.H.; Jung, S.W.; Choi, M.H.; Shin, H.K.; Yoo, J.I.; Oh, S.W.; Kim, J.Y.; Kim, H.G.; Lee, Y.J.; Youn, S.Y.; et al. Deep learning for end-to-end kidney cancer diagnosis on multi-phase abdominal computed tomography. NPJ Precis. Oncol. 2021, 5, 54. [Google Scholar] [CrossRef] [PubMed]
- Kocak, B.; Yardimci, A.H.; Bektas, C.T.; Turkcanoglu, M.H.; Erdim, C.; Yucetas, U.; Koca, S.B.; Kilickesmez, O. Textural differences between renal cell carcinoma subtypes: Machine learning-based quantitative computed tomography texture analysis with independent external validation. Eur. J. Radiol. 2018, 107, 149–157. [Google Scholar] [CrossRef]
- Sun, X.; Liu, L.; Xu, K.; Li, W.; Huo, Z.; Liu, H.; Shen, T.; Pan, F.; Jiang, Y.; Zhang, M. Prediction of ISUP grading of clear cell renal cell carcinoma using support vector machine model based on CT images. Medicine 2019, 98, e15022. [Google Scholar] [CrossRef]
- Shu, J.; Wen, D.; Xi, Y.; Xia, Y.; Cai, Z.; Xu, W.; Meng, X.; Liu, B.; Yin, H. Clear cell renal cell carcinoma: Machine learning-based computed tomography radiomics analysis for the prediction of WHO/ISUP grade. Eur. J. Radiol. 2019, 121, 108738. [Google Scholar] [CrossRef]
- Nikpanah, M.; Xu, Z.; Jin, D.; Farhadi, F.; Saboury, B.; Ball, M.W.; Gautam, R.; Merino, M.J.; Wood, B.J.; Turkbey, B.; et al. A deep-learning based artificial intelligence (AI) approach for differentiation of clear cell renal cell carcinoma from oncocytoma on multi-phasic MRI. Clin. Imaging 2021, 77, 291–298. [Google Scholar] [CrossRef]
- Arita, Y.; Yoshida, S.; Kwee, T.C.; Akita, H.; Okuda, S.; Iwaita, Y.; Mukai, K.; Matsumoto, S.; Ueda, R.; Ishii, R.; et al. Diagnostic value of texture analysis of apparent diffusion coefficient maps for differentiating fat-poor angiomyolipoma from non-clear-cell renal cell carcinoma. Eur. J. Radiol. 2021, 143, 109895. [Google Scholar] [CrossRef]
- Gündüz, N.; Eser, M.; Yıldırım, A.; Kabaalioğlu, A. Radiomics improves the utility of ADC for differentiation between renal oncocytoma and chromophobe renal cell carcinoma: Preliminary findings. Actas Urológicas Espa Nolas 2022, 46, 167–177. [Google Scholar] [CrossRef]
- Choi, J.W.; Hu, R.; Zhao, Y.; Purkayastha, S.; Wu, J.; McGirr, A.J.; Stavropoulos, S.W.; Silva, A.C.; Soulen, M.C.; Palmer, M.B.; et al. Preoperative prediction of the stage, size, grade, and necrosis score in clear cell renal cell carcinoma using MRI-based radiomics. Abdom. Radiol. 2021, 46, 2656–2664. [Google Scholar] [CrossRef] [PubMed]
- Hoang, U.N.; Malayeri, A.A.; Lay, N.S.; Summers, R.M.; Yao, J. Texture analysis of common renal masses in multiple MR sequences for prediction of pathology. In Proceedings of the Medical Imaging 2017: Computer-Aided Diagnosis, Orlando, FL, USA, 11–16 February 2017; SPIE: Bellingham, WA, USA; Volume 10134, pp. 917–929. [Google Scholar]
- Gatta, G.; Di Grezia, G.; Cuccurullo, V.; Sardu, C.; Iovino, F.; Comune, R.; Ruggiero, A.; Chirico, M.; La Forgia, D.; Fanizzi, A.; et al. MRI in pregnancy and precision medicine: A review from literature. J. Pers. Med. 2021, 12, 9. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.; Hung, S.C.; Rathmell, W.K.; Shen, L.; Wang, L.; Lin, W.; Fielding, J.R.; Khandani, A.H.; Woods, M.E.; Milowsky, M.I.; et al. Integrative radiomics expression predicts molecular subtypes of primary clear cell renal cell carcinoma. Clin. Radiol. 2018, 73, 782–791. [Google Scholar] [CrossRef] [PubMed]
- Bowen, L.; Xiaojing, L. Radiogenomics of clear cell renal cell carcinoma: Associations between mRNA-based subtyping and CT imaging features. Acad. Radiol. 2019, 26, e32–e37. [Google Scholar] [CrossRef] [PubMed]
- Kocak, B.; Durmaz, E.S.; Ates, E.; Ulusan, M.B. Radiogenomics in clear cell renal cell carcinoma: Machine learning–based high-dimensional quantitative CT texture analysis in predicting PBRM1 mutation status. Am. J. Roentgenol. 2019, 212, W55–W63. [Google Scholar] [CrossRef]
- Marigliano, C.; Badia, S.; Bellini, D.; Rengo, M.; Caruso, D.; Tito, C.; Miglietta, S.; Palleschi, G.; Pastore, A.L.; Carbone, A.; et al. Radiogenomics in clear cell renal cell carcinoma: Correlations between advanced CT imaging (texture analysis) and microRNAs expression. Technol. Cancer Res. Treat. 2019, 18, 1533033819878458. [Google Scholar] [CrossRef]
- Scrima, A.T.; Lubner, M.G.; Abel, E.J.; Havighurst, T.C.; Shapiro, D.D.; Huang, W.; Pickhardt, P.J. Texture analysis of small renal cell carcinomas at MDCT for predicting relevant histologic and protein biomarkers. Abdom. Radiol. 2019, 44, 1999–2008. [Google Scholar] [CrossRef]
- Yu, Z.; Ding, J.; Pang, H.; Fang, H.; He, F.; Xu, C.; Li, X.; Ren, K. CT Features in Differentiating Chromophobe Cell Renal Carcinoma from Renal Oncocytoma and CK7 Expression Evaluation: A Radiomics Analysis. Res. Sq. 2022. [Google Scholar] [CrossRef]

| Study | Data | Radiomics | Methods | Results | Findings |
|---|---|---|---|---|---|
| Main Goal(s): Benign vs. Malignant | |||||
| Yang et al. [27] |
|
|
|
| Radiomics extracted from unenhanced CT phase can be used to precisely discriminate AMLwvf from RCC using SVM |
| You et al. [28] |
|
|
|
| Radiomics of small renal masses extracted from multiphasic CECT can accurately differentiate between AMLwvf and ccRCC using SVM |
| Coy et al. [60] |
|
|
|
| Radiomics extracted from 3D VOI of the entire tumor shown a reasonable diagnostic accuracy using Phase 4 CECT and TL of GTf |
| Deng et al. [82] (Study 1) |
|
|
|
| Entropy had shown higher statistically significant values (p < 0.05) in RCC tumors and is a sufficient discriminatory radiomic marker |
| Zhou et al. [83] |
|
|
|
| Deep learning can potentially be used to identify malignant renal tumors using deep transfer learning |
| Kim et al. [61] |
|
|
|
| Entropy ≥ 4 differentiated RCC from benign renal tumors (AUC = 0.89). A better AUC of 0.92 is obtained using a combined model |
| Nie et al. [84] |
|
|
|
| Radiomics of multiphasic CECT distinguish AMLwvf from ccRCC. A combined model that integrates clinical factors, with a Nomo-score ≥ 1.451, provided higher diagnostic performance (Acc = 0.89, AUC = 0.95) |
| Tang et al. [57] |
|
|
|
| Integrating different radiomic markers is potentially helpful in distinguishing AMLwvf from RCC renal tumors |
| Lee et al. [53] (Study 1) |
|
|
|
| Proper selection and integration of optimal radiomic markers and machine learning-based classifiers could sufficiently differentiate between AMLwvf and ccRCC |
| Lee et al. [85] (Study 2) |
|
|
|
| A combined model integrating hand-crafted with deep radiomic markers provided an enhanced diagnostic performance than individual models and thus; has the potential to distinguish AMLwvf from ccRCC |
| Feng et al. [54] (Study 1) |
|
|
|
| Combination of SVM, RFE, and SMOTE can help selecting optimal radiomics that could accurately distinguish AMLwvf from RCC |
| Yan et al. [55] |
|
|
|
| Optimal radiomics of multiphasic CECT images can potentially be used to discriminate between AMLwvf, ccRCC, and paRCC |
| Hodgdon et al. [14] |
|
|
|
| Radiomic markers of unenhanced CT images can differentiate between AMLwvf and RCC |
| Tanaka et al. [62] |
|
|
|
| Deep learning can be used to identify malignant tumors, especially in Phase 2 CECT |
| Kunapuli et al. [86] |
|
|
|
| RFGB machine learning classifier and radiomic markers have the potential to identify the malignancy status of renal tumors |
| Yap et al. [59] |
|
|
|
| Combining shape and texture radiomic markers of multiphasic CECT can improve the overall diagnostic performance |
| Ma et al. [56] (Study 1) |
|
|
|
| Combined model integrating radiomics from different phases of CECT enhanced the final diagnostic performance when compared with individual models as well as unenhanced CT |
| Ma et al. [87] (Study 2) |
|
|
|
| The perirenal model that extracts radiomic markers from Phase 3 CECT is superior to other phases to distinguish between AMLwvf and ccRCC |
| Nassiri et al. [58] |
|
|
|
| Radiomic markers of Phase 3 CECT can sufficiently identify malignancy status. When combined, some clinical factors can improve the overall diagnostic performance |
| Zabihollahy et al. [66] |
|
|
|
| Semiautomated CNN showed the highest diagnostic performance in distinguishing RCC from benign renal tumors using CECT |
| Uhlig et al. [36] (Study 1) |
|
|
|
| Radiomic markers derived from Phase 3 CECT can successfully differentiate benign from malignant renal tumors using RF machine learning classifier |
| Li et al. [63] (Study 1) |
|
|
|
| Radiomics derived from multiphasic CECT can accurately differentiate chrRCC from ONC using SVM |
| Li et al. [64] (Study 2) |
|
|
|
| Radiomics of multiphasic CECT can differentiate ONC from ccRCC. By integrating clinical factors, enhanced diagnosis is obtained (Acc = 0.87, Sen = 0.86, Spe = 0.87, and AUC = 0.90) |
| Li et al. [65] (Study 3) |
|
|
|
| Radiomics of multiphasic CT can differentiate ONC from chrRCC. Clinical factors, when combined, with a Nomo-score ≥0.19 can enhance the diagnosis (Acc = 0.95, Sen = 0.90, Spe = 0.97, and AUC = 0.99) |
| Main Goal(s): Malignant Subtyping | |||||
| Uhlig et al. [70] (Study 2) |
|
|
|
| Radiomic markers extracted from Phase 3 CECT along with machine learning classifiers, can help distinguish different subtypes. Differentiation of ONCs remains challenging |
| Uhm et al. [88] |
|
|
|
| Deep learning outperformed radiological diagnosis of renal tumors using multiphasic CECT |
| Kocak et al. [89] |
|
|
|
| Combined radiomics extracted from phases 1 and 2 (Phase 2 is superior) can distinguish RCC major subtypes using machine learning. Distinguishing ccRCC, paRCC, chrRCC remains challenging |
| Zhang et al. [35] |
|
|
|
| Radiomic markers of Phase 2 CECT have the potential for RCC subtyping using SVM |
| Chen et al. [71] |
|
|
|
| Second-order radiomics integrated with nontexture markers of Phase 3 CECT provide the best RCC subtyping performance (AUC = 0.9) |
| Deng et al. [34] (Study 2) |
|
|
|
| Entropy had shown higher statistically significant values in ccRCC (p < 0.05) with high values being correlated with RCC’s high grade |
| Main Goal(s): Benign vs. Malignant and Malignant Subtyping | |||||
| Shehata et al. [68] |
|
|
|
| A MLP-ANN diagnostic model integrating shape, texture, and functional radiomic-based markers can identify malignant renal tumors as well as their subtypes. |
| Yu et al. [67] |
|
|
|
| Machine learning and 1st-Order radiomic markers (e.g., skewness, kurtosis, and median) demonstrates high diagnostic performance of different renal tumors’ types. |
| Varghese et al. [69] |
|
|
|
| With a significance level (p < 0.05), various radiomic markers are helpful in discriminating benign from malignant renal tumors as well as RCC subtypes |
| Cui et al. [52] |
|
|
|
| Machine learning-based radiomics techniques are comparable to radiological assessment and can precisely distinguish AMLwvf from RCC and its subtypes |
| Main Goal(s): Malignant Grading | |||||
| Sun et al. [90] |
|
|
|
| Radiomics extracted and combined from phases 2 and 3 of CECT have the potential to successfully grade ccRCC renal tumors using SVM |
| Feng et al. [37] (Study 2) |
|
|
|
| Statistically significant (p < 0.05) radiomics markers, such as entropy, STD, and kurtosis are superior to grade ccRCC renal tumors |
| Shu et al. [38] (Study 1) |
|
|
|
| Combined radiomic markers extracted from phases 2 and 3 of CECT are sufficient for ccRCC grading |
| Shu et al. [91] (Study 2) |
|
|
|
| Combined radiomic markers extracted of phases 2 and 3 of CECT along with machine learning could be sufficiently used for ccRCC grading |
| Ding et al. [72] |
|
|
|
| Radiomic markers of phases 2 and 3 of CECT are helpful in ccRCC grading |
| Bektas et al. [74] |
|
|
|
| SVM machine learning classifier and radiomic markers of Phase 3 CECT can be used in ccRCC grading |
| Lin et al. [75] |
|
|
|
| Using machine learning, combined radiomic markers from phases 1, 2, and 3 of CECT can sufficiently grade ccRCCs |
| He et al. [80] |
|
|
|
| Combined radiomic markers of phases 2 and 3 of CECT have the potential for RCC grading using ANN |
| Momenian et al. [76] |
|
|
|
| First-order radiomic markers extracted from Phase 2 CECT showed the best diagnostic performance in ccRCC grading using the RF classifier when compared with 2nd-order radiomic markers alone as well as combined radiomic markers. |
| Yin et al. [73] |
|
|
|
| 2nd-Order radiomic markers of Phase 2 CECT provided the highest ccRCC grading performance using SVM |
| Lai et al. [77] |
|
|
|
| Shape and 1st-Order radiomics extracted from Phase 1 CECT along with a Bagging classifier provided the highest ccRCC grading performance (AUC = 0.75) |
| Yi et al. [79] |
|
|
|
| Radiomic markers of Phase 1 CECT can successfully grade ccRCCs using SVM (AUC = 0.91) |
| Xu et al. [81] |
|
|
|
| Deep learning applied on Phase 2 CECT images has the potential to grade ccRCC renal tumors with an AUC of 0.88 using the combined (Ensamble) model outperforming all other individual models. |
| Luo et al. [78] |
|
|
|
| Shape and 1st-Order radiomics extracted from phase 1 and 4 of CECT along with an RF classifier demonstrated the highest diagnostic performance in ccRCC grading (AUC = 0.87) |
| Main Goal(s): Malignant Grading and Staging | |||||
| Demirjian et al. [39] |
|
|
|
| Radiomic markers of multiphasic CECT have the potential to grade and stage ccRCCs using RF (AUC = 0.73 and 0.77) |
| Study | Data | Radiomics | Methods | Results | Findings |
|---|---|---|---|---|---|
| Xu et al. [29] |
|
|
|
| Combined radiomic markers of multimodal MRIs can sufficiently identify the malignancy status of renal tumors by utilizing handcrafted-based RF or DL-based classification models |
| Oostenburgge et al. [30] |
|
|
|
| Radiomics extracted from 3D ADCs such as standard deviation and entropy can discriminate ONC from RCC when combined with tumor volume and gender |
| Li et al. [31] |
|
|
|
| Radiomic markers extracted from 3D ADCs of DW-MRIs are significantly higher (p < 0.05) in malignant than benign tumors |
| Razik et al. [23] |
|
|
|
| MPP and the mean value can distinguish RCC from AML as well as RCC from ONC with an AUC of 0.89 and 0.94 at s/mm2 and s/mm2 of DW-MRI, respectively |
| Nikpanah et al. [92] |
|
|
|
| Using multiphasic MRIs, DL-based system can provide high diagnostic performance that differentiates ONC from ccRCC renal tumors |
| Arita et al. [93] |
|
|
|
| Long-zone high grey-level emphasis is the most informative radiomic marker to distinguish between AML and nccRCC using an RF classifier with (AUC = 0.82) |
| Gunduz et al. [94] |
|
|
|
| Squared root of mean ADC and GLRLM radiomic markers of ADC maps can sufficiently differentiate between ONC and chrRCC |
| Matsumoto et al. [32] |
|
|
|
| Mean ADC, grey-level run emphasis, and long-run low grey-level, are the most dominant and important radiomic markers in distinguishing AML from ccRCC with an AUC of 0.87 |
| Hoang et al. [96] (Study 1) |
|
|
|
| Using an RF classification model, first-order radiomic markers of multiphasic CEMRI have the potential to identify RCC renal tumors |
| Main Goal(s): Benign vs. Malignant and Malignant Subtyping | |||||
| Hoang et al. [33] (Study 2) |
|
|
|
| First-order radiomic markers are important for identifying the malignancy status, while adding second-order markers helps in RCC subtyping |
| Main Goal(s): Malignant Grading | |||||
| Sun et al. [40] |
|
|
|
| Radiomic markers of SW-MRI can reliably differentiate low-grade from high-grade ccRCC |
| Chen et al. [41] |
|
|
|
| First- and second-order radiomic markers of Phase 2 CEMRI along with MLP-ANN classification model have the potential to grade ccRCC |
| Choi et al. [95] |
|
|
|
| Proper selection and integration of optimal radiomic markers of MRIs can potentially help grade ccRCCs |
| Main Goal(s): Malignant Subtyping and Grading | |||||
| Goyal et al. [22] |
|
|
|
| Multiple first-order radiomic markers of multiparametric MRIs are beneficial tools in both subtyping and grading of renal tumors |
| Study | Main Goal | Radiomics | Methods | Results | Findings |
|---|---|---|---|---|---|
| Bharwani et al. [43] | To find the radiomic markers extracted from diffusion-weighted MR (DW-MR) and dynamic contrast-enhanced MR (DCE-MR) images that correlate with responses to neoadjuvant sunitinib therapy, in particular overall survival (OS), in metastatic renal cell carcinoma (mRCC) patients (N = 20) |
|
|
| Patients with a tumour volume < median at baseline had a prolonged OS. A greater than median increase in AUClow of ADCs indicates reduced OS while a decrease in AUClow indicates a prolonged OS in mRCC. A positive correlation between mean ADC was found between the primary tumor and metastases |
| Antunes et al. [44] | To find the optimal radiomic markers on an integrated positron emission tomography (PET)/MRI that best describe early treatment response/changes in advanced mRCC undergoing sunitinib therapy (N = 2) |
|
|
| SUV from PET, T2w difference average from T2w, and ADC energy from DW-MRI ADC maps are ranked highest for reproducibility and for capturing treatment related changes/response |
| Lubner et al. [50] | To determine the radiomic-based texture markers extracted from CECT images on phases 1 and 3 of RCCs patients that are correlated with the histological finding and treatment response (N = 157) |
|
|
| 1st-Order texture markers (entropy, STD, and MPP) extracted from phases 1 and 3 of CECT are correlated with histologic type, nuclear grade, and clinical outcomes (time to recurrence and OS) in patients with RCC |
| Boos et al. [45] | To assess the ability of mean and median intensity attenuation (HU) using CECT images for predicting treatment response (response, stable, and progression) in patients with RCC tumors who received targeted therapy, namely VEGFR TKI (N = 19) |
|
|
| Median HU attenuation shift rather than mean yields better prediction accuracy and thus is preferable. It correlates well with clinical outcome in mRCC patients. A shift of median <–44 HU indicates a partial response while a shift of median >–41 HU indicates progression |
| Haider et al. [46] | To highlight potential radiomic predictors of progression-free survival (PFS) and overall survival (OS) that could be extracted from CECT images in RCC patients undergoing treatment with sunitinib (N = 40) |
|
|
| nSTD extracted from CECT before and after sunitinib treatment is positively correlated with both OS and PFS, while entropy and % size change are predictors of OS in RCC patients |
| Mains et al. [47] | To identify radiomic functional markers derived from CECT to act as potential predictors of OS and PFS in mRCC patients (N = 69) |
|
|
| Medians and modes of BVdeconv, BVpatlak, and BFdeconv are statistically significant (p < 0.05) and provide the strongest correlation with clinical outcome (PFS and OS) |
| Reynolds et al. [51] | To investigate the ability of radiomic markers extracted from DW-MRI (N = 12) and DCE-MRI (N = 10) as potential predictors of early treatment responses in RCC patients after stereotactic ablative body radiotherapy (SABR) |
|
|
| Statistically significant correlations between the change in percentage washout, change in mean IRE, and mean Ktrans, and the change in tumour volume (p < 0.05). Changes in ADC kurtosis showed statistically significant positive correlations with the percentage tumour volume change (p < 0.05) |
| Khodabakhshi et al. [48] | To explore the potential radiomic markers extracted from Phase 2 CECT and clinical biomarkers for the prediction of OS in RCC patients after partial or radical nephrectomy (N = 210) |
|
|
| Besides tumor heterogeneity, grade, and stage as clinical indicators for OS, flatness, area density, and median are the most significant radiomic-based predictors (p < 0.05) of OS |
| Zhang et al. [49] | To investigate the prediction potentials of radiomics-based markers extracted from CECT images and clinical markers that are linked to progression-free survival (PFS) after partial or radical nephrectomy in ccRCC patients (N = 175) |
|
|
| Radiomic-based markers extracted from CECT, especially Phase 2, demonstrated better prediction performance of PFS in ccRCC patients when combined with clinical markers (age, stage, and KPS score) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shehata, M.; Abouelkheir, R.T.; Gayhart, M.; Van Bogaert, E.; Abou El-Ghar, M.; Dwyer, A.C.; Ouseph, R.; Yousaf, J.; Ghazal, M.; Contractor, S.; et al. Role of AI and Radiomic Markers in Early Diagnosis of Renal Cancer and Clinical Outcome Prediction: A Brief Review. Cancers 2023, 15, 2835. https://doi.org/10.3390/cancers15102835
Shehata M, Abouelkheir RT, Gayhart M, Van Bogaert E, Abou El-Ghar M, Dwyer AC, Ouseph R, Yousaf J, Ghazal M, Contractor S, et al. Role of AI and Radiomic Markers in Early Diagnosis of Renal Cancer and Clinical Outcome Prediction: A Brief Review. Cancers. 2023; 15(10):2835. https://doi.org/10.3390/cancers15102835
Chicago/Turabian StyleShehata, Mohamed, Rasha T. Abouelkheir, Mallorie Gayhart, Eric Van Bogaert, Mohamed Abou El-Ghar, Amy C. Dwyer, Rosemary Ouseph, Jawad Yousaf, Mohammed Ghazal, Sohail Contractor, and et al. 2023. "Role of AI and Radiomic Markers in Early Diagnosis of Renal Cancer and Clinical Outcome Prediction: A Brief Review" Cancers 15, no. 10: 2835. https://doi.org/10.3390/cancers15102835
APA StyleShehata, M., Abouelkheir, R. T., Gayhart, M., Van Bogaert, E., Abou El-Ghar, M., Dwyer, A. C., Ouseph, R., Yousaf, J., Ghazal, M., Contractor, S., & El-Baz, A. (2023). Role of AI and Radiomic Markers in Early Diagnosis of Renal Cancer and Clinical Outcome Prediction: A Brief Review. Cancers, 15(10), 2835. https://doi.org/10.3390/cancers15102835