Simple Summary
It is known that socioeconomically disadvantaged people more often develop esophageal cancer. Therefore, we assumed that those patients more often have advanced tumor stages and comorbidities at the time of surgery and, thus, are more likely to suffer from postoperative complications and poorer survival. To clarify this, we used the purchasing power of the respective postal codes to estimate the socioeconomic status (SES) of 310 patients who had undergone surgery for esophageal cancer in our institution. Fortunately, it turns out that SES was not associated with tumor stage or comorbidities at the time of surgery. Moreover, SES was neither related to postoperative complications nor overall survival. In conclusion, socioeconomic inequalities of patients treated at a high-volume center do not affect treatment results.
Abstract
In Germany, socioeconomically deprived citizens more often develop esophageal carcinoma, since typical risk factors follow the social gradient. Therefore, we hypothesized that socioeconomic deprivation might also be associated with advanced tumor stages and comorbidities at the time of surgery. As a consequence, socioeconomic deprivation may be related to postoperative complications and reduced overall survival. Therefore, 310 patients who had undergone esophagectomy for cancer in curative intent between 2012 and 2020 at the University Medical Center Hamburg-Eppendorf (UKE) were included in this study. Socioeconomic status (SES) was estimated using the purchasing power of patients’ postal codes as a surrogate parameter. No association was found between SES and tumor stage or comorbidities at the time of surgery. Moreover, SES was neither associated with postoperative complications nor overall survival. In conclusion, socioeconomic inequalities of patients treated at a high-volume center do not affect treatment outcomes.
1. Introduction
Esophageal carcinoma is among the most common causes of cancer deaths in Germany. Each year, approximately 5710 new diagnoses are made in men and about 1840 in women. Unfortunately, most patients present at advanced stages and die in the course of the disease [1]. It has been demonstrated that not only in Germany [2] but also in other countries, e.g., Sweden [3], France [4] and Iran [5], esophageal carcinomas occur more frequently in socioeconomically deprived people.
The two main histological subtypes, squamous cell carcinoma and adenocarcinoma, have different risk factors. Among the most critical risk factors for esophageal squamous-cell carcinoma are nicotine and alcohol consumption. Obesity and the frequently associated gastroesophageal reflux disease favor the development of esophageal adenocarcinoma. In Germany, these risk factors are related to socioeconomic status (SES): people with a high SES more often exceed the recommended moderate amounts of alcohol [6] and economically weaker populations smoke more often and are less successful at quitting [7]. The prevalence of obesity also follows the social gradient: it is more common in people with low SES [8].
The treatment of esophageal cancer is complex and depends on the histologic subtype, tumor stage, and the patient’s operability. Endoscopic resection is performed in patients with an early carcinoma (Union for International Cancer Control (UICC) TNM stage cT1a, N0, M0) [9]. Operable patients with stage cT1b-T2 N0 primarily undergo a transthoracic radical en bloc esophageal resection with systematic lymph node dissection [9]. Neoadjuvant radiochemotherapy is recommended in patients with locally advanced esophageal squamous cell carcinoma (cT3-T4 or N1-N3 M0). In patients with locally advanced esophageal adenocarcinoma (cT3-T4 or N1-N3 M0), perioperative chemotherapy is recommended [10]. Regarding the surgical technique, open esophagectomy was the standard for decades. However, in recent years, laparoscopic, hybrid, and cost-intensive robotic esophagectomies have become well-established due to better short-term outcomes [11].
In Germany, health insurance is mandatory and provides comprehensive medical care, including state-of-the-art cancer treatment, to all citizens and legal residents. It is financed by statutory health insurance (SHI) and private health insurance (PHI). Around 85% of the population is covered by SHI, which is mandatory for individuals with an income below a certain threshold. Those who earn above this threshold can choose between SHI and PHI. Contributions of PHI members depend on their age, health status, and level of coverage [12]. Since the amount paid by PHI to the hospital is usually a multiple of the amount paid by SHI, privately insured patients often have advantages; for example, shorter waiting times for appointments.
Given this background, we hypothesized that, in Germany, esophageal cancer is not only characterized by a social determination of incidence, as shown by Hoebel et al. [2], but also with advanced tumor stages and comorbidities at the time of surgery. Thus, the risk for postoperative complications might be higher, and, as a consequence, the overall survival of socially deprived patients might be reduced. Unfortunately, due to the lack of digitalization and data exchange in the German health care system, there is no detailed German national register that captures the relevant confounders, such as comorbidity and surgical technique, to adequately clarify whether the SES is associated with treatment outcome after oncologic esophagectomy. Therefore, taking into account the relevant confounders, this retrospective single-center study is the first to investigate whether socioeconomic status is associated with treatment results after esophagectomy for cancer in Germany. If our hypothesis proves true, targeted prevention programs should be developed and provided.
2. Materials and Methods
2.1. Patient Selection
Inclusion criteria were (I) histologically confirmed esophageal malignancy with UICC stage I-III at the time of diagnosis and (II) transthoracic radical en bloc esophageal resection with systematic lymph node dissection between 2012 and 2020 at the Department of General, Visceral and Thoracic Surgery, University Medical Center Hamburg-Eppendorf (UKE). Patients with a history of former carcinoma were excluded. In total, 369 individuals have been assessed for eligibility. Of these, 35 patients have been noted as ineligible. Twenty-four individuals were eligible but refused to enter the study. A total of 310 patients were confirmed eligible and recruited.
2.2. Treatment
According to the German guideline for esophageal cancer [13], patients with cT1b-T2 N0 M0 carcinomas primarily underwent transthoracic radical en bloc esophageal resection with systematic lymph node dissection. Patients with locally advanced, non-metastatic esophageal squamous-cell carcinoma (cT3-T4 or N1-N3 M0) were treated with neoadjuvant radiochemotherapy following the CROSS protocol [10]. Patients with locally advanced, non-metastatic esophageal adenocarcinoma (cT3-T4 or N1-N3 M0) received perioperative chemotherapy following the FLOT protocol or neoadjuvant chemoradiotherapy [14]. If the pathological examination of the resected tissue unexpectedly revealed a locally advanced esophageal adenocarcinoma (cT3-T4 or N1-N3 MX), as opposed to the preoperative staging, adjuvant chemotherapy was administered. Patients who refused neoadjuvant radiochemotherapy or perioperative chemotherapy primarily underwent surgery. The UKE is a high-volume center for the treatment of esophageal carcinomas certified by the German Cancer Society.
2.3. Definition of Socioeconomic Status
As in previous studies, the purchasing power for the postal codes in the patients’ addresses was used as a surrogate parameter for SES [15,16,17]. The purchasing power data were acquired from Michael Bauer Research GmbH. Purchasing power reflects net household income. It encompasses all income from labor, capital investment, rents, leases after taxes and social security contributions and also includes transfers such as unemployment benefits, child allowances, and pensions. Income has previously been certified to correctly indicate socioeconomic status [17,18,19,20]. Based on the German GEDA study, a cut-off of EUR 24,000 per year was set as the threshold for a low or high socioeconomic status [21].
2.4. Further Data Retrieval
As part of the preoperative work-up, known comorbidities and typical risk factors such as nicotine or alcohol abuse were routinely recorded in our Clinical Information System (Soarian Clinicals®, Cerner, Kansas City, MO, USA). Nicotine abuse was defined as the consumption of more than 10 packyears. The number of packyears has been calculated by multiplying the number of cigarette packs smoked per day by the number of years estimated by the patient. Alcohol abuse was present when the patient, currently or in the history, fulfilled the general criteria of the dependence syndrome according to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition [22]. If the patients agreed to data collection, the patient and treatment characteristics were transferred from the Clinical Information System to our scientific database system (Ninox®, Berlin, Germany). Based on this, the preoperative Charlson Comorbidity Index before surgery was raised [23]. Postoperative complications were recorded as defined by the Esophagectomy Complications Consensus Group (ECCG) and classified according to Clavien–Dindo [24,25]. For overall survival, the time was recorded from the date of surgery to either the date of death of any cause according to the German National Population Register or the date of last access to the population register (last follow-up). The resulting median follow-up time was 20 months (range 0–108 months).
2.5. Statistical Analysis
The Statistical Package for Social Sciences (SPSS®) for Mac (Version 28) was used for the statistical analysis. Median and interquartile range (IQR) was used to describe the distribution of continuous and ordinal variables, and percentages were used for categorical variables. The chi-squared test was used to determine the univariate association between categorical variables. The Mann–Whitney U test was performed to compare continuous variables.
Survival curves for the overall survival of the patients were plotted (according to the Kaplan–Meier method) and analyzed by implementing the log-rank test as a univariate model. Cox proportional hazard regression was performed as a multivariate model of survival. The Cox proportional hazard regression model satisfied the proportional hazards assumptions. As variable entering method “Enter” was used. A p-value < 0.05 was considered to be statistically significant.
3. Results
3.1. Associations of Socioeconomic Status and Cohort Characteristics
The associations of SES with the cohort’s relevant clinical and pathological characteristics are shown in Table 1. The median estimated annual income of all 310 patients in the study was EUR 24,145 (IQR 21,399–26,474). A total of 154 patients (49.7%) with an estimated annual income of less than or equal to EUR 24,000 were assigned to the low SES group. Conversely, 156 patients (50.3%) with an estimated annual income of more than EUR 24,000 were assigned to the high SES group. The median annual income of low SES patients amounted to EUR 21,399 (IQR 20,057–22,496), and that of high SES patients was EUR 26,452 (24,717–28,293) per year. Regarding the type of health insurance, 260 patients (83.9%) had SHI, and 50 patients were privately insured (16.1%). As expected, patients with high SES were significantly more likely to have PHI (p = 0.035).

Table 1.
Associations of socioeconomic status and cohort characteristics. Data are given as median (interquartile range (IQR)) or N (%).
The median age was 64 (IQR 57–72) and showed no association with SES (p = 0.120). Likewise, the distribution of, in total, 242 men (78.1%) and 68 (21.9%) women did not differ between high and low SES (p = 0.445). Low SES patients were more likely to be smokers (p = 0.040) and tended towards a higher Body Mass Index (BMI, 25.2 (IQR 22.7–29.0) kg/m2, p = 0.054) compared to high SES patients (24.9 (IQR 21.8–27.5) kg/m2, p = 0.054). Regarding alcohol abuse, no significant difference was found between the two groups (p = 0.577). The mean Charlson Comorbidity Index (CCI) of all patients at the time of surgery was 4 (IQR 3–5). Interestingly, the CCI did not differ significantly between low and high SES (p = 0.815).
Concerning histological subtypes, patients with adenocarcinoma and squamous cell carcinoma were approximately equally distributed between the groups (p = 0.965). Regarding neoadjuvant treatment, 64 patients (20.6%) received chemotherapy according to the FLOT protocol, and 79 (25.5%) received radiochemotherapy following the CROSS protocol. The frequency of neoadjuvant therapy did not differ significantly between high and low SES (p = 0.662).
Open surgery was performed in 139 patients (44.8%) and 89 (28.7%) underwent laparoscopic surgery. Hybrid procedures (laparoscopic abdominal surgery, open thoracic surgery) were performed in 48 patients (15.5%), and 34 patients (11.0%) underwent complete robotic esophagectomy. No differences were found between high and low SES in the frequency of surgical techniques used (p = 0.839). Ninety patients (29.0%) developed a severe postoperative complication (Clavien–Dindo grades IIIb–IVb) and forty-four patients (14.2%) died during the hospital stay. The severity and frequency of postoperative complications (Clavien–Dindo) and in-hospital mortality did not differ between high and low SES (p = 0.443).
The UICC tumor stage also did not differ between groups (p = 0.542). Likewise, there was no significant difference in pathological tumor staging (p = 0.490), lymph node staging (p = 0.433), and resection status either (p = 0.284). Additionally, no differences were found in the frequency of adjuvant radiotherapy (p = 0.845) or chemotherapy between high and low SES (p = 0.582).
3.2. Associations of Socioeconomic Status and Survival
Univariate survival analysis revealed no association between socioeconomic status and survival (p = 0.748, Figure 1A). Similarly, the type of health insurance (SHI vs. PHI) was not associated with overall survival (p = 0.903, Figure 1B).
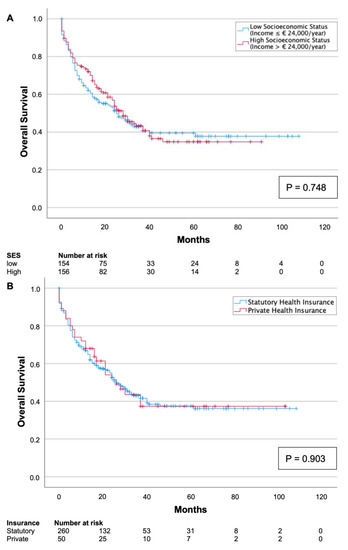
Figure 1.
Kaplan–Meier survival curves split by low and high socioeconomic status (A) and type of health insurance (B). Neither socioeconomic status (p = 0.748) nor type of health insurance (p = 0.903) was associated with overall survival.
As expected, univariate survival analysis confirmed a significant association with the CCI (p = 0.007, Table 2): The median survival of patients with a low CCI (≤4) was 50 (95%CI 44–57) months, whereas the median survival for patients with a high CCI (>5) was 42 (95%CI 34–51) months. Moreover, patients who underwent laparoscopic surgery showed significantly longer survival in univariate analysis with 59 (95%CI 49–56) months compared to patients who underwent open surgery (p = 0.014, Table 2). Additionally, patients with an advanced tumor stage showed poorer survival: the median survival for patients with UICC stage IV was 13 (95%CI 9–17) months, while the median survival for patients with UICC stage I was 77 (95%CI 66–89) months (p < 0.001, Figure 2A). Furthermore, the median survival of patients with minor postoperative complications (Clavien–Dindo grades I–IIIa) was more favorable (65 months (95%CI 57–72)) than the survival of patients with severe postoperative complications (Clavien–Dindo grades IIB–IVb) with a median of 36 (95%CI 28–43) months (p < 0.001, Figure 2B). No significant difference in survival was observed for the histological type (adenocarcinoma vs. squamous-cell carcinoma, p = 0.325, Table 2).

Table 2.
Univariate and multivariate survival analysis.
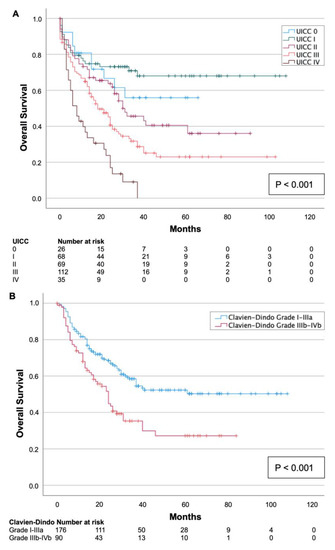
Figure 2.
Kaplan–Meier survival curves split by UICC tumor stage (A) and severity of postoperative complication according to Clavien–Dindo (B). An advanced tumor stage and severe postoperative complication (Clavien–Dindo grade IIIb–IVb) were related to shorter overall survival (p < 0.001).
4. Discussion
The present retrospective single-center study was the first study conducted in Germany to examine whether SES is associated with treatment outcomes after oncologic esophagectomy: First, it was found that socioeconomically deprived esophageal cancer patients were not more likely to have advanced tumor stages or severe comorbidities at the time of surgery. Second, no associations were found between SES and preoperative treatment, surgical technique, postoperative complications, in-hospital mortality, or adjuvant therapy. Last, neither SES nor the type of health insurance (SHI vs. PHI) had an influence on overall survival.
As no German study on this issue has been published so far, the results of the present study can only be compared with studies from other countries with considerably different healthcare systems. Those previous studies are usually based on national cancer registries and therefore have a higher number of patients. However, the analysis of registries is often accompanied by the problem that detailed information—for example, comorbidities, surgical technique, and postoperative complications—has not been systematically recorded and these details are, therefore, missing as confounders in the analysis.
For example, a French study using data from the French Network of Cancer Registries (FRANCIM) was published in 2021 and included 3250 esophageal cancer patients. It was found that the prognosis of esophageal cancer patients was markedly worsened by socioeconomic deprivation. More precisely, the hazard ratio of death in the lowest socioeconomic quintile compared to the highest quintile was 1.44 (95%CI 1.13–183). In this study, the tumor stage, tumor stage, comorbidities, surgical technique, and severity of postoperative complications were not considered as confounders in the analysis [26].
An analysis of Canadian databases, including 2125 esophageal adenocarcinoma patients diagnosed between 1993 and 2012, showed no association between SES and tumor stage at diagnosis or conducted therapy (surgery, chemotherapy, or radiotherapy). Moreover, the multivariate survival analysis showed no association between SES and the survival of patients with esophageal adenocarcinoma. In this study, comorbidity was estimated using the Johns Hopkins Adjusted Clinical Groups (ACG) case-mix system. Again, detailed information on the performed treatment, such as surgical technique and the severity of postoperative complications, was lacking in this study [27].
An analysis of the UK Hospital Episode Statistics (HES) database included 6282 patients who had received esophagectomy for cancer from 1998–2002. The authors found that the highest levels of socioeconomic deprivation had significantly higher mortality rates than those in areas with lower levels of deprivation. Precisely, the 30-day risk of death in patients in the lowest quintile for deprivation after esophagectomy was increased 1.37-fold (95%CI 1.03–1.85) compared to the highest quintile. However, in this study, important confounders are unknown to the authors, e.g., no records were available on whether the surgery was performed with palliative or curative intent. [28].
This study analyzed 4097 patients from Taiwan’s National Health Insurance Research Database (NHIRD) diagnosed with esophageal cancer who underwent any hospital treatment for their disease between 2002 and 2006. Here, the authors distinguished between individual SES based on the enrollee category and neighborhood SES. It was found that 5-year overall survival rates were poorest amongst individuals with low individual SES living in deprived neighborhoods. Although Taiwan has a universal healthcare system, patients with high individual SES from deprived areas were more likely to undergo surgery [29].
In a study from the USA, published in 2017, 11,599 patients from the National Cancer Data Base (NCDB) who underwent esophagectomy for cancer between 2003 and 2011 were analyzed. The multivariate analysis, which included tumor stage and the Charlson comorbidity index, showed that patients in the highest income quartile had better overall survival than those in the lowest quartile (HR 0.803, 95%CI 0.743–0.867) [30].
In a further study from the USA, which evaluated 60,621 patients with UICC stages I–III between 2004 and 2015, results demonstrated that black patients, uninsured patients, and patients living in areas with lower levels of education receive surgical interventions or any other kind of therapy less often. Consequently, patients receiving surgical treatment, compared to both, no treatment and definitive chemoradiation, had significantly better long-term survival. However, the researchers did not investigate whether socioeconomically disadvantaged patients within the group of surgically treated patients had a worse outcome [31].
A Dutch study by Bus et al. based on the Eindhoven Cancer Registry (ECR) found that patients with low SES were diagnosed with a more advanced tumor stage (13% vs. 10%, stage T4). The researchers found no significant difference in survival within the curative treatment group, which included 708 patients. The authors systematically recorded the comorbidity using the Charlson Comorbidity Index; relevant factors such as surgical technique and severity of postoperative complications were not recorded [32].
A limitation of the present study is its nature as a single-center study: First, SES had no significant influence on the outcome of patients treated at only one single high-volume center. Second, the number of patients is low compared to the above-mentioned register of studies from other countries. Therefore, a minor association between SES and postoperative outcomes cannot be ruled out with certainty. Of course, an evaluation at the German national level would be desirable to increase the number of cases and include all relevant institutions. However, unfortunately, as important confounders were not systematically recorded in the German national cancer registry until today, the authors do not believe this is feasible in the near future.
5. Conclusions
Once socioeconomically deprived patients gained access to the treatment at a high-volume center, fortunately, no significant differences in treatment outcomes compared to socioeconomically privileged patients were found.
Author Contributions
Conceptualization, M.K., J.W., S.W. and M.R.; data curation, M.K., J.Z., J.-K.G.; validation, J.R.I., N.M., S.W. and M.R.; statistical analysis, M.K., J.Z., S.W. and M.R.; writing—original draft preparation, M.K. and J.Z.; writing—review and editing, all authors.; visualization, M.K. and J.Z.; supervision, J.R.I.; project administration, J.R.I., S.W. and M.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the ethics committee of the Hamburg Medical Chamber, Germany approval number: PV3548; date of approval: 10 June 2020.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Robert-Koch-Institut. Population-Based Cancer Registry Dataset from the German Center for Cancer Registry Data; Robert-Koch-Institut: Berlin, Germany, 2019. [Google Scholar] [CrossRef]
- Hoebel, J.; Kroll, L.E.; Fiebig, J.; Lampert, T.; Katalinic, A.; Barnes, B.; Kraywinkel, K. Socioeconomic Inequalities in Total and Site-Specific Cancer Incidence in Germany: A Population-Based Registry Study. Front. Oncol. 2018, 8, 402. [Google Scholar] [CrossRef] [PubMed]
- Ljung, R.; Drefahl, S.; Andersson, G.; Lagergren, J. Socio-Demographic and Geographical Factors in Esophageal and Gastric Cancer Mortality in Sweden. PLoS ONE 2013, 8, e62067. [Google Scholar] [CrossRef] [PubMed]
- Bryere, J.; Dejardin, O.; Launay, L.; Colonna, M.; Grosclaude, P.; Launoy, G.; French Network of Cancer Registries (FRANCIM). Socioeconomic Status and Site-Specific Cancer Incidence, a Bayesian Approach in a French Cancer Registries Network Study. Eur. J. Cancer Prev. 2018, 27, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Islami, F.; Kamangar, F.; Nasrollahzadeh, D.; Aghcheli, K.; Sotoudeh, M.; Abedi-Ardekani, B.; Merat, S.; Nasseri-Moghaddam, S.; Semnani, S.; Sepehr, A.; et al. Socio-Economic Status and Oesophageal Cancer: Results from a Population-Based Case–Control Study in a High-Risk Area. Int. J. Epidemiol. 2009, 38, 978–988. [Google Scholar] [CrossRef]
- German Federal Health Survey: Alcohol. Available online: https://www.gbe-bund.de/pdf/alkohol.pdf (accessed on 18 May 2022).
- Kotz, D.; West, R. Explaining the Social Gradient in Smoking Cessation: It’s Not in the Trying, but in the Succeeding. Tob. Control 2008, 18, 43–46. [Google Scholar] [CrossRef] [PubMed]
- Kuntz, B.; Lampert, T. Socioeconomic Factors and Obesity. Dtsch. Arztebl. Int. 2010, 107, 517–522. [Google Scholar] [CrossRef]
- Hölscher, A.; Meyer, H. New S3 Guideline for Esophageal Cancer: Important Surgical Aspects. Chirurg 2016, 87, 865–872. [Google Scholar] [CrossRef]
- van Hagen, P.; Hulshof, M.C.C.M.; van Lanschot, J.J.B.; Steyerberg, E.W.; van Berge Henegouwen, M.I.; Wijnhoven, B.P.L.; Richel, D.J.; Nieuwenhuijzen, G.A.P.; Hospers, G.A.P.; Bonenkamp, J.J.; et al. Preoperative Chemoradiotherapy for Esophageal or Junctional Cancer. New Engl. J. Med. 2012, 366, 2074–2084. [Google Scholar] [CrossRef]
- Biere, S.S.A.Y.; van Berge Henegouwen, M.I.; Maas, K.W.; Bonavina, L.; Rosman, C.; Garcia, J.R.; Gisbertz, S.S.; Klinkenbijl, J.H.G.; Hollmann, M.W.; de Lange, E.S.M.; et al. Minimally Invasive versus Open Oesophagectomy for Patients with Oesophageal Cancer: A Multicentre, Open-Label, Randomised Controlled Trial. Lancet 2012, 379, 1887–1892. [Google Scholar] [CrossRef]
- The German Healthcare System, Bundesgesundheitsministerium. Available online: https://www.bundesgesundheitsministerium.de/fileadmin/Dateien/5_Publikationen/Gesundheit/Broschueren/200629_BMG_Das_deutsche_Gesundheitssystem_DE.pdf (accessed on 3 May 2023).
- German S3 Guideline: Diagnosis and Therapy of Squamous Cell Carcinomas and Adenocarcinomas of the Esophagus. Available online: https://www.leitlinienprogramm-onkologie.de/fileadmin/user_upload/Downloads/Leitlinien/Oesophaguskarzinom/Version_3/LL_%C3%96sophaguskarzinom_Leitlinienreport_3.1.pdf (accessed on 18 May 2022).
- Al-Batran, S.-E.; Homann, N.; Pauligk, C.; Goetze, T.O.; Meiler, J.; Kasper, S.; Kopp, H.-G.; Mayer, F.; Haag, G.M.; Luley, K.; et al. Perioperative Chemotherapy with Fluorouracil plus Leucovorin, Oxaliplatin, and Docetaxel versus Fluorouracil or Capecitabine plus Cisplatin and Epirubicin for Locally Advanced, Resectable Gastric or Gastro-Oesophageal Junction Adenocarcinoma (FLOT4): A Randomised, Phase 2/3 Trial. Lancet 2019, 393, 1948–1957. [Google Scholar] [CrossRef]
- Carden, A.; Blum, K.; Arbaugh, C.J.; Trickey, A.; Eisenberg, D. Low Socioeconomic Status Is Associated with Lower Weight-Loss Outcomes 10-Years after Roux-En-Y Gastric Bypass. Surg. Endosc. 2019, 33, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Roswall, J.; Almqvist-Tangen, G.; Holmén, A.; Alm, B.; Bergman, S.; Dahlgren, J.; Strömberg, U. Overweight at Four Years of Age in a Swedish Birth Cohort: Influence of Neighbourhood-Level Purchasing Power. BMC Public Health 2016, 16, 546. [Google Scholar] [CrossRef] [PubMed]
- Wagner, J.; Zanker, N.; Duprée, A.; Mann, O.; Izbicki, J.; Wolter, S. Higher Socioeconomic Status Is Associated with Improved Outcomes After Obesity Surgery Among Women in Germany. World J. Surg. 2021, 45, 3330–3340. [Google Scholar] [CrossRef] [PubMed]
- van den Berg, I.; Buettner, S.; van den Braak, R.R.J.C.; Ultee, K.H.J.; Lingsma, H.F.; van Vugt, J.L.A.; Ijzermans, J.N.M. Low Socioeconomic Status Is Associated with Worse Outcomes After Curative Surgery for Colorectal Cancer: Results from a Large, Multicenter Study. J. Gastrointest. Surg. 2020, 24, 2628–2636. [Google Scholar] [CrossRef]
- Lantz, P.M.; House, J.S.; Lepkowski, J.M.; Williams, D.R.; Mero, R.P.; Chen, J. Socioeconomic Factors, Health Behaviors, and Mortality: Results from a Nationally Representative Prospective Study of US Adults. JAMA 1998, 279, 1703–1708. [Google Scholar] [CrossRef]
- Sabanayagam, C.; Shankar, A. Income Is a Stronger Predictor of Mortality than Education in a National Sample of US Adults. J. Heal. Popul. Nutr. 2012, 30, 82–86. [Google Scholar] [CrossRef]
- Lampert, T.; Kroll, L.E.; Müters, S.; Stolzenberg, H. Messung Des Sozioökonomischen Status in Der Studie Gesundheit in Deutschland Aktuell (GEDA). Bundesgesundheitsblatt Gesundh. Gesundh. 2012, 56, 131–143. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5, 5th ed.; American Psychiatric Publishing: Washington, DC, USA, 2013. [Google Scholar]
- Quan, H.; Li, B.; Couris, C.M.; Fushimi, K.; Graham, P.; Hider, P.; Januel, J.-M.; Sundararajan, V. Updating and Validating the Charlson Comorbidity Index and Score for Risk Adjustment in Hospital Discharge Abstracts Using Data From 6 Countries. Am. J. Epidemiol. 2011, 173, 676–682. [Google Scholar] [CrossRef]
- Low, D.E.; Alderson, D.; Cecconello, I.; Chang, A.C.; Darling, G.E.; DʼJourno, X.B.; Griffin, S.M.; Hölscher, A.H.; Hofstetter, W.L.; Jobe, B.A.; et al. International Consensus on Standardization of Data Collection for Complications Associated with Esophagectomy: Esophagectomy Complications Consensus Group (ECCG). Ann. Surg. 2015, 262, 286–294. [Google Scholar] [CrossRef]
- Dindo, D.; Demartines, N.; Clavien, P.-A. Classification of Surgical Complications: A New Proposal with Evaluation in a Cohort of 6336 Patients and Results of a Survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Tron, L.; Fauvernier, M.; Bouvier, A.-M.; Robaszkiewicz, M.; Bouvier, V.; Cariou, M.; Jooste, V.; Dejardin, O.; Remontet, L.; Alves, A.; et al. Socioeconomic Environment and Survival in Patients with Digestive Cancers: A French Population-Based Study. Cancers 2021, 13, 5156. [Google Scholar] [CrossRef]
- Thein, H.-H.; Anyiwe, K.; Jembere, N.; Yu, B.; De, P.; Earle, C.C. Effects of Socioeconomic Status on Esophageal Adenocarcinoma Stage at Diagnosis, Receipt of Treatment, and Survival: A Population-Based Cohort Study. PLoS ONE 2017, 12, e0186350. [Google Scholar] [CrossRef]
- Leigh, Y.; Seagroatt, V.; Goldacre, M.; McCulloch, P. Impact of Socio-Economic Deprivation on Death Rates after Surgery for Upper Gastrointestinal Tract Cancer. Brit. J. Cancer 2006, 95, 940–943. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-C.; Chang, C.-M.; Hsu, T.-W.; Lee, C.-H.; Chen, J.-H.; Huang, C.-Y.; Lee, C.-C. The Effect of Individual and Neighborhood Socioeconomic Status on Esophageal Cancer Survival in Working-Age Patients in Taiwan. Medicine 2016, 95, e4140. [Google Scholar] [CrossRef] [PubMed]
- Erhunmwunsee, L.; Gulack, B.C.; Rushing, C.; Niedzwiecki, D.; Berry, M.F.; Hartwig, M.G. Socioeconomic Status, Not Race, Is Associated with Reduced Survival in Esophagectomy Patients. Ann. Thorac. Surg. 2017, 104, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Schlottmann, F.; Gaber, C.; Strassle, P.D.; Herbella, F.A.M.; Molena, D.; Patti, M.G. Disparities in Esophageal Cancer: Less Treatment, Less Surgical Resection, and Poorer Survival in Disadvantaged Patients. Dis. Esophagus 2019, 33, doz045. [Google Scholar] [CrossRef]
- Bus, P.; Aarts, M.J.; Lemmens, V.E.P.P.; van Oijen, M.G.; Creemers, G.-J.; Nieuwenhuijzen, G.A.; van Baal, J.W.; Siersema, P.D. The Effect of Socioeconomic Status on Staging and Treatment Decisions in Esophageal Cancer. J. Clin. Gastroenterol. 2012, 46, 833–839. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).