Beyond Corticoresistance, A Paradoxical Corticosensitivity Induced by Corticosteroid Therapy in Pediatric Acute Lymphoblastic Leukemias
Abstract
Simple Summary
Abstract
1. Introduction
2. Glucocorticoids in Leukemia Treatment
3. Glucocorticoids and Leukemic Cell Death: Mechanisms
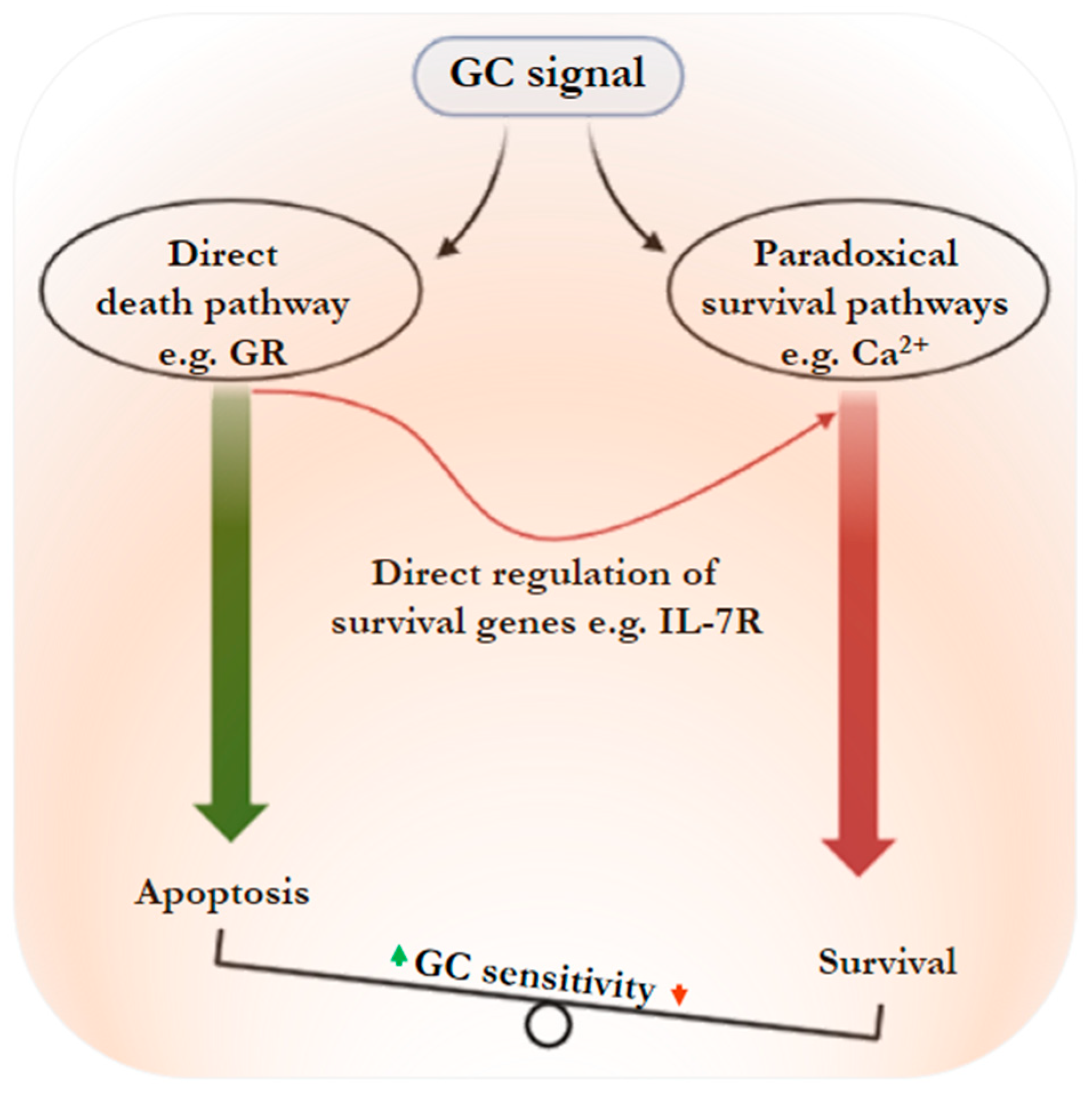
4. Glucocorticoids Stimulate Leukemic Cell Resistance: The Paradox
4.1. Calcium Signaling
4.2. IL-7 Dependent Pathway
4.3. PI3K/AKT/mTOR Signaling
4.4. MAPK Signaling Pathway
4.5. Lck Signaling Pathway
4.6. Hedgehog Signaling
4.7. Metabolic Reprogramming
5. Inhibition of Paradoxical Signaling Nodes to Overcome GC Resistance
5.1. Inhibition of Ca2+ and MAPK Signaling
5.2. Inhibitor of PI3/AKT/mTOR Pathway
5.3. Inhibitor of IL-7R and BCL-2 Signaling
5.4. Inhibition of Hedgehog Signaling
5.5. Lck Inhibition
6. What Direction to Go in to Decipher this Paradox and Move the Field Forward
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wei, G.; Twomey, D.; Lamb, J.; Schlis, K.; Agarwal, J.; Stam, R.W.; Opferman, J.T.; Sallan, S.E.; den Boer, M.L.; Pieters, R.; et al. Gene Expression-Based Chemical Genomics Identifies Rapamycin as a Modulator of MCL1 and Glucocorticoid Resistance. Cancer Cell 2006, 10, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Serafin, V.; Capuzzo, G.; Milani, G.; Minuzzo, S.A.; Pinazza, M.; Bortolozzi, R.; Bresolin, S.; Porcù, E.; Frasson, C.; Indraccolo, S.; et al. Glucocorticoid Resistance Is Reverted by LCK Inhibition in Pediatric T-Cell Acute Lymphoblastic Leukemia. Blood 2017, 130, 2750–2761. [Google Scholar] [CrossRef] [PubMed]
- Bhojwani, D.; Yang, J.J.; Pui, C.-H. Biology of Childhood Acute Lymphoblastic Leukemia. Pediatr. Clin. N. Am. 2015, 62, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Pui, C.-H.; Robison, L.L.; Look, A.T. Acute Lymphoblastic Leukaemia. Lancet 2008, 371, 1030–1043. [Google Scholar] [CrossRef]
- Rafei, H.; Kantarjian, H.M.; Jabbour, E.J. Recent Advances in the Treatment of Acute Lymphoblastic Leukemia. Leuk. Lymphoma 2019, 60, 2606–2621. [Google Scholar] [CrossRef]
- Hunger, S.P.; Lu, X.; Devidas, M.; Camitta, B.M.; Gaynon, P.S.; Winick, N.J.; Reaman, G.H.; Carroll, W.L. Improved Survival for Children and Adolescents with Acute Lymphoblastic Leukemia Between 1990 and 2005: A Report from the Children’s Oncology Group Listen to the Podcast by Dr Silverman at Www.Jco.Org/Podcasts. J. Clin. Oncol. 2012, 30, 1663–1669. [Google Scholar] [CrossRef]
- Pui, C.-H.; Yang, J.J.; Hunger, S.P.; Pieters, R.; Schrappe, M.; Biondi, A.; Vora, A.; Baruchel, A.; Silverman, L.B.; Schmiegelow, K.; et al. Childhood Acute Lymphoblastic Leukemia: Progress Through Collaboration. J. Clin. Oncol. 2015, 33, 2938–2948. [Google Scholar] [CrossRef]
- Chan, K.W. Acute Lymphoblastic Leukemia. Curr. Probl. Pediatr. Adolesc. Health Care 2002, 32, 40–49. [Google Scholar] [CrossRef]
- Van Vlierberghe, P.; Pieters, R.; Beverloo, H.B.; Meijerink, J.P.P. Molecular-Genetic Insights in Paediatric T-Cell Acute Lymphoblastic Leukaemia. Br. J. Haematol. 2008, 143, 153–168. [Google Scholar] [CrossRef]
- De Smedt, R.; Morscio, J.; Goossens, S.; Van Vlierberghe, P. Targeting Steroid Resistance in T-Cell Acute Lymphoblastic Leukemia. Blood Rev. 2019, 38, 100591. [Google Scholar] [CrossRef]
- Piovan, E.; Yu, J.; Tosello, V.; Herranz, D.; Ambesi-Impiombato, A.; DaSilva, A.C.; Sanchez-Martin, M.; Perez-Garcia, A.; Rigo, I.; Castillo, M.; et al. Direct Reversal of Glucocorticoid Resistance by AKT Inhibition in Acute Lymphoblastic Leukemia. Cancer Cell 2013, 24, 766–776. [Google Scholar] [CrossRef] [PubMed]
- Wittmann, M.; Riccardi, C.; Timmermans, S.; Souffriau, J.; Libert, C. A General Introduction to Glucocorticoid Biology. Front. Immunol. 2019, 1, 1545. [Google Scholar] [CrossRef]
- Tissing, W.J.E.; Meijerink, J.P.P.; den Boer, M.L.; Pieters, R. Molecular Determinants of Glucocorticoid Sensitivity and Resistance in Acute Lymphoblastic Leukemia. Leukemia 2003, 17, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Inaba, H.; Pui, C.H. Glucocorticoid Use in Acute Lymphoblastic Leukaemia. Lancet Oncol. 2010, 11, 1096–1106. [Google Scholar] [CrossRef]
- Montgomery, B.; Cheng, H.H.; Drechsler, J.; Mostaghel, E.A. Glucocorticoids and Prostate Cancer Treatment: Friend or Foe? Asian J. Androl. 2014, 16, 354–358. [Google Scholar] [CrossRef]
- Möricke, A.; Zimmermann, M.; Valsecchi, M.G.; Stanulla, M.; Biondi, A.; Mann, G.; Locatelli, F.; Cazzaniga, G.; Niggli, F.; Aricò, M.; et al. Dexamethasone vs Prednisone in Induction Treatment of Pediatric ALL: Results of the Randomized Trial AIEOP-BFM ALL 2000. Blood 2016, 127, 2101–2112. [Google Scholar] [CrossRef]
- Uckun, F.M.; Gaynon, P.S.; Stram, D.O.; Sensel, M.G.; Sarquis, M.B.; Willoughby, M. Bone Marrow Leukemic Progenitor Cell Content in Pediatric T-Lineage Acute Lymphoblastic Leukemia Patients with an Isolated Extramedullary First Relapse. Leuk. Lymphoma 2001, 40, 279–285. [Google Scholar] [CrossRef]
- Uckun, F.M.; Gaynon, P.S.; Stram, D.O.; Sensel, M.G.; Sarquis, M.B.; Lazarus, K.H.; Willoughby, M. Paucity of Leukemic Progenitor Cells in the Bone Marrow of Pediatric B- Lineage Acute Lymphoblastic Leukemia Patients with an Isolated Extramedullary First Relapse. Clin. Cancer Res. 1999, 5, 2415–2420. [Google Scholar]
- Liu, X.; Zou, Y.; Chen, X.; Wang, S.; Guo, Y.; Yang, W.; Zhang, L.; Chen, Y.; Zhang, Y.; Zhu, X. Minimal Residual Disease Surveillance at Day 90 Predicts Long-Term Survival in Pediatric Patients with T-Cell Acute Lymphoblastic Leukemia. Leuk. Lymphoma 2020, 61, 3460–3467. [Google Scholar] [CrossRef]
- Bostrom, B.C.; Sensel, M.R.; Sather, H.N.; Gaynon, P.S.; La, M.K.; Johnston, K.; Erdmann, G.R.; Gold, S.; Heerema, N.A.; Hutchinson, R.J.; et al. Dexamethasone versus Prednisone and Daily Oral versus Weekly Intravenous Mercaptopurine for Patients with Standard-Risk Acute Lymphoblastic Leukemia: A Report from the Children’s Cancer Group. Blood 2003, 101, 3809–3817. [Google Scholar] [CrossRef]
- Larsen, E.C.; Devidas, M.; Chen, S.; Salzer, W.L.; Raetz, E.A.; Loh, M.L.; Mattano, L.A.; Cole, C.; Eicher, A.; Haugan, M.; et al. Dexamethasone and High-Dose Methotrexate Improve Outcome for Children and Young Adults with High-Risk B-Acute Lymphoblastic Leukemia: A Report From Children’s Oncology Group Study AALL0232. J. Clin. Oncol. 2016, 34, 2380–2388. [Google Scholar] [CrossRef] [PubMed]
- Domenech, C.; Suciu, S.; De Moerloose, B.; Mazingue, F.; Plat, G.; Ferster, A.; Uyttebroeck, A.; Sirvent, N.; Lutz, P.; Yakouben, K.; et al. Dexamethasone (6 Mg/M2/Day) and Prednisolone (60 Mg/M2/Day) Were Equally Effective as Induction Therapy for Childhood Acute Lymphoblastic Leukemia in the EORTC CLG 58951 Randomized Trial. Haematologica 2014, 99, 1220–1227. [Google Scholar] [CrossRef]
- Erlacher, M.; Knoflach, M.; Stec, I.E.M.; Böck, G.; Wick, G.; Wiegers, G.J. TCR Signaling Inhibits Glucocorticoid-Induced Apoptosis in Murine Thymocytes Depending on the Stage of Development. Eur. J. Immunol. 2005, 35, 3287–3296. [Google Scholar] [CrossRef] [PubMed]
- Jamieson, C.A.M.; Yamamoto, K.R. Crosstalk Pathway for Inhibition of Glucocorticoid-Induced Apoptosis by T Cell Receptor Signaling. Proc. Natl. Acad. Sci. USA 2000, 97, 7319–7324. [Google Scholar] [CrossRef]
- Tsitoura, D.C.; Rothman, P.B. Enhancement of MEK/ERK Signaling Promotes Glucocorticoid Resistance in CD4+ T Cells. J. Clin. Investig. 2004, 113, 619–627. [Google Scholar] [CrossRef] [PubMed]
- DeFranco, D.B. Role of Molecular Chaperones in Subnuclear Trafficking of Glucocorticoid Receptors. Kidney Int. 2000, 57, 1241–1249. [Google Scholar] [CrossRef] [PubMed]
- Clarisse, D.; Offner, F.; De Bosscher, K. Latest Perspectives on Glucocorticoid-Induced Apoptosis and Resistance in Lymphoid Malignancies. Biochim. Biophys. Acta Rev. Cancer 2020, 1874, 188430. [Google Scholar] [CrossRef]
- Brown, J.A.; Ferrando, A. Glucocorticoid Resistance in Acute Lymphoblastic Leukemia: BIM Finally. Cancer Cell 2018, 34, 869–871. [Google Scholar] [CrossRef]
- Schmidt, S.; Rainer, J.; Ploner, C.; Presul, E.; Riml, S.; Kofler, R. Glucocorticoid-Induced Apoptosis and Glucocorticoid Resistance: Molecular Mechanisms and Clinical Relevance. Cell Death Differ. 2004, 11, 45–55. [Google Scholar] [CrossRef]
- Mullighan, C.G.; Zhang, J.; Kasper, L.H.; Lerach, S.; Payne-Turner, D.; Phillips, L.A.; Heatley, S.L.; Holmfeldt, L.; Collins-Underwood, J.R.; Ma, J.; et al. CREBBP Mutations in Relapsed Acute Lymphoblastic Leukaemia. Nature 2011, 471, 235–239. [Google Scholar] [CrossRef]
- Nicolaides, N.C.; Charmandari, E. Novel Insights into the Molecular Mechanisms Underlying Generalized Glucocorticoid Resistance and Hypersensitivity Syndromes. Hormones 2017, 16, 124–138. [Google Scholar] [PubMed]
- Hala, M.; Hartmann, B.L.; Bock, G.; Geley, S.; Kofler, R. Glucocorticoid-Receptor-Gene Defects and Resistance to Glucocorticoid-Induced Apoptosis in Human Leukemic Cell Lines. Int. J. Cancer 1996, 68, 663–668. [Google Scholar] [CrossRef]
- Strasser-Wozak, E.M.C.; Hattmannstorfer, R.; Hála, M.; Hartmann, B.L.; Fiegl, M.; Geley, S.; Kofler, R. Splice Site Mutation in the Glucocorticoid Receptor Gene Causes Resistance to Glucocorticoid-Induced Apoptosis in a Human Acute Leukemic Cell Line. Cancer Res. 1995, 55, 348–353. [Google Scholar]
- Klumper, E.; Pieters, R.; Veerman, A.J.P.; Huismans, D.R.; Loonen, A.H.; Hählen, K.; Kaspers, G.J.L.; Van Wering, E.R.; Hartmann, R.; Henze, G. In Vitro Cellular Drug Resistance in Children with Relapsed/Refractory Acute Lymphoblastic Leukemia. Blood 1995, 86, 3861–3868. [Google Scholar] [CrossRef] [PubMed]
- Lauten, M.; Möricke, A.; Beier, R.; Zimmermann, M.; Stanulla, M.; Meissner, B.; Odenwald, E.; Attarbaschi, A.; Niemeyer, C.; Niggli, F.; et al. Prediction of Outcome by Early Bone Marrow Response in Childhood Acute Lymphoblastic Leukemia Treated in the ALL-BFM 95 Trial: Differential Effects in Precursor B-Cell and T-Cell Leukemia. Haematologica 2012, 97, 1048–1056. [Google Scholar] [CrossRef] [PubMed]
- Conter, V.; Bartram, C.R.; Valsecchi, M.G.; Schrauder, A.; Panzer-Grümayer, R.; Möricke, A.; Aricò, M.; Zimmermann, M.; Mann, G.; De Rossi, G.; et al. Molecular Response to Treatment Redefines All Prognostic Factors in Children and Adolescents with B-Cell Precursor Acute Lymphoblastic Leukemia: Results in 3184 Patients of the AIEOP-BFM ALL 2000 Study. Blood 2010, 115, 3206–3214. [Google Scholar] [CrossRef] [PubMed]
- Abdoul-Azize, S.; Dubus, I.; Vannier, J.P. Improvement of Dexamethasone Sensitivity by Chelation of Intracellular Ca2+ in Pediatric Acute Lymphoblastic Leukemia Cells through the Prosurvival Kinase ERK1/2 Deactivation. Oncotarget 2017, 8, 27339–27352. [Google Scholar] [CrossRef]
- Abdoul-Azize, S.; Buquet, C.; Vannier, J.-P.; Dubus, I. Pyr3, a TRPC3 Channel Blocker, Potentiates Dexamethasone Sensitivity and Apoptosis in Acute Lymphoblastic Leukemia Cells by Disturbing Ca2+ Signaling, Mitochondrial Membrane Potential Changes and Reactive Oxygen Species Production. Eur. J. Pharmacol. 2016, 784, 90–98. [Google Scholar] [CrossRef]
- Meyer, L.K.; Huang, B.J.; Delgado-Martin, C.; Roy, R.P.; Hechmer, A.; Wandler, A.M.; Vincent, T.L.; Fortina, P.; Olshen, A.B.; Wood, B.L.; et al. Glucocorticoids Paradoxically Facilitate Steroid Resistance in T Cell Acute Lymphoblastic Leukemias and Thymocytes. J. Clin. Investig. 2020, 130, 863–876. [Google Scholar] [CrossRef]
- Delgado-Martin, C.; Meyer, L.K.; Huang, B.J.; Shimano, K.A.; Zinter, M.S.; Nguyen, J.V.; Smith, G.A.; Taunton, J.; Winter, S.S.; Roderick, J.R.; et al. JAK/STAT Pathway Inhibition Overcomes IL7-Induced Glucocorticoid Resistance in a Subset of Human T-Cell Acute Lymphoblastic Leukemias. Leukemia 2017, 31, 2568–2576. [Google Scholar] [CrossRef]
- De Groot, A.P.; Saito, Y.; Kawakami, E.; Hashimoto, M.; Aoki, Y.; Ono, R.; Ogahara, I.; Fujiki, S.; Kaneko, A.; Sato, K.; et al. Targeting Critical Kinases and Anti-Apoptotic Molecules Overcomes Steroid Resistance in MLL-Rearranged Leukaemia. EBioMedicine 2021, 64, 103235. [Google Scholar] [CrossRef] [PubMed]
- Bodaar, K.; Yamagata, N.; Barthe, A.; Landrigan, J.; Chonghaile, T.N.; Burns, M.; Stevenson, K.E.; Devidas, M.; Loh, M.L.; Hunger, S.P.; et al. JAK3 Mutations and Mitochondrial Apoptosis Resistance in T-Cell Acute Lymphoblastic Leukemia. Leukemia 2022, 36, 1499–1507. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Beckett, M.C.; Blair, H.J.; Tirtakusuma, R.; Nakjang, S.; Enshaei, A.; Halsey, C.; Vormoor, J.; Heidenreich, O.; Krippner-Heidenreich, A.; et al. Phase II-like Murine Trial Identifies Synergy between Dexamethasone and Dasatinib in T-Cell Acute Lymphoblastic Leukemia. Haematologica 2021, 106, 1056–1066. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, M.C.; Baatar, D.; Collins, G.; Carter, A.; Indig, F.; Biragyn, A.; Taub, D.D. Dexamethasone Augments CXCR4-Mediated Signaling in Resting Human T Cells via the Activation of the Src Kinase Lck. Blood 2009, 113, 575–584. [Google Scholar] [CrossRef]
- Fazio, G.; Bresolin, S.; Silvestri, D.; Quadri, M.; Saitta, C.; Vendramini, E.; Buldini, B.; Palmi, C.; Bardini, M.; Grioni, A.; et al. PAX5 Fusion Genes Are Frequent in Poor Risk Childhood Acute Lymphoblastic Leukaemia and Can Be Targeted with BIBF1120. EBioMedicine 2022, 83, 104224. [Google Scholar] [CrossRef]
- Jin, Q.; Gutierrez Diaz, B.; Pieters, T.; Zhou, Y.; Narang, S.; Fijalkwoski, I.; Borin, C.; Van Laere, J.; Payton, M.; Cho, B.-K.; et al. Oncogenic Deubiquitination Controls Tyrosine Kinase Signaling and Therapy Response in Acute Lymphoblastic Leukemia. Sci. Adv. 2022, 8, eabq8437. [Google Scholar] [CrossRef]
- Spijkers-Hagelstein, J.A.P.; Mimoso Pinhanços, S.; Schneider, P.; Pieters, R.; Stam, R.W. Src Kinase-Induced Phosphorylation of Annexin A2 Mediates Glucocorticoid Resistance in MLL-Rearranged Infant Acute Lymphoblastic Leukemia. Leukemia 2013, 27, 1063–1071. [Google Scholar] [CrossRef]
- Xie, M.; Yang, A.; Ma, J.; Wu, M.; Xu, H.; Wu, K.; Jin, Y.; Xie, Y. Akt2 Mediates Glucocorticoid Resistance in Lymphoid Malignancies through FoxO3a/Bim Axis and Serves as a Direct Target for Resistance Reversal. Cell Death Dis. 2019, 9, 1013. [Google Scholar] [CrossRef]
- Miller, A.L.; Garza, A.S.; Johnson, B.H.; Thompson, E.B. Pathway Interactions between MAPKs, MTOR, PKA, and the Glucocorticoid Receptor in Lymphoid Cells. Cancer Cell Int. 2007, 7, 3. [Google Scholar] [CrossRef]
- Nicholson, L.; Evans, C.A.; Matheson, E.; Minto, L.; Keilty, C.; Sanichar, M.; Case, M.; Schwab, C.; Williamson, D.; Rainer, J.; et al. Quantitative Proteomic Analysis Reveals Maturation as a Mechanism Underlying Glucocorticoid Resistance in B Lineage ALL and Re-Sensitization by JNK Inhibition. Br. J. Haematol. 2015, 171, 595–605. [Google Scholar] [CrossRef]
- Ji, Z.; Mei, F.C.; Johnson, B.H.; Thompson, E.B.; Cheng, X. Protein Kinase A, Not Epac, Suppresses Hedgehog Activity and Regulates Glucocorticoid Sensitivity in Acute Lymphoblastic Leukemia Cells. J. Biol. Chem. 2007, 282, 37370–37377. [Google Scholar] [CrossRef]
- Bongiovanni, D.; Tosello, V.; Saccomani, V.; Dalla Santa, S.; Amadori, A.; Zanovello, P.; Piovan, E. Crosstalk between Hedgehog Pathway and the Glucocorticoid Receptor Pathway as a Basis for Combination Therapy in T-Cell Acute Lymphoblastic Leukemia. Oncogene 2020, 39, 6544–6555. [Google Scholar] [CrossRef] [PubMed]
- Prevarskaya, N.; Skryma, R.; Shuba, Y. Calcium in Tumour Metastasis: New Roles for Known Actors. Nat. Rev. Cancer 2011, 11, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Monteith, G.R.; McAndrew, D.; Faddy, H.M.; Roberts-Thomson, S.J. Calcium and Cancer: Targeting Ca2+ Transport. Nat. Rev. Cancer 2007, 7, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Roderick, H.L.; Cook, S.J. Ca2+ Signalling Checkpoints in Cancer: Remodelling Ca2+ for Cancer Cell Proliferation and Survival. Nat. Rev. Cancer 2008, 8, 361–375. [Google Scholar] [CrossRef] [PubMed]
- Olivas-Aguirre, M.; Pérez-Chávez, J.; Torres-López, L.; Hernández-Cruz, A.; Pottosin, I.; Dobrovinskaya, O. Dexamethasone-Induced Fatty Acid Oxidation and Autophagy/Mitophagy Are Essential for T-ALL Glucocorticoid Resistance. Cancers 2023, 15, 445. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Hao, D.; Hu, H. High Doses of Dexamethasone Induce Endoplasmic Reticulum Stress-Mediated Apoptosis by Promoting Calcium Ion Influx-Dependent CHOP Expression in Osteoblasts. Mol. Biol. Rep. 2021, 48, 7841–7851. [Google Scholar] [CrossRef]
- Feske, S.; Skolnik, E.Y.; Prakriya, M. Ion Channels and Transporters in Lymphocyte Function and Immunity. Nat. Rev. Immunol. 2012, 12, 532–547. [Google Scholar] [CrossRef]
- Dobrovinskaya, O.; Delgado-Enciso, I.; Quintero-Castro, L.J.; Best-Aguilera, C.; Rojas-Sotelo, R.M.; Pottosin, I. Placing Ion Channels into a Signaling Network of T Cells: From Maturing Thymocytes to Healthy T Lymphocytes or Leukemic T Lymphoblasts. Biomed Res. Int. 2015, 2015, 750203. [Google Scholar] [CrossRef]
- Sarang, Z.; Gyurina, K.; Scholtz, B.; Kiss, C.; Szegedi, I. Altered Expression of Autophagy-Related Genes Might Contribute to Glucocorticoid Resistance in Precursor B-Cell-Type Acute Lymphoblastic Leukemia. Eur. J. Haematol. 2016, 97, 453–460. [Google Scholar] [CrossRef]
- Eylenstein, A.; Gehring, E.; Heise, N.; Shumilina, E.; Schmidt, S.; Szteyn, K.; Münzer, P.; Nurbaeva, M.K.; Eichenmüller, M.; Tyan, L.; et al. Stimulation of Ca2+-Channel Orai1/STIM1 by Serum- and Glucocorticoid-Inducible Kinase 1 (SGK1). FASEB J. 2011, 25, 2012–2021. [Google Scholar] [CrossRef] [PubMed]
- Lang, F.; Pelzl, L.; Hauser, S.; Hermann, A.; Stournaras, C.; Schöls, L. To Die or Not to Die SGK1-Sensitive ORAI/STIM in Cell Survival. Cell Calcium 2018, 74, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Honisch, S.; Schmidt, S.; Yan, J.; Schmid, E.; Alkahtani, S.; Alkahtane, A.A.; Alarifi, S.; Stournaras, C.; Lang, F. Chorein Sensitive Orai1 Expression and Store Operated Ca2+ Entry in Rhabdomyosarcoma Cells. Cell. Physiol. Biochem. 2016, 40, 1141–1152. [Google Scholar] [CrossRef] [PubMed]
- Lang, F.; Böhmer, C.; Palmada, M.; Seebohm, G.; Strutz-Seebohm, N.; Vallon, V. (Patho)Physiological Significance of the Serum- and Glucocorticoid-Inducible Kinase Isoforms. Physiol. Rev. 2006, 86, 1151–1178. [Google Scholar] [CrossRef]
- Lang, F.; Voelkl, J. Therapeutic Potential of Serum and Glucocorticoid Inducible Kinase Inhibition. Expert Opin. Investig. Drugs 2013, 22, 701–714. [Google Scholar] [CrossRef]
- Valle-Reyes, S.; Valencia-Cruz, G.; Liñan-Rico, L.; Pottosin, I.; Dobrovinskaya, O. Differential Activity of Voltage- and Ca2+-Dependent Potassium Channels in Leukemic T Cell Lines: Jurkat Cells Represent an Exceptional Case. Front. Physiol. 2018, 9, 499. [Google Scholar] [CrossRef]
- Valle, J.S.; Castellanos, R.; Olivas, M.Á.; Pottosin, I.; Dobrovinskaya, O.; Schnoor, M. Kv1.3 Channel Is a Potential Marker for B Acute Lymphoblastic Leukemia. FASEB J. 2020, 34, 1. [Google Scholar] [CrossRef]
- Henke, G.; Maier, G.; Wallisch, S.; Boehmer, C.; Lang, F. Regulation of the Voltage Gated K+ Channel Kv1.3 by the Ubiquitin Ligase Nedd4-2 and the Serum and Glucocorticoid Inducible Kinase SGK1. J. Cell. Physiol. 2004, 199, 194–199. [Google Scholar] [CrossRef]
- Ribeiro, D.; Melão, A.; van Boxtel, R.; Santos, C.I.; Silva, A.; Silva, M.C.; Cardoso, B.A.; Coffer, P.J.; Barata, J.T. STAT5 Is Essential for IL-7-Mediated Viability, Growth, and Proliferation of T-Cell Acute Lymphoblastic Leukemia Cells. Blood Adv. 2018, 2, 2199–2213. [Google Scholar] [CrossRef]
- Silva, A.; Laranjeira, A.B.A.; Martins, L.R.; Cardoso, B.A.; Demengeot, J.; Yunes, J.A.; Seddon, B.; Barata, J.T. IL-7 Contributes to the Progression of Human T-Cell Acute Lymphoblastic Leukemias. Cancer Res. 2011, 71, 4780–4789. [Google Scholar] [CrossRef]
- Jing, D.; Bhadri, V.A.; Beck, D.; Thoms, J.A.I.; Yakob, N.A.; Wong, J.W.H.; Knezevic, K.; Pimanda, J.E.; Lock, R.B. Opposing Regulation of BIM and BCL2 Controls Glucocorticoid-Induced Apoptosis of Pediatric Acute Lymphoblastic Leukemia Cells. Blood 2015, 125, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Buijs-Gladdines, J.G.C.A.M.; Canté-Barrett, K.; Stubbs, A.P.; Vroegindeweij, E.M.; Smits, W.K.; van Marion, R.; Dinjens, W.N.M.; Horstmann, M.; Kuiper, R.P.; et al. IL-7 Receptor Mutations and Steroid Resistance in Pediatric T Cell Acute Lymphoblastic Leukemia: A Genome Sequencing Study. PLoS Med. 2016, 13, e1002200. [Google Scholar] [CrossRef] [PubMed]
- Barata, J.T.; Cardoso, A.A.; Nadler, L.M.; Boussiotis, V.A. Interleukin-7 Promotes Survival and Cell Cycle Progression of T-Cell Acute Lymphoblastic Leukemia Cells by down-Regulating the Cyclin-Dependent Kinase Inhibitor P27kip1. Blood 2001, 98, 1524–1531. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Li, W.Q.; Hofmeister, R.R.; Young, H.A.; Hodge, D.R.; Keller, J.R.; Khaled, A.R.; Durum, S.K. Distinct Regions of the Interleukin-7 Receptor Regulate Different Bcl2 Family Members. Mol. Cell. Biol. 2004, 24, 6501–6513. [Google Scholar] [CrossRef] [PubMed]
- Kontro, M.; Kuusanmäki, H.; Eldfors, S.; Burmeister, T.; Andersson, E.I.; Bruserud; Brümmendorf, T.H.; Edgren, H.; Gjertsen, B.T.; Itälä-Remes, M.; et al. Novel Activating STAT5B Mutations as Putative Drivers of T-Cell Acute Lymphoblastic Leukemia. Leukemia 2014, 28, 1738–1742. [Google Scholar] [CrossRef]
- De Smedt, R.; Morscio, J.; Reunes, L.; Roels, J.; Bardelli, V.; Lintermans, B.; Van Loocke, W.; Almeida, A.; Cheung, L.C.; Kotecha, R.S.; et al. Targeting Cytokine- and Therapy-Induced PIM1 Activation in Preclinical Models of T-Cell Acute Lymphoblastic Leukemia and Lymphoma. Blood 2020, 135, 1685–1695. [Google Scholar] [CrossRef]
- Lin, Y.-W.; Beharry, Z.M.; Hill, E.G.; Song, J.H.; Wang, W.; Xia, Z.; Zhang, Z.; Aplan, P.D.; Aster, J.C.; Smith, C.D.; et al. A Small Molecule Inhibitor of Pim Protein Kinases Blocks the Growth of Precursor T-Cell Lymphoblastic Leukemia/Lymphoma. Blood 2010, 115, 824–833. [Google Scholar] [CrossRef]
- Van der Zwet, J.C.G.; Buijs-Gladdines, J.G.C.A.M.; Cordo’, V.; Debets, D.O.; Smits, W.K.; Chen, Z.; Dylus, J.; Zaman, G.J.R.; Altelaar, M.; Oshima, K.; et al. MAPK-ERK Is a Central Pathway in T-Cell Acute Lymphoblastic Leukemia That Drives Steroid Resistance. Leukemia 2021, 35, 3394–3405. [Google Scholar] [CrossRef]
- Oliveira, M.L.; Akkapeddi, P.; Ribeiro, D.; Melão, A.; Barata, J.T. IL-7R-Mediated Signaling in T-Cell Acute Lymphoblastic Leukemia: An Update. Adv. Biol. Regul. 2019, 71, 88–96. [Google Scholar] [CrossRef]
- Barata, J.T.; Silva, A.; Brandao, J.G.; Nadler, L.M.; Cardoso, A.A.; Boussiotis, V.A. Activation of PI3K Is Indispensable for Interleukin 7-Mediated Viability, Proliferation, Glucose Use, and Growth of T Cell Acute Lymphoblastic Leukemia Cells. J. Exp. Med. 2004, 200, 659–669. [Google Scholar] [CrossRef]
- Zenatti, P.P.; Ribeiro, D.; Li, W.; Zuurbier, L.; Silva, M.C.; Paganin, M.; Tritapoe, J.; Hixon, J.A.; Silveira, A.B.; Cardoso, B.A.; et al. Oncogenic IL7R Gain-of-Function Mutations in Childhood T-Cell Acute Lymphoblastic Leukemia. Nat. Genet. 2011, 43, 932–941. [Google Scholar] [CrossRef] [PubMed]
- Canté-Barrett, K.; Spijkers-Hagelstein, J.A.P.; Buijs-Gladdines, J.G.C.A.M.; Uitdehaag, J.C.M.; Smits, W.K.; Van Der Zwet, J.; Buijsman, R.C.; Zaman, G.J.R.; Pieters, R.; Meijerink, J.P.P. MEK and PI3K-AKT Inhibitors Synergistically Block Activated IL7 Receptor Signaling in T-Cell Acute Lymphoblastic Leukemia. Leukemia 2016, 30, 1832–1843. [Google Scholar] [CrossRef] [PubMed]
- Van der Zwet, J.C.G.; Cordo’, V.; Buijs-Gladdines, J.G.C.A.M.; Hagelaar, R.; Smits, W.K.; Vroegindeweij, E.; Graus, L.T.M.; Poort, V.; Nulle, M.; Pieters, R.; et al. STAT5 Does Not Drive Steroid Resistance in T-Cell Acute Lymphoblastic Leukemia despite the Activation of BCL2 and BCLXL Following Glucocorticoid Treatment. Haematologica 2022, 108, 732–746. [Google Scholar] [CrossRef]
- Goossens, S.; Van Vlierberghe, P. Overcoming Steroid Resistance in T Cell Acute Lymphoblastic Leukemia. PLoS Med. 2016, 13, e1002208. [Google Scholar] [CrossRef] [PubMed]
- So, L.; Fruman, D.A. PI3K Signalling in B- and T-Lymphocytes: New Developments and Therapeutic Advances. Biochem. J. 2012, 442, 465–481. [Google Scholar] [CrossRef]
- Liu, P.; Cheng, H.; Roberts, T.M.; Zhao, J.J. Targeting the Phosphoinositide 3-Kinase Pathway in Cancer. Nat. Rev. Drug Discov. 2009, 8, 627–644. [Google Scholar] [CrossRef]
- Bornhauser, B.C.; Bonapace, L.; Lindholm, D.; Martinez, R.; Cario, G.; Schrappe, M.; Niggli, F.K.; Schäfer, B.W.; Bourquin, J.P. Low-Dose Arsenic Trioxide Sensitizes Glucocorticoid-Resistant Acute Lymphoblastic Leukemia Cells to Dexamethasone via an Akt-Dependent Pathway. Blood 2007, 110, 2084–2091. [Google Scholar] [CrossRef]
- Datta, S.R.; Dudek, H.; Xu, T.; Masters, S.; Haian, F.; Gotoh, Y.; Greenberg, M.E. Akt Phosphorylation of BAD Couples Survival Signals to the Cell-Intrinsic Death Machinery. Cell 1997, 91, 231–241. [Google Scholar] [CrossRef]
- Beesley, A.H.; Firth, M.J.; Ford, J.; Weller, R.E.; Freitas, J.R.; Perera, K.U.; Kees, U.R. Glucocorticoid Resistance in T-Lineage Acute Lymphoblastic Leukaemia Is Associated with a Proliferative Metabolism. Br. J. Cancer 2009, 100, 1926–1936. [Google Scholar] [CrossRef]
- Toscan, C.E.; Jing, D.; Mayoh, C.; Lock, R.B. Reversal of Glucocorticoid Resistance in Paediatric Acute Lymphoblastic Leukaemia Is Dependent on Restoring BIM Expression. Transl. Ther. Br. J. Cancer 2020, 122, 1769–1781. [Google Scholar] [CrossRef]
- Gutierrez, A.; Sanda, T.; Grebliunaite, R.; Carracedo, A.; Salmena, L.; Ahn, Y.; Dahlberg, S.; Neuberg, D.; Moreau, L.A.; Winter, S.S.; et al. High Frequency of PTEN, PI3K, and AKT Abnormalities in T-Cell Acute Lymphoblastic Leukemia. Blood 2009, 114, 647–650. [Google Scholar] [CrossRef] [PubMed]
- Arancibia, S.; Benítez, D.; Núñez, L.E.; Jewell, C.M.; Langjahr, P.; Candia, E.; Zapata-Torres, G.; Cidlowski, J.A.; González, M.-J.; Hermoso, M.A. Phosphatidylinositol 3-Kinase Interacts with the Glucocorticoid Receptor upon TLR2 Activation. J. Cell. Mol. Med. 2011, 15, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.J.; Kobayashi, S.; Izawa, K.; Ishida, T.; Watanabe, T.; Umezawa, K.; Lin, S.F.; Tojo, A. Bioimaging Analysis of Nuclear Factor-ΚB Activity in Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia Cells Reveals Its Synergistic Upregulation by Tumor Necrosis Factor-α-Stimulated Changes to the Microenvironment. Cancer Sci. 2011, 102, 2014–2021. [Google Scholar] [CrossRef] [PubMed]
- Birkenkamp, K.U.; Coffer, P.J. Regulation of Cell Survival and Proliferation by the FOXO (Forkhead Box, Class O) Subfamily of Forkhead Transcription Factors. Biochem. Soc. Trans. 2003, 31, 292–297. [Google Scholar] [CrossRef]
- Ma, J.; Xie, Y.; Shi, Y.; Qin, W.; Zhao, B.; Jin, Y. Glucocorticoid-Induced Apoptosis Requires FOXO3A Activity. Biochem. Biophys. Res. Commun. 2008, 377, 894–898. [Google Scholar] [CrossRef]
- Kruth, K.A.; Fang, M.; Shelton, D.N.; Abu-Halawa, O.; Mahling, R.; Yang, H.; Weissman, J.S.; Loh, M.L.; Müschen, M.; Tasian, S.K.; et al. Suppression of B-Cell Development Genes Is Key to Glucocorticoid Efficacy in Treatment of Acute Lymphoblastic Leukemia. Blood 2017, 129, 3000–3008. [Google Scholar] [CrossRef]
- Weigelt, B.; Downward, J. Genomic Determinants of PI3K Pathway Inhibitor Response in Cancer. Front. Oncol. 2012, 2, 109. [Google Scholar] [CrossRef]
- Miller, A.L.; Webb, M.S.; Copik, A.J.; Wang, Y.; Johnson, B.H.; Kumar, R.; Thompson, E.B. P38 Mitogen-Activated Protein Kinase (MAPK) Is a Key Mediator in Glucocorticoid-Induced Apoptosis of Lymphoid Cells: Correlation between P38 MAPK Activation and Site-Specific Phosphorylation of the Human Glucocorticoid Receptor at Serine 211. Mol. Endocrinol. 2005, 19, 1569–1583. [Google Scholar] [CrossRef]
- Jones, C.L.; Gearheart, C.M.; Fosmire, S.; Delgado-Martin, C.; Evensen, N.A.; Bride, K.; Waanders, A.J.; Pais, F.; Wang, J.; Bhatla, T.; et al. MAPK Signaling Cascades Mediate Distinct Glucocorticoid Resistance Mechanisms in Pediatric Leukemia. Blood 2015, 126, 2202–2212. [Google Scholar] [CrossRef]
- Di, J.; Huang, H.; Qu, D.; Tang, J.; Cao, W.; Lu, Z.; Cheng, Q.; Yang, J.; Bai, J.; Zhang, Y.; et al. Rap2B Promotes Proliferation, Migration, and Invasion of Human Breast Cancer through Calcium-Related ERK1/2 Signaling Pathway. Sci. Rep. 2015, 5, 12363. [Google Scholar] [CrossRef]
- Cazzaniga, V.; Bugarin, C.; Bardini, M.; Giordan, M.; te Kronnie, G.; Basso, G.; Biondi, A.; Fazio, G.; Cazzaniga, G. LCK Over-Expression Drives STAT5 Oncogenic Signaling in PAX5 Translocated BCP-ALL Patients. Oncotarget 2015, 6, 1569–1581. [Google Scholar] [CrossRef] [PubMed]
- Bommhardt, U.; Schraven, B.; Simeoni, L. Beyond TCR Signaling: Emerging Functions of Lck in Cancer and Immunotherapy. Int. J. Mol. Sci. 2019, 20, 3500. [Google Scholar] [CrossRef] [PubMed]
- Accordi, B.; Espina, V.; Giordan, M.; VanMeter, A.; Milani, G.; Galla, L.; Ruzzene, M.; Sciro, M.; Trentin, L.; De Maria, R.; et al. Functional Protein Network Activation Mapping Reveals New Potential Molecular Drug Targets for Poor Prognosis Pediatric BCP-ALL. PLoS ONE 2010, 5, e13552. [Google Scholar] [CrossRef] [PubMed]
- Conter, V.; Valsecchi, M.G.; Buldini, B.; Parasole, R.; Locatelli, F.; Colombini, A.; Rizzari, C.; Putti, M.C.; Barisone, E.; Lo Nigro, L.; et al. Early T-Cell Precursor Acute Lymphoblastic Leukaemia in Children Treated in AIEOP Centres with AIEOP-BFM Protocols: A Retrospective Analysis. Lancet. Haematol. 2016, 3, e80–e86. [Google Scholar] [CrossRef] [PubMed]
- Bartis, D.; Boldizsár, F.; Szabó, M.; Pálinkás, L.; Németh, P.; Berki, T. Dexamethasone Induces Rapid Tyrosine-Phosphorylation of ZAP-70 in Jurkat Cells. J. Steroid Biochem. Mol. Biol. 2006, 98, 147–154. [Google Scholar] [CrossRef]
- Foà, R.; Bassan, R.; Vitale, A.; Elia, L.; Piciocchi, A.; Puzzolo, M.-C.; Canichella, M.; Viero, P.; Ferrara, F.; Lunghi, M.; et al. Dasatinib-Blinatumomab for Ph-Positive Acute Lymphoblastic Leukemia in Adults. N. Engl. J. Med. 2020, 383, 1613–1623. [Google Scholar] [CrossRef]
- Harr, M.W.; Caimi, P.F.; McColl, K.S.; Zhong, F.; Patel, S.N.; Barr, P.M.; Distelhorst, C.W. Inhibition of Lck Enhances Glucocorticoid Sensitivity and Apoptosis in Lymphoid Cell Lines and in Chronic Lymphocytic Leukemia. Cell Death Differ. 2010, 17, 1381–1391. [Google Scholar] [CrossRef]
- Palacios, E.H.; Weiss, A. Function of the Src-Family Kinases, Lck and Fyn, in T-Cell Development and Activation. Oncogene 2004, 23, 7990–8000. [Google Scholar] [CrossRef]
- Lewis, R.S. Calcium Signaling Mechanisms in T Lymphocytes. Annu. Rev. Immunol. 2003, 19, 497–521. [Google Scholar] [CrossRef]
- Crabtree, G.R. Generic Signals and Specific Minireview Outcomes: Signaling through Ca2+, Calcineurin, and NF-AT. Cell 1999, 96, 611–614. [Google Scholar] [CrossRef]
- Zhao, Y.; Tozawa, Y.; Iseki, R.; Mukai, M.; Iwata, M. Calcineurin Activation Protects T Cells from Glucocorticoid-Induced Apoptosis. J. Immunol. 1995, 154, 6346–6354. [Google Scholar] [CrossRef] [PubMed]
- Burns, M.A.; Liao, Z.W.; Yamagata, N.; Pouliot, G.P.; Stevenson, K.E.; Neuberg, D.S.; Thorner, A.R.; Ducar, M.; Silverman, E.A.; Hunger, S.P.; et al. Hedgehog Pathway Mutations Drive Oncogenic Transformation in High-Risk T-Cell Acute Lymphoblastic Leukemia. Leukemia 2018, 32, 2126–2137. [Google Scholar] [CrossRef] [PubMed]
- Dagklis, A.; Demeyer, S.; DeBie, J.; Radaelli, E.; Pauwels, D.; Degryse, S.; Gielen, O.; Vicente, C.; Vandepoel, R.; Geerdens, E.; et al. Hedgehog Pathway Activation in T-Cell Acute Lymphoblastic Leukemia Predicts Response to SMO and GLI1 Inhibitors. Blood 2016, 128, 2642–2654. [Google Scholar] [CrossRef] [PubMed]
- Regl, G.; Kasper, M.; Schnidar, H.; Eichberger, T.; Neill, G.W.; Philpott, M.P.; Esterbauer, H.; Hauser-Kronberger, C.; Frischauf, A.-M.; Aberger, F. Activation of the BCL2 Promoter in Response to Hedgehog/GLI Signal Transduction Is Predominantly Mediated by GLI2. Cancer Res. 2004, 64, 7724–7731. [Google Scholar] [CrossRef] [PubMed]
- Heine, V.M.; Rowitch, D.H. Hedgehog Signaling Has a Protective Effect in Glucocorticoid-Induced Mouse Neonatal Brain Injury through an 11betaHSD2-Dependent Mechanism. J. Clin. Investig. 2009, 119, 267–277. [Google Scholar] [CrossRef]
- Martelli, A.M.; Paganelli, F.; Truocchio, S.; Palumbo, C.; Chiarini, F.; McCubrey, J.A. Understanding the Roles of the Hedgehog Signaling Pathway during T-Cell Lymphopoiesis and in T-Cell Acute Lymphoblastic Leukemia (T-ALL). Int. J. Mol. Sci. 2023, 24, 2962. [Google Scholar] [CrossRef]
- Deftos, M.L.; He, Y.W.; Ojala, E.W.; Bevan, M.J. Correlating Notch Signaling with Thymocyte Maturation. Immunity 1998, 9, 777–786. [Google Scholar] [CrossRef]
- Palomero, T.; Lim, W.K.; Odom, D.T.; Sulis, M.L.; Real, P.J.; Margolin, A.; Barnes, K.C.; O’Neil, J.; Neuberg, D.; Weng, A.P.; et al. NOTCH1 Directly Regulates C-MYC and Activates a Feed-Forward-Loop Transcriptional Network Promoting Leukemic Cell Growth. Proc. Natl. Acad. Sci. USA 2006, 103, 18261–18266. [Google Scholar] [CrossRef]
- Kamdje, A.H.N.; Krampera, M. Notch Signaling in Acute Lymphoblastic Leukemia: Any Role for Stromal Microenvironment? Blood 2011, 118, 6506–6514. [Google Scholar] [CrossRef]
- Aster, J.C.; Pear, W.S.; Blacklow, S.C. Notch Signaling in Leukemia. Annu. Rev. Pathol. Mech. Dis. 2008, 3, 587–613. [Google Scholar] [CrossRef]
- Palomero, T.; Sulis, M.L.; Cortina, M.; Real, P.J.; Barnes, K.; Ciofani, M.; Caparros, E.; Buteau, J.; Brown, K.; Perkins, S.L.; et al. Mutational Loss of PTEN Induces Resistance to NOTCH1 Inhibition in T-Cell Leukemia. Nat. Med. 2007, 13, 1203–1210. [Google Scholar] [CrossRef] [PubMed]
- Cramer, S.D.; Aplan, P.D.; Durum, S.K. Therapeutic Targeting of IL-7Rα Signaling Pathways in ALL Treatment. Blood 2016, 128, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Okuhashi, Y.; Itoh, M.; Nara, N.; Tohda, S. Effects of Combination of Notch Inhibitor plus Hedgehog Inhibitor or Wnt Inhibitor on Growth of Leukemia Cells. Anticancer Res. 2011, 31, 893–896. [Google Scholar] [PubMed]
- Tosello, V.; Bongiovanni, D.; Liu, J.; Pan, Q.; Yan, K.K.; Saccomani, V.; Van Trimpont, M.; Pizzi, M.; Mazzoni, M.; Dei Tos, A.P.; et al. Cross-Talk between GLI Transcription Factors and FOXC1 Promotes T-Cell Acute Lymphoblastic Leukemia Dissemination. Leukemia 2021, 35, 984–1000. [Google Scholar] [CrossRef]
- Hou, X.; Chen, X.; Zhang, P.; Fan, Y.; Ma, A.; Pang, T.; Song, Z.; Jin, Y.; Hao, W.; Liu, F.; et al. Inhibition of Hedgehog Signaling by GANT58 Induces Apoptosis and Shows Synergistic Antitumor Activity with AKT Inhibitor in Acute T Cell Leukemia Cells. Biochimie 2014, 101, 50–59. [Google Scholar] [CrossRef]
- Bardwell, A.J.; Wu, B.; Sarin, K.Y.; Waterman, M.L.; Atwood, S.X.; Bardwell, L. ERK2 MAP Kinase Regulates SUFU Binding by Multisite Phosphorylation of GLI1. Life Sci. Alliance 2022, 5, e202101353. [Google Scholar] [CrossRef]
- Tissing, W.J.E.; Meijerink, J.P.P.; Den Boer, M.L.; Brinkhof, B.; Van Rossum, E.F.C.; Van Wering, E.R.; Koper, J.W.; Sonneveld, P.; Pieters, R. Genetic Variations in the Glucocorticoid Receptor Gene Are Not Related to Glucocorticoid Resistance in Childhood Acute Lymphoblastic Leukemia. Clin. Cancer Res. 2005, 11, 6050–6056. [Google Scholar] [CrossRef]
- Bachmann, P.S.; Gorman, R.; MacKenzie, K.L.; Lutze-Mann, L.; Lock, R.B. Dexamethasone Resistance in B-Cell Precursor Childhood Acute Lymphoblastic Leukemia Occurs Downstream of Ligand-Induced Nuclear Translocation of the Glucocorticoid Receptor. Blood 2005, 105, 2519–2526. [Google Scholar] [CrossRef]
- Olivas-Aguirre, M.; Torres-López, L.; Pottosin, I.; Dobrovinskaya, O. Overcoming Glucocorticoid Resistance in Acute Lymphoblastic Leukemia: Repurposed Drugs Can Improve the Protocol. Front. Oncol. 2021, 11, 617937. [Google Scholar] [CrossRef]
- Abdoul-Azize, S.; Dubus, I.; Vannier, J.P. Modulation of Glucocorticoid Sensitivity in Acute Lymphoblastic Leukemia: Pyr3, a New Therapeutic Tool? Med. Sci. 2017, 33, 130–132. [Google Scholar] [CrossRef]
- Polak, A.; Kiliszek, P.; Sewastianik, T.; Szydłowski, M.; Jabłońska, E.; Białopiotrowicz, E.; Górniak, P.; Markowicz, S.; Nowak, E.; Grygorowicz, M.A.; et al. MEK Inhibition Sensitizes Precursor B-Cell Acute Lymphoblastic Leukemia (B-ALL) Cells to Dexamethasone through Modulation of MTOR Activity and Stimulation of Autophagy. PLoS ONE 2016, 11, e0155893. [Google Scholar] [CrossRef] [PubMed]
- Follini, E.; Marchesini, M.; Roti, G. Strategies to Overcome Resistance Mechanisms in T-Cell Acute Lymphoblastic Leukemia. Int. J. Mol. Sci. 2019, 20, 3021. [Google Scholar] [CrossRef] [PubMed]
- Silveira, A.B.; Laranjeira, A.B.A.; Rodrigues, G.O.L.; Leal, P.C.; Cardoso, B.A.; Barata, J.T.; Yunes, R.A.; Zanchin, N.I.T.; Brandalise, S.R.; Yunes, J.A. PI3K Inhibition Synergizes with Glucocorticoids but Antagonizes with Methotrexate in T-Cell Acute Lymphoblastic Leukemia. Oncotarget 2015, 6, 13105–13118. [Google Scholar] [CrossRef] [PubMed]
- Janes, M.R.; Limon, J.J.; So, L.; Chen, J.; Lim, R.J.; Chavez, M.A.; Vu, C.; Lilly, M.B.; Mallya, S.; Ong, S.T.; et al. Effective and Selective Targeting of Leukemia Cells Using a TORC1/2 Kinase Inhibitor. Nat. Med. 2010, 16, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Chiarini, F.; Grimaldi, C.; Ricci, F.; Tazzari, P.L.; Evangelisti, C.; Ognibene, A.; Battistelli, M.; Falcieri, E.; Melchionda, F.; Pession, A.; et al. Activity of the Novel Dual Phosphatidylinositol 3-Kinase/Mammalian Target of Rapamycin Inhibitor NVP-BEZ235 against T-Cell Acute Lymphoblastic Leukemia. Cancer Res. 2010, 70, 8097–8107. [Google Scholar] [CrossRef]
- Hall, C.P.; Reynolds, C.P.; Kang, M.H. Modulation of Glucocorticoid Resistance in Pediatric T-Cell Acute Lymphoblastic Leukemia by Increasing BIM Expression with the PI3K/MTOR Inhibitor BEZ235. Clin. Cancer Res. 2016, 22, 621–632. [Google Scholar] [CrossRef]
- Meyer, L.K.; Delgado-Martin, C.; Sharp, P.P.; Huang, B.J.; McMinn, D.; Vincent, T.L.; Ryan, T.; Horton, T.M.; Wood, B.L.; Teachey, D.T.; et al. Inhibition of the Sec61 Translocon Overcomes Cytokine-Induced Glucocorticoid Resistance in T-Cell Acute Lymphoblastic Leukaemia. Br. J. Haematol. 2022, 198, 137–141. [Google Scholar] [CrossRef]
- Tian, Y.; Ai, H.; Ji, X.; Wei, X.D.; Song, Y.P.; Yin, Q.S. Efficacy Analysis of Venetoclax Combined with TKI and Dexamethasone-Containing Low-Dose Chemotherapy for Relapsed/Refractory Ph+acute B-Lymphoblastic Leukemia. Zhonghua Yi Xue Za Zhi 2022, 102, 745–748. [Google Scholar] [CrossRef]
- Kawashima-Goto, S.; Imamura, T.; Tomoyasu, C.; Yano, M.; Yoshida, H.; Fujiki, A.; Tamura, S.; Osone, S.; Ishida, H.; Morimoto, A.; et al. BCL2 Inhibitor (ABT-737): A Restorer of Prednisolone Sensitivity in Early T-Cell Precursor-Acute Lymphoblastic Leukemia with High MEF2C Expression? PLoS ONE 2015, 10, e0132926. [Google Scholar] [CrossRef]
- Hui, P.Y.; Chen, Y.H.; Qin, J.; Jiang, X.H. PON2 Blockade Overcomes Dexamethasone Resistance in Acute Lymphoblastic Leukemia. Hematology 2022, 27, 32–42. [Google Scholar] [CrossRef]
- Cialfi, S.; Palermo, R.; Manca, S.; Checquolo, S.; Bellavia, D.; Pelullo, M.; Quaranta, R.; Dominici, C.; Gulino, A.; Screpanti, I.; et al. Glucocorticoid Sensitivity of T-Cell Lymphoblastic Leukemia/Lymphoma Is Associated with Glucocorticoid Receptor-Mediated Inhibition of Notch1 Expression. Leukemia 2013, 27, 485–488. [Google Scholar] [CrossRef]
- Real, P.J.; Ferrando, A.A. NOTCH Inhibition and Glucocorticoid Therapy in T-Cell Acute Lymphoblastic Leukemia. Leukemia 2009, 23, 1374–1377. [Google Scholar] [CrossRef] [PubMed]
- Real, P.J.; Tosello, V.; Palomero, T.; Castillo, M.; Hernando, E.; De Stanchina, E.; Sulis, M.L.; Barnes, K.; Sawai, C.; Homminga, I.; et al. γ-Secretase Inhibitors Reverse Glucocorticoid Resistance in T Cell Acute Lymphoblastic Leukemia. Nat. Med. 2008, 15, 50–58. [Google Scholar] [CrossRef]
- Bachmann, P.S.; Gorman, R.; Papa, R.A.; Bardell, J.E.; Ford, J.; Kees, U.R.; Marshall, G.M.; Lock, R.B. Divergent Mechanisms of Glucocorticoid Resistance in Experimental Models of Pediatric Acute Lymphoblastic Leukemia. Cancer Res. 2007, 67, 4482–4490. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Liu, W.J. Prognostic Value of the Response to Prednisone for Children with Acute Lymphoblastic Leukemia: A Meta-Analysis. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 7858–7866. [Google Scholar] [CrossRef]
- Irving, J.A.E. Towards an Understanding of the Biology and Targeted Treatment of Paediatric Relapsed Acute Lymphoblastic Leukaemia. Br. J. Haematol. 2016, 172, 655–666. [Google Scholar] [CrossRef] [PubMed]

| Name | GC Potency | Mineral Corticoid Potency | Plasma Half-life | Dosing in Treatment | References |
|---|---|---|---|---|---|
| Prednisone | 3.5–4 | 0.8 | 12–36 h | 40–60 mg/m2 | [14,15] |
| Dexamethasone | 25–80 | 0 | 36–54 h | 6–10 mg/m2 | [14] |
| Trials | n | GC | Dose | Duration of Treatment | Disease | Study Arm/Group | Results | Refs |
|---|---|---|---|---|---|---|---|---|
NCT00613457 | 2039 | Dex Pred | 10 mg/m2/day 60 mg/m2/day | 14 days 20 days | ALL | Pred vs. Dex | Dex reduced the incidence of better salvageable relapses Significant survival benefit from dex only for patients with T-cell ALL | [16] |
NCT03390387 | 4000 | Dex Pred | 6 mg/m2/day 60 mg/m2/day | 28 days or day 1–15 and 22–29 28 days | ALL | Dex intermittent vs Dex continue vs. Pred | Recruiting | NA |
NCT00002816 Phase 3 | 120 | Dex | - | - | Relapsed ALL | Early relapse vs. late relapse for drugs association | After reinduction, LPC counts were lower than in patients treated for an overt BM first relapse. | [17,18] |
| NCT00707083 Phase 3 | 2231 | Dex | - | Days 1–5 and 29–33 | ALL | Bone marrow suppression and liver toxicity | [19] | |
(CCG)-1922 | 1060 | Dex Pred | 6 mg/m2/day 40 mg/m2/day | 28 days 28 days | ALL | 6 MP + Oral Pred vs. 6 MP + Intravenous Pred vs. 6 MP + Oral dex vs. 6 MP + Intravenous Dex | Dex provided a 34% reduction in risk of relapse | [20] |
AALL0232 | 3154 | Dex Pred | 10 mg/m2/day 60 mg/m2/day | 14 days 28 days | High risk B-ALL | Pred vs. Dex | Higher rate of subsequent osteonecrosis with Dex-treated patient | [21] |
NCT00003728 | 1947 | Dex Pred | 6 mg/m2/day 60 mg/m2/day | - | ALL | Pred vs. Dex | EVS similar Decreased cumulative incidence of CNS relapse with dex. | [22] |
| NCT01324180 | 14 | Dex | 10 mg/m2/day | - | Relapsed/ refractory ALL | Met + VPLD | - | NA |
NCT03613428 | 12 | Pred | 1 mg/kg | 28 days | Relapse/refractory T-ALL | Rux + Pred | - | NA |
NCT03817320 | 31 | Dex | 10 mg/m2/day | 14 days | Relapsed /refractory ALL | Ixa + VXLD | Recruiting | NA |
| Pathways | Drug | Activation Mode | Inhibitor (s) | Leukemic Cells | Consequences | References |
|---|---|---|---|---|---|---|
Ca2+ signaling | Dex | intracellular release | BAPTA-AM Pyr3 thapsigargin | Reh Nalm-6 | increases GC sensitivity | [37,38] |
IL7R signaling | Dex | IL-7R upregulation | Ruxolitinib JAK3i | CCRF-CEM Patient samples PDX samples | overcomes GC resistance | [39,40] |
BCL2 signaling | Dex | BCL2 upregulation | ABT-199 Ruxolitinib Tofacitinib | PDX samples Patient samples | overcomes GC resistance | [39,41,42] |
LCK signaling | Dex/Pred | LCK phosphorylation | Dasatinib Bosutinib Nintedanib WH-4-023 shRNA | Patient samples CCRF-CEM Jurkat TALL-1 SUPT1 LK203 PDX samples | overcomes GC resistance | [2,43,44,45,46,47] |
AKT signaling | Dex | AKT phosphorylation | MK2206 Akt inhibitor IV | Patient samples CCRF-CEM MOLT3 PF382 | increases GC sensitivity | [11,48] |
ERK signaling JNK signaling | Dex | ERK, JNK phosphorylation | U0126 SP600125 ip | CEM-C1-15 R3F9 | overcomes GC resistance | [49,50] |
Hedgehog signaling | Dex | Hh activation via GLI1 and PTCH mRNAs | mPKI GANT61 | CEM-C7-14 T-ALL cell lines PDX samples | increases GC sensitivity | [51,52] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Angot, L.; Schneider, P.; Vannier, J.-P.; Abdoul-Azize, S. Beyond Corticoresistance, A Paradoxical Corticosensitivity Induced by Corticosteroid Therapy in Pediatric Acute Lymphoblastic Leukemias. Cancers 2023, 15, 2812. https://doi.org/10.3390/cancers15102812
Angot L, Schneider P, Vannier J-P, Abdoul-Azize S. Beyond Corticoresistance, A Paradoxical Corticosensitivity Induced by Corticosteroid Therapy in Pediatric Acute Lymphoblastic Leukemias. Cancers. 2023; 15(10):2812. https://doi.org/10.3390/cancers15102812
Chicago/Turabian StyleAngot, Laure, Pascale Schneider, Jean-Pierre Vannier, and Souleymane Abdoul-Azize. 2023. "Beyond Corticoresistance, A Paradoxical Corticosensitivity Induced by Corticosteroid Therapy in Pediatric Acute Lymphoblastic Leukemias" Cancers 15, no. 10: 2812. https://doi.org/10.3390/cancers15102812
APA StyleAngot, L., Schneider, P., Vannier, J.-P., & Abdoul-Azize, S. (2023). Beyond Corticoresistance, A Paradoxical Corticosensitivity Induced by Corticosteroid Therapy in Pediatric Acute Lymphoblastic Leukemias. Cancers, 15(10), 2812. https://doi.org/10.3390/cancers15102812





