Androgen Receptor Is Expressed in the Majority of Breast Cancer Brain Metastases and Is Subtype-Dependent
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Immunohistochemistry
2.3. Statistical Analysis
2.4. Systematized Review of Active Clinical Trials Investigating AR Targeted Therapies in Advanced and Metastatic Breast Cancer
3. Results
3.1. Baseline Patient Characteristics
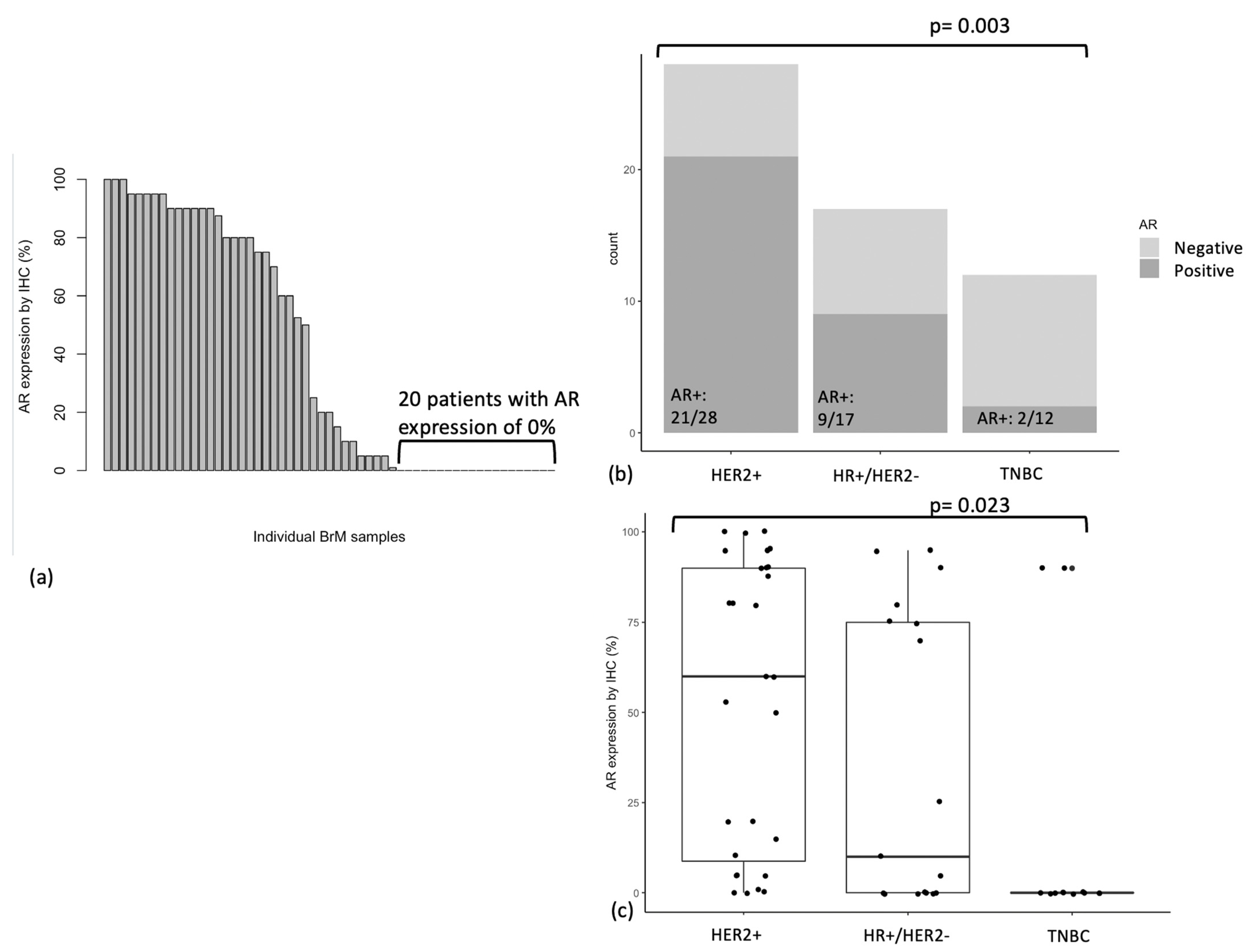
3.2. AR Expression in BrM
3.3. AR Expression by Breast Cancer Subtype
3.4. AR Expression in Relation to Ki-67 Expression and GATA3 Expression
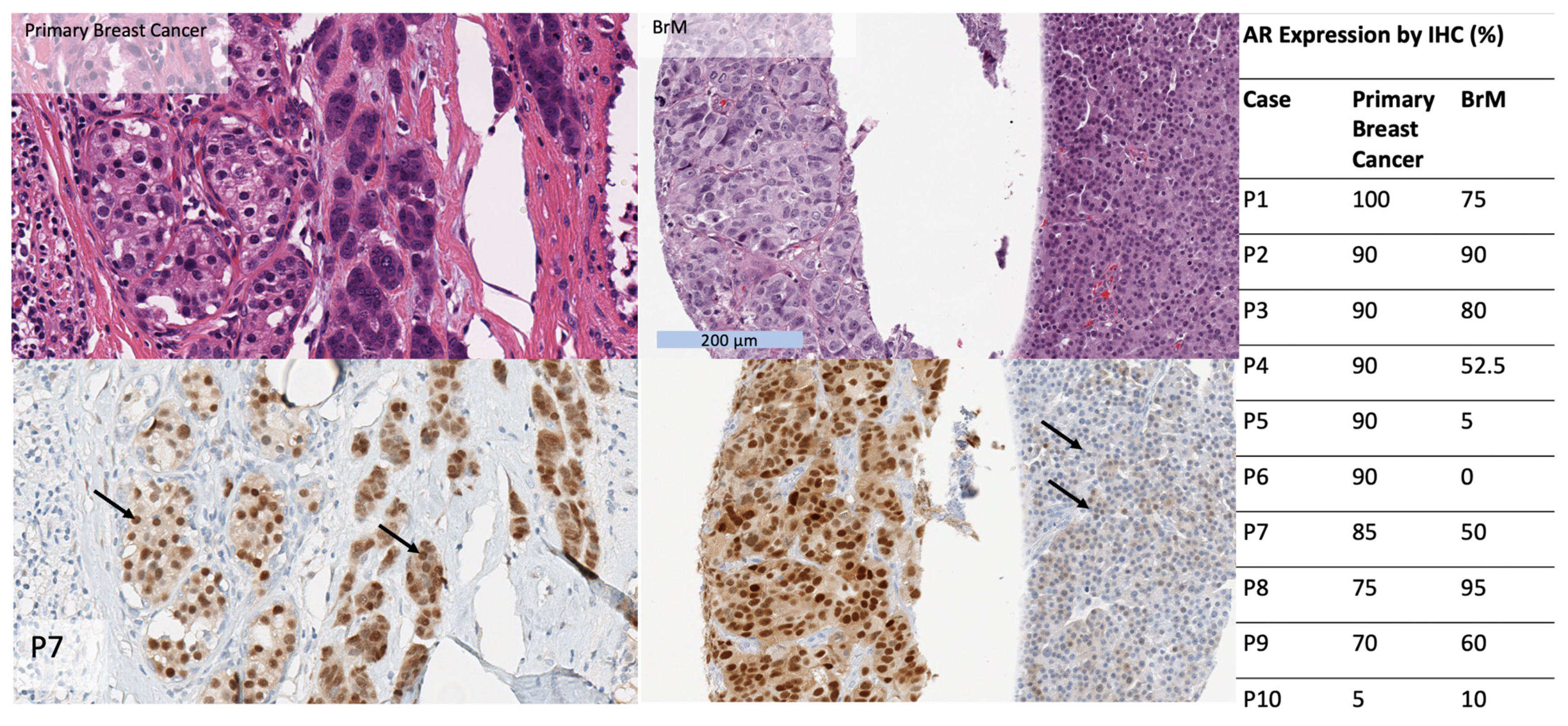
3.5. AR Expression in Matched Primary Breast Cancers
3.6. Associations between AR Expression and Clinical Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gerratana, L.; Basile, D.; Buono, G.; De Placido, S.; Giuliano, M.; Minichillo, S.; Coinu, A.; Martorana, F.; De Santo, I.; Del Mastro, L.; et al. Androgen Receptor in Triple Negative Breast Cancer: A Potential Target for the Targetless Subtype. Cancer Treat. Rev. 2018, 68, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.D.; Gucalp, A.; Traina, T.A. The Role of the Androgen Receptor in Triple-Negative Breast Cancer. Womens Health 2013, 9, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Koo, J.; Park, H.S.; Kim, J.-H.; Choi, S.-Y.; Lee, J.H.; Park, B.-W.; Lee, K.S. Expression of Androgen Receptors in Primary Breast Cancer. Ann. Oncol. 2010, 21, 488–492. [Google Scholar] [CrossRef] [PubMed]
- Isola, J.J. Immunohistochemical Demonstration of Androgen Receptor in Breast Cancer and Its Relationship to Other Prognostic Factors. J. Pathol. 1993, 170, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Collins, L.C.; Cole, K.S.; Marotti, J.D.; Hu, R.; Schnitt, S.J.; Tamimi, R.M. Androgen Receptor Expression in Breast Cancer in Relation to Molecular Phenotype: Results from the Nurses’ Health Study. Mod. Pathol. 2011, 24, 924–931. [Google Scholar] [CrossRef]
- Vera-Badillo, F.E.; Templeton, A.J.; de Gouveia, P.; Diaz-Padilla, I.; Bedard, P.L.; Al-Mubarak, M.; Seruga, B.; Tannock, I.F.; Ocana, A.; Amir, E. Androgen Receptor Expression and Outcomes in Early Breast Cancer: A Systematic Review and Meta-Analysis. J. Natl. Cancer Inst. 2014, 106, djt319. [Google Scholar] [CrossRef]
- Luo, X.; Shi, Y.-X.; Li, Z.-M.; Jiang, W.-Q. Expression and Clinical Significance of Androgen Receptor in Triple Negative Breast Cancer. Chin. J. Cancer 2010, 29, 585–590. [Google Scholar] [CrossRef]
- Kumar, V.; Yu, J.; Phan, V.; Tudor, I.C.; Peterson, A.; Uppal, H. Androgen Receptor Immunohistochemistry as a Companion Diagnostic Approach to Predict Clinical Response to Enzalutamide in Triple-Negative Breast Cancer. JCO Precis. Oncol. 2017, 1, 1–19. [Google Scholar] [CrossRef]
- Bonnefoi, H.; Grellety, T.; Tredan, O.; Saghatchian, M.; Dalenc, F.; Mailliez, A.; L’Haridon, T.; Cottu, P.; Abadie-Lacourtoisie, S.; You, B.; et al. A Phase II Trial of Abiraterone Acetate plus Prednisone in Patients with Triple-Negative Androgen Receptor Positive Locally Advanced or Metastatic Breast Cancer (UCBG 12-1). Ann. Oncol. 2016, 27, 812–818. [Google Scholar] [CrossRef]
- Gucalp, A.; Tolaney, S.; Isakoff, S.J.; Ingle, J.N.; Liu, M.C.; Carey, L.A.; Blackwell, K.; Rugo, H.; Nabell, L.; Forero, A.; et al. Phase II Trial of Bicalutamide in Patients with Androgen Receptor–Positive, Estrogen Receptor–Negative Metastatic Breast Cancer. Clin. Cancer Res. 2013, 19, 5505–5512. [Google Scholar] [CrossRef]
- Kolyvas, E.A.; Caldas, C.; Kelly, K.; Ahmad, S.S. Androgen Receptor Function and Targeted Therapeutics across Breast Cancer Subtypes. Breast Cancer Res. 2022, 24, 79. [Google Scholar] [CrossRef]
- Traina, T.A.; Miller, K.; Yardley, D.A.; Eakle, J.; Schwartzberg, L.S.; O’Shaughnessy, J.; Gradishar, W.; Schmid, P.; Winer, E.; Kelly, C.; et al. Enzalutamide for the Treatment of Androgen Receptor–Expressing Triple-Negative Breast Cancer. J. Clin. Oncol. 2018, 36, 884–890. [Google Scholar] [CrossRef]
- Wardley, A.; Cortes, J.; Provencher, L.; Miller, K.; Chien, A.J.; Rugo, H.S.; Steinberg, J.; Sugg, J.; Tudor, I.C.; Huizing, M.; et al. The Efficacy and Safety of Enzalutamide with Trastuzumab in Patients with HER2+ and Androgen Receptor-Positive Metastatic or Locally Advanced Breast Cancer. Breast Cancer Res. Treat. 2021, 187, 155–165. [Google Scholar] [CrossRef]
- Li, W.; O’Shaughnessy, J.; Hayes, D.; Campone, M.; Bondarenko, I.; Zbarskaya, I.; Brain, E.; Stenina, M.; Ivanova, O.; Graas, M.-P.; et al. Biomarker Associations with Efficacy of Abiraterone Acetate and Exemestane in Postmenopausal Patients with Estrogen Receptor–Positive Metastatic Breast Cancer. Clin. Cancer Res. 2016, 22, 6002–6009. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.U.; Bellon, J.R.; Winer, E.P. CNS Metastases in Breast Cancer. J. Clin. Oncol. 2004, 22, 3608–3617. [Google Scholar] [CrossRef] [PubMed]
- Moilanen, A.-M.; Riikonen, R.; Oksala, R.; Ravanti, L.; Aho, E.; Wohlfahrt, G.; Nykänen, P.S.; Törmäkangas, O.P.; Palvimo, J.J.; Kallio, P.J. Discovery of ODM-201, A New-Generation Androgen Receptor Inhibitor Targeting Resistance Mechanisms to Androgen Signaling-Directed Prostate Cancer Therapies. Sci. Rep. 2015, 5, 12007. [Google Scholar] [CrossRef]
- Bergen, E.S.; Berghoff, A.S.; Steindl, A.; Rajky, O.; Mercea, P.A.; Kiesel, B.; Tendl-Schulz, K.; Bago-Horvath, Z.; Exner, R.; Fitzal, F.; et al. Androgen Receptor Is Expressed in Breast Cancer Brain Metastases. Appl. Immunohistochem. Mol. Morphol. 2021, 29, 728–733. [Google Scholar] [CrossRef] [PubMed]
- Allison, K.H.; Hammond, M.E.H.; Dowsett, M.; McKernin, S.E.; Carey, L.A.; Fitzgibbons, P.L.; Hayes, D.F.; Lakhani, S.R.; Chavez-MacGregor, M.; Perlmutter, J.; et al. Estrogen and Progesterone Receptor Testing in Breast Cancer: ASCO/CAP Guideline Update. J. Clin. Oncol. 2020, 38, 1346–1366. [Google Scholar] [CrossRef]
- Micello, D.; Marando, A.; Sahnane, N.; Riva, C.; Capella, C.; Sessa, F. Androgen Receptor Is Frequently Expressed in HER2-Positive, ER/PR-Negative Breast Cancers. Virchows Arch. 2010, 457, 467–476. [Google Scholar] [CrossRef]
- Qi, J.; Yang, Y.; Zhu, H.; Wang, J.; Jia, Y.; Liu, N.; Song, Y.; Zan, L.; Zhang, X.; Zhou, M.; et al. Expression of the Androgen Receptor and Its Correlation with Molecular Subtypes in 980 Chinese Breast Cancer Patients. Breast Cancer Basic Clin. Res. 2012, 6, BCBCR.S8323. [Google Scholar] [CrossRef]
- Bronte, G.; Bravaccini, S.; Ravaioli, S.; Puccetti, M.; Scarpi, E.; Andreis, D.; Tumedei, M.M.; Sarti, S.; Cecconetto, L.; Pietri, E.; et al. Androgen Receptor Expression in Breast Cancer: What Differences Between Primary Tumor and Metastases? Transl. Oncol. 2018, 11, 950–956. [Google Scholar] [CrossRef] [PubMed]
- Govindan, S.; Siraganahalli Eswaraiah, M.; Basavaraj, C.; Adinarayan, M.; Sankaran, S.; Bakre, M. Androgen Receptor MRNA Levels Determine the Prognosis in Triple-Negative Breast Cancer Patients. BMC Cancer 2020, 20, 745. [Google Scholar] [CrossRef]
- Jinna, N.; Alsten, S.V.; Rida, P.; Seewaldt, V.; Troester, M. Molecular Features of Androgen-Receptor Low, Estrogen Receptor-Negative Breast Cancers in the Carolina Breast Cancer Study. Res. Sq. 2023. [Google Scholar] [CrossRef]
- Palmieri, C.; Linden, H.M.; Birrell, S.; Lim, E.; Schwartzberg, L.S.; Rugo, H.S.; Cobb, P.W.; Jain, K.; Vogel, C.L.; O’Shaughnessy, J.; et al. Efficacy of Enobosarm, a Selective Androgen Receptor (AR) Targeting Agent, Correlates with the Degree of AR Positivity in Advanced AR+/Estrogen Receptor (ER)+ Breast Cancer in an International Phase 2 Clinical Study. J. Clin. Oncol. 2021, 39, 1020. [Google Scholar] [CrossRef]
- Kraby, M.R.; Valla, M.; Opdahl, S.; Haugen, O.A.; Sawicka, J.E.; Engstrøm, M.J.; Bofin, A.M. The Prognostic Value of Androgen Receptors in Breast Cancer Subtypes. Breast Cancer Res. Treat. 2018, 172, 283–296. [Google Scholar] [CrossRef]
- Hu, R.; Dawood, S.; Holmes, M.D.; Collins, L.C.; Schnitt, S.J.; Cole, K.; Marotti, J.D.; Hankinson, S.E.; Colditz, G.A.; Tamimi, R.M. Androgen Receptor Expression and Breast Cancer Survival in Postmenopausal Women. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2011, 17, 1867–1874. [Google Scholar] [CrossRef]
- Hickey, T.E.; Selth, L.A.; Chia, K.M.; Laven-Law, G.; Milioli, H.H.; Roden, D.; Jindal, S.; Hui, M.; Finlay-Schultz, J.; Ebrahimie, E.; et al. The Androgen Receptor Is a Tumor Suppressor in Estrogen Receptor–Positive Breast Cancer. Nat. Med. 2021, 27, 310–320. [Google Scholar] [CrossRef]
- Astvatsaturyan, K.; Yue, Y.; Walts, A.E.; Bose, S. Androgen Receptor Positive Triple Negative Breast Cancer: Clinicopathologic, Prognostic, and Predictive Features. PLoS ONE 2018, 13, e0197827. [Google Scholar] [CrossRef]
- Thike, A.A.; Yong-Zheng Chong, L.; Cheok, P.Y.; Li, H.H.; Wai-Cheong Yip, G.; Huat Bay, B.; Tse, G.M.-K.; Iqbal, J.; Tan, P.H. Loss of Androgen Receptor Expression Predicts Early Recurrence in Triple-Negative and Basal-like Breast Cancer. Mod. Pathol. 2014, 27, 352–360. [Google Scholar] [CrossRef]
- Loibl, S.; Müller, B.M.; von Minckwitz, G.; Schwabe, M.; Roller, M.; Darb-Esfahani, S.; Ataseven, B.; du Bois, A.; Fissler-Eckhoff, A.; Gerber, B.; et al. Androgen Receptor Expression in Primary Breast Cancer and Its Predictive and Prognostic Value in Patients Treated with Neoadjuvant Chemotherapy. Breast Cancer Res. Treat. 2011, 130, 477–487. [Google Scholar] [CrossRef]
- McGhan, L.J.; McCullough, A.E.; Protheroe, C.A.; Dueck, A.C.; Lee, J.J.; Nunez-Nateras, R.; Castle, E.P.; Gray, R.J.; Wasif, N.; Goetz, M.P.; et al. Androgen Receptor-Positive Triple Negative Breast Cancer: A Unique Breast Cancer Subtype. Ann. Surg. Oncol. 2014, 21, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.E.; Kang, S.H.; Lee, S.J.; Bae, Y.K. Androgen Receptor Expression Predicts Decreased Survival in Early Stage Triple-Negative Breast Cancer. Ann. Surg. Oncol. 2015, 22, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.L.L.; Macarthur, S.; Ross-Innes, C.S.; Tilley, W.D.; Neal, D.E.; Mills, I.G.; Carroll, J.S. Androgen Receptor Driven Transcription in Molecular Apocrine Breast Cancer Is Mediated by FoxA1. EMBO J. 2011, 30, 3019–3027. [Google Scholar] [CrossRef] [PubMed]
- Kono, M.; Fujii, T.; Lim, B.; Karuturi, M.S.; Tripathy, D.; Ueno, N.T. Androgen Receptor Function and Androgen Receptor–Targeted Therapies in Breast Cancer: A Review. JAMA Oncol. 2017, 3, 1266. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, B.D.; Jovanović, B.; Chen, X.; Estrada, M.V.; Johnson, K.N.; Shyr, Y.; Moses, H.L.; Sanders, M.E.; Pietenpol, J.A. Refinement of Triple-Negative Breast Cancer Molecular Subtypes: Implications for Neoadjuvant Chemotherapy Selection. PLoS ONE 2016, 11, e0157368. [Google Scholar] [CrossRef] [PubMed]
- Gordon, M.A.; D’Amato, N.C.; Gu, H.; Babbs, B.; Wulfkuhle, J.; Petricoin, E.F.; Gallagher, I.; Dong, T.; Torkko, K.; Liu, B.; et al. Synergy between Androgen Receptor Antagonism and Inhibition of MTOR and HER2 in Breast Cancer. Mol. Cancer Ther. 2017, 16, 1389–1400. [Google Scholar] [CrossRef]
- Inwald, E.C.; Klinkhammer-Schalke, M.; Hofstädter, F.; Zeman, F.; Koller, M.; Gerstenhauer, M.; Ortmann, O. Ki-67 Is a Prognostic Parameter in Breast Cancer Patients: Results of a Large Population-Based Cohort of a Cancer Registry. Breast Cancer Res. Treat. 2013, 139, 539–552. [Google Scholar] [CrossRef]
- Asch-Kendrick, R.; Cimino-Mathews, A. The Role of GATA3 in Breast Carcinomas: A Review. Hum. Pathol. 2016, 48, 37–47. [Google Scholar] [CrossRef]
- Yoon, N.K.; Maresh, E.L.; Shen, D.; Elshimali, Y.; Apple, S.; Horvath, S.; Mah, V.; Bose, S.; Chia, D.; Chang, H.R.; et al. Higher Levels of GATA3 Predict Better Survival in Women with Breast Cancer. Hum. Pathol. 2010, 41, 1794–1801. [Google Scholar] [CrossRef]
- Kouros-Mehr, H.; Bechis, S.K.; Slorach, E.M.; Littlepage, L.E.; Egeblad, M.; Ewald, A.J.; Pai, S.-Y.; Ho, I.-C.; Werb, Z. GATA-3 Links Tumor Differentiation and Dissemination in a Luminal Breast Cancer Model. Cancer Cell 2008, 13, 141–152. [Google Scholar] [CrossRef]


| NCT Identifier | Trial Status | Phase | Patient Population | AR Status | Drug Category | Drug Class | Drug Name | Combination Drug(s) | Inclusion of BrM |
|---|---|---|---|---|---|---|---|---|---|
| NCT05673694 | Not yet recruiting | 1a/1b | Metastatic or recurrent ER+/HER2− breast cancer | AR positive | AR agonist | Non-steroidal androgen receptor agonist | EG017 | Controlled BrM only | |
| NCT02971761 | Active, not recruiting | 2 | Metastatic TNBC | AR positive (≥50%) | AR agonist | Selective androgen receptor modulator | Enobosarm | Pembrolizumab | Controlled BrM only |
| NCT05573126 | Not yet recruiting | 1 and 2 | Metastatic or locally advanced ER+/HER2− breast cancer | AR positive (≥30% using SP107) | AR agonist | Selective androgen receptor modulator | EP0062 | Included | |
| NCT04869943 | Recruiting | 3 | Metastatic ER+/HER2− breast cancer | AR positive (≥40%) | AR agonist | Selective androgen receptor modulator | Enobosarm | Controlled BrM only | |
| NCT05065411 | Recruiting | 3 | Metastatic ER+/HER2− breast cancer | AR positive (≥40%) | AR agonist | Selective androgen receptor modulator | Enobosarm | abemaciclib | Controlled BrM only |
| NCT02067741 | Active, not recruiting | 2 | Metastatic or locally advanced ER+/HER2− or TNBC | TNBC cases need to be AR+ | AR agonist | Testosterone analogue | CR1447 | Controlled BrM only | |
| NCT01889238 | Active, not recruiting | 2 | Advanced TNBC | AR positive | AR antagonist | Non-steroidal antiandrogen | Enzalutamide | Excluded | |
| NCT05095207 | Recruiting | 1 and 2 | Metastatic HER2− | AR positive (≥1%) | AR antagonist | Non-steroidal antiandrogen | Bicalutamide | Abemaciclib | Controlled BrM only |
| NCT03207529 | Recruiting | 1 | Metastatic PTEN+, TNBC or HR+/HER2−, breast cancer | AR positive (≥1%) | AR antagonist | Non-steroidal antiandrogen | Enzalutamide | Alpelisib | Controlled BrM only |
| NCT02091960 | Active, not recruiting | 2 | Locally advanced or metastatic HER2+ breast cancer | AR positive | AR antagonist | Non-steroidal antiandrogen | Enzalutamide | Traztuzumab | Excluded |
| NCT03090165 | Recruiting | 1 and 2 | Metastatic TNBC | AR positive (≥10%) | AR antagonist | Non-steroidal antiandrogen | Bicalutamide | Ribociclib | Controlled BrM only |
| NCT02605486 | Active, not recruiting | 1 and 2 | Metastatic TNBC | AR positive (≥1% by AR441) | AR antagonist | Non-steroidal antiandrogen | Bicalutamide | Palbociclib | Controlled BrM only |
| NCT03650894 | Recruiting | 2 | Metastatic or locally advanced HER2− breast cancer | TNBC cases need to be AR+ | AR antagonist | Non-steroidal antiandrogen | Bicalutamide | Nivolumab and Ipilimumab | Controlled BrM only |
| NCT02955394 | Active, not recruiting | 2 | T2 or greater ER+/HER2− breast cancer | AR positive | AR antagonist | Non-steroidal antiandrogen | Enzalutamide | Fulvestrant | Excluded |
| NCT02007512 | Active, not recruiting | 2 | Advanced ER+ (and/or PR+) HER2− breast cancer | No requirement | AR antagonist | Non-steroidal antiandrogen | Enzalutamide | Exemestane | Excluded |
| NCT04947189 | Not yet recruiting | 1 and 2 | Metastatic TNBC | AR positive (>0% by IHC or gene classifier molecular testing) | AR antagonist | nonsteroidal CYP17A1 inhibitor | Seviteronel | Docetaxel | Controlled BrM only |
| Characteristic | N = 57 |
|---|---|
| Age at BrM diagnosis (years) | |
| Median (IQR) | 51.8 (14) |
| Range | 32–85 |
| BrM subtype | |
| Triple negative | 12 (21%) |
| HER2+ | 28 (49%) |
| HR+/HER2− | 17 (30%) |
| Number of BrM | |
| One | 35 (61%) |
| More than 1 | 22 (39%) |
| BrM size (cm) | |
| Median (IQR) | 3 (1.4) |
| Range | 0.3–6.2 |
| BrM location | |
| Frontal | 15 (26%) |
| Parietal | 13 (23%) |
| Temporal | 3 (5%) |
| Cerebellar | 25 (44%) |
| Occipital | 1 (2%) |
| BrM grade | |
| 1 | 16 (28%) |
| 2 | 20 (35%) |
| 3 | 15 (26%) |
| Unknown | 6 (11%) |
| Symptomatic BrM | |
| Yes | 50 (88%) |
| No | 7 (12%) |
| Sites of extra-cranial metastatic disease | |
| Lung | 17 (30%) |
| Liver | 14 (25%) |
| Lymph node | 11 (19%) |
| Bone | 22 (39%) |
| Chest wall | 2 (4%) |
| Other | 4 (7%) |
| Radiotherapy for BrM | |
| Yes | 51 (90%) |
| No | 3 (5%) |
| Unknown | 3 (5%) |
| Systemic therapy for metastatic disease prior to BrM | |
| Chemotherapy | 9 (16%) |
| Trastuzumab-based treatment | 10 (18%) |
| Endocrine therapy | 4 (7%) |
| Unknown | 34 (60%) |
| Primary breast cancer subtype | |
| Triple negative | 4 (7%) |
| HER2+ | 16 (28%) |
| HR+/HER2− | 12 (21%) |
| Unknown | 25 (44%) |
| Breast cancer stage at presentation | |
| I | 12(21%) |
| II | 14 (25%) |
| III | 9 (16%) |
| IV | 1 (2%) |
| Unknown | 21 (37%) |
| Characteristic | Patients | Number (%) of AR+ Cases | p |
|---|---|---|---|
| Overall | 57 | 32 (56%) | |
| BrM subtype | 0.003 | ||
| Triple negative | 12 | 2 (17%) | |
| HER2+ | 28 | 21 (75%) | |
| HR+/HER2− | 17 | 9 (53%) | |
| Ki-67 expression | 0.02 | ||
| Low (1–24%) | 17 | 8 (47%) | |
| Intermediate (25–49%) | 21 | 16 (76%) | |
| High (≥50%) | 16 | 5 (31%) | |
| Unknown | 3 | 3 (100%) | |
| GATA3 expression | 0.003 | ||
| Negative (0%) | 19 | 6 (32%) | |
| Low (1–24%) | 4 | 0 (0%) | |
| Intermediate (25–49%) | 6 | 4 (67%) | |
| High (≥50%) | 25 | 19 (76%) | |
| Unknown | 3 | 3 (100%) | |
| Age at BrM (years) | 0.64 | ||
| <50 | 23 | 14 (61%) | |
| ≥50 | 33 | 17 (52%) | |
| Unknown | 1 | 1 (100%) | |
| BrM location | 0.95 | ||
| Frontal | 15 | 8 (53%) | |
| Parietal | 13 | 8 (62%) | |
| Temporal | 3 | 2 (67%) | |
| Cerebellar | 25 | 14 (56%) | |
| Occipital | 1 | 0 (0%) | |
| BrM size (cm) | 0.25 | ||
| <3.0 | 26 | 12 (46% | |
| ≥3.0 | 27 | 19 (70%) | |
| Unknown | 4 | 1 (25%) | |
| BrM grade | 0.29 | ||
| 1 | 16 | 7 (44%) | |
| 2 | 20 | 14 (70%) | |
| 3 | 15 | 5 (33%) | |
| Unknown | 6 | 6 (100%) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, K.Y.; Chehade, R.; Qazi, M.; Moravan, V.; Nofech-Mozes, S.; Jerzak, K.J. Androgen Receptor Is Expressed in the Majority of Breast Cancer Brain Metastases and Is Subtype-Dependent. Cancers 2023, 15, 2748. https://doi.org/10.3390/cancers15102748
Fan KY, Chehade R, Qazi M, Moravan V, Nofech-Mozes S, Jerzak KJ. Androgen Receptor Is Expressed in the Majority of Breast Cancer Brain Metastases and Is Subtype-Dependent. Cancers. 2023; 15(10):2748. https://doi.org/10.3390/cancers15102748
Chicago/Turabian StyleFan, Kevin Yijun, Rania Chehade, Maleeha Qazi, Veronika Moravan, Sharon Nofech-Mozes, and Katarzyna J. Jerzak. 2023. "Androgen Receptor Is Expressed in the Majority of Breast Cancer Brain Metastases and Is Subtype-Dependent" Cancers 15, no. 10: 2748. https://doi.org/10.3390/cancers15102748
APA StyleFan, K. Y., Chehade, R., Qazi, M., Moravan, V., Nofech-Mozes, S., & Jerzak, K. J. (2023). Androgen Receptor Is Expressed in the Majority of Breast Cancer Brain Metastases and Is Subtype-Dependent. Cancers, 15(10), 2748. https://doi.org/10.3390/cancers15102748






