Targeting Hypoxia-Inducible Factor-1α for the Management of Hepatocellular Carcinoma
Abstract
Simple Summary
Abstract
1. Introduction
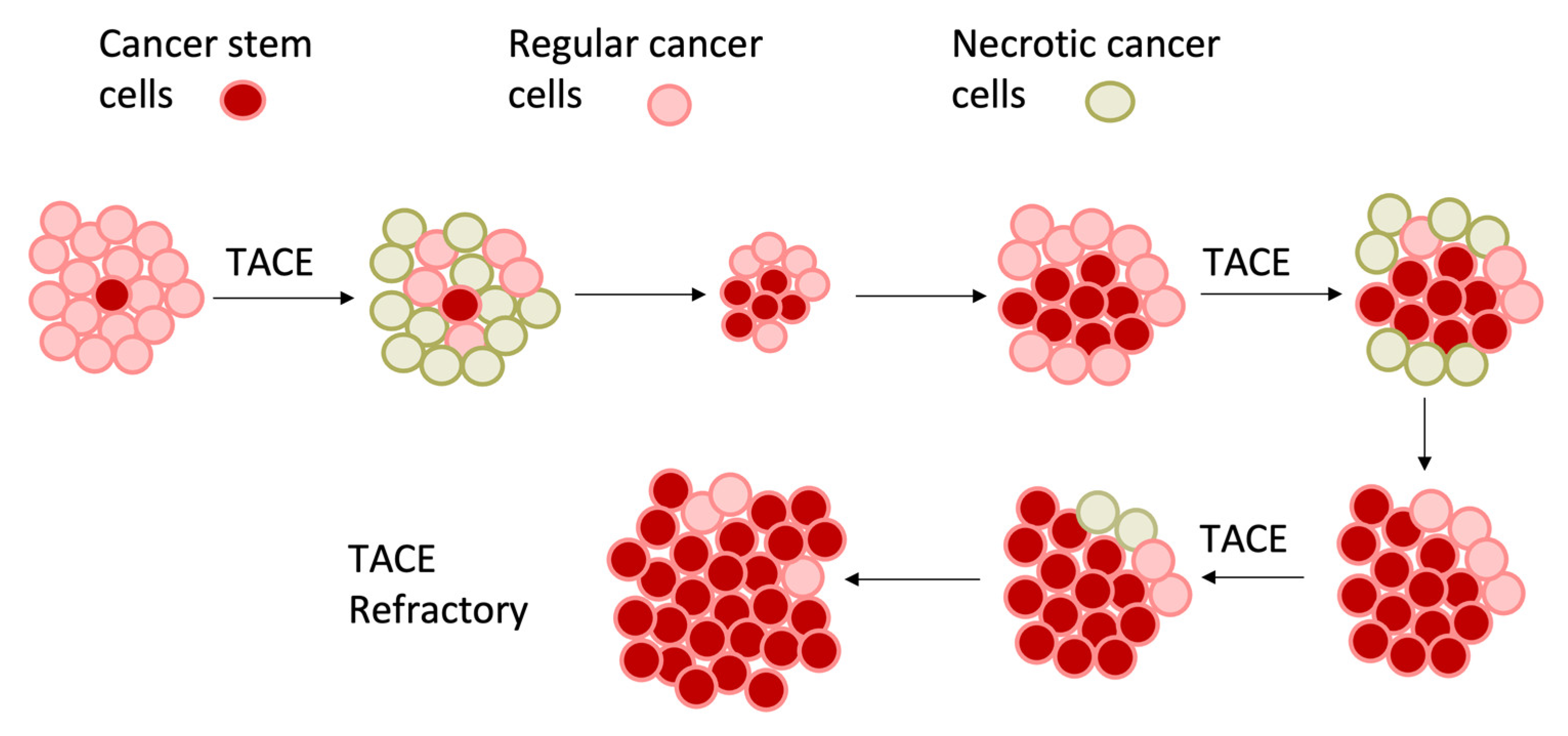
2. The Role of Hypoxia in Tumor Progression
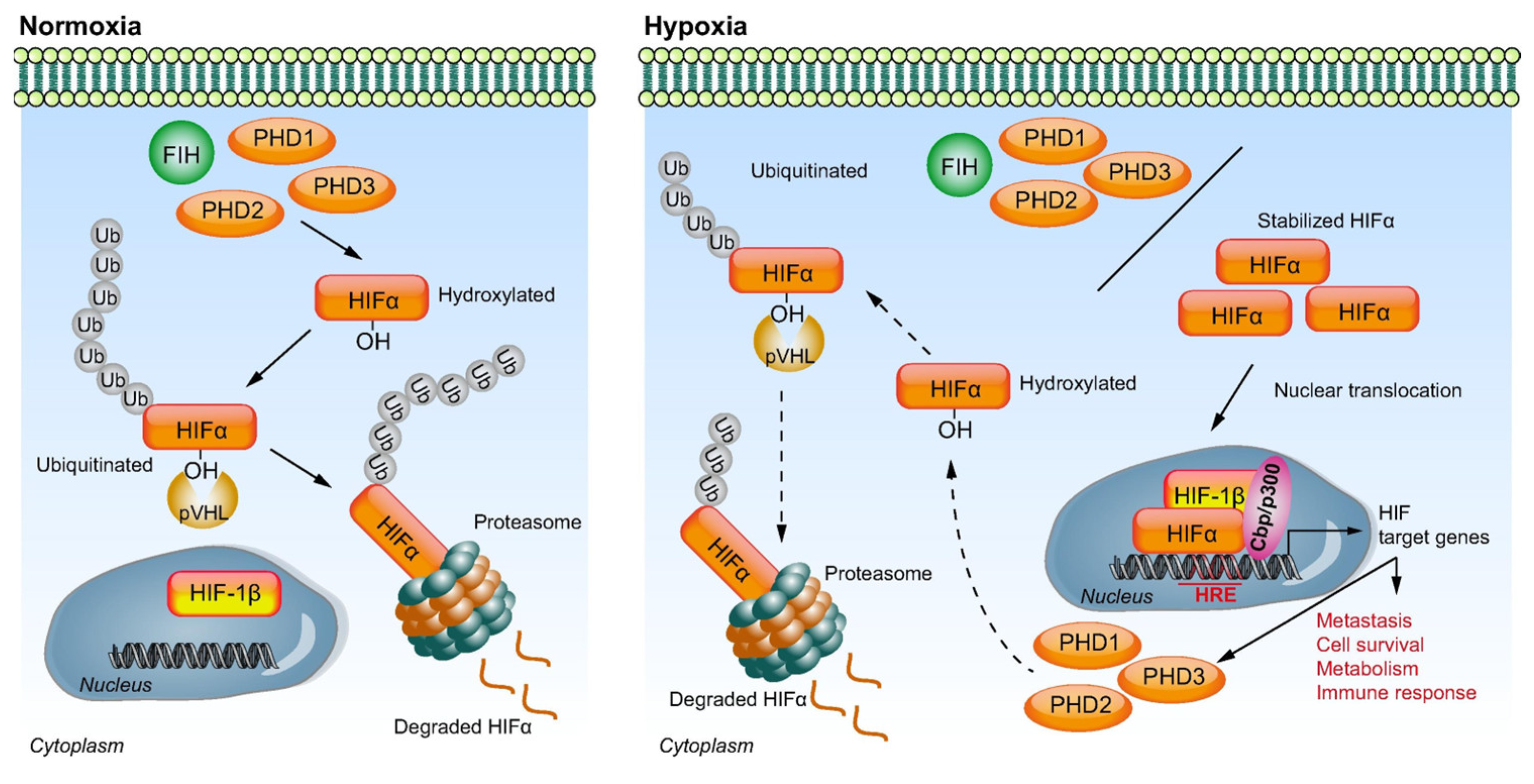
3. Molecular Pathways of Hypoxia
4. Significance of Serum HIF-1α Levels in the Biology of HCC
5. Intra-Vascular Liver-Directed Therapies for the Treatment of HCC
6. Therapeutic Strategies That Target Tumor Hypoxia
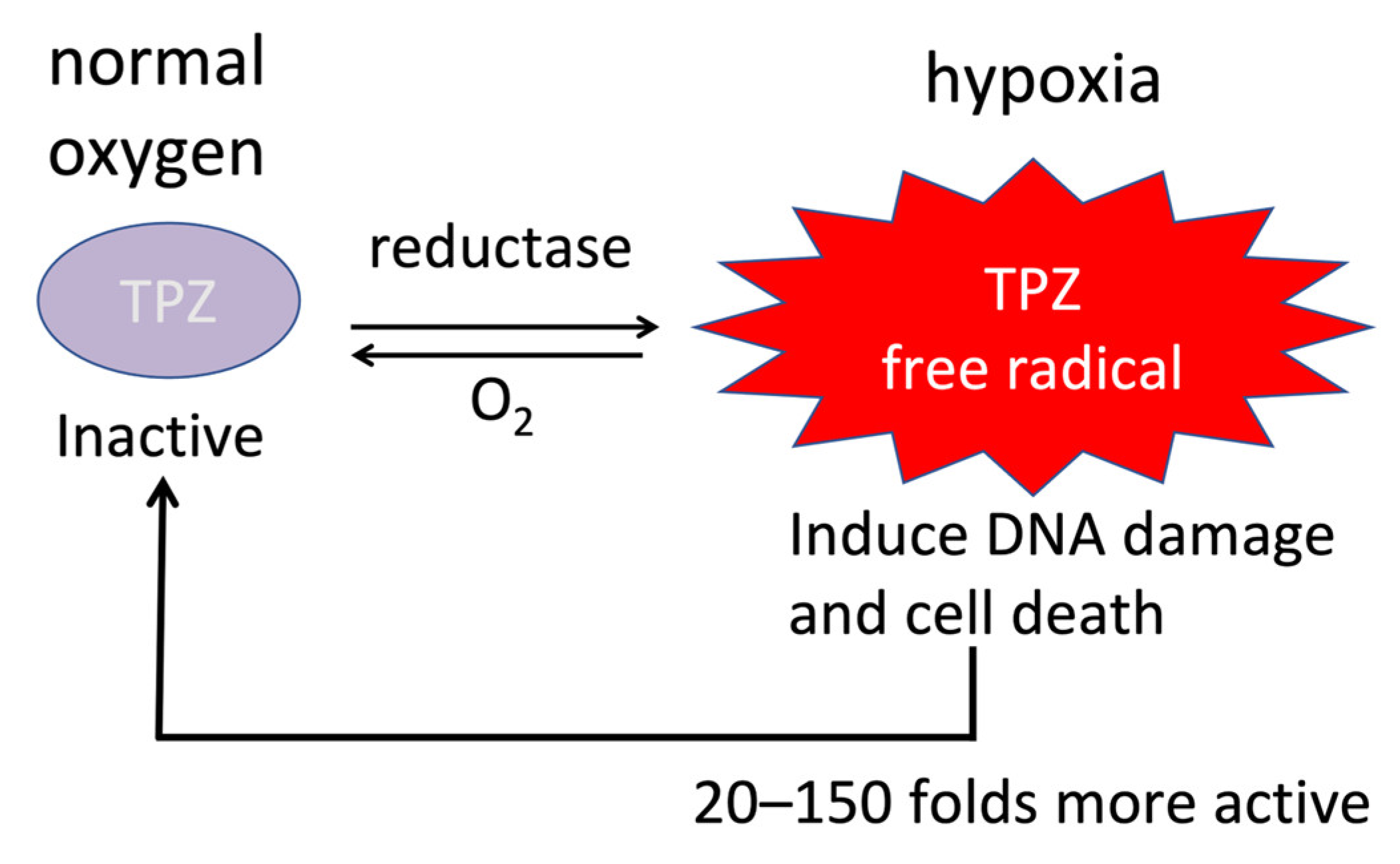
6.1. Aromatic Prodrugs
6.2. Nitro-Based Prodrugs
6.3. Aliphatic Prodrugs
6.4. Quinone Prodrugs
6.5. Other Strategies Targeting Tumor Hypoxia
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rumgay, H.; Arnold, M.; Ferlay, J.; Lesi, O.; Cabasag, C.J.; Vignat, J.; Laversanne, M.; McGlynn, K.A.; Soerjomataram, I. Global burden of primary liver cancer in 2020 and predictions to 2040. J. Hepatol. 2022, 77, 1598–1606. [Google Scholar] [CrossRef] [PubMed]
- Kulik, L.; El-Serag, H.B. Epidemiology and Management of Hepatocellular Carcinoma. Gastroenterology 2019, 156, 477–491.e1. [Google Scholar] [CrossRef]
- Leung, T.W.T.; Tang, A.M.Y.; Zee, B.C.-Y.; Yu, S.C.H.; Lai, P.B.S.; Lau, W.Y.; Johnson, P.J. Factors predicting response and survival in 149 patients with unresectable hepatocellular carcinoma treated by combination cisplatin, interferon-alpha, doxorubicin and 5-fluorouracil chemotherapy. Cancer 2002, 94, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Simonetti, R.G.; Liberati, A.; Angiolini, C.; Pagliaro, L. Treatment of hepatocellular carcinoma: A systematic review of randomized controlled trials. Ann. Oncol. 1997, 8, 117–136. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.X.; Blaszkowsky, L.S.; Ryan, D.P.; Clark, J.W.; Muzikansky, A.; Horgan, K.; Sheehan, S.; Hale, K.E.; Enzinger, P.C.; Bhargava, P.; et al. Phase II Study of Gemcitabine and Oxaliplatin in Combination with Bevacizumab in Patients with Advanced Hepatocellular Carcinoma. J. Clin. Oncol. 2006, 24, 1898–1903. [Google Scholar] [CrossRef] [PubMed]
- Mistry, I.N.; Thomas, M.; Calder, E.D.; Conway, S.J.; Hammond, E.M. Clinical Advances of Hypoxia-Activated Prodrugs in Combination with Radiation Therapy. Int. J. Radiat. Oncol. 2017, 98, 1183–1196. [Google Scholar] [CrossRef]
- Lohitesh, K.; Chowdhury, R.; Mukherjee, S. Resistance a major hindrance to chemotherapy in hepatocellular carcinoma: An insight. Cancer Cell Int. 2018, 18, 44. [Google Scholar] [CrossRef]
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Primers 2021, 7, 6. [Google Scholar] [CrossRef]
- Vaupel, P.; Höckel, M.; Mayer, A. Detection and Characterization of Tumor Hypoxia Using pO2 Histography. Antioxid. Redox Signal 2007, 9, 1221–1236. [Google Scholar] [CrossRef]
- Wu, X.-Z.; Xie, G.-R.; Chen, D. Hypoxia and hepatocellular carcinoma: The therapeutic target for hepatocellular carcinoma. J. Gastroenterol. Hepatol. 2007, 22, 1178–1182. [Google Scholar] [CrossRef]
- Jia, Z.-Z.; Jiang, G.-M.; Feng, Y.-L. Serum HIF-1α and VEGF Levels Pre- and Post-TACE in Patients with Primary Liver Cancer. Chin. Med. Sci. J. 2011, 26, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Yang, L.; Zhang, X.-M.; Zhou, Y.; Zhu, T.; Miao, N.-D.; Ren, Y.-J.; Xu, H.; Min, X.-L.; Peng, J.; et al. HIF-1α and VEGF levels for monitoring hepatocellular carcinoma treatment response to transcatheter arterial chemoembolization. Transl. Cancer Res. 2017, 6, 1043–1049. [Google Scholar] [CrossRef]
- Gai, X.; Zhou, P.; Xu, M.; Liu, Z.; Zheng, X.; Liu, Q. Hyperactivation of IL-6/STAT3 pathway leaded to the poor prognosis of post-TACE HCCs by HIF-1α/SNAI1 axis-induced epithelial to mesenchymal transition. J. Cancer 2020, 11, 570–582. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Huang, G.; Li, X.; Zhang, Y.; Jiang, Y.; Shen, J.; Liu, J.; Wang, Q.; Zhu, J.; Feng, X.; et al. Hypoxia induces epithelial-mesenchymal transition via activation of SNAI1 by hypoxia-inducible factor -1α in hepatocellular carcinoma. BMC Cancer 2013, 13, 108. [Google Scholar] [CrossRef]
- Virmani, S.; Rhee, T.K.; Ryu, R.K.; Sato, K.T.; Lewandowski, R.J.; Mulcahy, M.F.; Kulik, L.M.; Szolc-Kowalska, B.; Woloschak, G.E.; Yang, G.-Y.; et al. Comparison of Hypoxia-inducible Factor-1α Expression before and after Transcatheter Arterial Embolization in Rabbit VX2 Liver Tumors. J. Vasc. Interv. Radiol. 2008, 19, 1483–1489. [Google Scholar] [CrossRef]
- Huang, M.; Wang, L.; Chen, J.; Bai, M.; Zhou, C.; Liu, S.; Lin, Q. Regulation of COX-2 expression and epithelial-to-mesenchymal transition by hypoxia-inducible factor-1α is associated with poor prognosis in hepatocellular carcinoma patients post TACE surgery. Int. J. Oncol. 2016, 48, 2144–2154. [Google Scholar] [CrossRef]
- Vaupel, P.; Thews, O.; Hoeckel, M. Introduction Treatment Resistance of Solid Tumors Role of Hypoxia and Anemia. Med. Oncol. 2001, 18, 243–259. [Google Scholar] [CrossRef]
- Jing, X.; Yang, F.; Shao, C.; Wei, K.; Xie, M.; Shen, H.; Shu, Y. Role of hypoxia in cancer therapy by regulating the tumor microenvironment. Mol. Cancer 2019, 18, 157. [Google Scholar] [CrossRef]
- Nejad, A.E.; Najafgholian, S.; Rostami, A.; Sistani, A.; Shojaeifar, S.; Esparvarinha, M.; Nedaeinia, R.; Javanmard, S.H.; Taherian, M.; Ahmadlou, M.; et al. The role of hypoxia in the tumor microenvironment and development of cancer stem cell: A novel approach to developing treatment. Cancer Cell Int. 2021, 21, 62. [Google Scholar] [CrossRef]
- Semenza, G.L. Hypoxia-Inducible Factors in Physiology and Medicine. Cell 2012, 148, 399–408. [Google Scholar] [CrossRef]
- Brown, J.M.; Wilson, W.R. Exploiting tumour hypoxia in cancer treatment. Nat. Rev. Cancer 2004, 4, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Cosse, J.-P.; Michiels, C. Anti-Cancer Agents in Medicinal Chemistry. Anti Cancer Agents Med. Chem. 2008, 8, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.-S.; Liao, M.-B.; Zhang, M.-Y.; Xie, Y.; Xu, L.; Zhang, Y.; Zheng, X.F.S.; Wang, H.-Y.; Chen, Y.-F. Synergistic Inhibitory Effect of Hyperbaric Oxygen Combined with Sorafenib on Hepatoma Cells. PLoS ONE 2014, 9, e100814. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Ma, J.; Zhan, Y.; Xu, X.; Zeng, Q.; Liang, J.; Chen, X. Hypoxia-activated prodrugs and redox-responsive nanocarriers. Int. J. Nanomed. 2018, 13, 6551–6574. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, L.; Li, X.-F. Targeting Hypoxia: Hypoxia-Activated Prodrugs in Cancer Therapy. Front. Oncol. 2021, 11, 700407. [Google Scholar] [CrossRef]
- Kaseb, A.O.; Hanbali, A.; Cotant, M.; Hassan, M.M.; Wollner, I.; Philip, P.A. Vascular endothelial growth factor in the management of hepatocellular carcinoma: A review of literature. Cancer 2009, 115, 4895–4906. [Google Scholar] [CrossRef]
- Abi-Jaoudeh, N.; Dayyani, F.; Chen, P.J.; Fernando, D.; Fidelman, N.; Javan, H.; Liang, P.-C.; Hwang, J.-I.; Imagawa, D.K. Phase I Trial on Arterial Embolization with Hypoxia Activated Tirapazamine for Unresectable Hepatocellular Carcinoma. J. Hepatocell. Carcinoma 2021, 8, 421–434. [Google Scholar] [CrossRef]
- Liu, C.-H.; Peng, C.-M.; Hwang, J.-I.; Liang, P.-C.; Chen, P.-J.; Abi-Jaoudeh, N.; Giiang, L.-H.; Tyan, Y.-S. Phase I Dose-Escalation Study of Tirapazamine Chemoembolization for Unresectable Early- and Intermediate-Stage Hepatocellular Carcinoma. J. Vasc. Interv. Radiol. 2022, 33, 926–933.e1. [Google Scholar] [CrossRef]
- Jungermann, K.; Kietzmann, T. Oxygen: Modulator of metabolic zonation and disease of the liver. Hepatology 2000, 31, 255–260. [Google Scholar] [CrossRef]
- Xiong, X.X.; Qiu, X.Y.; Hu, D.X.; Chen, X.Q. Advances in Hypoxia-Mediated Mechanisms in Hepatocellular Carcinoma. Mol. Pharmacol. 2017, 92, 246–255. [Google Scholar] [CrossRef]
- Choi, S.H.; Park, J.Y. Regulation of the hypoxic tumor environment in hepatocellular carcinoma using RNA interference. Cancer Cell Int. 2017, 17, 3. [Google Scholar] [CrossRef] [PubMed]
- Yeom, C.J.; Goto, Y.; Zhu, Y.; Hiraoka, M.; Harada, H. Microenvironments and Cellular Characteristics in the Micro Tumor Cords of Malignant Solid Tumors. Int. J. Mol. Sci. 2012, 13, 13949–13965. [Google Scholar] [CrossRef] [PubMed]
- Guise, C.P.; Mowday, A.M.; Ashoorzadeh, A.; Yuan, R.; Lin, W.-H.; Wu, D.-H.; Smaill, J.B.; Patterson, A.V.; Ding, K. Bioreductive prodrugs as cancer therapeutics: Targeting tumor hypoxia. Chin. J. Cancer 2014, 33, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Wilson, G.K.; Tennant, D.A.; McKeating, J.A. Hypoxia inducible factors in liver disease and hepatocellular carcinoma: Current understanding and future directions. J. Hepatol. 2014, 61, 1397–1406. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Zen, Y.; Sato, Y.; Kozaka, K.; Matsui, O.; Harada, K.; Nakanuma, Y. Vascular endothelial growth factor, its receptor Flk-1, and hypoxia inducible factor-1α are involved in malignant transformation in dysplastic nodules of the liver. Hum. Pathol. 2007, 38, 1532–1546. [Google Scholar] [CrossRef] [PubMed]
- Poon, R.T.-P.; Fan, S.-T.; Wong, J. Clinical Implications of Circulating Angiogenic Factors in Cancer Patients. J. Clin. Oncol. 2001, 19, 1207–1225. [Google Scholar] [CrossRef]
- Van Pelt, J.F.; van Malenstein, H.; Nevens, F.; Verslype, C. Chronic Hypoxia Emerging as One of the Driving Forces behind Gene Expression and Prognosis of Hepatocellular Carcinoma. ISRN Pathol. 2011, 2011, 273924. [Google Scholar] [CrossRef]
- Liu, K.; Sun, B.; Zhao, X.; Wang, X.; Li, Y.; Qiu, Z.; Liu, T.; Gu, Q.; Dong, X.; Zhang, Y.; et al. Hypoxia promotes vasculogenic mimicry formation by the Twist1-Bmi1 connection in hepatocellular carcinoma. Int. J. Mol. Med. 2015, 36, 783–791. [Google Scholar] [CrossRef]
- Carreres, L.; Mercey-Ressejac, M.; Kurma, K.; Ghelfi, J.; Fournier, C.; Manches, O.; Chuffart, F.; Rousseaux, S.; Minoves, M.; Decaens, T.; et al. Chronic Intermittent Hypoxia Increases Cell Proliferation in Hepatocellular Carcinoma. Cells 2022, 11, 2051. [Google Scholar] [CrossRef]
- Li, X.; Feng, G.-S.; Zheng, C.-S.; Zhuo, C.-K.; Liu, X. Expression of plasma vascular endothelial growth factor in patients with hepatocellular carcinoma and effect of transcatheter arterial chemoembolization therapy on plasma vascular endothelial growth factor level. World J. Gastroenterol. 2004, 10, 2878–2882. [Google Scholar] [CrossRef]
- Lamouille, S.; Xu, J.; Derynck, R. Molecular mechanisms of epithelial–mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2014, 15, 178–196. [Google Scholar] [CrossRef] [PubMed]
- White, J.A.; Gray, S.H.; Li, P.; Simpson, H.N.; Mcguire, B.M.; Eckhoff, D.E.; Kamel, A.M.; Aal, A.; Saddekni, S.; Dubay, D.A. Current Guidelines for Chemoembolization for Hepatocellular Carcinoma: Room for Improvement? Hepatol. Commun. 2017, 1, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.T.; Do, R.K.; Gonen, M.; Covey, A.M.; Getrajdman, G.I.; Sofocleous, C.T.; Jarnagin, W.R.; D’angelica, M.I.; Allen, P.J.; Erinjeri, J.P.; et al. Randomized Trial of Hepatic Artery Embolization for Hepatocellular Carcinoma Using Doxorubicin-Eluting Microspheres Compared with Embolization with Microspheres Alone. J. Clin. Oncol. 2016, 34, 2046–2053. [Google Scholar] [CrossRef] [PubMed]
- Lammer, J.; Malagari, K.; Vogl, T.; Pilleul, F.; Denys, A.; Watkinson, A.; Pitton, M.; Sergent, G.; Pfammatter, T.; Terraz, S.; et al. Prospective Randomized Study of Doxorubicin-Eluting-Bead Embolization in the Treatment of Hepatocellular Carcinoma: Results of the PRECISION V Study. Cardiovasc. Interv. Radiol. 2010, 33, 41–52. [Google Scholar] [CrossRef]
- Katsanos, K.; Kitrou, P.; Spiliopoulos, S.; Maroulis, I.; Petsas, T.; Karnabatidis, D. Comparative effectiveness of different transarterial embolization therapies alone or in combination with local ablative or adjuvant systemic treatments for unresectable hepatocellular carcinoma: A network meta-analysis of randomized controlled trials. PLoS ONE 2017, 12, e0184597. [Google Scholar] [CrossRef]
- Golfieri, R.; on behalf of the PRECISION ITALIA STUDY GROUP; Giampalma, E.; Renzulli, M.; Cioni, R.; Bargellini, I.; Bartolozzi, C.; Breatta, A.D.; Gandini, G.; Nani, R.; et al. Randomised controlled trial of doxorubicin-eluting beads vs conventional chemoembolisation for hepatocellular carcinoma. Br. J. Cancer 2014, 111, 255–264. [Google Scholar] [CrossRef]
- Golfieri, R.; Renzulli, M.; Mosconi, C.; Forlani, L.; Giampalma, E.; Piscaglia, F.; Trevisani, F.; Bolondi, L. Hepatocellular Carcinoma Responding to Superselective Transarterial Chemoembolization: An Issue of Nodule Dimension? J. Vasc. Interv. Radiol. 2013, 24, 509–517. [Google Scholar] [CrossRef]
- Lencioni, R.; De Baere, T.; Soulen, M.C.; Rilling, W.S.; Geschwind, J.F.H. Lipiodol Transarterial Chemoembolization for Hepatocellular Carcinoma: A Systematic Review of Efficacy and Safety Data. Hepatology 2016, 64, 106–116. [Google Scholar] [CrossRef]
- Wang, B.; Xu, H.; Gao, Z.Q.; Ning, H.F.; Sun, Y.Q.; Cao, G.W. Increased expression of vascular endothelial growth factor in hepatocellular carcinoma after transcatheter arterial chemoembolization. Acta Radiol. 2008, 49, 523–529. [Google Scholar] [CrossRef]
- Xiao, E.-H.; Guo, D.; Bian, D.-J. Effect of preoperative transcatheter arterial chemoembolization on angiogenesis of hepatocellular carcinoma cells. World J. Gastroenterol. 2009, 15, 4582–4586. [Google Scholar] [CrossRef]
- Schicho, A.; Hellerbrand, C.; Krüger, K.; Beyer, L.P.; Wohlgemuth, W.; Niessen, C.; Hohenstein, E.; Stroszczynski, C.; Pereira, P.L.; Wiggermann, P. Impact of Different Embolic Agents for Transarterial Chemoembolization (TACE) Procedures on Systemic Vascular Endothelial Growth Factor (VEGF) Levels. J. Clin. Transl. Hepatol. 2016, 4, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Fan, D.; Adah, D.; Wu, Z.; Liu, R.; Yan, Q.; Zhang, Y.; Du, Z.; Wang, D.; Li, Y.; et al. CRISPR/Cas9-mediated hypoxia inducible factor-1α knockout enhances the antitumor effect of transarterial embolization in hepatocellular carcinoma. Oncol. Rep. 2018, 40, 2547–2557. [Google Scholar] [CrossRef] [PubMed]
- Chao, Y.; Wu, C.-Y.; Kuo, C.-Y.; Wang, J.P.; Luo, J.-C.; Kao, C.-H.; Lee, R.-C.; Lee, W.-P.; Li, C.-P. Cytokines are associated with postembolization fever and survival in hepatocellular carcinoma patients receiving transcatheter arterial chemoembolization. Hepatol. Int. 2013, 7, 883–892. [Google Scholar] [CrossRef]
- Lin, W.H.; Yeh, S.H.; Yeh, K.H.; Chen, K.W.; Cheng, Y.W.; Su, T.H.; Jao, P.; Ni, L.C.; Chen, P.J.; Chen, D.S. Hypoxia-activated cytotoxic agent tirapazamine enhances hepatic artery ligation-induced killing of liver tumor in HBx transgenic mice. Proc. Natl. Acad. Sci. USA 2016, 113, 11937–11942. [Google Scholar] [CrossRef] [PubMed]
- Duran, R.; Mirpour, S.; Pekurovsky, V.; Ganapathy-Kanniappan, S.; Brayton, C.F.; Cornish, T.C.; Gorodetski, B.; Reyes, J.; Chapiro, J.; Schernthaner, R.E.; et al. Preclinical Benefit of Hypoxia-Activated Intra-arterial Therapy with Evofosfamide in Liver Cancer. Clin. Cancer Res. 2017, 23, 536–548. [Google Scholar] [CrossRef]
- Zhang, H.; Xiong, F.; Qian, K.; Liu, Y.; Liang, B.; Xiong, B.; Yang, F.; Zheng, C. Transcatheter arterial embolization combined with hypoxia-replicative oncolytic adenovirus perfusion enhances the therapeutic effect of hepatic carcinoma. Cancer Manag. Res. 2019, 11, 981–996. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.H.; Foster, N.R.; Mahipal, A.; Byrne, T.; Hubbard, J.; Silva, A.; Mody, K.; Alberts, S.; Borad, M.J. Phase IB study of sorafenib and evofosfamide in patients with advanced hepatocellular and renal cell carcinomas (NCCTG N1135, Alliance). Investig. New Drugs 2021, 39, 1072–1080. [Google Scholar] [CrossRef]
- Lim, A.M.; Rischin, D.; Fisher, R.; Cao, H.; Kwok, K.; Truong, D.; McArthur, G.A.; Young, R.J.; Giaccia, A.; Peters, L.; et al. Prognostic Significance of Plasma Osteopontin in Patients with Locoregionally Advanced Head and Neck Squamous Cell Carcinoma Treated on TROG 02.02 Phase III Trial. Clin. Cancer Res. 2012, 18, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Rischin, D.; Peters, L.J.; O’Sullivan, B.; Giralt, J.; Fisher, R.; Yuen, K.; Trotti, A.; Bernier, J.; Bourhis, J.; Ringash, J.; et al. Tirapazamine, Cisplatin, and Radiation Versus Cisplatin and Radiation for Advanced Squamous Cell Carcinoma of the Head and Neck (TROG 02.02, HeadSTART): A Phase III Trial of the Trans-Tasman Radiation Oncology Group. J. Clin. Oncol. 2010, 28, 2989–2995. [Google Scholar] [CrossRef]
- DiSilvestro, P.A.; Ali, S.; Craighead, P.S.; Lucci, J.A.; Lee, Y.C.; Cohn, D.E.; Spirtos, N.M.; Tewari, K.S.; Muller, C.; Gajewski, W.H.; et al. Phase III Randomized Trial of Weekly Cisplatin and Irradiation Versus Cisplatin and Tirapazamine and Irradiation in Stages IB2, IIA, IIB, IIIB, and IVA Cervical Carcinoma Limited to the Pelvis: A Gynecologic Oncology Group Study. J. Clin. Oncol. 2014, 32, 458–464. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, L.; Li, X.-F. The Hypoxia-Activated Prodrug TH-302: Exploiting Hypoxia in Cancer Therapy. Front. Pharmacol. 2021, 12, 636892. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, J.; Li, J.; Chen, D.; Matteucci, M.; Curd, J.; Duan, J.-X. Inhibition of Both Thioredoxin Reductase and Glutathione Reductase may Contribute to the Anticancer Mechanism of TH-302. Biol. Trace Element Res. 2009, 136, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.D.; Liu, Q.; Wang, J.; Ahluwalia, D.; Ferraro, D.; Wang, Y.; Duan, J.-X.; Ammons, W.S.; Curd, J.G.; Matteucci, M.D.; et al. Selective Tumor Hypoxia Targeting by Hypoxia-Activated Prodrug TH-302 Inhibits Tumor Growth in Preclinical Models of Cancer. Clin. Cancer Res. 2012, 18, 758–770. [Google Scholar] [CrossRef] [PubMed]
- Abou-Alfa, G.K.; Chan, S.L.; Lin, C.-C.; Chiorean, E.G.; Holcombe, R.F.; Mulcahy, M.F.; Carter, W.D.; Patel, K.; Wilson, W.R.; Melink, T.J.; et al. PR-104 plus sorafenib in patients with advanced hepatocellular carcinoma. Cancer Chemother. Pharmacol. 2011, 68, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Abbattista, M.R.; Jamieson, S.M.F.; Gu, Y.; Nickel, J.E.; Pullen, S.M.; Patterson, A.V.; Wilson, W.R.; Guise, C.P. Pre-clinical activity of PR-104 as monotherapy and in combination with sorafenib in hepatocellular carcinoma. Cancer Biol. Ther. 2015, 16, 610–622. [Google Scholar] [CrossRef]
- Patterson, L.H.; McKeown, S.R.; Ruparelia, K.; Double, J.A.; Bibby, M.C.; Cole, S.; Stratford, I.J. Enhancement of chemotherapy and radiotherapy of murine tumours by AQ4N, a bioreductively activated anti-tumour agent. Br. J. Cancer 2000, 82, 1984–1990. [Google Scholar] [CrossRef]
- Yamada, R.; Bassaco, B.; Bracewell, S.; Gillen, K.; Kocher, M.; Collins, H.; Anderson, M.B.; Guimaraes, M. Long-term follow-up after conventional transarterial chemoembolization (c-TACE) with mitomycin for hepatocellular carcinoma (HCC). J. Gastrointest. Oncol. 2019, 10, 348–353. [Google Scholar] [CrossRef]
- Haffty, B.G.; Wilson, L.D.; Son, H.-Y.; Cho, E.I.; Papac, R.J.; Fischer, D.B.; Rockwell, S.; Sartorelli, A.C.; Ross, D.A.; Sasaki, C.T.; et al. Concurrent chemo-radiotherapy with mitomycin C compared with porfiromycin in squamous cell cancer of the head and neck: Final results of a randomized clinical trial. Int. J. Radiat. Oncol. 2005, 61, 119–128. [Google Scholar] [CrossRef]
- Phillips, R.M.; Hendriks, H.R.; Peters, G.J.; on behalf of the EORTC-Pharmacology and Molecular Mechanism Group. EO9 (Apaziquone): From the clinic to the laboratory and back again. Br. J. Pharmacol. 2013, 168, 11–18. [Google Scholar] [CrossRef]
- Loadman, P.; Bibby, M.C.; Phillips, R.M. Pharmacological approach towards the development of indolequinone bioreductive drugs based on the clinically inactive agent EO9. Br. J. Pharmacol. 2002, 137, 701–709. [Google Scholar] [CrossRef]
- Bahrami, A.; Atkin, S.L.; Majeed, M.; Sahebkar, A. Effects of curcumin on hypoxia-inducible factor as a new therapeutic target. Pharmacol. Res. 2018, 137, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Fang, J.; Xia, C.; Shi, X.; Jiang, B.-H. trans- 3,4,5′-Trihydroxystibene Inhibits Hypoxia-Inducible Factor 1α and Vascular Endothelial Growth Factor Expression in Human Ovarian Cancer Cells. Clin. Cancer Res. 2004, 10, 5253–5263. [Google Scholar] [CrossRef] [PubMed]
- Su, Q.; Fan, M.; Wang, J.; Ullah, A.; Ghauri, M.A.; Dai, B.; Zhan, Y.; Zhang, D.; Zhang, Y. Sanguinarine inhibits epithelial–mesenchymal transition via targeting HIF-1α/TGF-β feed-forward loop in hepatocellular carcinoma. Cell Death Dis. 2019, 10, 939. [Google Scholar] [CrossRef]
- Li, X.; Tsauo, J.; Geng, C.; Zhao, H.; Lei, X. Ginsenoside Rg3 Decreases NHE1 Expression via Inhibiting EGF-EGFR-ERK1/2-HIF-1α Pathway in Hepatocellular Carcinoma: A Novel Antitumor Mechanism. Am. J. Chin. Med. 2018, 46, 1915–1931. [Google Scholar] [CrossRef]
- Ullah, A.; Aziz, T.; Ullah, N.; Nawaz, T. Molecular mechanisms of Sanguinarine in cancer prevention and treatment. Anti-Cancer Agents Med. Chem. 2023, 23, 765–778. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef]
- Afzal, O.; Altamimi, A.S.A.; Nadeem, M.S.; Alzarea, S.I.; Almalki, W.H.; Tariq, A.; Mubeen, B.; Murtaza, B.N.; Iftikhar, S.; Riaz, N.; et al. Nanoparticles in Drug Delivery: From History to Therapeutic Applications. Nanomaterials 2022, 12, 4494. [Google Scholar] [CrossRef]
- Atabakhshi-Kashi, M.; Geranpayehvaghei, M.; Wang, Y.; Akhbariyoon, H.; Taleb, M.; Zhang, Y.; Khajeh, K.; Nie, G. Recent Advances of Nanocarriers for Effective Delivery of Therapeutic Peptides. Precis. Nanomed. 2020, 3, 549–576. [Google Scholar] [CrossRef]
- Pollak, M. The insulin and insulin-like growth factor receptor family in neoplasia: An update. Nat. Rev. Cancer 2012, 12, 159–169. [Google Scholar] [CrossRef]
- Saito, T.; Chiba, T.; Yuki, K.; Zen, Y.; Oshima, M.; Koide, S.; Motoyama, T.; Ogasawara, S.; Suzuki, E.; Ooka, Y.; et al. Metformin, a Diabetes Drug, Eliminates Tumor-Initiating Hepatocellular Carcinoma Cells. PLoS ONE 2013, 8, e70010. [Google Scholar] [CrossRef]
- Zhang, H.; Gao, C.; Fang, L.; Zhao, H.-C.; Yao, S.-K. Metformin and reduced risk of hepatocellular carcinoma in diabetic patients: A meta-analysis. Scand. J. Gastroenterol. 2013, 48, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Chandel, N.S.; Avizonis, D.; Reczek, C.R.; Weinberg, S.E.; Menz, S.; Neuhaus, R.; Christian, S.; Haegebarth, A.; Algire, C.; Pollak, M. Are Metformin Doses Used in Murine Cancer Models Clinically Relevant? Cell Metab. 2016, 23, 569–570. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-L.; Wu, C.-X.; Zhang, J.-B.; Zhang, H.; Sun, Y.-D.; Tian, S.-L.; Han, J.-J. Transarterial chemoembolization combined with metformin improves the prognosis of hepatocellular carcinoma patients with type 2 diabetes. Front. Endocrinol. 2022, 13, 996228. [Google Scholar] [CrossRef] [PubMed]
- Jung, W.J.; Jang, S.; Choi, W.J.; Park, J.; Choi, G.H.; Jang, E.S.; Jeong, S.-H.; Lee, J.H.; Yoon, C.J.; Kim, J.-W. Metformin administration is associated with enhanced response to transarterial chemoembolization for hepatocellular carcinoma in type 2 diabetes patients. Sci. Rep. 2022, 12, 14482. [Google Scholar] [CrossRef] [PubMed]
- Kamimura, K.; Yokoo, T.; Abe, H.; Terai, S. Gene Therapy for Liver Cancers: Current Status from Basic to Clinics. Cancers 2019, 11, 1865. [Google Scholar] [CrossRef]
| Study | Phase of Trial | Cancer of Interest | Intervention | PFS | mOS | ORR | |
|---|---|---|---|---|---|---|---|
| Lin 2016 [54] | P | HCC in HBx transgenic mice | HAL alone DXR/HAL TPZ/HAL | - | - | - | >99% necrosis in TPZ/HAL ~5% necrosis in DXR/HAL No detectable necrosis in HAL alone |
| Duran 2017 [55] | P | VX2 tumor-bearing rabbits | TH-302 alone cTACE alone TH-302/cTACE | - | - | - | Higher necrotic fraction, tumor shrinkage, and lower tumor growth rate in TH-302/cTACE on day 14 compared to TH-302 or cTACE alone (p < 0.05) |
| Abi-Jaoudeh 2021 [27] (n = 27) | I | HCC | TPZ/TAE | 80.5% at 6 months | 52 months | 84.0% | |
| Tran 2021 [57] (n = 18) | Ib | HCC (n = 12), RCC (n = 6) | TH-302/sorafenib | 6.3 months (HCC) | 13.9 months (HCC) | 55.6% | |
| Liu 2022 [28] (n = 17) | I | HCC | TPZ/TAE | 72.6% at 6 months | 29.3 months | 64.7% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huynh, K.N.; Rao, S.; Roth, B.; Bryan, T.; Fernando, D.M.; Dayyani, F.; Imagawa, D.; Abi-Jaoudeh, N. Targeting Hypoxia-Inducible Factor-1α for the Management of Hepatocellular Carcinoma. Cancers 2023, 15, 2738. https://doi.org/10.3390/cancers15102738
Huynh KN, Rao S, Roth B, Bryan T, Fernando DM, Dayyani F, Imagawa D, Abi-Jaoudeh N. Targeting Hypoxia-Inducible Factor-1α for the Management of Hepatocellular Carcinoma. Cancers. 2023; 15(10):2738. https://doi.org/10.3390/cancers15102738
Chicago/Turabian StyleHuynh, Kenneth N., Sriram Rao, Bradley Roth, Theodore Bryan, Dayantha M. Fernando, Farshid Dayyani, David Imagawa, and Nadine Abi-Jaoudeh. 2023. "Targeting Hypoxia-Inducible Factor-1α for the Management of Hepatocellular Carcinoma" Cancers 15, no. 10: 2738. https://doi.org/10.3390/cancers15102738
APA StyleHuynh, K. N., Rao, S., Roth, B., Bryan, T., Fernando, D. M., Dayyani, F., Imagawa, D., & Abi-Jaoudeh, N. (2023). Targeting Hypoxia-Inducible Factor-1α for the Management of Hepatocellular Carcinoma. Cancers, 15(10), 2738. https://doi.org/10.3390/cancers15102738





