Early Intervention with a Compression Sleeve in Mild Breast Cancer-Related Arm Lymphedema: A 12-Month Prospective Observational Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Method
2.1. Study Design
2.2. Participants
2.3. Ethical Approval
2.4. Procedures
2.5. Randomization
2.6. Description of the Interventions
2.7. Background Data
2.8. Primary Outcome
2.9. Secondary Outcomes
2.10. Measurements
2.10.1. Arm Volume
2.10.2. Local Tissue Water
2.10.3. Self-Care
2.11. Statistical Power and Analysis
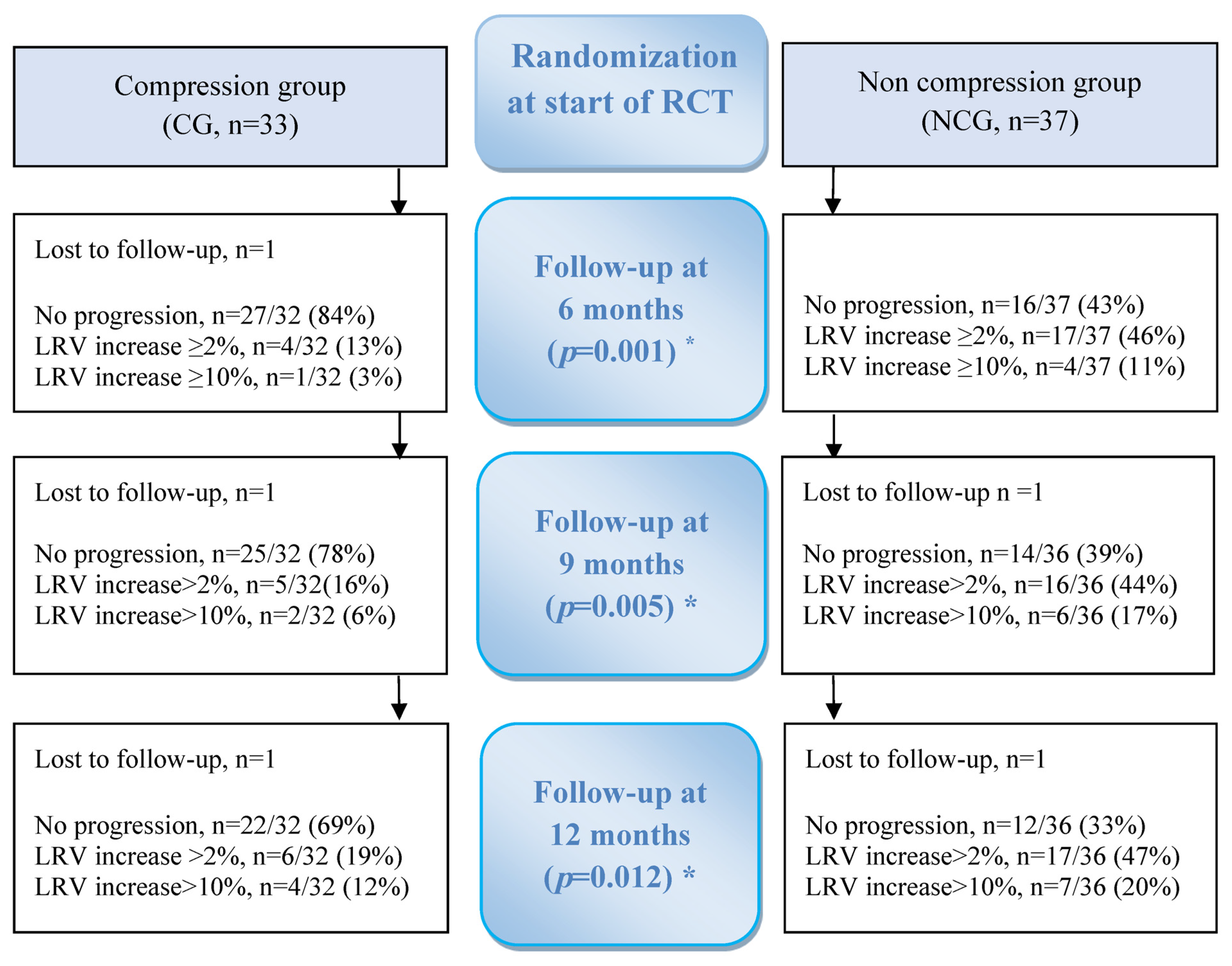
3. Results
3.1. Differences between the Groups at Start of the RCT and at 12-Months Follow-Up
3.2. Use of Compression Sleeves
3.3. Primary Outcome
Proportion of Progression/No Progression of BCRL for 12 Months
3.4. Secondary Outcomes
3.4.1. Lymphedema Relative Volume for 12 Months
3.4.2. Changes in Local Tissue Water for 12 Months
4. Discussion
5. Strength and Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, K.; Beeraka, N.M.; Zhang, J.; Reshetov, I.V.; Nikolenko, V.N.; Sinelnikov, M.Y. Efficacy of da Vinci robot-assisted lymph node surgery than conventional axillary lymph node dissection in breast cancer—A comparative study. Int. J. Med. Robot. 2021, 17, e2307. [Google Scholar] [CrossRef]
- DiSipio, T.; Rye, S.; Newman, B.; Hayes, S. Incidence of unilateral arm lymphoedema after breast cancer: A systematic review and meta-analysis. Lancet Oncol. 2013, 14, 500–515. [Google Scholar] [CrossRef] [PubMed]
- Stanton, A.W.; Modi, S.; Mellor, R.H.; Levick, J.R.; Mortimer, P.S. Recent advances in breast cancer-related lymphedema of the arm: Lymphatic pump failure and predisposing factors. Lymphat. Res. Biol. 2009, 7, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Johansson, K.; Branje, E. Arm lymphoedema in a cohort of breast cancer survivors 10 years after diagnosis. Acta Oncol. 2010, 49, 166–735. [Google Scholar] [CrossRef] [PubMed]
- Kilgore, L.J.; Korentager, S.S.; Hangge, A.N.; Amin, A.L.; Balanoff, C.R.; Larson, K.E.; Mitchell, M.P.; Chen, J.G.; Burgen, E.; Khan, Q.J.; et al. Reducing Breast Cancer-Related Lymphedema (BCRL) Through Prospective Surveillance Monitoring Using Bioimpedance Spectroscopy (BIS) and Patient Directed Self-Interventions. Ann. Surg. Oncol. 2018, 25, 2948–2952. [Google Scholar] [CrossRef]
- McNeely, M.L.; Peddle, C.J.; Yurick, J.L.; Dayes, I.S.; Mackey, J.R. Conservative and dietary interventions for cancer-related lymphedema: A systematic review and meta-analysis. Cancer 2011, 117, 1136–1148. [Google Scholar] [CrossRef]
- Casley-Smith, J. Alterations of untreated lymphedema and it’s grades over time. Lymphology 1995, 28, 174–185. [Google Scholar]
- Bar Ad, V.; Cheville, A.; Solin, L.J.; Dutta, P.; Both, S.; Harris, E.E. Time course of mild arm lymphedema after breast conservation treatment for early-stage breast cancer. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, 85–90. [Google Scholar] [CrossRef]
- Soran, A.; Ozmen, T.; McGuire, K.P.; Diego, E.J.; McAuliffe, P.F.; Bonaventura, M.; Ahrendt, G.M.; DeGore, L.; Johnson, R. The importance of detection of subclinical lymphedema for the prevention of breast cancer-related clinical lymphedema after axillary lymph node dissection; a prospective observational study. Lymphat. Res. Biol. 2014, 12, 289–294. [Google Scholar] [CrossRef]
- Kaufman, D.I.; Shah, C.; Vicini, F.A.; Rizzi, M. Utilization of bioimpedance spectroscopy in the prevention of chronic breast cancer-related lymphedema. Breast Cancer Res. Treat. 2017, 166, 809–815. [Google Scholar] [CrossRef]
- Bundred, N.J.; Barrett, E.; Todd, C.; Morris, J.; Watterson, D.; Purushotham, A.; Riches, K.; Evans, A.; Skene, A.; Keeley, V.; et al. Prevention of lymphoedema after axillary clearance by external compression sleeves PLACE randomised trial results. Effects of high BMI. Cancer Med. 2023, 12, 5506–5516. [Google Scholar] [CrossRef] [PubMed]
- Ramos, S.; O´Donnell, L.; Knight, G. Edema volume, not timing, is the key to success in lymphedema treatment. Am. J. Surg. 1999, 178, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Bundred, N.; Foden, P.; Todd, C.; Morris, J.; Watterson, D.; Purushotham, A.; Bramley, M.; Riches, K.; Hodgkiss, T.; Evans, A.; et al. Increases in arm volume predict lymphoedema and quality of life deficits after axillary surgery: A prospective cohort study. Br. J. Cancer 2020, 123, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Mazor, M.; Smoot, B.J.; Mastick, J.; Mausisa, G.; Paul, S.M.; Kober, K.M.; Elboim, C.; Singh, K.; Conley, Y.P.; Mickevicius, G.; et al. Assessment of local tissue water in the arms and trunk of breast cancer survivors with and without upper extremity lymphoedema. Clin. Physiol. Funct. Imaging 2019, 39, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Stout, N.L.; Pfalzer, L.A.; Levy, E.; McGarvey, C.; Springer, B.; Gerber, L.H.; Soballe, P. Segmental limb volume change as a predictor of the onset of lymphedema in women with early breast cancer. PM&R 2011, 3, 1098–1105. [Google Scholar] [CrossRef]
- Thomis, S.; Dams, L.; Fourneau, I.; De Vrieze, T.; Nevelsteen, I.; Neven, P.; Gebruers, N.; Devoogdt, N. Correlation Between Clinical Assessment and Lymphofluoroscopy in Patients with Breast Cancer-Related Lymphedema: A Study of Concurrent Validity. Lymphat. Res. Biol. 2020, 18, 539–548. [Google Scholar] [CrossRef]
- Suami, H. Anatomical Theories of the Pathophysiology of Cancer-Related Lymphoedema. Cancers 2020, 12, 1338. [Google Scholar] [CrossRef]
- Karlsson, K.; Nilsson-Wikmar, L.; Brogardh, C.; Johansson, K. Palpation of Increased Skin and Subcutaneous Thickness, Tissue Dielectric Constant, and Water Displacement Method for Diagnosis of Early Mild Arm Lymphedema. Lymphat. Res. Biol. 2020, 18, 219–225. [Google Scholar] [CrossRef]
- Blom, K.Y.; Johansson, K.I.; Nilsson-Wikmar, L.B.; Brogårdh, C.B. Early intervention with compression garments prevents progression in mild breast cancer-related arm lymphedema: A randomized controlled trial. Acta Oncol. 2022, 61, 897–905. [Google Scholar] [CrossRef]
- Johansson, K.; Ingvar, C.; Albertsson, M.; Ekdahl, C. Arm lymphedema, shoulder mobility and muscle strength after breast cancer treatment-a prospective 2 year study. Adv. Physiother. 2001, 3, 55–66. [Google Scholar] [CrossRef]
- Lahtinen, T.; Seppala, J.; Viren, T.; Johansson, K. Experimental and Analytical Comparisons of Tissue Dielectric Constant (TDC) and Bioimpedance Spectroscopy (BIS) in Assessment of Early Arm Lymphedema in Breast Cancer Patients after Axillary Surgery and Radiotherapy. Lymphat. Res. Biol. 2015, 13, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Mayrovitz, H.N.; Weingrad, D.N.; Lopez, L. Assessing localized skin-to-fat water in arms of women with breast cancer via tissue dielectric constant measurements in pre- and post-surgery patients. Ann. Surg. Oncol. 2015, 22, 1483–1489. [Google Scholar] [CrossRef] [PubMed]
- Specht, M.C.; Miller, C.L.; Russell, T.A.; Horick, N.; Skolny, M.N.; O’Toole, J.A.; Jammallo, L.S.; Niemierko, A.; Sadek, B.T.; Shenouda, M.N.; et al. Defining a threshold for intervention in breast cancer-related lymphedema: What level of arm volume increase predicts progression? Breast Cancer Res. Treat. 2013, 140, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Mahamaneerat, W.K.; Shyu, C.-R.; Stewart, B.R.; Armer, J.M. Breast cancer treatment, BMI, post-op swelling/lymphedema. J. Lymphoedema 2008, 3, 38–44. [Google Scholar]
- Ibrahim, E.M.; Al-Homaidh, A. Physical activity and survival after breast cancer diagnosis: Meta-analysis of published studies. Med. Oncol. 2011, 28, 753–765. [Google Scholar] [CrossRef]
- Lahart, I.M.; Metsios, G.S.; Nevill, A.M.; Carmichael, A.R. Physical activity, risk of death and recurrence in breast cancer survivors: A systematic review and meta-analysis of epidemiological studies. Acta Oncol. 2015, 54, 635–654. [Google Scholar] [CrossRef]
- Johansson, K.; Ohlsson, K.; Ingvar, C.; Albertsson, M. Factors associated with the development of arm lymphedema following breast cancer treatment: A match pair case-control study. Lymphology 2002, 35, 59–71. [Google Scholar]
- Ridner, S.; Deng, J.; Fu, M.R.; Radina, E.; Thiadens, S.R.; Weiss, J.; Dietrich, M.S.; Cormier, J.N.; Tuppo, C.M.; Armer, J.M. Symptom burden and infection occurrence among individuals with extremity lymphedema. Lymphology 2012, 45, 113–123. [Google Scholar]
- Moayedi, M.; Davis, K.D. Theories of pain: From specificity to gate control. J. Neurophysiol. 2013, 109, 5–12. [Google Scholar] [CrossRef]
- Karges, J.; Mark, B.; Stikeleather, S.; Worrell, T. Concurrent validity of upper extremity volume estimates: Comparison of calculated volume derived from girth measurements and water displacement volume. Phys. Ther. 2003, 83, 134–145. [Google Scholar] [CrossRef]
- Hidding, J.T.; Viehoff, P.B.; Beurskens, C.H.; van Laarhoven, H.W.; Nijhuis-van der Sanden, M.W.; van der Wees, P.J. Measurement properties of instruments for measuring of lymphedema:systematic review. Phys Ther. 2016, 96, 1965–1981. [Google Scholar] [CrossRef] [PubMed]
- ISL. The diagnosis and treatment of peripheral lymphedema: 2020 Consensus document of the International Society of Lymphology. Lymphology 2020, 53, 3–19. [Google Scholar]
- Taylor, R.; Jayasinghe, U.; Koelmeyer, L.; Ung, O.A.; Boyages, J. Reliability and Validity of arm volume measurements for assessment of lymphedema. Phys. Ther. 2006, 86, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Mayrovitz, H.N.; Weingrad, D.N.; Brlit, F.; Lopez, L.B.; Desfor, R. Tissue dielectric constant (Water) as an index of localized arm skin water: Differences between measuring probes and genders. Lymphology 2015, 48, 15–23. [Google Scholar] [PubMed]
- Mayrovitz, H.N.; Davey, S.; Shapiro, E. Suitability of single tissue dielectric constant measurements to assess local tissue water in normal and lymphedematous skin. Clin. Physiol. Funct. Imaging 2009, 29, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Mayrovitz, H.N.; Weingrad, D.N.; Davey, S. Local tissue water in at-risk and contralateral forearms of women with and without breast cancer treatment related lymphedema. Lymphat. Res. Biol. 2009, 7, 153–158. [Google Scholar] [CrossRef]
- Frändin, K.; Grimby, G. Assessment of physical activity, fitness and performance in 76 year olds. Scand. J. Med. Sci. Sport 1994, 4, 41–46. [Google Scholar] [CrossRef]
- Akita, S.; Nakamura, R.; Yamamoto, N.; Tokumoto, H.; Ishigaki, T.; Yamaji, Y.; Yoshitaro, S.; Yoshitaka, K.; Nobuyuki, M.; Kaneshige, S. Early Detection of Lymphatic Disorder and Treatment for Lymphedema following Breast Cancer. Plast. Reconstr. Surg. 2016, 138, 192e–202e. [Google Scholar] [CrossRef]
- Martin-Almedina, S.; Mortimer, P.S.; Ostergaard, P. Development and physiological functions of the lymphatic system: Insights from human genetic studies of primary lymphedema. Physiol. Rev. 2021, 101, 1809–1871. [Google Scholar] [CrossRef]
- Kilbreath, S.L.; Lee, M.J.; Refshauge, K.M.; Beith, J.M.; Ward, L.C.; Simpson, J.M.; Black, D. Transient swelling versus lymphoedema in the first year following surgery for breast cancer. Support. Care Cancer 2013, 21, 2207–2215. [Google Scholar] [CrossRef]
- Stout Gergich, N.L.; Pfalzer, L.A.; McGarvey, C.; Springer, B.; Gerber, L.H.; Soballe, P. Preoperative assessment enables the early diagnosis and successful treatment of lymphedema. Cancer 2008, 112, 2809–2819. [Google Scholar] [CrossRef] [PubMed]
- Bernas, M.; Witte, C.; Belch, D.; Summers, P. Limb volume measurments in lymphedema issues and standards. Lymphology 1996, 29, 199–202. [Google Scholar]
- Jonsson, C.; Johansson, K.; Bjurberg, M.; Brogardh, C. Circumferential Measurements to Calculate Lower Limb Volume in Persons with Lymphedema: What Segment Length Is to Be Recommended? Lymphat. Res. Biol. 2022, 54, 1389–1399. [Google Scholar] [CrossRef] [PubMed]
- Hayes, S.C.; Singh, B.; Reul-Hirche, H.; Bloomquist, K.; Johansson, K.; Jonsson, C.; Plinsinga, M. The Effect of Exercise for the Prevention and Treatment of Cancer-Related Lymphedema: A Systematic Review with Meta-analysis. Med. Sci. Sport. Exerc. 2022, 54, 1389–1399. [Google Scholar] [CrossRef]
- Huang, T.; Tseng, S.; Lin, C.; Bai, C.; Chen, C.; Hung, C.; Huang, C.; Wu, C.; Tam, K. Effects of manual lymphatic drainage on breast cancer-related lymphedema: A systematic review and meta-analysis of randomized controlled trial. World J. Surg. Oncol. 2013, 11, 15. [Google Scholar] [CrossRef]
- Liang, M.; Chen, Q.; Peng, K.; Deng, L.; He, L.; Hou, Y.; Zhang, Y.; Guo, J.; Mei, Z.; Li, Z. Manual lymphatic drainage for lymphedema in patients after breast cancer surgery: A systematic review and meta-analysis of randomized controlled trials. Medicine 2020, 99, e23192. [Google Scholar] [CrossRef]
- De Vrieze, T.; Gebruers, N.; Nevelsteen, I.; Fieuws, S.; Thomis, S.; De Groef, A.; Tjalma, W.A.; Belgrado, J.-P.; Vandermeeren, L.; Monten, C.; et al. Manual lymphatic drainage with or without fluoroscopy guidance did not substantially improve the effect of decongestive lymphatic therapy in people with breast cancer-related lymphoedema (EFforT-BCRL trial): A multicentre randomised trial. J. Physiother. 2022, 68, 110–122. [Google Scholar] [CrossRef]
- Lymphoedema Framework. Best Practice for the Management of Lymphoedema. International Consensus London: MEP Ltd., BP PAGESfinjune8 bQ5. Available online: lympho.org (accessed on 1 March 2023).
- Karlsson, K.; Biguet, G.; Johansson, K.; Nilsson-Wikmar, L. Perceptions of lymphoedema treatment in patients with breast cancer—A patient perspective. Scand. J. Caring Sci. 2015, 29, 110–117. [Google Scholar] [CrossRef]
- Zaleska, M.T.; Olszewski, W.L. Indocyanine green near-infrared lymphangiography for evaluation of effectiveness of edema fluid flow under therapeutic compression. J. Biophotonics 2018, 11, 1–9. [Google Scholar] [CrossRef]
- Suami, H.; Koelmeyer, L.; Mackie, H.; Boyages, J. Patterns of lymphatic drainage after axillary node dissection impact arm lymphoedema severity: A review of animal and clinical imaging studies. Surg. Oncol. 2018, 27, 743–750. [Google Scholar] [CrossRef]
| CG n = 33 | NCG n = 37 | p-Value * | |
|---|---|---|---|
| Age in years at diagnosis, mean (SD) | 57.9(13.8) | 57.0(12.5) | 0.795 |
| BMI kg/m2, mean (SD) | 26.1(4.8) | 27.2(5.4) | 0.390 |
| Surgery | 0.033 | ||
| Mastectomy and ALND, n (%) | 20(61) | 13(35) | |
| Lumpectomy and ALND, n (%) | 13(39) | 24(65) | |
| Lymph nodes | |||
| Removed at surgery, mean (SD) | 14.9(5.8) | 16.0(5.5) | 0.397 |
| With metastasis, mean (SD) | 2.3(3.0) | 2.6(2.4) | 0.655 |
| Adjuvant treatment | |||
| Radiotherapy, n (%) | 32(97) | 37(100) | 0.471 |
| Chemotherapy, n (%) | 27(82) | 33(89) | 0.499 |
| Hormone therapy, n (%) | 25(76) | 28(76) | 0.994 |
| Affected side | |||
| Right/left, n | 18/15 | 19/18 | 0.789 |
| Dominant a, n (%) | 19(58) | 17(47) | 0.390 |
| Lymphedema | |||
| Time from surgery to onset, months, mean (SD) | 6.1(5.5) | 6.6(5.2) | 0.721 |
| Duration, months, mean (SD) | 1.0(1.3) | 1.0(1.5) | 0.834 |
| In hand, self-rated a, n (%) | 9(27) | 9(25) | 0.830 |
| LRV % a | CG | NCG | p-Value * |
|---|---|---|---|
| At start of RCT | n = 33 | n = 37 | |
| Mean ± SD | 4.4 ± 3.1 | 3.8 ± 3.5 | 0.456 |
| At 6 months | n = 30 | n = 33 | |
| Mean ± SD | 0.7 ± 3.0 | 3.6 ± 4.5 | 0.004 |
| Change from start | |||
| Mean diff (CI) | −3.8 (−5.0 to −2.5) | 0.1 (−1.1 to 1.2) | <0.001 |
| At 9 months | n = 30 | n = 28 | |
| Mean ± SD | 3.2 (3.3) | 3.5 (5.0) | 0.799 |
| Change from start | |||
| Mean diff (CI) | −1.3 (−2.7 to 0.1) | 0.2 (−1.3 to 1.7) | 0.143 |
| At 12 months | n = 30 | n = 25 | |
| Mean ± SD | 4.2 (3.6) | 2.6 (4.2) | 0.143 |
| Change from start | |||
| Mean diff (CI) | −0.3 (−1.8 to 1.1) | −0.4 (−1.9 to 1.0) | 0.900 |
| TDC a | CG | NCG | p-Value * |
|---|---|---|---|
| At start of RCT | n = 33 | n = 37 | |
| Median (min–max) | 1.50 (1.09 to 1.96) | 1.55 (1.05 to 2.15) | 0.805 |
| Mean ± SD | 1.53 ± 0.21 | 1.51 ± 0.30 | |
| At 6 months | n = 29 | n = 31 | |
| Median (min–max) | 1.12 (0.77 to 1.84) | 1.18 (0.91 to 1.90) | 0.689 |
| Mean ± SD | 1.23 ± 0.24 | 1.28 ± 0.29 | |
| Change from start | |||
| Median diff (min–max) | −0.28 (−1.04 to 0.30) | −0.16 (−1.00 to 0.40) | 0.375 |
| Mean diff (CI) | −0.29 (−0.39 to −0.20) | −0.25 (−0.36 to −0.13) | |
| At 9 months | n = 28 | n = 27 | |
| Median (min–max) | 1.18 (0.73 to 2.14) | 1.24 (0.97 to 1.74) | 0.266 |
| Mean ± SD | 1.21 ± 0.29 | 1.26 ± 0.22 | |
| Change from start | |||
| Median diff (min–max) | −0.32 (−0.91 to 0.29) | −0.21 (−1.15 to 0.50) | 0.567 |
| Mean diff (CI) | −0.31 (−0.42 to −0.20) | −0.27 (−0.40 to −0.14) | |
| At 12 months | n = 29 | n = 25 | |
| Median (min–max) | 1.12 (0.96 to 2.35) | 1.21 (0.76 to 1.80) | 0.340 |
| Mean ± SD | 1.19 ± 0.28 | 1.23 ± 0.26 | |
| Change from start | |||
| Median diff (min–max) | −0.33 (−0.82 to 0.68) | −0.32 (−1.15 to 0.30) | 0.709 |
| Mean diff (CI) | −0.33 (−0.45 to −0.21) | −0.32 (−0.46 to −0.17) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johansson, K.; Blom, K.; Nilsson-Wikmar, L.; Brogårdh, C. Early Intervention with a Compression Sleeve in Mild Breast Cancer-Related Arm Lymphedema: A 12-Month Prospective Observational Study. Cancers 2023, 15, 2674. https://doi.org/10.3390/cancers15102674
Johansson K, Blom K, Nilsson-Wikmar L, Brogårdh C. Early Intervention with a Compression Sleeve in Mild Breast Cancer-Related Arm Lymphedema: A 12-Month Prospective Observational Study. Cancers. 2023; 15(10):2674. https://doi.org/10.3390/cancers15102674
Chicago/Turabian StyleJohansson, Karin, Katarina Blom, Lena Nilsson-Wikmar, and Christina Brogårdh. 2023. "Early Intervention with a Compression Sleeve in Mild Breast Cancer-Related Arm Lymphedema: A 12-Month Prospective Observational Study" Cancers 15, no. 10: 2674. https://doi.org/10.3390/cancers15102674
APA StyleJohansson, K., Blom, K., Nilsson-Wikmar, L., & Brogårdh, C. (2023). Early Intervention with a Compression Sleeve in Mild Breast Cancer-Related Arm Lymphedema: A 12-Month Prospective Observational Study. Cancers, 15(10), 2674. https://doi.org/10.3390/cancers15102674








