Updated Mortality Analysis of SELTINE, the French Cohort of Nuclear Workers, 1968–2014
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Vital Status and Outcome Determination
2.3. Radiation Dose Reconstruction
2.4. Mortality Analysis (Comparison with External Reference)
2.5. Analysis of the Dose–Risk Associations (with Internal Reference)
3. Results
3.1. Study Population
| Characteristics | Male | Female | Total | |||
|---|---|---|---|---|---|---|
| Number of workers | 69,487 | 10,861 | 80,348 | |||
| Number of person-years | 2,202,492 | 352,062 | 2,554,554 | |||
| Median year of birth (range) | 1951 (1893–1984) | 1947 (1896–1983) | 1951 (1893–1984) | |||
| Follow-up (1968–2014), in years (SD) | ||||||
| Mean duration | 31.7 | (11.4) | 32.4 | (13.0) | 31.8 | (11.6) |
| Mean age at beginning of follow-up | 31.1 | (8.1) | 31.0 | (8.8) | 31.1 | (8.2) |
| Mean age at end of follow-up | 62.8 | (13.6) | 63.4 | (15.3) | 62.9 | (13.9) |
| Vital status on 31 December 2014, n (%) | ||||||
| Alive | 55,134 | (79.3) | 9164 | (84.4) | 64,298 | (80.0) |
| Deceased | 14,131 | (20.4) | 1564 | (14.4) | 15,695 | (19.5) |
| Lost to follow-up | 222 | (0.3) | 133 | (1.2) | 355 | (0.5) |
| Employment | ||||||
| Median year of hiring (range) | 1977 | 1938–2004 | 1974 | 1940–2003 | 1977 | 1938–2004 |
| Mean age at hiring, in years (SD) | 26.4 | (6.3) | 26.1 | (7.0) | 26.3 | (6.4) |
| Mean duration, in years (SD) | 26.5 | (9.0) | 24.4 | (10.6) | 26.2 | (9.2) |
| Hired after 1956, n (%) | 66,003 | (95.0) | 10,239 | (94.3) | 76,242 | (94.9) |
| Number of person-years by principal * employing company (%) | ||||||
| CEA | 1,133,865 | (51.5) | 284,956 | (80.9) | 1,418,821 | (55.5) |
| Orano | 232,415 | (10.5) | 17,247 | (4.9) | 249,662 | (9.8) |
| EDF | 768,889 | (34.9) | 39,299 | (11.2) | 808,188 | (31.6) |
| Other ** | 67,323 | (3.1) | 10,560 | (3.0) | 77,883 | (3.1) |
| Socioeconomic status, n (%) | ||||||
| Managers and engineers | 13,466 | (19.4) | 2492 | (22.9) | 15,958 | (19.9) |
| Administrative employees | 2909 | (4.2) | 3952 | (36.4) | 6861 | (8.5) |
| Skilled workers | 40,537 | (58.3) | 3448 | (31.8) | 43,985 | (54.7) |
| Unskilled workers | 11,787 | (17.0) | 827 | (7.6) | 12,614 | (15.7) |
| Unknown | 788 | (1.1) | 142 | (1.3) | 930 | (1.2) |
| Monitoring of external radiation exposure, in years | ||||||
| Mean duration (SD) | 21.2 | (9.9) | 15.0 | (9.8) | 20.3 | (10.1) |
| Mean age at last monitoring (SD) | 50.0 | (9.7) | 43.7 | (11.5) | 49.2 | (10.2) |
| Number of exposed *** workers (%) | 49,995 | (71.9) | 4585 | (42.2) | 54,580 | (67.9) |
| Cumulative Hp(10) dose, in milliSieverts (SD) | ||||||
| Mean (SD), whole cohort | 17.7 | (39.7) | 3.1 | (13.7) | 15.7 | (37.5) |
| Median/IQR/maximum, whole cohort | 2.1/0.0–16.1/668.6 | 0.0/0.0–0.9/573.9 | 1.3/0.0–13.0/668.6 | |||
| Mean (SD), exposed *** workers only | 24.7 | (44.9) | 7.3 | (20.4) | 23.1 | (43.6) |
| Person-years by categories of 10-years lagged cumulative Hp(10) dose in milliSievert, n (%) | ||||||
| <2.5 | 1,487,620 | (67.6) | 308,589 | (87.7) | 1,796,209 | (70.3) |
| 2.5 –4.9 | 128,096 | (5.8) | 13,295 | (3.8) | 141,391 | (5.5) |
| 5.0 –9.9 | 135,109 | (6.1) | 10,724 | (3.0) | 145,833 | (5.7) |
| 10.0–19.9 | 142,935 | (6.5) | 9470 | (2.7) | 152,405 | (6.0) |
| 20.0–49.9 | 169,571 | (7.7) | 7281 | (2.0) | 176,852 | (6.9) |
| 50.0–99.9 | 85,740 | (3.9) | 1865 | (0.5) | 87,605 | (3.4) |
| 100.0–199.9 | 42,162 | (1.9) | 641 | (0.2) | 42,803 | (1.7) |
| ≥200.0 | 11,259 | (0.5) | 197 | (0.1) | 11,456 | (0.5) |
| Person-years by status of neutron exposure, n (%) | ||||||
| Not exposed | 2,159,095 | (98.0) | 349,872 | (99.4) | 2,508,967 | (98.2) |
| Potentially exposed | 43,397 | (2.0) | 2190 | (0.6) | 45,587 | (1.8) |
| Person-years by status of potential for internal contamination, n (%) | ||||||
| No | 2,127,408 | (96.6) | 340,711 | (96.8) | 2,468,119 | (96.6) |
| Low/medium potential | 63,372 | (2.9) | 8780 | (2.5) | 72,152 | (2.8) |
| High potential | 11,712 | (0.5) | 2571 | (0.7) | 14,283 | (0.6) |
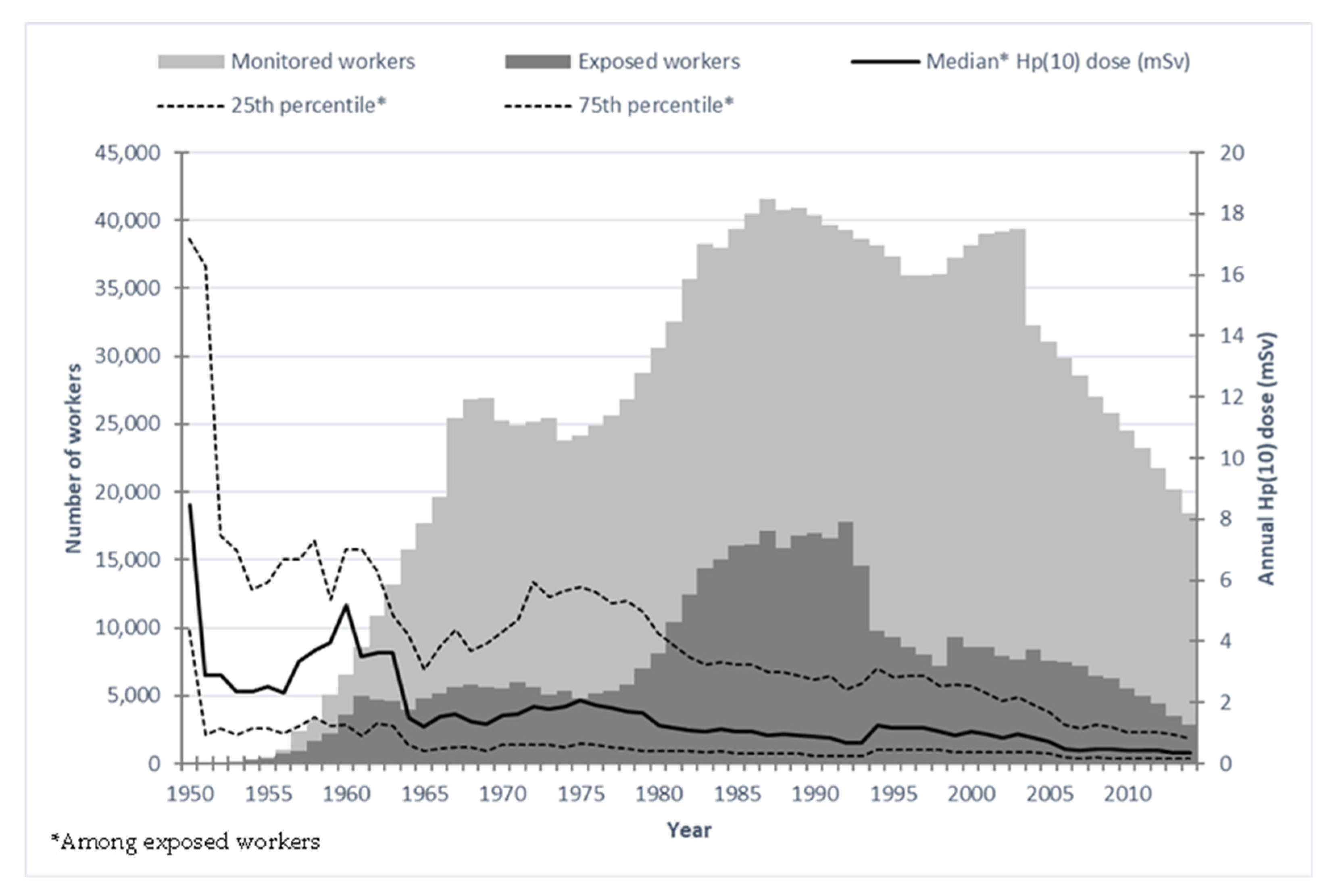
3.2. Mortality Analysis (Comparison with External Reference)
3.3. Analysis of the Dose–Risk Associations (with Internal Reference)
3.3.1. Solid Cancers
| Cause of Death | Target Tissue | Observed Deaths | ERR/Gy | 95% CI | p-Value |
|---|---|---|---|---|---|
| All cancers | Colon | 5,618 | 0.53 | −0.40; 1.55 | 0.28 |
| All cancers excluding leukaemia | Colon | 5,414 | 0.46 | −0.48; 1.50 | 0.35 |
| Solid cancers | Colon | 5,130 | 0.71 | −0.28; 1.80 | 0.16 |
| Solid cancers excluding lung cancer | Colon | 3,778 | 0.45 | −0.65; 1.69 | 0.44 |
| Oral cavity cancer | Skin | 201 | 4.54 | −0.92; 12.8 | 0.12 |
| Oesophagus cancer | Stomach | 206 | 1.26 | ne; 7.97 | 0.63 |
| Stomach cancer | Stomach | 198 | 2.82 | ne; 10.7 | 0.32 |
| Colon cancer | Colon | 383 | 1.48 | −1.71; 6.03 | 0.42 |
| Rectum cancer | Colon | 142 | 4.31 | −1.24; 13.1 | 0.15 |
| Liver cancer | Liver | 286 | 1.20 | ne; 7.17 | 0.63 |
| Gallbladder cancer | Gallbladder | 35 | NC | NC | NC |
| Pancreas cancer | Pancreas | 338 | −2.41 | ne; 1.19 | 0.15 |
| Peritoneum cancer | Colon | 74 | 6.58 | ne; 23.9 | 0.18 |
| Nasal cancer | Skin | 45 | 8.87 | ne; 33.5 | 0.15 |
| Larynx cancer | Stomach | 102 | 5.23 | ne; 18.6 | 0.22 |
| Lung cancer | Lung | 1,352 | 1.09 | −0.83; 3.39 | 0.29 |
| Pleural cancer | Lung | 95 | −1.79 | −2.14; 7.03 | 0.61 |
| Bones, connective, and other soft tissues cancers | Colon | 48 | 3.67 | ne; 22.8 | 0.52 |
| Melanoma | Skin | 86 | 3.68 | ne; 17.0 | 0.37 |
| Prostate cancer | Bladder | 473 | −1.66 | ne; 1.42 | 0.25 |
| Bladder cancer | Bladder | 212 | −1.14 ** | ne; 4.63 | 0.65 |
| Kidney cancer | Bladder | 160 | 3.59 | ne; 13.8 | 0.32 |
| Brain and central nervous system cancer | Brain | 169 | −1.68 | ne; 5.31 | 0.56 |
| Brain and central nervous system tumours including benign tumours | Brain | 250 | 0.87 | ne; 7.71 | 0.75 |
3.3.2. Hematopoietic and Lymphatic Cancers
3.3.3. Non-Cancer Diseases
| Cause of Death | Target Tissue | Observed Deaths | ERR/Gy or ERR/Sv | 95% CI | p-Value |
|---|---|---|---|---|---|
| Non-cancer diseases (all) | Hp(10) | 8577 | 0.02 | −0.49; 0.57 | 0.95 |
| Diabetes mellitus | Hp(10) | 169 | −0.91 | ne; 2.62 | 0.54 |
| Mental and behavioural disorders | Brain | 235 | 2.74 | −1.95; 10.33 | 0.32 |
| Diseases of the nervous system | Brain | 531 | 3.47 | −0.48; 8.56 | 0.09 |
| Dementia, Alzheimer’s disease, Parkinson’s disease, motoneuron disease | Brain | 469 | 4.99 | 0.74; 10.52 | 0.02 |
| Dementia, Alzheimer’s disease | Brain | 269 | 9.62 | 3.05; 18.68 | <0.01 |
| Parkinson’s disease | Brain | 124 | −1.30 | ne; 7.44 | 0.71 |
| Circulatory diseases | Hp(10) | 3261 | 0.09 | −0.72; 1.03 | 0.83 |
| Ischemic diseases | Hp(10) | 1258 | −0.23 | ne; 1.38 | 0.76 |
| Cerebrovascular diseases | Hp(10) | 684 | 1.41 | ne; 4.05 | 0.19 |
| Hypertensive diseases | Hp(10) | 120 | 2.31 | ne; 9.98 | 0.37 |
| Respiratory diseases | Lung | 558 | 0.22 | ne; 3.71 | 0.88 |
| Chronic obstructive pulmonary diseases | Lung | 164 | 0.15 | ne; 7.52 | 0.96 |
| Digestive diseases | Colon | 583 | 0.96 | −1.72; 4.58 | 0.53 |
| Cirrhosis | Liver | 166 | 3.72 | ne; 13.64 | 0.29 |
4. Discussion
4.1. General Strengths and Limitations
4.2. Mortality Analyses (Comparison with External Reference)
4.3. Dose–Risk Relationships (with Internal Reference)
4.3.1. Solid Cancers
4.3.2. Leukaemia Excluding CLL
4.3.3. Circulatory Diseases
4.3.4. Dementia
4.4. Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Agency for Research on Cancer. Monographs on the Evaluation of Carcinogenic Risks to Humans. X and Gamma Radiation; IARC: Lyon, France, 2012; Volume 100D-7.
- United Nations Scientific Committee on the Effects of Atomic Radiation. 2012 Report to the General Assembly with Scientific Annexes, Sources and Effects of Ionizing Radiation. Scientific Annex A Attributing Health Effects to Ionizing Radiation Exposure and Inferring Risks; United Nations: New York, NY, USA, 2015; Volume 2.
- Brenner, A.V.; Preston, D.L.; Sakata, R.; Cologne, J.; Sugiyama, H.; Utada, M.; Cahoon, E.K.; Grant, E.; Mabuchi, K.; Ozasa, K. Comparison of All Solid Cancer Mortality and Incidence Dose-Response in the Life Span Study of Atomic Bomb Survivors, 1958–2009. Radiat. Res. 2022, 197, 491–508. [Google Scholar] [CrossRef] [PubMed]
- Shore, R.E.; Beck, H.L.; Boice, J.D.; Caffrey, E.A.; Davis, S.; Grogan, H.A.; Mettler, F.A.; Preston, R.J.; Till, J.E.; Wakeford, R.; et al. Implications of recent epidemiologic studies for the linear nonthreshold model and radiation protection. J. Radiol. Prot. 2018, 38, 1217–1233. [Google Scholar] [CrossRef] [PubMed]
- Hauptmann, M.; Daniels, R.D.; Cardis, E.; Cullings, H.M.; Kendall, G.; Laurier, D.; Linet, M.S.; Little, M.P.; Lubin, J.H.; Preston, D.L.; et al. Epidemiological Studies of Low-Dose Ionizing Radiation and Cancer: Summary Bias Assessment and Meta-Analysis. J. Natl. Cancer Inst. Monogr. 2020, 2020, 188–200. [Google Scholar] [CrossRef]
- Tapio, S.; Little, M.P.; Kaiser, J.C.; Impens, N.; Hamada, N.; Georgakilas, A.G.; Simar, D.; Salomaa, S. Ionizing radiation-induced circulatory and metabolic diseases. Environ. Int. 2021, 146, 106235. [Google Scholar] [CrossRef] [PubMed]
- Gillies, M.; Richardson, D.B.; Cardis, E.; Daniels, R.D.; O’Hagan, J.A.; Haylock, R.; Laurier, D.; Leuraud, K.; Moissonnier, M.; Schubauer-Berigan, M.K.; et al. Mortality from Circulatory Diseases and other Non-Cancer Outcomes among Nuclear Workers in France, the United Kingdom and the United States (INWORKS). Radiat. Res. 2017, 188, 276–290. [Google Scholar] [CrossRef]
- Little, M.P.; Azizova, T.V.; Bazyka, D.; Bouffler, S.D.; Cardis, E.; Chekin, S.; Chumak, V.V.; Cucinotta, F.A.; de Vathaire, F.; Hall, P.; et al. Systematic review and meta-analysis of circulatory disease from exposure to low-level ionizing radiation and estimates of potential population mortality risks. Environ. Health Perspect. 2012, 120, 1503–1511. [Google Scholar] [CrossRef]
- Wakeford, R. Risk of diseases of the circulatory system after low-level radiation exposure-an assessment of evidence from occupational exposures. J. Radiol. Prot. 2022, 42. [Google Scholar] [CrossRef]
- Pasqual, E.; Boussin, F.; Bazyka, D.; Nordenskjold, A.; Yamada, M.; Ozasa, K.; Pazzaglia, S.; Roy, L.; Thierry-Chef, I.; de Vathaire, F.; et al. Cognitive effects of low dose of ionizing radiation—Lessons learned and research gaps from epidemiological and biological studies. Environ. Int. 2021, 147, 106295. [Google Scholar] [CrossRef]
- Lopes, J.; Leuraud, K.; Klokov, D.; Durand, C.; Bernier, M.-O.; Baudin, C. Risk of Developing Non-Cancerous Central Nervous System Diseases Due to Ionizing Radiation Exposure during Adulthood: Systematic Review and Meta-Analyses. Brain Sci. 2022, 12, 984. [Google Scholar] [CrossRef]
- Cardis, E.; Vrijheid, M.; Blettner, M.; Gilbert, E.; Hakama, M.; Hill, C.; Howe, G.; Kaldor, J.; Muirhead, C.R.; Schubauer-Berigan, M.; et al. The 15-Country Collaborative Study of Cancer Risk among Radiation Workers in the Nuclear Industry: Estimates of Radiation-Related Cancer Risks. Radiat. Res. 2007, 167, 396–416. [Google Scholar] [CrossRef]
- Richardson, D.B.; Cardis, E.; Daniels, R.D.; Gillies, M.; O’Hagan, J.A.; Hamra, G.B.; Haylock, R.; Laurier, D.; Leuraud, K.; Moissonnier, M.; et al. Risk of cancer from occupational exposure to ionising radiation: Retrospective cohort study of workers in France, the United Kingdom, and the United States (INWORKS). BMJ 2015, 351, h5359. [Google Scholar] [CrossRef] [PubMed]
- Boice, J.D., Jr.; Cohen, S.S.; Mumma, M.T.; Ellis, E.D. The Million Person Study, whence it came and why. Int. J. Radiat. Biol. 2022, 98, 537–550. [Google Scholar] [CrossRef] [PubMed]
- Richardson, D.B.; Cardis, E.; Daniels, R.D.; Gillies, M.; Haylock, R.; Leuraud, K.; Laurier, D.; Moissonnier, M.; Schubauer-Berigan, M.K.; Thierry-Chef, I.; et al. Site-specific Solid Cancer Mortality After Exposure to Ionizing Radiation: A Cohort Study of Workers (INWORKS). Epidemiology 2018, 29, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Haylock, R.G.E.; Gillies, M.; Hunter, N.; Zhang, W.; Phillipson, M. Cancer mortality and incidence following external occupational radiation exposure: An update of the 3rd analysis of the UK national registry for radiation workers. Br. J. Cancer 2018, 119, 631–637. [Google Scholar] [CrossRef]
- Azizova, T.V.; Moseeva, M.B.; Grigoryeva, E.S.; Hamada, N. Incidence risks for cerebrovascular diseases and types of stroke in a cohort of Mayak PA workers. Radiat. Environ. Biophys. 2022, 61, 5–16. [Google Scholar] [CrossRef]
- Hunter, N.; Haylock, R.G.E.; Gillies, M.; Zhang, W. Extended analysis of solid cancer incidence among the Nuclear Industry Workers in the UK: 1955–2011. Radiat. Res. 2022, 198, 1–17. [Google Scholar] [CrossRef]
- Telle-Lamberton, M.; Samson, E.; Caer, S.; Bergot, D.; Bard, D.; Bermann, F.; Gelas, J.M.; Giraud, J.M.; Hubert, P.; Metz-Flamant, C.; et al. External radiation exposure and mortality in a cohort of French nuclear workers. Occup. Environ. Med. 2007, 64, 694–700. [Google Scholar] [CrossRef]
- Metz-Flamant, C.; Samson, E.; Caer-Lorho, S.; Acker, A.; Laurier, D. Solid cancer mortality associated with chronic external radiation exposure at the French atomic energy commission and nuclear fuel company. Radiat. Res. 2011, 176, 115–127. [Google Scholar] [CrossRef]
- Metz-Flamant, C.; Samson, E.; Caer-Lorho, S.; Acker, A.; Laurier, D. Leukemia risk associated with chronic external exposure to ionizing radiation in a French cohort of nuclear workers. Radiat. Res. 2012, 178, 489–498. [Google Scholar] [CrossRef]
- Rogel, A.; Joly, K.; Metz-Flamant, C.; Laurent, O.; Tirmarche, M.; Hubert, D.; Garcier, Y.; Laurier, D. Mortality in nuclear workers of the French electricity company: Period 1968–2003. Rev. Epidemiol. Sante Publique 2009, 57, 257–265. [Google Scholar] [CrossRef]
- Laurent, O.; Metz-Flamant, C.; Rogel, A.; Hubert, D.; Riedel, A.; Garcier, Y.; Laurier, D. Relationship between occupational exposure to ionizing radiation and mortality at the French electricity company, period 1961–2003. Int. Arch. Occup. Environ. Health 2010, 83, 935–944. [Google Scholar] [CrossRef] [PubMed]
- Leuraud, K.; Fournier, L.; Samson, E.; Caer, S.; Laurier, D. Mortality in the French cohort of nuclear workers. Radioprotection 2017, 52, 199–210. [Google Scholar] [CrossRef]
- Schubauer-Berigan, M.K.; Daniels, R.D.; Bertke, S.J.; Tseng, C.Y.; Richardson, D.B. Cancer Mortality through 2005 among a Pooled Cohort of U.S. Nuclear Workers Exposed to External Ionizing Radiation. Radiat. Res. 2015, 183, 620–631. [Google Scholar] [CrossRef] [PubMed]
- Leuraud, K.; Richardson, D.B.; Cardis, E.; Daniels, R.D.; Gillies, M.; O’Hagan, J.A.; Hamra, G.B.; Haylock, R.; Laurier, D.; Moissonnier, M.; et al. Ionising radiation and risk of death from leukaemia and lymphoma in radiation-monitored workers (INWORKS): An international cohort study. Lancet Haematol. 2015, 2, e276–e281. [Google Scholar] [CrossRef]
- Rage, E.; Caer-Lorho, S.; Laurier, D. Low radon exposure and mortality among Jouac uranium miners: An update of the French cohort (1946–2007). J. Radiol. Prot. 2018, 38, 92–108. [Google Scholar] [CrossRef]
- Bouet, S.; Samson, E.; Jovanovic, I.; Laurier, D.; Laurent, O. First mortality analysis in the French cohort of uranium millers (F-Millers), period 1968–2013. Int. Arch. Occup. Environ. Health 2018, 91, 23–33. [Google Scholar] [CrossRef]
- Scanff, P.; Crescini, D.; Roy, H.; Billarand, Y.; Rannou, A. National Dose Register in France within the National Information System Siseri. Radiat. Prot. Dosimetry 2016, 170, 429–432. [Google Scholar] [CrossRef]
- Thierry-Chef, I.; Marshall, M.; Fix, J.J.; Bermann, F.; Gilbert, E.S.; Hacker, C.; Heinmiller, B.; Murray, W.; Pearce, M.S.; Utterback, D.; et al. The 15-Country Collaborative Study of Cancer Risk among Radiation Workers in the Nuclear Industry: Study of errors in dosimetry. Radiat. Res. 2007, 167, 380–395. [Google Scholar] [CrossRef]
- Thierry-Chef, I.; Richardson, D.B.; Daniels, R.D.; Gillies, M.; Hamra, G.B.; Haylock, R.; Kesminiene, A.; Laurier, D.; Leuraud, K.; Moissonnier, M.; et al. Dose Estimation for a Study of Nuclear Workers in France, the United Kingdom and the United States of America: Methods for the International Nuclear Workers Study (INWORKS). Radiat. Res. 2015, 183, 632–642. [Google Scholar] [CrossRef]
- Fournier, L.; Laurent, O.; Samson, E.; Caer-Lorho, S.; Laroche, P.; Le Guen, B.; Laurier, D.; Leuraud, K. External radiation dose and cancer mortality among French nuclear workers: Considering potential confounding by internal radiation exposure. Int. Arch. Occup. Environ. Health 2016, 89, 1183–1191. [Google Scholar] [CrossRef]
- Breslow, N.; Day, N. Statistical Methods in Cancer Research; IARC: Lyon, France, 1980.
- Preston, D.; Lubin, J.; Pierce, D.; McConney, M. Epicure User’s Guide; H.I. Corporation: Seattle, WA, USA, 1993. [Google Scholar]
- Wakeford, R. The growing importance of radiation worker studies. Br. J. Cancer 2018, 119, 527–529. [Google Scholar] [CrossRef] [PubMed]
- Wakeford, R. Overview of epidemiological studies of nuclear workers: Opportunities, expectations, and limitations. J. Radiol. Prot. 2021, 41, 1075–1092. [Google Scholar] [CrossRef] [PubMed]
- Merzenich, H.; Hammer, G.P.; Troltzsch, K.; Ruecker, K.; Buncke, J.; Fehringer, F.; Blettner, M. Mortality risk in a historical cohort of nuclear power plant workers in Germany: Results from a second follow-up. Radiat. Environ. Biophys. 2014, 53, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Samson, E.; Telle-Lamberton, M.; Caer-Lorho, S.; Bard, D.; Giraud, J.M.; Metz-Flamant, C.; Neron, M.O.; Quesne, B.; Acker, A.; Tirmarche, M.; et al. Cancer mortality among two different populations of French nuclear workers. Int. Arch. Occup. Environ. Health 2011, 84, 627–634. [Google Scholar] [CrossRef]
- Baillargeon, J. Characteristics of the healthy worker effect. Occup. Med. 2001, 16, 359–366. [Google Scholar]
- Li, C.Y.; Sung, F.C. A review of the healthy worker effect in occupational epidemiology. Occup. Med. 1999, 49, 225–229. [Google Scholar] [CrossRef]
- Metz-Flamant, C.; Guseva Canu, I.; Laurier, D. Malignant pleural mesothelioma risk among nuclear workers: A review. J. Radiol. Prot. 2011, 31, 9–23. [Google Scholar] [CrossRef]
- Mumma, M.T.; Sirko, J.L.; Boice, J.D., Jr.; Blot, W.J. Mesothelioma mortality within two radiation monitored occupational cohorts. Int. J. Radiat. Biol. 2022, 98, 786–794. [Google Scholar] [CrossRef]
- Leuraud, K.; Richardson, D.B.; Cardis, E.; Daniels, R.D.; Gillies, M.; Haylock, R.; Moissonnier, M.; Schubauer-Berigan, M.K.; Thierry-Chef, I.; Kesminiene, A.; et al. Risk of cancer associated with low-dose radiation exposure: Comparison of results between the INWORKS nuclear workers study and the A-bomb survivors study. Radiat. Environ. Biophys. 2021, 60, 23–39. [Google Scholar] [CrossRef]
- Furuta, H.; Kudo, S.; Ishizawa, N.; Saigusa, S. Reanalysis of cancer mortality using reconstructed organ-absorbed dose: J-EPISODE 19912010. J. Radiol. Prot. 2022, 42, 2761. [Google Scholar] [CrossRef]
- Boice, J.D.; Cohen, S.S.; Mumma, M.T.; Hagemeyer, D.A.; Chen, H.; Golden, A.P.; Yoder, R.C.; Dauer, L.T. Mortality from leukemia, cancer and heart disease among U.S. nuclear power plant workers, 1957–2011. Int. J. Radiat. Biol. 2022, 98, 657–678. [Google Scholar] [CrossRef] [PubMed]
- Gillies, M.; Haylock, R.; Hunter, N.; Zhang, W. Risk of Leukemia Associated with Protracted Low-Dose Radiation Exposure: Updated Results from the National Registry for Radiation Workers Study. Radiat. Res. 2019, 192, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Haylock, R.G.E.; Gillies, M.; Hunter, N. Mortality from heart diseases following occupational radiation exposure: Analysis of the National Registry for Radiation Workers (NRRW) in the United Kingdom. J. Radiol. Prot. 2019, 39, 327–353. [Google Scholar] [CrossRef] [PubMed]
- Hinksman, C.A.; Haylock, R.G.E.; Gillies, M. Cerebrovascular Disease Mortality after occupational Radiation Exposure among the UK National Registry for Radiation Workers Cohort. Radiat. Res. 2022, 197, 459–470. [Google Scholar] [CrossRef] [PubMed]
- de Vocht, F.; Hidajat, M.; Martin, R.M.; Agius, R.; Wakeford, R. Ischemic Heart Disease Mortality and Occupational Radiation Exposure in a Nested Matched Case-Control Study of British Nuclear Fuel Cycle Workers: Investigation of Confounding by Lifestyle, Physiological Traits and Occupational Exposures. Radiat. Res. 2020, 194, 431–444. [Google Scholar] [CrossRef]
- Zhivin, S.; Guseva Canu, I.; Davesne, E.; Blanchardon, E.; Garsi, J.P.; Samson, E.; Niogret, C.; Zablotska, L.B.; Laurier, D. Circulatory disease in French nuclear fuel cycle workers chronically exposed to uranium: A nested case-control study. Occup. Environ. Med. 2018, 75, 270–276. [Google Scholar] [CrossRef]
- Drubay, D.; Caer-Lorho, S.; Laroche, P.; Laurier, D.; Rage, E. Mortality from Circulatory System Diseases among French Uranium Miners: A Nested Case-Control Study. Radiat. Res. 2015, 183, 550–562. [Google Scholar] [CrossRef]
- Kreuzer, M.; Dufey, F.; Sogl, M.; Schnelzer, M.; Walsh, L. External gamma radiation and mortality from cardiovascular diseases in the German WISMUT uranium miners cohort study, 1946–2008. Radiat. Environ. Biophys. 2013, 52, 37–46. [Google Scholar] [CrossRef]
- Sibley, R.F.; Moscato, B.S.; Wilkinson, G.S.; Natarajan, N. Nested case-control study of external ionizing radiation dose and mortality from dementia within a pooled cohort of female nuclear weapons workers. Am. J. Ind. Med. 2003, 44, 351–358. [Google Scholar] [CrossRef]
- Yamada, M.; Landes, R.D.; Mimori, Y.; Nagano, Y.; Sasaki, H. Radiation Effects on Cognitive Function Among Atomic Bomb Survivors Exposed at or After Adolescence. Am. J. Med. 2016, 129, 586–591. [Google Scholar] [CrossRef]
- Yamada, M.; Kasagi, F.; Mimori, Y.; Miyachi, T.; Ohshita, T.; Sasaki, H. Incidence of dementia among atomic-bomb survivors—Radiation Effects Research Foundation Adult Health Study. J. Neurol. Sci. 2009, 281, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Begum, N.; Wang, B.; Mori, M.; Vares, G. Does ionizing radiation influence Alzheimer’s disease risk? J. Radiat. Res. 2012, 53, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Stephen, R.; Barbera, M.; Peters, R.; Ee, N.; Zheng, L.; Lehtisalo, J.; Kulmala, J.; Hakansson, K.; Chowdhary, N.; Dua, T.; et al. Development of the First WHO Guidelines for Risk Reduction of Cognitive Decline and Dementia: Lessons Learned and Future Directions. Front. Neurol. 2021, 12, 763573. [Google Scholar] [CrossRef] [PubMed]

| Cause of Death | Observed Deaths | Lag | ERR/Gy | 95% CI | p-Value |
|---|---|---|---|---|---|
| Leukaemia excluding CLL | 157 | 2 | 3.31 | 0.94; 13.38 | <0.01 |
| Acute myeloid leukaemia | 59 | 2 | 5.26 | ne; 24.17 | 0.05 |
| Acute myeloid leukaemia and MDS | 96 | 2 | 3.86 | 0.43; 21.36 | 0.02 |
| Leukaemia (excluding CLL) and MDS | 194 | 2 | 2.87 | 0.95; 11.55 | 0.01 |
| Chronic lymphocytic leukaemia | 44 | 10 | NC | NC | NC |
| Non-Hodgkin lymphoma | 184 | 10 | NC | NC | NC |
| Multiple myeloma | 80 | 10 | −0.57 | ne; 11.94 | 0.90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laurent, O.; Samson, E.; Caër-Lorho, S.; Fournier, L.; Laurier, D.; Leuraud, K. Updated Mortality Analysis of SELTINE, the French Cohort of Nuclear Workers, 1968–2014. Cancers 2023, 15, 79. https://doi.org/10.3390/cancers15010079
Laurent O, Samson E, Caër-Lorho S, Fournier L, Laurier D, Leuraud K. Updated Mortality Analysis of SELTINE, the French Cohort of Nuclear Workers, 1968–2014. Cancers. 2023; 15(1):79. https://doi.org/10.3390/cancers15010079
Chicago/Turabian StyleLaurent, Olivier, Eric Samson, Sylvaine Caër-Lorho, Lucie Fournier, Dominique Laurier, and Klervi Leuraud. 2023. "Updated Mortality Analysis of SELTINE, the French Cohort of Nuclear Workers, 1968–2014" Cancers 15, no. 1: 79. https://doi.org/10.3390/cancers15010079
APA StyleLaurent, O., Samson, E., Caër-Lorho, S., Fournier, L., Laurier, D., & Leuraud, K. (2023). Updated Mortality Analysis of SELTINE, the French Cohort of Nuclear Workers, 1968–2014. Cancers, 15(1), 79. https://doi.org/10.3390/cancers15010079






