Thioredoxin Reductase-1 as a Potential Biomarker in Fibroblast-Associated HCT116 Cancer Cell Progression and Dissemination in a Zebrafish Model
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Fibroblast-Cultured Medium Preparation
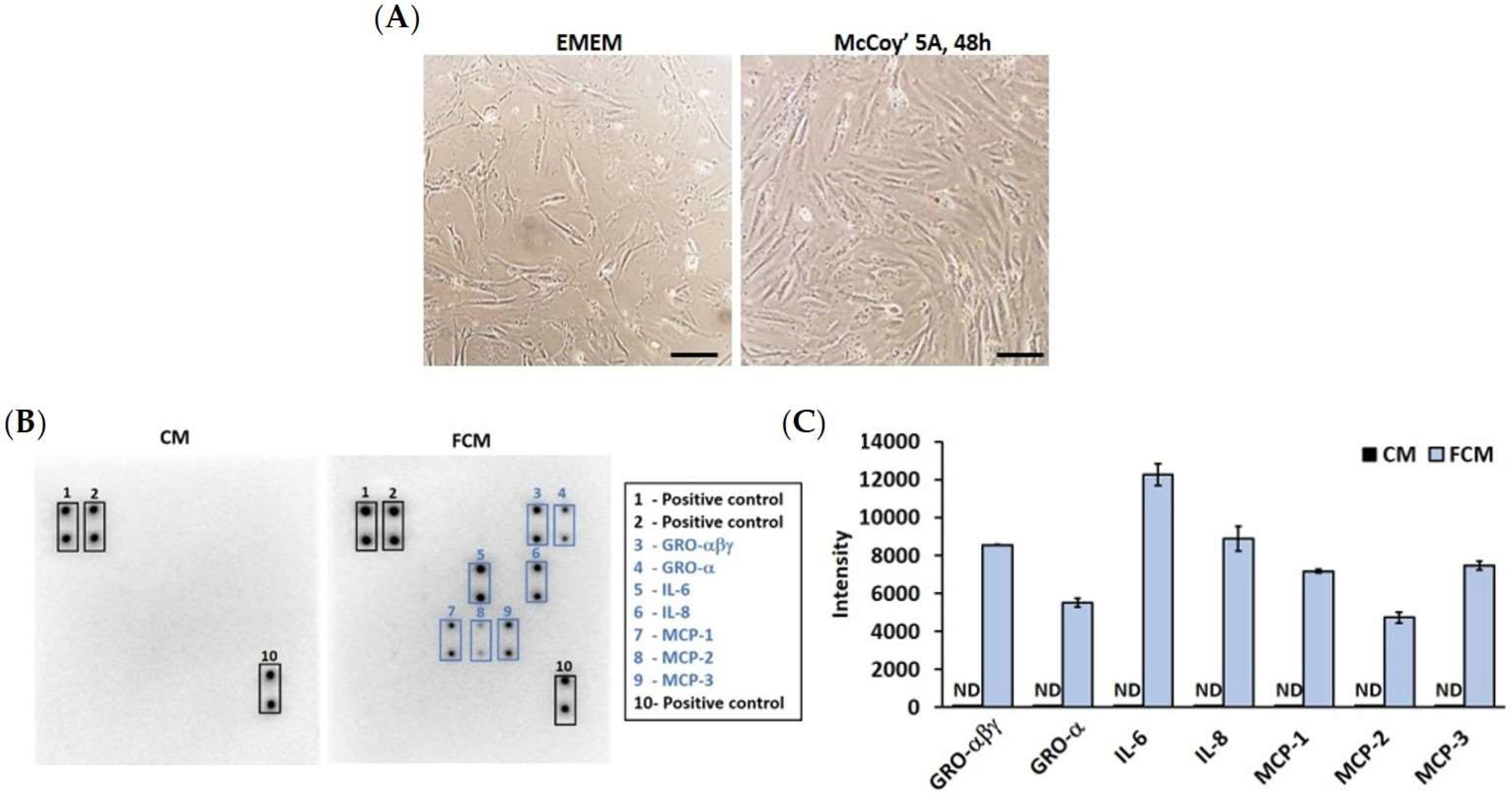
2.2. Cytokine Array
2.3. Cell Viability by MTT Assay
2.4. Wound-Healing Assay
2.5. Boyden Chamber Assay
2.6. Indirect Immunofluorescence Assay
2.7. Morphology Change
2.8. DCFH-DA Assay
2.9. Western Blotting
2.10. Zebrafish Model
2.11. Xenograft Procedure
2.12. Histology Examination and Immunohistochemistry
2.13. Statistical Analysis
3. Results
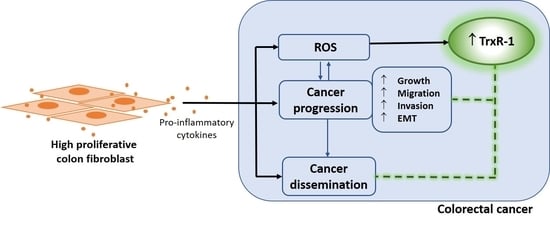
3.1. High Proliferation of Colon Fibroblast Secretes Pro-Inflammatory Cytokines
3.2. Fibroblast-Cultured Media Induce HCT116 Progression
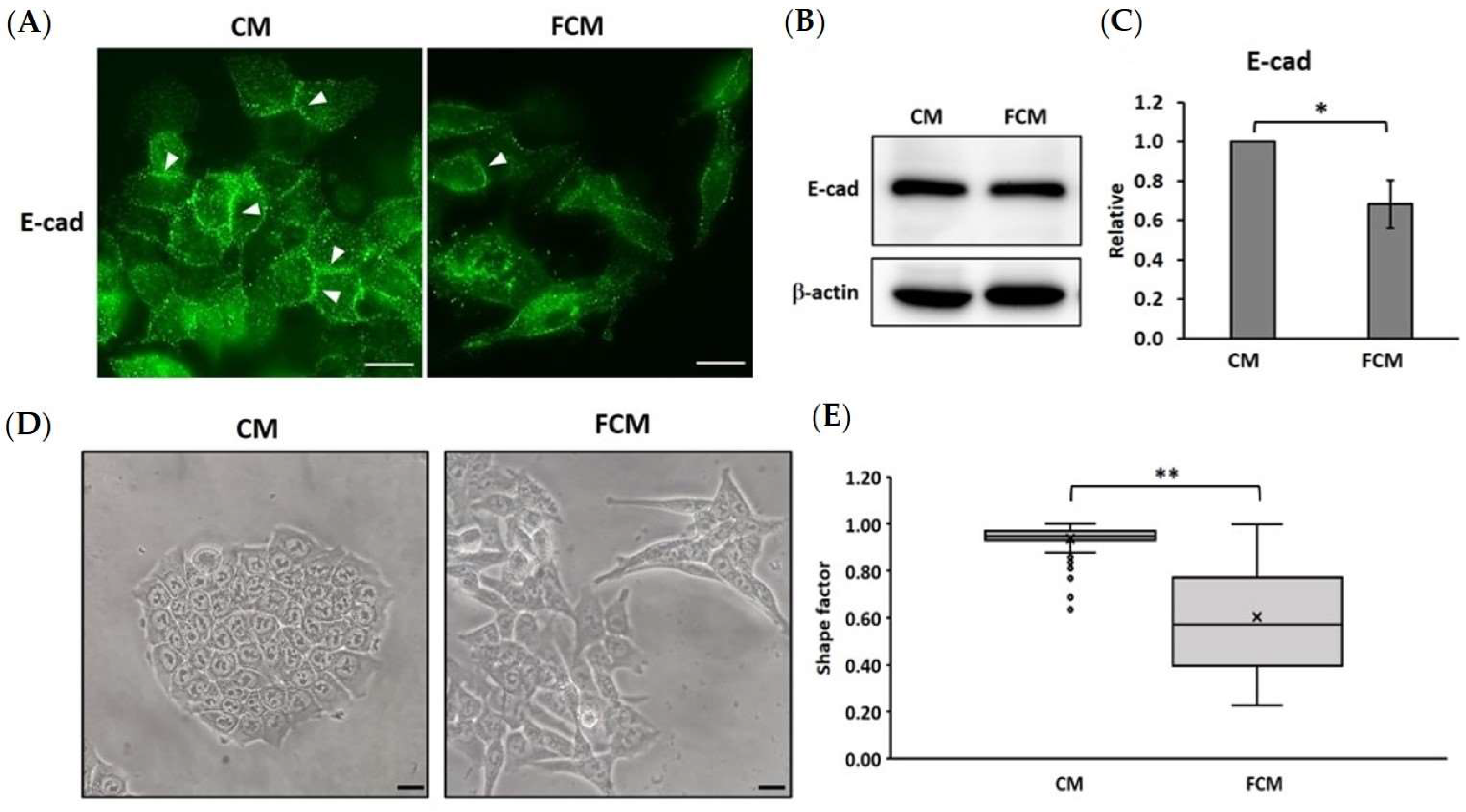
3.3. Fibroblast-Cultured Media Induce the EMT Process in HCT116 Cells
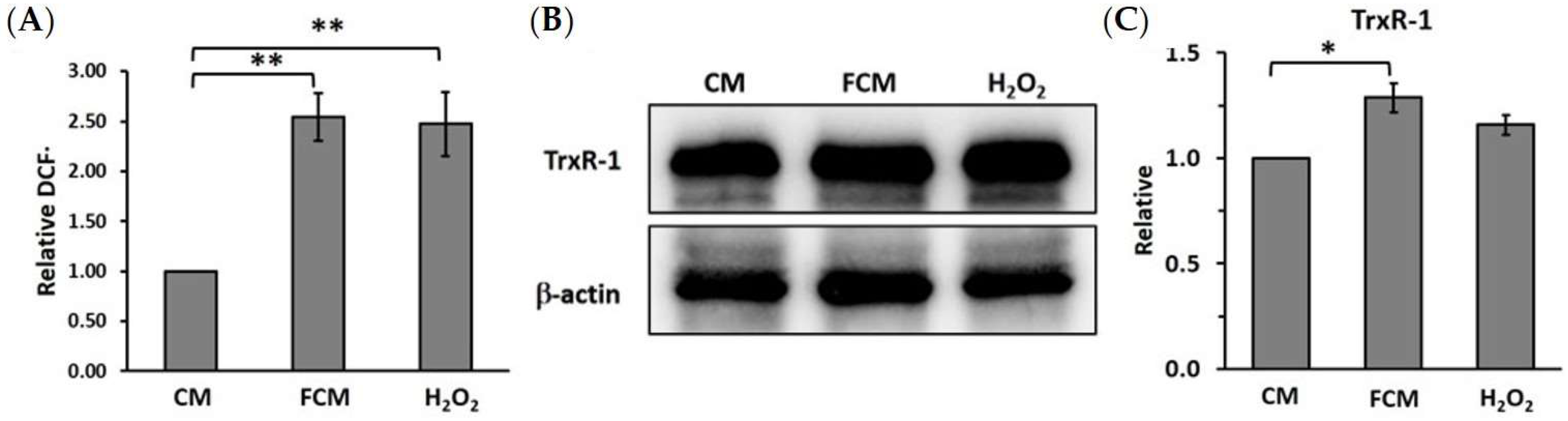
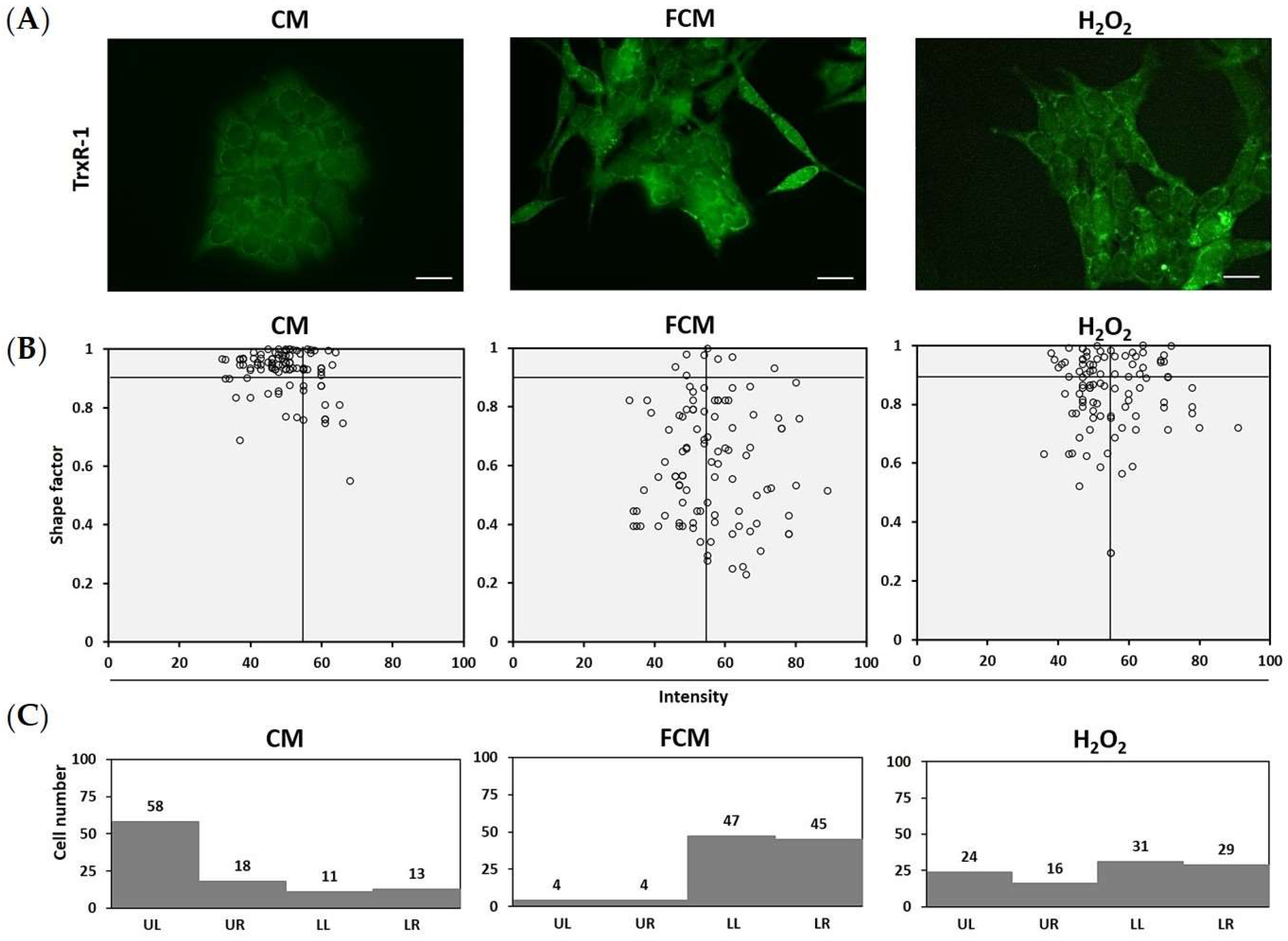
3.4. Fibroblast-Cultured Media Increase HCT116 Oxidative Stress Related to Thioredoxin Reductase-1
3.5. TrxR-1 Relates to Mesenchymal Morphology in Fibroblast-Induced HCT116 Cells
3.6. TrxR-1 Indicates HCT116 Dissemination in the Adult Zebrafish Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Fares, J.; Fares, M.Y.; Khachfe, H.H.; Salhab, H.A.; Fares, Y. Molecular principles of metastasis: A hallmark of cancer revisited. Signal Transduct. Target Ther. 2020, 5, 28. [Google Scholar] [CrossRef]
- Welch, D.R.; Hurst, D.R. Defining the Hallmarks of Metastasis. Cancer Res. 2019, 79, 3011–3027. [Google Scholar] [CrossRef] [PubMed]
- Za’abi, M.A.; Ali, B.H.; ALOthman, Z.A.; Ali, I. Analyses of acute kidney injury biomarkers by ultra-high performance liquid chromatography with mass spectrometry. J. Sep. Sci. 2016, 39, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Lakatos, P.L.; Lakatos, L. Risk for colorectal cancer in ulcerative colitis: Changes, causes and management strategies. World J. Gastroenterol. 2008, 14, 3937–3947. [Google Scholar] [CrossRef]
- Gordon, I.O.; Agrawal, N.; Willis, E.; Goldblum, J.R.; Lopez, R.; Allende, D.; Liu, X.; Patil, D.Y.; Yerian, L.; El-Khider, F.; et al. Fibrosis in ulcerative colitis is directly linked to severity and chronicity of mucosal inflammation. Aliment Pharmacol Ther 2018, 47, 922–939. [Google Scholar] [CrossRef]
- Chandler, C.; Liu, T.; Buckanovich, R.; Coffman, L.G. The double edge sword of fibrosis in cancer. Transl. Res. 2019, 209, 55–67. [Google Scholar] [CrossRef]
- Piersma, B.; Hayward, M.K.; Weaver, V.M. Fibrosis and cancer: A strained relationship. Biochim. Biophys. Acta Rev. Cancer 2020, 1873, 188356. [Google Scholar] [CrossRef]
- Cruz-Bermúdez, A.; Laza-Briviesca, R.; Vicente-Blanco, R.J.; García-Grande, A.; Coronado, M.J.; Laine-Menéndez, S.; Alfaro, C.; Sanchez, J.C.; Franco, F.; Calvo, V.; et al. Cancer-associated fibroblasts modify lung cancer metabolism involving ROS and TGF-β signaling. Free Radic. Biol. Med. 2019, 130, 163–173. [Google Scholar] [CrossRef]
- Martinez-Outschoorn, U.E.; Balliet, R.M.; Rivadeneira, D.B.; Chiavarina, B.; Pavlides, S.; Wang, C.; Whitaker-Menezes, D.; Daumer, K.M.; Lin, Z.; Witkiewicz, A.K.; et al. Oxidative stress in cancer associated fibroblasts drives tumor-stroma co-evolution: A new paradigm for understanding tumor metabolism, the field effect and genomic instability in cancer cells. Cell Cycle 2010, 9, 3256–3276. [Google Scholar] [CrossRef] [PubMed]
- Liou, G.-Y.; Storz, P. Reactive oxygen species in cancer. Free Radic. Res. 2010, 44, 479–496. [Google Scholar] [CrossRef] [PubMed]
- Arnér, E.S.; Holmgren, A. Physiological functions of thioredoxin and thioredoxin reductase. Eur. J. Biochem. 2000, 267, 6102–6109. [Google Scholar] [CrossRef]
- Holmgren, A. Thioredoxin. Annu. Rev. Biochem. 1985, 54, 237–271. [Google Scholar] [CrossRef]
- Arnér, E.S.; Holmgren, A. The thioredoxin system in cancer. Semin. Cancer Biol. 2006, 16, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Raffel, J.; Bhattacharyya, A.K.; Gallegos, A.; Cui, H.; Einspahr, J.G.; Alberts, D.S.; Powis, G. Increased expression of thioredoxin-1 in human colorectal cancer is associated with decreased patient survival. J. Lab. Clin. Med. 2003, 142, 46–51. [Google Scholar] [CrossRef]
- Yoo, M.-H.; Xu, X.-M.; Carlson, B.A.; Patterson, A.D.; Gladyshev, V.N.; Hatfield, D.L. Targeting Thioredoxin Reductase 1 Reduction in Cancer Cells Inhibits Self-Sufficient Growth and DNA Replication. PLoS ONE 2007, 2, e1112. [Google Scholar] [CrossRef]
- Zhuge, W.; Chen, R.; Vladimir, K.; Dong, X.; Zia, K.; Sun, X.; Dai, X.; Bao, M.; Shen, X.; Liang, G. Costunolide specifically binds and inhibits thioredoxin reductase 1 to induce apoptosis in colon cancer. Cancer Lett. 2018, 412, 46–58. [Google Scholar] [CrossRef]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Analysis of Cell Viability by the MTT Assay. Cold Spring Harb. Protoc. 2018, 6, pdb.prot095505. [Google Scholar] [CrossRef]
- Rodriguez, L.G.; Wu, X.; Guan, J.L. Wound-healing assay. Methods Mol. Biol. 2005, 294, 23–29. [Google Scholar]
- Guy, J.B.; Espenel, S.; Vallard, A.; Battiston-Montagne, P.; Wozny, A.S.; Ardail, D.; Alphonse, G.; Rancoule, C.; Rodriguez-Lafrasse, C.; Magne, N. Evaluation of the Cell Invasion and Migration Process: A Comparison of the Video Microscope-based Scratch Wound Assay and the Boyden Chamber Assay. J. Vis. Exp. 2017, 129, e56337. [Google Scholar] [CrossRef] [PubMed]
- Kasioumi, P.; Vrazeli, P.; Vezyraki, P.; Zerikiotis, S.; Katsouras, C.; Damalas, A.; Angelidis, C. Hsp70 (HSP70A1A) downregulation enhances the metastatic ability of cancer cells. Int. J. Oncol. 2018, 54, 821–832. [Google Scholar] [CrossRef] [PubMed]
- Pincus, Z.; Theriot, J.A. Comparison of quantitative methods for cell-shape analysis. J. Microsc. 2007, 227, 140–156. [Google Scholar] [CrossRef] [PubMed]
- Analysis of Cell Morphology. Available online: https://vlab.amrita.edu/?sub=3&brch=278&sim=1465&cnt=6 (accessed on 6 April 2021).
- Aranda, A.; Sequedo, L.; Tolosa, L.; Quintas, G.; Burello, E.; Castell, J.V.; Gombau, L. Dichloro-dihydro-fluorescein diacetate (DCFH-DA) assay: A quantitative method for oxidative stress assessment of nanoparticle-treated cells. Toxicol. In Vitro 2013, 27, 954–963. [Google Scholar] [CrossRef]
- Lee, C. Western blotting. Methods Mol. Biol. 2007, 362, 391–399. [Google Scholar]
- Aleström, P.; D’Angelo, L.; Midtlyng, P.J.; Schorderet, D.F.; Schulte-Merker, S.; Sohm, F.; Warner, S. Zebrafish: Housing and husbandry recommendations. Lab Anim. 2020, 54, 213–224. [Google Scholar] [CrossRef]
- Mendonça-Gomes, J.M.; Valverde, T.M.; Martins, T.M.d.M.; Charlie-Silva, I.; Padovani, B.N.; Fénero, C.M.; da Silva, E.M.; Domingues, R.Z.; Melo-Hoyos, D.C.; Corrêa-Junior, J.D.; et al. Long-term dexamethasone treatment increases the engraftment efficiency of human breast cancer cells in adult zebrafish. Fish Shellfish. Immunol. Rep. 2021, 2, 100007. [Google Scholar] [CrossRef]
- Fan, R.-Y.; Wu, J.-Q.; Liu, Y.-Y.; Liu, X.-Y.; Qian, S.-T.; Li, C.-Y.; Wei, P.; Song, Z.; He, M.-F. Zebrafish xenograft model for studying mechanism and treatment of non-small cell lung cancer brain metastasis. J. Exp. Clin. Cancer Res. 2021, 40, 371. [Google Scholar] [CrossRef]
- Bickel, M. The role of interleukin-8 in inflammation and mechanisms of regulation. J. Periodontol. 1993, 64 (Suppl. S5), 456–460. [Google Scholar]
- Wu, C.-L.; Yin, R.; Wang, S.-N.; Ying, R. A Review of CXCL1 in Cardiac Fibrosis. Front. Cardiovasc. Med. 2021, 8. [Google Scholar] [CrossRef]
- Wu, X.; Tao, P.; Zhou, Q.; Li, J.; Yu, Z.; Wang, X.; Li, J.; Li, C.; Yan, M.; Zhu, Z.; et al. IL-6 secreted by cancer-associated fibroblasts promotes epithelial-mesenchymal transition and metastasis of gastric cancer via JAK2/STAT3 signaling pathway. Oncotarget 2017, 8, 20741–20750. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Liu, J.; Zhang, L.; Xiao, X.; Li, W. Curcumin inhibits H2O2-induced invasion and migration of human pancreatic cancer via suppression of the ERK/NF-κB pathway. Oncol. Rep. 2016, 36, 2245–2251. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Sun, Y.; Hu, X.; Liu, L. Gigantol inhibits proliferation and enhanced oxidative stress-mediated apoptosis through modulating of Wnt/β-catenin signaling pathway in HeLa cells. J. Biochem. Mol. Toxicol. 2022, 36, e22944. [Google Scholar] [CrossRef] [PubMed]
- Murthy, S.; Ryan, A.; He, C.; Mallampalli, R.K.; Carter, A.B. Rac1-mediated mitochondrial H2O2 generation regulates MMP-9 gene expression in macrophages via inhibition of SP-1 and AP-1. J. Biol. Chem. 2010, 285, 25062–25073. [Google Scholar] [CrossRef] [PubMed]
- Chavda, V.; Chaurasia, B.; Garg, K.; Deora, H.; Umana, G.E.; Palmisciano, P.; Scalia, G.; Lu, B. Molecular mechanisms of oxidative stress in stroke and cancer. Brain Disord. 2022, 5, 100029. [Google Scholar] [CrossRef]
- Mohamed, H.T.; El-Ghonaimy, E.A.; El-Shinawi, M.; Hosney, M.; Götte, M.; Woodward, W.A.; El-Mamlouk, T.; Mohamed, M.M. IL-8 and MCP-1/CCL2 regulate proteolytic activity in triple negative inflammatory breast cancer a mechanism that might be modulated by Src and Erk1/2. Toxicol. Appl. Pharmacol. 2020, 401, 115092. [Google Scholar] [CrossRef]
- Blomberg, O.S.; Spagnuolo, L.; de Visser, K.E. Immune regulation of metastasis: Mechanistic insights and therapeutic opportunities. Dis. Model Mech. 2018, 11, dmm036236. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, B.P. Inflammation: A driving force speeds cancer metastasis. Cell Cycle 2009, 8, 3267–3273. [Google Scholar] [CrossRef]
- Powis, G.; Mustacich, D.; Coon, A. The role of the redox protein thioredoxin in cell growth and cancer. Free Radic. Biol. Med. 2000, 29, 312–322. [Google Scholar] [CrossRef]
- Yoon, Y.-S.; Lee, J.-H.; Hwang, S.-C.; Choi, K.S.; Yoon, G. TGF β1 induces prolonged mitochondrial ROS generation through decreased complex IV activity with senescent arrest in Mv1Lu cells. Oncogene 2005, 24, 1895–1903. [Google Scholar] [CrossRef]
- Tsuji, P.A.; Carlson, B.A.; Yoo, M.-H.; Naranjo-Suarez, S.; Xu, X.-M.; He, Y.; Asaki, E.; Seifried, H.E.; Reinhold, W.C.; Davis, C.D.; et al. The 15kDa Selenoprotein and Thioredoxin Reductase 1 Promote Colon Cancer by Different Pathways. PLoS ONE 2015, 10, e0124487. [Google Scholar] [CrossRef] [PubMed]
- Lechner, S.; Müller-Ladner, U.; Neumann, E.; Spöttl, T.; Schlottmann, K.; Rüschoff, J.; Schölmerich, J.; Kullmann, F. Thioredoxin Reductase 1 Expression in Colon Cancer: Discrepancy between In Vitro and In Vivo Findings. Lab. Investig. 2003, 83, 1321–1331. [Google Scholar] [CrossRef] [PubMed][Green Version]

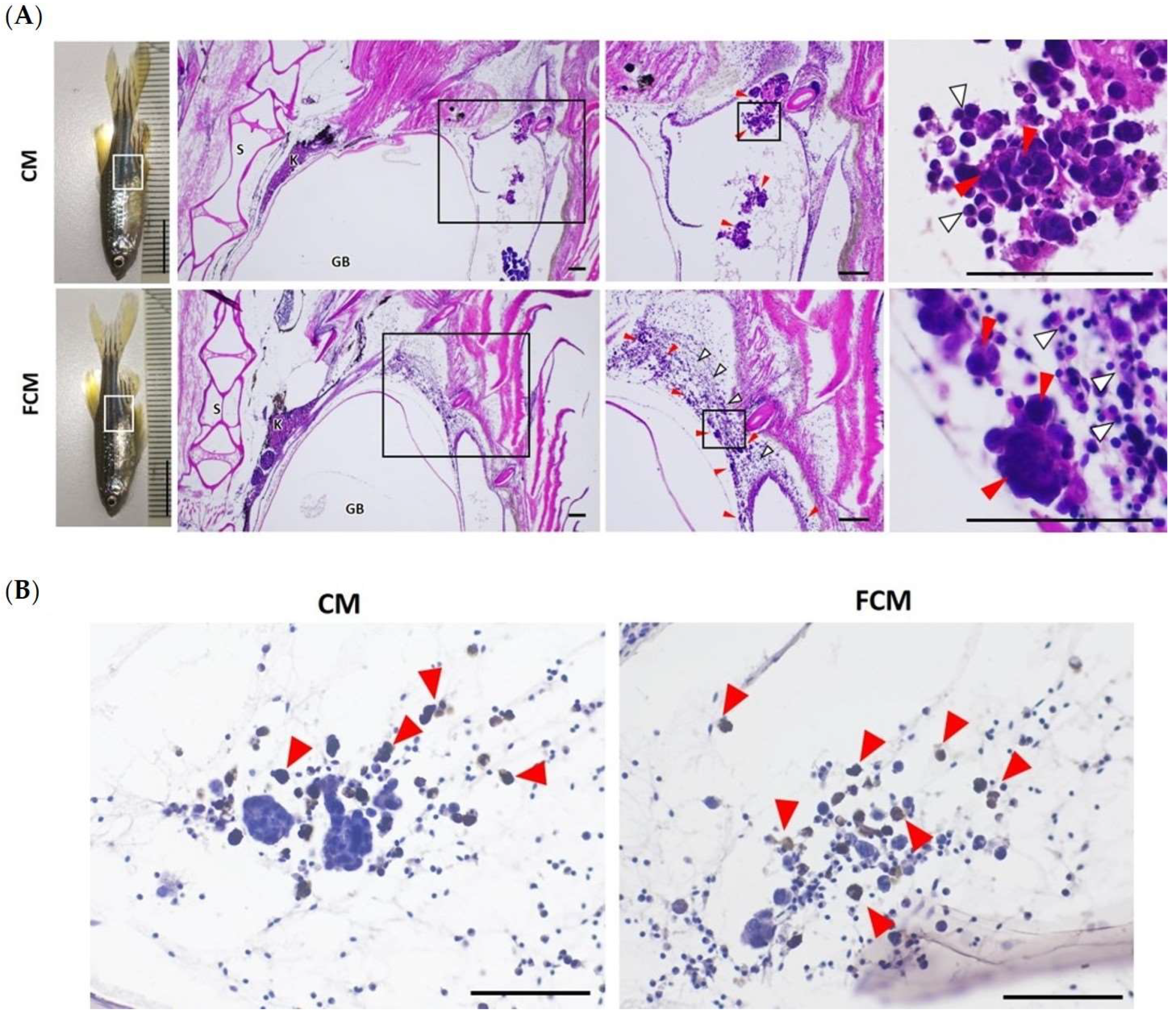
| Number of Microtumor/HPF (n = 6) | Microtumor Size (μm2) | Number of Lymphocyte/HPF | |
|---|---|---|---|
| CM | 4.33 ± 1.51 (3–7) | 2870.35 ± 1197.59 (426.66–9887.63) | 20.83 ± 12.38 (4–40) |
| FCM | 25.00 ± 13.18 * (14–47) | 198.71 ± 100.83 * (30.31–1223.06) | 169.00 ± 56.16 * (73–211) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muangthong, T.; Chusangnin, P.; Hassametto, A.; Tanomrat, R.; Suwannalert, P. Thioredoxin Reductase-1 as a Potential Biomarker in Fibroblast-Associated HCT116 Cancer Cell Progression and Dissemination in a Zebrafish Model. Cancers 2023, 15, 56. https://doi.org/10.3390/cancers15010056
Muangthong T, Chusangnin P, Hassametto A, Tanomrat R, Suwannalert P. Thioredoxin Reductase-1 as a Potential Biomarker in Fibroblast-Associated HCT116 Cancer Cell Progression and Dissemination in a Zebrafish Model. Cancers. 2023; 15(1):56. https://doi.org/10.3390/cancers15010056
Chicago/Turabian StyleMuangthong, Tharathip, Pornnapat Chusangnin, Artchaya Hassametto, Rataya Tanomrat, and Prasit Suwannalert. 2023. "Thioredoxin Reductase-1 as a Potential Biomarker in Fibroblast-Associated HCT116 Cancer Cell Progression and Dissemination in a Zebrafish Model" Cancers 15, no. 1: 56. https://doi.org/10.3390/cancers15010056
APA StyleMuangthong, T., Chusangnin, P., Hassametto, A., Tanomrat, R., & Suwannalert, P. (2023). Thioredoxin Reductase-1 as a Potential Biomarker in Fibroblast-Associated HCT116 Cancer Cell Progression and Dissemination in a Zebrafish Model. Cancers, 15(1), 56. https://doi.org/10.3390/cancers15010056