Physical Exercise and the Hallmarks of Breast Cancer: A Narrative Review
Abstract
Simple Summary
Abstract
1. Introduction
2. The Effects of Physical Exercise on the Hallmarks Associated with Breast Cancer
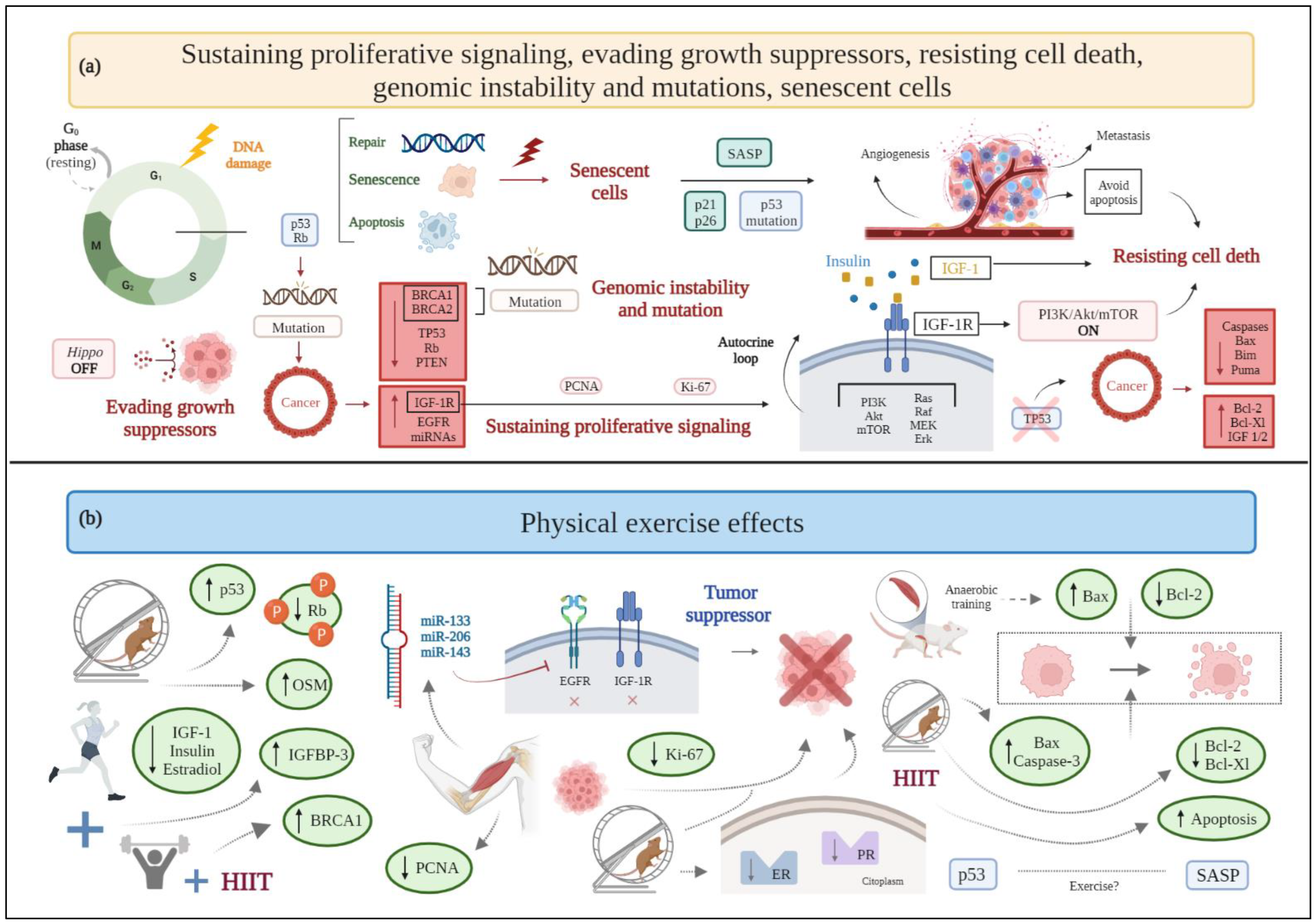
2.1. Sustaining Proliferative Signaling
2.2. Evading Growth Suppressors
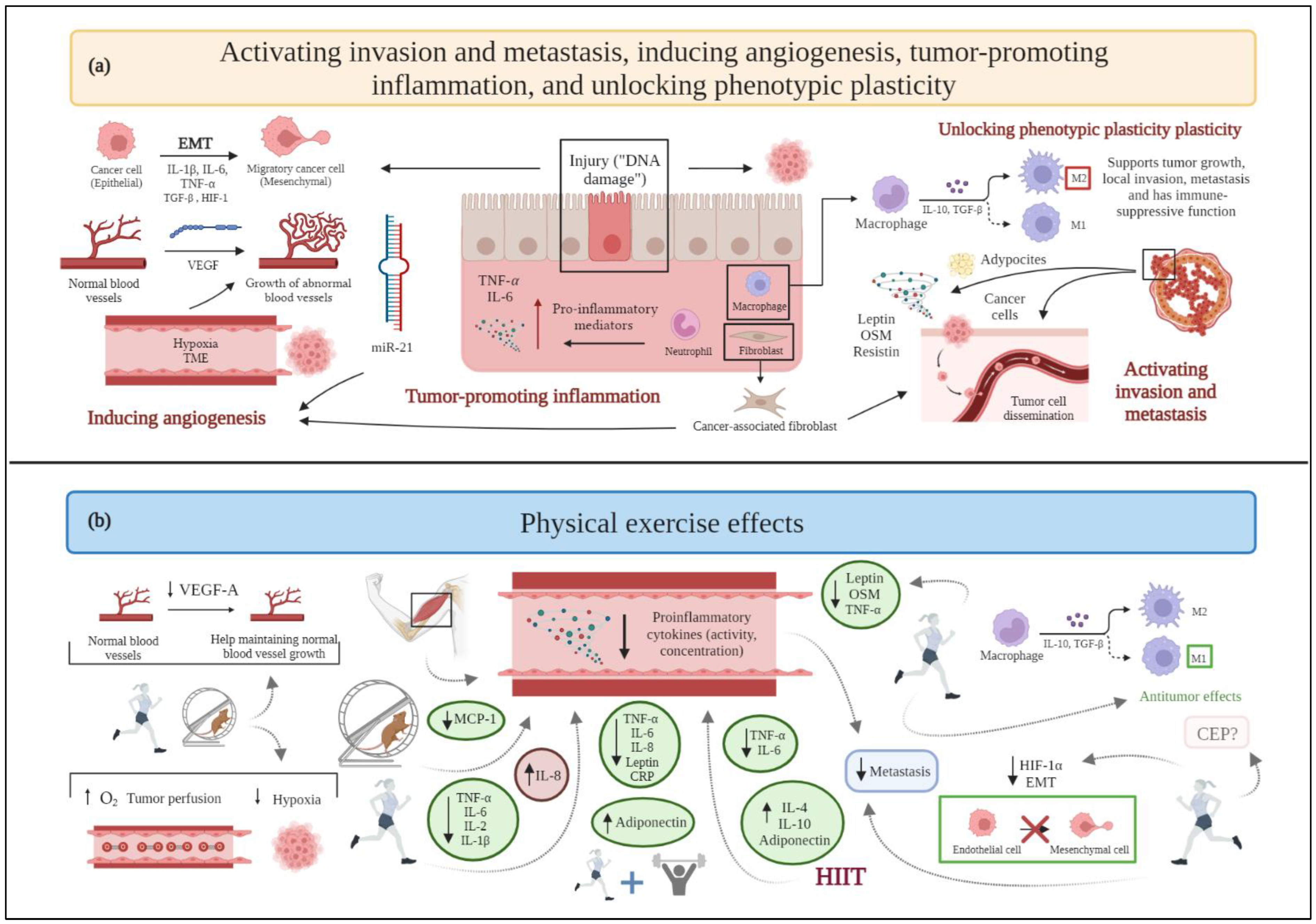
2.3. Activating Invasion and Metastasis
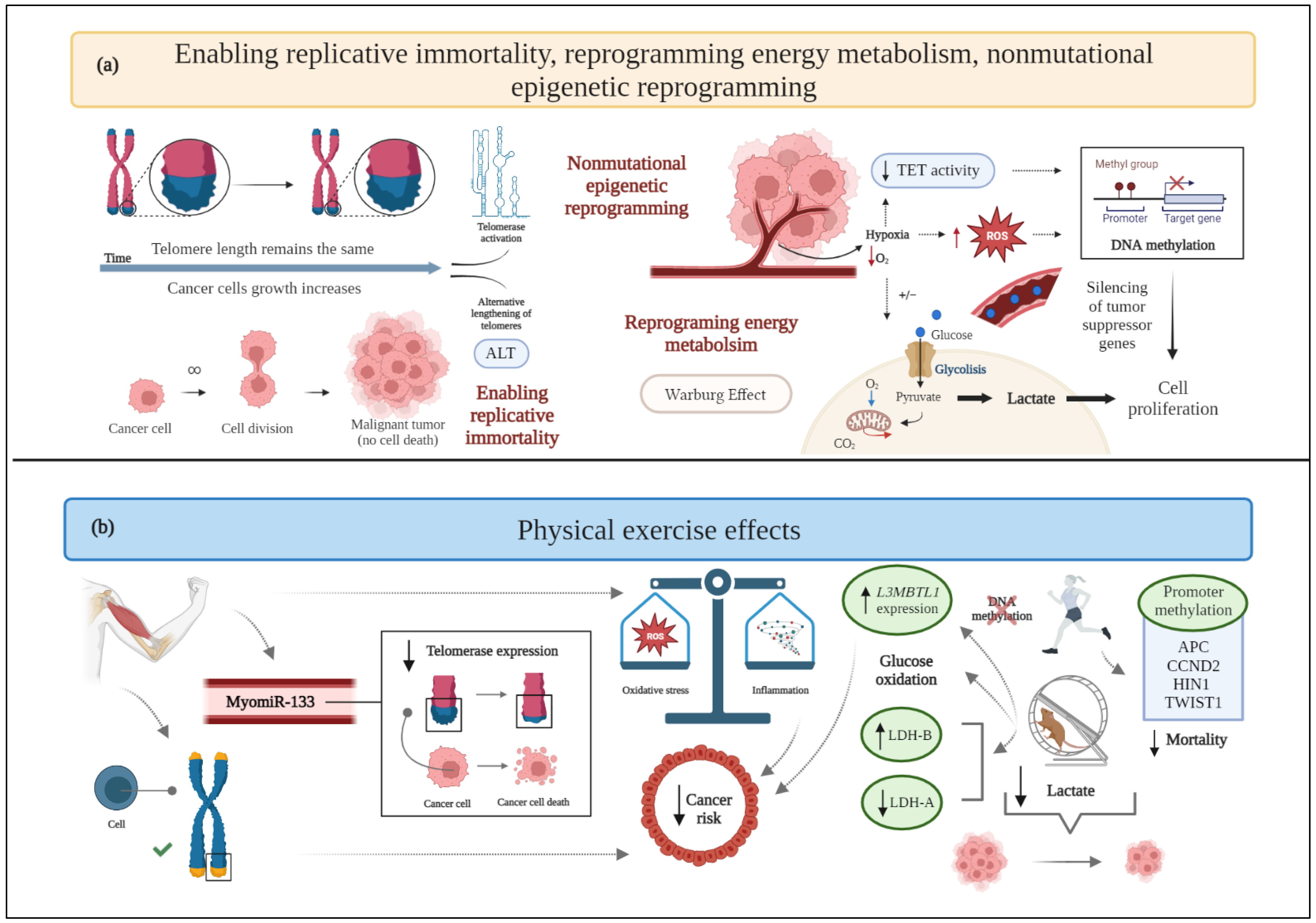
2.4. Enabling Replicative Immortality
2.5. Inducing Angiogenesis
2.6. Resisting Cell Death
2.7. Reprogramming Energy Metabolism
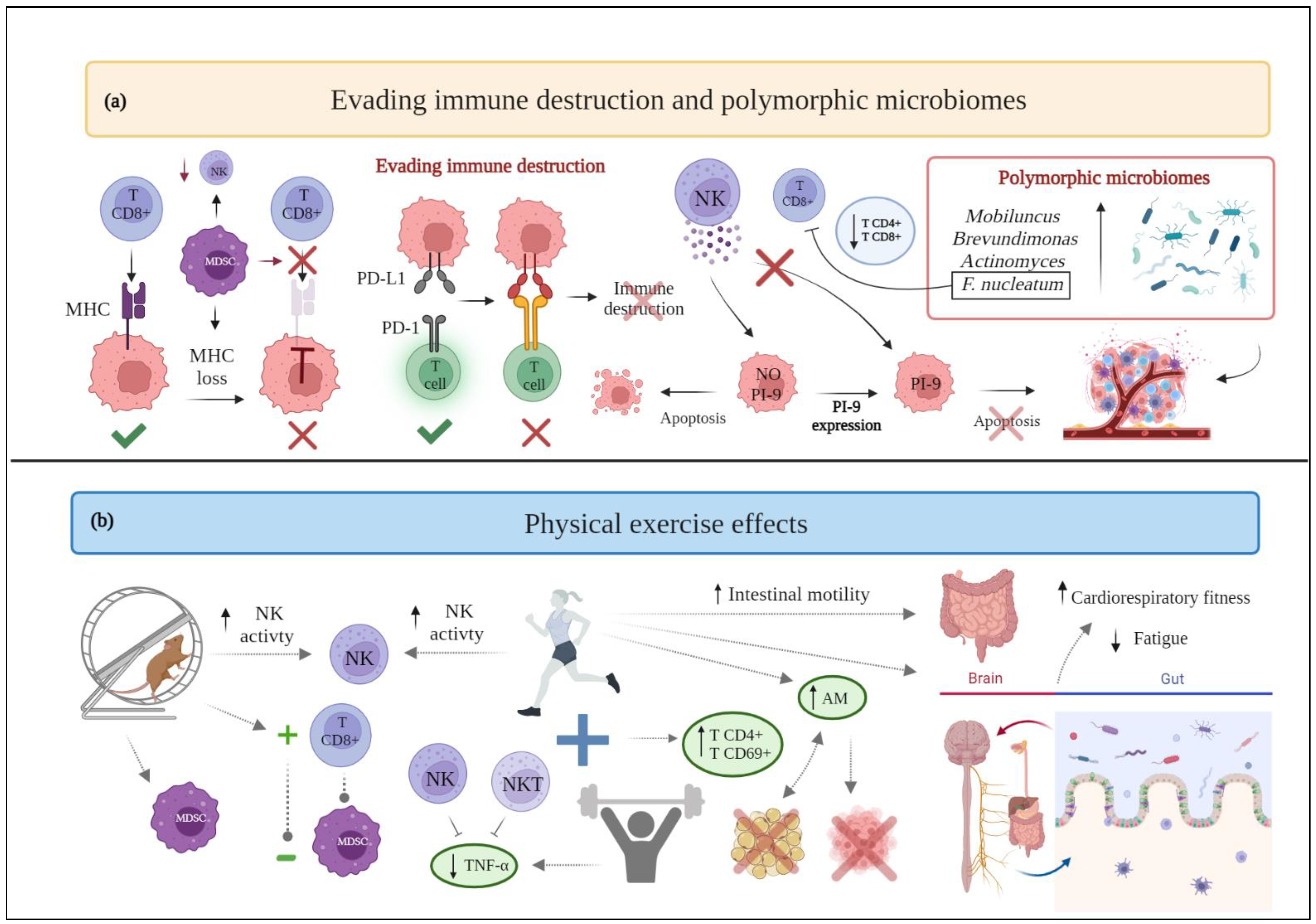
2.8. Evading Immune Destruction
2.9. Genomic Instability and Mutation
2.10. Tumor-Promoting Inflammation
2.11. Unlocking Phenotypic Plasticity
2.12. Nonmutational Epigenetic Reprogramming
2.13. Polymorphic Microbiomes
2.14. Senescent Cells
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Breast Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/breast-cancer (accessed on 19 October 2022).
- Hanahan, D.; Weinberg, R.A. The Hallmarks of Cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Spezia, M.; Huang, S.; Yuan, C.; Zeng, Z.; Zhang, L.; Ji, X.; Liu, W.; Huang, B.; Luo, W.; et al. Breast Cancer Devel-opment and Progression: Risk Factors, Cancer Stem Cells, Signaling Pathways, Genomics, and Molecular Pathogenesis. Genes Dis. 2018, 5, 77–106. [Google Scholar] [CrossRef] [PubMed]
- Ataollahi, M.R.; Sharifi, J.; Paknahad, M.R.; Paknahad, A. Breast Cancer and Associated Factors: A Review. J. Med. Life 2015, 8, 6–11. [Google Scholar] [PubMed]
- Sharma, G.N.; Dave, R.; Sanadya, J.; Sharma, P.; Sharma, K. Various Types and Management of Breast Cancer: An Overview. J. Adv. Pharm. Technol. Res. 2010, 1, 109–126. [Google Scholar]
- Shah, R.; Rosso, K.; David Nathanson, S. Pathogenesis, Prevention, Diagnosis and Treatment of Breast Cancer. World J. Clin. Oncol. 2014, 5, 283–298. [Google Scholar] [CrossRef] [PubMed]
- Momenimovahed, Z.; Salehiniya, H. Epidemiological Characteristics of and Risk Factors for Breast Cancer in the World. Breast Cancer 2019, 11, 151–164. [Google Scholar] [CrossRef]
- Rojas, K.; Stuckey, A. Breast Cancer Epidemiology and Risk Factors. Clin. Obstet. Gynecol. 2016, 59, 651–672. [Google Scholar] [CrossRef]
- Howell, A.; Anderson, A.S.; Clarke, R.B.; Duffy, S.W.; Evans, D.G.; Garcia-Closas, M.; Gescher, A.J.; Key, T.J.; Saxton, J.M.; Harvie, M.N. Risk Determination and Prevention of Breast Cancer. Breast Cancer Res. 2014, 16, 446. [Google Scholar] [CrossRef]
- Abdulkareem, I.H. Aetio-Pathogenesis of Breast Cancer. Niger. Med. J. 2013, 54, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhao, Z.; Yang, Z.; Xu, F.; Lu, H.; Zhu, Z.; Shi, W. Risk Factors and Preventions of Breast Cancer. Int. J. Biol. Sci. 2017, 13, 1387–1397. [Google Scholar] [CrossRef] [PubMed]
- Sauter, E.R. Breast Cancer Prevention: Current Approaches and Future Directions. Eur. J. Breast Health 2018, 14, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Mokhtari-Hesari, P.; Montazeri, A. Health-Related Quality of Life in Breast Cancer Patients: Review of Reviews from 2008 to 2018. Health Qual. Life Outcomes 2020, 18, 1–25. [Google Scholar] [CrossRef]
- Juvet, L.K.; Thune, I.; Elvsaas, I.K.Ø.; Fors, E.A.; Lundgren, S.; Bertheussen, G.; Leivseth, G.; Oldervoll, L.M. The Effect of Exercise on Fatigue and Physical Functioning in Breast Cancer Patients during and after Treatment and at 6 Months Follow-up: A Meta-Analysis. Breast 2017, 33, 166–177. [Google Scholar] [CrossRef] [PubMed]
- Dasso, N.A. How Is Exercise Different from Physical Activity? A Concept Analysis. Nurs. Forum 2019, 54, 45–52. [Google Scholar] [CrossRef]
- Cannioto, R.A.; Hutson, A.; Dighe, S.; McCann, W.; McCann, S.E.; Zirpoli, G.R.; Barlow, W.; Kelly, K.M.; DeNysschen, C.A.; Hershman, D.L.; et al. Physical Activity before, during, and after Chemotherapy for High-Risk Breast Cancer: Relationships with Survival. J. Natl. Cancer Inst. 2021, 113, 54–63. [Google Scholar] [CrossRef]
- Palesh, O.; Kamen, C.; Sharp, S.; Golden, A.; Neri, E.; Spiegel, D.; Koopman, C. Physical Activity and Survival in Women with Advanced Breast Cancer. Cancer Nurs. 2018, 41, E31–E38. [Google Scholar] [CrossRef]
- Cormie, P.; Zopf, E.M.; Zhang, X.; Schmitz, K.H. The Impact of Exercise on Cancer Mortality, Recurrence, and Treatment-Related Adverse Effects. Epidemiol. Rev. 2017, 39, 71–92. [Google Scholar] [CrossRef]
- Irwin, M.L.; Crumley, D.; McTiernan, A.; Bernstein, L.; Baumgartner, R.; Gilliland, F.D.; Kriska, A.; Ballard-Barbash, R. Physical Activity Levels before and after a Diagnosis of Breast Carcinoma: The Health, Eating, Activity, and Lifestyle (HEAL) Study. Cancer 2003, 97, 1746–1757. [Google Scholar] [CrossRef]
- Schmitz, K.H.; Courneya, K.S.; Matthews, C.; Demark-Wahnefried, W.; Galvão, D.A.; Pinto, B.M.; Irwin, M.L.; Wolin, K.Y.; Segal, R.J.; Lucia, A.; et al. American College of Sports Medicine Roundtable on Exercise Guidelines for Cancer Survivors. Med. Sci. Sport. Exerc. 2010, 42, 1409–1426. [Google Scholar] [CrossRef]
- Jones, L.W.; Courneya, K.S.; Mackey, J.R.; Muss, H.B.; Pituskin, E.N.; Scott, J.M.; Hornsby, W.E.; Coan, A.D.; Herndon, J.E.; Douglas, P.S.; et al. Cardiopulmonary Function and Age-Related Decline across the Breast Cancer: Survivorship Continuum. J. Clin. Oncol. 2012, 30, 2530–2537. [Google Scholar] [CrossRef]
- Invernizzi, M.; Lippi, L.; Folli, A.; Turco, A.; Zattoni, L.; Maconi, A.; de Sire, A.; Fusco, N. Integrating molecular biomarkers in breast cancer rehabilitation. What is the current evidence? A systematic review of randomized controlled trials. Front. Mol. Biosci. 2022, 9, 930361. [Google Scholar] [CrossRef]
- Maginador, G.; Lixandrão, M.E.; Bortolozo, H.I.; Vechin, F.C.; Sarian, L.O.; Derchain, S.; Telles, G.D.; Zopf, E.; Ugrinowitsch, C.; Conceição, M.S. Aerobic Exercise-Induced Changes in Cardiorespiratory Fitness in Breast Cancer Patients Receiving Chemotherapy: A Systematic Review and Meta-Analysis. Cancers 2020, 12, 2240. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.-Y.; Yao, S.-S.; Shi, Y.-Y.; Lu, N.-N.; Cheng, F. Effect of Aerobic Exercise on Cardiotoxic Outcomes in Women with Breast Cancer Undergoing Anthracycline or Trastuzumab Treatment: A Systematic Review and Meta-Analysis. Support. Care Cancer 2022, 30, 10323–10334. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Casado, A.; Martín-Ruiz, A.; Pérez, L.M.; Provencio, M.; Fiuza-Luces, C.; Lucia, A. Exercise and the Hallmarks of Cancer. Trends Cancer 2017, 3, 423–441. [Google Scholar] [CrossRef] [PubMed]
- Koedoot, E.; Wolters, L.; van de Water, B.; le Dévédec, S.E. Splicing Regulatory Factors in Breast Cancer Hallmarks and Disease Progression. Oncotarget 2019, 10, 6021–6037. [Google Scholar] [CrossRef]
- Dai, X.; Xiang, L.; Li, T.; Bai, Z. Cancer Hallmarks, Biomarkers and Breast Cancer Molecular Subtypes. J. Cancer 2016, 7, 1281–1294. [Google Scholar] [CrossRef]
- Donepudi, M.S.; Kondapalli, K.; Amos, S.J.; Venkanteshan, P. Breast Cancer Statistics and Markers. J. Cancer Res. Ther. 2014, 10, 506–511. [Google Scholar]
- Tian, J.M.; Ran, B.; Zhang, C.L.; Yan, D.M.; Li, X.H. Estrogen and Progesterone Promote Breast Cancer Cell Proliferation by Inducing Cyclin G1 Expression. Braz. J. Med. Biol. Res. 2018, 51, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Goh, J.; Tsai, J.; Bammler, T.K.; Farin, F.M.; Endicott, E.; Ladiges, W.C. Exercise Training in Transgenic Mice Is Associated with Attenuation of Early Breast Cancer Growth in a Dose-Dependent Manner. PLoS ONE 2013, 8, e80123. [Google Scholar] [CrossRef]
- Malicka, I.; Siewierska, K.; Pula, B.; Kobierzycki, C.; Haus, D.; Paslawska, U.; Cegielski, M.; Dziegiel, P.; Podhorska-Okolow, M.; Wozniewski, M. The Effect of Physical Training on the N-Methyl-N-Nitrosourea-Induced Mammary Carcinogenesis of Sprague–Dawley Rats. Exp. Biol. Med. 2015, 240, 1408–1415. [Google Scholar] [CrossRef] [PubMed]
- Siewierska, K.; Malicka, I.; Kobierzycki, C.; Paslawska, U.; Cegielski, M.; Grzegrzolka, J.; Piotrowska, A.; Podhorska-Okolow, M.; Dziegiel, P.; Wozniewski, M. The Impact of Exercise Training on Breast Cancer. Vivo 2018, 32, 249–254. [Google Scholar]
- Kim, M.K.; Kim, Y.; Park, S.H.; Kim, E.; Kim, Y.; Kim, Y.; Kim, J.H. Effects of Steady Low-Intensity Exercise on High-Fat Diet Stimulated Breast Cancer Progression Via the Alteration of Macrophage Polarization. Integr. Cancer 2020, 19, 1534735420949678. [Google Scholar] [CrossRef] [PubMed]
- Siewierska, K.; Malicka, I.; Kobierzycki, C.; Grzegrzolka, J.; Piotrowska, A.; Paslawska, U.; Cegielski, M.; Podhorska-Okolow, M.; Dziegiel, P.; Wozniewski, M. Effect of Physical Training on the Levels of Sex Hormones and the Expression of Their Receptors in Rats with Induced Mammary Cancer in Secondary Prevention Model–Preliminary Study. Vivo 2020, 34, 495–501. [Google Scholar] [CrossRef]
- de Lima, C.; Alves, L.; Iagher, F.; Machado, A.F.; Kryczyk, M.; Yamazaki, R.K.; Brito, G.A.P.; Nunes, E.A.; Naliwaiko, K.; Fernandes, L.C. Tumor Growth Reduction in Walker 256 Tumorbearing Rats Performing Anaerobic Exercise: Participation of Bcl-2, Bax, Apoptosis, and Peroxidation. Appl. Physiol. Nutr. Metab. 2011, 36, 533–538. [Google Scholar] [CrossRef]
- Dieli-Conwright, C.M.; Courneya, K.S.; Demark-Wahnefried, W.; Sami, N.; Lee, K.; Buchanan, T.A.; Spicer, D.; Tripathy, D.; Bernstein, L.; Mortimer, J.E. Effects of Aerobic and Resistance Exercise on Metabolic Syndrome, Sarcopenic Obesity, and Circulating Biomarkers in Overweight or Obese Survivors of Breast Cancer: A Randomized Controlled Trial. J. Clin. Oncol. 2018, 36, 875–883. [Google Scholar] [CrossRef] [PubMed]
- Rigiracciolo, D.C.; Nohata, N.; Lappano, R.; Cirillo, F.; Talia, M.; Scordamaglia, D.; Gutkind, J.S.; Maggiolini, M. IGF-1/IGF-1R/FAK/YAP Transduction Signaling Prompts Growth E Ff Ects in Triple-Negative Breast Cancer (TNBC) Cells. Cells 2020, 9, 1010. [Google Scholar] [CrossRef]
- Ligibel, J.A.; Campbell, N.; Partridge, A.; Chen, W.Y.; Salinardi, T.; Chen, H.; Adloff, K.; Keshaviah, A.; Winer, E.P. Impact of a Mixed Strength and Endurance Exercise Intervention on Insulin Levels in Breast Cancer Survivors. J. Clin. Oncol. 2008, 26, 907–912. [Google Scholar] [CrossRef]
- Irwin, M.L.; Varma, K.; Alvarez-Reeves, M.; Cadmus, L.; Wiley, A.; Chung, G.G.; DiPietro, L.; Mayne, S.T.; Yu, H. Randomized Controlled Trial of Aerobic Exercise on Insulin and Insulin-like Growth Factors in Breast Cancer Survivors: The Yale Exercise and Survivorship Study. Cancer Epidemiol. Biomark. Prev. 2009, 18, 306–313. [Google Scholar] [CrossRef]
- Juríková, M.; Danihel, Ľ.; Polák, Š.; Varga, I. Ki67, PCNA, and MCM Proteins: Markers of Proliferation in the Diagnosis of Breast Cancer. Acta Histochem. 2016, 118, 544–552. [Google Scholar] [CrossRef]
- Dela Cruz, M.A.; Roy, P.; Chowdhury, S.; Chan, S.; Roy, H.K. Abstract P3-07-18: Exercise and Triple Negative Breast Cancer: Unravelling the Anti-Neoplastic Molecular Factors through Novel Culture Method. Cancer Res. 2017, 77, P3-07-18. [Google Scholar] [CrossRef]
- Wang, L.H.; Wu, C.F.; Rajasekaran, N.; Shin, Y.K. Loss of Tumor Suppressor Gene Function in Human Cancer: An Overview. Cell Physiol. Biochem. 2019, 51, 2647–2693. [Google Scholar] [CrossRef] [PubMed]
- Amin, A.R.M.R.; Karpowicz, P.A.; Carey, T.E.; Arbiser, J.; Nahta, R.; Chen, Z.G.; Dong, J.T.; Kucuk, O.; Khan, G.N.; Huang, G.S.; et al. Evasion of Anti-Growth Signaling: A Key Step in Tumorigenesis and Potential Target for Treatment and Prophylaxis by Natural Compounds. Semin. Cancer Biol. 2015, 35, S55–S77. [Google Scholar] [CrossRef] [PubMed]
- Tung, N.M.; Garber, J.E. BRCA1/2 Testing: Therapeutic Implications for Breast Cancer Management. Br. J. Cancer 2018, 119, 141–152. [Google Scholar] [CrossRef]
- Papadopetraki, A.; Maridaki, M.; Zagouri, F.; Dimopoulos, M.A.; Koutsilieris, M.; Philippou, A. Physical Exercise Restrains Cancer Progression through Muscle-Derived Factors. Cancers 2022, 14, 1892. [Google Scholar] [CrossRef]
- Loh, H.Y.; Norman, B.P.; Lai, K.S.; Rahman, N.M.A.N.A.; Alitheen, N.B.M.; Osman, M.A. The Regulatory Role of MicroRNAs in Breast Cancer. Int. J. Mol. Sci. 2019, 20, 4940. [Google Scholar] [CrossRef] [PubMed]
- Agostini, D.; Natalucci, V.; Baldelli, G.; de Santi, M.; Zeppa, S.D.; Vallorani, L.; Annibalini, G.; Lucertini, F.; Federici, A.; Izzo, R.; et al. New Insights into the Role of Exercise in Inhibiting MTOR Signaling in Triple-Negative Breast Cancer. Oxidative Med. Cell. Longev. 2018, 2018, 5896786. [Google Scholar] [CrossRef]
- Cai, Q.; Dozmorov, M.; Oh, Y. IGFBP-3/IGFBP-3 Receptor System as an Anti-Tumor and Anti-Metastatic Signaling in Cancer. Cells 2020, 9, 1261. [Google Scholar] [CrossRef]
- Minami, A.; Murai, T.; Nakanishi, A.; Kitagishi, Y.; Ichimura, M.; Matsuda, S. Cell Cycle Regulation via the P53, PTEN, and BRCA1 Tumor Suppressors. In New Aspects in Molecular and Cellular Mechanisms of Human Carcinogenesis; IntechOpen: London, UK, 2016. [Google Scholar]
- Biello, F.; Platini, F.; D’avanzo, F.; Cattrini, C.; Mennitto, A.; Genestroni, S.; Martini, V.; Marzullo, P.; Aimaretti, G.; Gennari, A. Insulin/IGF Axis in Breast Cancer: Clinical Evidence and Translational Insights. Biomolecules 2021, 11, 125. [Google Scholar] [CrossRef]
- Ianza, A.; Sirico, M.; Bernocchi, O.; Generali, D. Role of the IGF-1 Axis in Overcoming Resistance in Breast Cancer. Front. Cell Dev. Biol. 2021, 9, 41449. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Yin, Z.; Tao, K.; Wang, G.; Gao, J. Function of Insulin-like Growth Factor 1 Receptor in Cancer Resistance to Chemotherapy. Oncol. Lett. 2018, 15, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Vulczak, A.; Souza, A.D.O.; Ferrari, G.D.; Azzolini, A.E.C.S.; Pereira-Da-Silva, G.; Alberici, L.C. Moderate Exercise Modulates Tumor Metabolism of Triple-Negative Breast Cancer. Cells 2020, 9, 628. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Jiang, W.; Sells, J.L.; Neil, E.S.; Mcginley, J.N.; Thompson, J. Effect of Nonmotorized Wheel Running on Mammary Carcinogenesis: Circulating Biomarkers, Cellular Processes, and Molecular Mechanisms in Rats. Cancer Epidemiol. Biomark. Prev. 2009, 17, 1920–1929. [Google Scholar] [CrossRef]
- Alizadeh, S.; Isanejad, A.; Sadighi, S.; Khalighfard, S.; Alizadeh, A.M. Effect of a High-Intensity Interval Training on Serum MicroRNA Levels in Women with Breast Cancer Undergoing Hormone Therapy. A Single-Blind Randomized Trial. Ann. Phys. Rehabil. Med. 2019, 62, 329–335. [Google Scholar] [CrossRef]
- Hojman, P.; Dethlefsen, C.; Brandt, C.; Hansen, J.; Pedersen, L.; Pedersen, B.K. Exercise-Induced Muscle-Derived Cytokines Inhibit Mammary Cancer Cell Growth. Am. J. Physiol. Endocrinol. Metab. 2011, 301, 504–510. [Google Scholar] [CrossRef]
- Zhang, D.; Tan, X.; Tang, N.; Huang, F.; Chen, Z.; Shi, G. Review of Research on the Role of Irisin in Tumors. OncoTargets Ther. 2020, 13, 4423–4430. [Google Scholar] [CrossRef]
- Gannon, N.P.; Vaughan, R.A.; Garcia-Smith, R.; Bisoffi, M.; Trujillo, K.A. Effects of the Exercise-Inducible Myokine Irisin on Malignant and Non-Malignant Breast Epithelial Cell Behavior in Vitro. Int. J. Cancer 2015, 136, E197–E202. [Google Scholar] [CrossRef]
- Dethlefsen, C.; Hansen, L.S.; Lillelund, C.; Andersen, C.; Gehl, J.; Christensen, J.F.; Pedersen, B.K.; Hojman, P. Exercise-Induced Catecholamines Activate the Hippo Tumor Suppressor Pathway to Reduce Risks of Breast Cancer Development. Cancer Res. 2017, 77, 4894–4904. [Google Scholar]
- Mittal, S.; Brown, N.J.; Holen, I. The Breast Tumor Microenvironment: Role in Cancer Development, Progression and Response to Therapy. Expert Rev. Mol. Diagn. 2018, 18, 227–243. [Google Scholar] [CrossRef]
- Anderson, N.M.; Simon, M.C. The Tumor Microenvironment. Curr. Biol. 2020, 30, R921–R925. [Google Scholar] [CrossRef]
- Goh, J.; Kirk, E.A.; Lee, S.X.; Ladiges, W.C. Exercise, Physical Activity and Breast Cancer: The Role of Tumor-Associated Macrophages. Eexrc. Immunol. Rev. 2012, 18, 158–176. [Google Scholar]
- Samuel, S.M.; Varghese, E.; Varghese, S.; Büsselberg, D. Challenges and Perspectives in the Treatment of Diabetes Associated Breast Cancer. Cancer Treat. Rev. 2018, 70, 98–111. [Google Scholar] [CrossRef] [PubMed]
- Vona-Davis, L.; Rose, D.P. Angiogenesis, Adipokines and Breast Cancer. Cytokine Growth Factor Rev. 2009, 20, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Christodoulatos, G.S.; Spyrou, N.; Kadillari, J.; Psallida, S.; Dalamaga, M. The Role of Adipokines in Breast Cancer: Current Evidence and Perspectives. Curr. Obes. Rep. 2019, 8, 413–433. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.J.; Semenza, G.L.; Zhang, H. feng Hypoxia-Inducible Factor 1 and Breast Cancer Metastasis. J. Zhejiang Univ. Sci. B 2015, 16, 32–43. [Google Scholar] [CrossRef]
- Masuda, H.; Zhang, D.; Bartholomeusz, C.; Doihara, H.; Hortobagyi, G.N.; Ueno, N.T. Role of Epidermal Growth Factor Receptor in Breast Cancer. Breast Cancer Res. Treat. 2012, 136, 331–345. [Google Scholar] [CrossRef]
- Avtanski, D.; Garcia, A.; Caraballo, B.; Thangeswaran, P.; Marin, S.; Bianco, J.; Lavi, A.; Poretsky, L. Resistin Induces Breast Cancer Cells Epithelial to Mesenchymal Transition (EMT) and Stemness through Both Adenylyl Cyclase-Associated Protein 1 (CAP1)-Dependent and CAP1-Independent Mechanisms. Cytokine 2019, 120, 155–164. [Google Scholar] [CrossRef]
- Colak, S.; ten Dijke, P. Targeting TGF-β Signaling in Cancer. Trends Cancer 2017, 3, 56–71. [Google Scholar] [CrossRef]
- van Doorslaer de Ten Ryen, S.; Deldicque, L. The Regulation of the Metastatic Cascade by Physical Activity: A Narrative Review. Cancers 2020, 12, 153. [Google Scholar] [CrossRef]
- Alvarado, A.; Gil da Costa, R.M.; Faustino-Rocha, A.I.; Ferreira, R.; Lopes, C.; Oliveira, P.A.; Colaço, B. Effects of Exercise Training on Breast Cancer Metastasis in a Rat Model. Int. J. Exp. Pathol. 2017, 98, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Smeda, M.; Przyborowski, K.; Proniewski, B.; Zakrzewska, A.; Kaczor, D.; Stojak, M.; Buczek, E.; Nieckarz, Z.; Zoladz, J.A.; Wietrzyk, J.; et al. Breast Cancer Pulmonary Metastasis Is Increased in Mice Undertaking Spontaneous Physical Training in the Running Wheel; a Call for Revising Beneficial Effects of Exercise on Cancer Progression. Am. J. Cancer Res. 2017, 7, 1926–1936. [Google Scholar]
- Jones, L.W.; Fels, D.R.; West, M.; Allen, J.D.; Broadwater, G.; Barry, W.T.; Wilke, L.G.; Masko, E.; Douglas, P.S.; Dash, R.C.; et al. Modulation of Circulating Angiogenic Factors and Tumor Biology by Aerobic Training in Breast Cancer Patients Receiving Neoadjuvant Chemotherapy. Cancer Prev. Res. 2013, 6, 925–937. [Google Scholar] [CrossRef]
- Provatopoulou, X.; Georgiou, G.P.; Kalogera, E.; Kalles, V.; Matiatou, M.A.; Papapanagiotou, I.; Sagkriotis, A.; Zografos, G.C.; Gounaris, A. Serum Irisin Levels Are Lower in Patients with Breast Cancer: Association with Disease Diagnosis and Tumor Characteristics. BMC Cancer 2015, 15, 898. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.P.; Zhang, X.F.; Li, H.; Liu, T.J.; Zhao, Q.P.; Huang, L.H.; Cao, Z.J.; He, L.M.; Hao, D.J. Serum Irisin Associates with Breast Cancer to Spinal Metastasis. Medicine 2018, 97, e0524. [Google Scholar] [CrossRef]
- Saxena, N.K.; Sharma, D. Metastasis Suppression by Adiponectin: LKB1 Rises up to the Challenge. Cell Adhes. Migr. 2010, 4, 358–362. [Google Scholar] [CrossRef] [PubMed]
- Ray, A.; Cleary, M.P. The Potential Role of Leptin in Tumor Invasion and Metastasis. Cytokine Growth Factor Rev. 2017, 38, 80–97. [Google Scholar] [CrossRef]
- Ham, B.; Fernandez, M.C.; D’costa, Z.; Brodt, P. The Diverse Roles of the TNF Axis in Cancer Progression and Metastasis. Trends Cancer Res. 2016, 11, 1–27. [Google Scholar] [PubMed]
- Lindholm, M.E.; Rundqvist, H. Skeletal Muscle Hypoxia-Inducible Factor-1 and Exercise. Exp. Physiol. 2016, 101, 28–32. [Google Scholar] [CrossRef]
- Boimel, P.J.; Smirnova, T.; Ni Zhou, Z.; Wyckoff, J.; Park, H.; Coniglio, S.J.; Qian, B.-Z.; Richard Stanley, E.; Cox, D.; Pollard, J.W.; et al. Contribution of CXCL12 Secretion to Invasion of Breast Cancer Cells. Breast Cancer Res. 2012, 14, R23. [Google Scholar] [CrossRef]
- Okamoto, K.; Seimiya, H. Revisiting Telomere Shortening in Cancer. Cells 2019, 8, 107. [Google Scholar] [CrossRef] [PubMed]
- Arsenis, N.C.; You, T.; Ogawa, E.F.; Tinsley, G.M.; Zuo, L. Physical Activity and Telomere Length: Impact of Aging and Potential Mechanisms of Action. Oncotarget 2017, 8, 45008–45019. [Google Scholar] [CrossRef] [PubMed]
- Gillman, A.S.; Helmuth, T.; Koljack, C.E.; Hutchison, K.E.; Kohrt, W.M.; Bryan, A.D. The Effects of Exercise Duration and Intensity on Breast Cancer-Related Dna Methylation: A Randomized Controlled Trial. Cancers 2021, 13, 4128. [Google Scholar] [CrossRef] [PubMed]
- Grazioli, E.; Dimauro, I.; Mercatelli, N.; Wang, G.; Pitsiladis, Y.; di Luigi, L.; Caporossi, D. Physical Activity in the Prevention of Human Diseases: Role of Epigenetic Modifications. BMC Genom. 2017, 18, 802. [Google Scholar] [CrossRef]
- Mandujano-Tinoco, E.A.; García-Venzor, A.; Melendez-Zajgla, J.; Maldonado, V. New Emerging Roles of MicroRNAs in Breast Cancer. Breast Cancer Res. Treat. 2018, 171, 247–259. [Google Scholar] [CrossRef]
- Hrdličková, R.; Nehyba, J.; Bargmann, W.; Bose, H.R. Multiple Tumor Suppressor MicroRNAs Regulate Telomerase and TCF7, an Important Transcriptional Regulator of the Wnt Pathway. PLoS ONE 2014, 9, e86990. [Google Scholar] [CrossRef]
- Sanft, T.; Usiskin, I.; Harrigan, M.; Cartmel, B.; Lu, L.; Li, F.Y.; Zhou, Y.; Chagpar, A.; Ferrucci, L.M.; Pusztai, L.; et al. Randomized Controlled Trial of Weight Loss versus Usual Care on Telomere Length in Women with Breast Cancer: The Lifestyle, Exercise, and Nutrition (LEAN) Study. Breast Cancer Res. Treat. 2018, 172, 105–112. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, H.; Wang, M.; Schmid, T.; Xin, Z.; Kozhuharova, L.; Yu, W.K.; Huang, Y.; Cai, F.; Biskup, E. Hypoxia in Breast Cancer—Scientific Translation to Therapeutic and Diagnostic Clinical Applications. Front. Oncol. 2021, 11, 652266. [Google Scholar] [CrossRef]
- Madu, C.O.; Wang, S.; Madu, C.O.; Lu, Y. Angiogenesis in Breast Cancer Progression, Diagnosis, and Treatment. J. Cancer 2020, 11, 4474–4494. [Google Scholar] [CrossRef]
- Nenclares, P.; Harrington, K.J. The Biology of Cancer. Medicine 2020, 48, 67–72. [Google Scholar] [CrossRef]
- Emami Nejad, A.; Najafgholian, S.; Rostami, A.; Sistani, A.; Shojaeifar, S.; Esparvarinha, M.; Nedaeinia, R.; Haghjooy Javanmard, S.; Taherian, M.; Ahmadlou, M.; et al. The Role of Hypoxia in the Tumor Microenvironment and Development of Cancer Stem Cell: A Novel Approach to Developing Treatment. Cancer Cell Int. 2021, 21, 62. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Croce, C.M. The Role of MicroRNAs in Human Cancer. Signal Transduct. Target. Ther. 2016, 1, 15004. [Google Scholar] [CrossRef] [PubMed]
- Nagy, J.A.; Chang, S.H.; Dvorak, A.M.; Dvorak, H.F. Why Are Tumour Blood Vessels Abnormal and Why Is It Important to Know? Br. J. Cancer 2009, 100, 865–869. [Google Scholar] [CrossRef] [PubMed]
- Betof, A.S.; Lascola, C.D.; Weitzel, D.; Landon, C.; Scarbrough, P.M.; Devi, G.R.; Palmer, G.; Jones, L.W.; Dewhirst, M.W. Modulation of Murine Breast Tumor Vascularity, Hypoxia, and Chemotherapeutic Response by Exercise. J. Natl. Cancer Inst. 2015, 107, djv40. [Google Scholar] [CrossRef]
- Jones, L.W.; Viglianti, B.L.; Tashjian, J.A.; Kothadia, S.M.; Keir, S.T.; Freedland, S.J.; Potter, M.Q.; Moon, J.; Schroeder, T.; Herndon, J.E.; et al. Effect of Aerobic Exercise on Tumor Physiology in an Animal Model of Human Breast Cancer. J. Appl. Physiol. 2010, 108, 343–348. [Google Scholar] [CrossRef]
- Scott, J.M.; Jones, L.W.; Hornsby, W.E.; Koelwyn, G.J.; Khouri, M.G.; Joy, A.A.; Douglas, P.S.; Lakoski, S.G. Cancer Therapy-Induced Autonomic Dysfunction in Early Breast Cancer: Implications for Aerobic Exercise Training. Int. J. Cardiol. 2014, 171, e50-1. [Google Scholar] [CrossRef]
- Magnussen, A.L.; Mills, I.G. Vascular Normalisation as the Stepping Stone into Tumour Microenvironment Transformation. Br. J. Cancer 2021, 125, 324–336. [Google Scholar] [CrossRef]
- Amani Shalamzari, S.; Agha-Alinejad, H.; Alizadeh, S.; Shahbazi, S.; Kashani Khatib, Z.; Kazemi, A.; Saei, M.A.; Minayi, N. The Effect of Exercise Training on the Level of Tissue IL-6 and Vascular Endothelial Growth Factor in Breast Cancer Bearing Mice. Iran J. Basic Med. Sci. 2014, 17, 231–236. [Google Scholar]
- Faustino-Rocha, A.I.; Silva, A.; Gabriel, J.; Gil da Costa, R.M.; Moutinho, M.; Oliveira, P.A.; Gama, A.; Ferreira, R.; Ginja, M. Long-Term Exercise Training as a Modulator of Mammary Cancer Vascularization. Biomed. Pharmacother. 2016, 81, 273–280. [Google Scholar] [CrossRef]
- Pakiz, B.; Flatt, S.W.; Bardwell, W.A.; Rock, C.L.; Mills, P.J. Effects of a Weight Loss Intervention on Body Mass, Fitness, and Inflammatory Biomarkers in Overweight or Obese Breast Cancer Survivors. Int. J. Behav. Med. 2011, 18, 333–341. [Google Scholar] [CrossRef]
- Ergun, M.; Eyigor, S.; Karaca, B.; Kisim, A.; Uslu, R. Effects of Exercise on Angiogenesis and Apoptosis-Related Molecules, Quality of Life, Fatigue and Depression in Breast Cancer Patients. Eur. J. Cancer Care 2013, 22, 626–637. [Google Scholar] [CrossRef]
- Jan, R.; Chaudhry, G.E. Understanding Apoptosis and Apoptotic Pathways Targeted Cancer Therapeutics. J. Cardiovasc. Thorac. Res. 2019, 9, 205–218. [Google Scholar] [CrossRef]
- Wong, R.S. Apoptosis in Cancer: From Pathogenesis to Treatment. J. Exp. Clin. Cancer Res. 2011, 30, 87. [Google Scholar] [CrossRef]
- Goldar, S.; Khaniani, M.S.; Derakhshan, S.M.; Baradaran, B. Molecular Mechanisms of Apoptosis and Roles in Cancer Development and Treatment. Asian Pac. J. Cancer Prev. 2015, 16, 2129–2144. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, A.M.; Heydari, Z.; Rahimi, M.; Bazgir, B.; Shirvani, H.; Alipour, S.; Heidarian, Y.; Khalighfard, S.; Isanejad, A. Oxytocin Mediates the Beneficial Effects of the Exercise Training on Breast Cancer. Exp. Physiol. 2018, 103, 222–235. [Google Scholar] [CrossRef] [PubMed]
- Liberti, M.V.; Locasale, J.W. The Warburg Effect: How Does It Benefit Cancer Cells? Trends Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef]
- Kroemer, G.; Pouyssegur, J. Tumor Cell Metabolism: Cancer’s Achilles’ Heel. Cancer Cell 2008, 13, 472–482. [Google Scholar] [CrossRef] [PubMed]
- Fernández, L.P.; Gómez de Cedrón, M.; Ramírez de Molina, A. Alterations of Lipid Metabolism in Cancer: Implications in Prognosis and Treatment. Front. Oncol. 2020, 10, 577420. [Google Scholar]
- Broadfield, L.A.; Pane, A.A.; Talebi, A.; Swinnen, J.V.; Fendt, S.-M. Lipid Metabolism in Cancer: New Perspectives and Emerging Mechanisms. Dev. Cell 2021, 56, 1363–1393. [Google Scholar] [CrossRef]
- Hojman, P.; Gehl, J.; Christensen, J.F.; Pedersen, B.K. Molecular Mechanisms Linking Exercise to Cancer Prevention and Treatment. Cell Metab. 2018, 27, 10–21. [Google Scholar] [CrossRef]
- Aveseh, M.; Nikooie, R.; Aminaie, M. Exercise-Induced Changes in Tumour LDH-B and MCT1 Expression Are Modulated by Oestrogen-Related Receptor Alpha in Breast Cancer-Bearing BALB/c Mice. J. Physiol. 2015, 593, 2635–2648. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Y.; Loo, T.Y.; Shen, J.G.; Wang, N.; Wang, D.M.; Yang, D.P.; Mo, S.L.; Guan, X.Y.; Chen, J.P. LDH-A Silencing Suppresses Breast Cancer Tumorigenicity through Induction of Oxidative Stress Mediated Mitochondrial Pathway Apoptosis. Breast Cancer Res. Treat. 2012, 131, 791–800. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, E.L.; Lee, Y.K.; Park, C.B.; Kim, B.W.; Wang, H.J.; Yoon, C.H.; Lee, S.J.; Yoon, G. Decreased Lactate Dehydrogenase B Expression Enhances Claudin 1-Mediated Hepatoma Cell Invasiveness via Mitochondrial Defects. Exp. Cell Res. 2011, 317, 1108–1118. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Shapiro, D.J. The Immune System and Inflammation in Breast Cancer. Mol. Cell Endocrinol. 2014, 382, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Nicolini, A.; Ferrari, P.; Carpi, A. Immune Checkpoint Inhibitors and Other Immune Therapies in Breast Cancer: A New Paradigm for Prolonged Adjuvant Immunotherapy. Biomedicines 2022, 10, 2511. [Google Scholar] [CrossRef]
- Jurdana, M. Physical Activity and Cancer Risk. Actual Knowledge and Possible Biological Mechanisms. Radiol. Oncol. 2021, 55, 7–17. [Google Scholar] [CrossRef]
- Wennerberg, E.; Lhuillier, C.; Rybstein, M.D.; Dannenberg, K.; Rudqvist, N.P.; Koelwyn, G.J.; Jones, L.W.; Demaria, S. Exercise Reduces Immune Suppression and Breast Cancer Progression in a Preclinical Model. Oncotarget 2020, 11, 452–461. [Google Scholar] [CrossRef]
- Fairey, A.S.; Courneya, K.S.; Field, C.J.; Bell, G.J.; Jones, L.W.; Mackey, J.R. Randomized Controlled Trial of Exercise and Blood Immune Function in Postmenopausal Breast Cancer Survivors. J. Appl. Physiol. 2005, 98, 1534–1540. [Google Scholar] [CrossRef]
- Hagstrom, A.D.; Marshall, P.W.M.; Lonsdale, C.; Papalia, S.; Cheema, B.S.; Toben, C.; Baune, B.T.; Fiatarone Singh, M.A.; Green, S. The Effect of Resistance Training on Markers of Immune Function and Inflammation in Previously Sedentary Women Recovering from Breast Cancer: A Randomized Controlled Trial. Breast Cancer Res. Treat. 2016, 155, 471–482. [Google Scholar] [CrossRef]
- Schmidt, T.; Jonat, W.; Wesch, D.; Oberg, H.H.; Adam-Klages, S.; Keller, L.; Röcken, C.; Mundhenke, C. Influence of Physical Activity on the Immune System in Breast Cancer Patients during Chemotherapy. J. Cancer Res. Clin. Oncol. 2018, 144, 579–586. [Google Scholar] [CrossRef]
- Valenzuela, P.L.; Saco-Ledo, G.; Santos-Lozano, A.; Morales, J.S.; Castillo-García, A.; Simpson, R.J.; Lucia, A.; Fiuza-Luces, C. Exercise Training and Natural Killer Cells in Cancer Survivors: Current Evidence and Research Gaps Based on a Systematic Review and Meta-Analysis. Sports Med. Open 2022, 8, 36. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C.; Cook, V.D.; Henson, D.A.; Suttles, J.; Rejeski, W.J.; Ribisl, P.M.; Fagoaga, O.R.; Nehlsen-Cannarella, S.L. Moderate Exercise Training and Natural Killer Cell Cytotoxic Activity in Breast Cancer Patients. Int. J. Sports Med. 1995, 16, 334–337. [Google Scholar] [CrossRef] [PubMed]
- Hutnick, N.A.; Williams, N.I.; Kraemer, W.J.; Orsega-Smith, E.; Dixon, R.H.; Bleznak, A.D.; Mastro, A.M. Excercise and Lymphocyte Activation Following Chemotherapy for Breast Cancer. Med. Sci. Sports Exerc. 2005, 37, 1827–1835. [Google Scholar] [CrossRef] [PubMed]
- Saxton, J.M.; Scott, E.J.; Daley, A.J.; Woodroofe, M.N.; Mutrie, N.; Crank, H.; Powers, H.J.; Coleman, R.E. Effects of an Exercise and Hypocaloric Healthy Eating Intervention on Indices of Psychological Health Status, Hypothalamic-Pituitary-Adrenal Axis Regulation and Immune Function after Early-Stage Breast Cancer: A Randomised Controlled Trial. Breast Cancer Res. 2014, 16, R39. [Google Scholar] [CrossRef]
- Kwei, K.A.; Kung, Y.; Salari, K.; Holcomb, I.N.; Pollack, J.R. Genomic Instability in Breast Cancer: Pathogenesis and Clinical Implications. Mol. Oncol. 2010, 4, 255–266. [Google Scholar] [CrossRef]
- Duijf, P.H.G.; Nanayakkara, D.; Nones, K.; Srihari, S.; Kalimutho, M.; Khanna, K.K. Mechanisms of Genomic Instability in Breast Cancer. Trends Mol. Med. 2019, 25, 595–611. [Google Scholar] [CrossRef]
- Costa, M.; Saldanha, P. Risk Reduction Strategies in Breast Cancer Prevention. Eur. J. Breast Health 2016, 13, 103–112. [Google Scholar] [CrossRef]
- Kehm, R.D.; Genkinger, J.M.; MacInnis, R.J.; John, E.M.; Phillips, K.A.; Dite, G.S.; Milne, R.L.; Zeinomar, N.; Liao, Y.; Knight, J.A.; et al. Recreational Physical Activity Is Associated with Reduced Breast Cancer Risk in Adult Women at High Risk for Breast Cancer: A Cohort Study of Women Selected for Familial and Genetic Risk. Cancer Res. 2020, 80, 116–125. [Google Scholar] [CrossRef]
- Pettapiece-Phillips, R.; Narod, S.A.; Kotsopoulos, J. The Role of Body Size and Physical Activity on the Risk of Breast Cancer in BRCA Mutation Carriers. Cancer Causes Control 2015, 26, 333–344. [Google Scholar] [CrossRef]
- Bizjak, D.A.; Schulz, S.V.W.; Schumann, U.; Otto, S.; Kirsten, J.; Ebner, F.; Leinert, E.; Huober, J.; Janni, W.; Steinacker, J.M. Beneficial Molecular Adaptations in BRCA-Mutation Carriers by Combined HIT/HIRT Intervention: Results from a Pilot Study. Cancers 2020, 12, 1526. [Google Scholar] [CrossRef]
- Munn, L. Cancer and Inflammation. Wiley Interdiscip. Rev. Syst. Biol. Med. 2017, 9, e1370. [Google Scholar] [CrossRef] [PubMed]
- Danforth, D.N. The Role of Chronic Inflammation in the Development of Breast Cancer. Cancers 2021, 13, 3918. [Google Scholar] [CrossRef] [PubMed]
- Mills, R.C. Breast Cancer Survivors, Common Markers of Inflammation, and Exercise: A Narrative Review. Breast Cancer 2017, 11, 1178223417743976. [Google Scholar] [CrossRef] [PubMed]
- Murphy, E.A.; Davis, J.M.; Barrilleaux, T.L.; McClellan, J.L.; Steiner, J.L.; Carmichael, M.D.; Pena, M.M.; Hebert, J.R.; Green, J.E. Benefits of Exercise Training on Breast Cancer Progression and Inflammation in C3(1)SV40Tag Mice. Cytokine 2012, 55, 274–279. [Google Scholar] [CrossRef]
- Isanejad, A.; Alizadeh, A.M.; Amani Shalamzari, S.; Khodayari, H.; Khodayari, S.; Khori, V.; Khojastehnjad, N. MicroRNA-206, Let-7a and MicroRNA-21 Pathways Involved in the Anti-Angiogenesis Effects of the Interval Exercise Training and Hormone Therapy in Breast Cancer. Life Sci. 2016, 151, 30–40. [Google Scholar] [CrossRef]
- Jones, S.B.; Thomas, G.A.; Hesselsweet, S.D.; Alvarez-reeves, M.; Yu, H.; Irwin, M.L. Effect of Exercise on Markers of Inflammation in Breast Cancer Survivors: The Yale Exercise and Survivorship Study. Cancer Prev. Res. 2013, 6, 109–118. [Google Scholar] [CrossRef]
- Hooshmand Moghadam, B.; Golestani, F.; Bagheri, R.; Cheraghloo, N.; Eskandari, M.; Wong, A.; Nordvall, M.; Suzuki, K.; Pournemati, P. The Effects of High-Intensity Interval Training vs. Moderate-Intensity Continuous Training on Inflammatory Markers, Body Composition, and Physical Fitness in Overweight/Obese Survivors of Breast Cancer: A Randomized Controlled Clinical Trial. Cancers 2021, 13, 4386. [Google Scholar] [CrossRef]
- Alizadeh, A.M.; Isanejad, A.; Sadighi, S.; Mardani, M.; Kalaghchi, B.; Hassan, Z.M. High-Intensity Interval Training Can Modulate the Systemic Inflammation and HSP70 in the Breast Cancer: A Randomized Control Trial. J. Cancer Res. Clin. Oncol. 2019, 145, 2583–2593. [Google Scholar] [CrossRef]
- Kim, S.H.; Song, Y.K.; Han, J.; Ko, Y.H.; Lee, H.; Kang, M.J.; Park, H.; Lee, H.; Kim, S. Pro-Inflammatory Cytokine Levels and Cancer-Related Fatigue in Breast Cancer Survivors: Effects of an Exercise Adherence Program. J. Breast Cancer 2020, 23, 205–217. [Google Scholar] [CrossRef]
- Hiensch, A.E.; Mijwel, S.; Bargiela, D.; Wengström, Y.; May, A.M.; Rundqvist, H. Inflammation Mediates Exercise Effects on Fatigue in Patients with Breast Cancer. Med. Sci. Sports Exerc. 2021, 53, 496–504. [Google Scholar] [CrossRef]
- Schmidt, M.E.; Meynköhn, A.; Habermann, N.; Wiskemann, J.; Oelmann, J.; Hof, H.; Wessels, S.; Klassen, O.; Debus, J.; Potthoff, K.; et al. Resistance Exercise and Inflammation in Breast Cancer Patients Undergoing Adjuvant Radiation Therapy: Mediation Analysis from a Randomized, Controlled Intervention Trial. Int. J. Radiat. Oncol. Biol. Phys. 2016, 94, 329–337. [Google Scholar] [CrossRef]
- Rogers, L.Q.; Fogleman, A.; Trammell, R.; Hopkins-Price, P.; Vicari, S.; Rao, K.; Edson, B.; Verhulst, S.; Courneya, K.S.; Hoelzer, K. Effects of a Physical Activity Behavior Change Intervention on Inflammation and Related Health Outcomes in Breast Cancer Survivors: Pilot Randomized Trial. Integr. Cancer 2013, 12, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Dieli-Conwright, C.M.; Parmentier, J.H.; Sami, N.; Lee, K.; Spicer, D.; Mack, W.J.; Sattler, F.; Mittelman, S.D. Adipose Tissue Inflammation in Breast Cancer Survivors: Effects of a 16-Week Combined Aerobic and Resistance Exercise Training Intervention. Breast Cancer Res. Treat. 2018, 168, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Lüönd, F.; Tiede, S.; Christofori, G. Breast Cancer as an Example of Tumour Heterogeneity and Tumour Cell Plasticity during Malignant Progression. Br. J. Cancer 2021, 125, 164–175. [Google Scholar] [CrossRef]
- Smallbone, K.; Maini, P.K.; Gatenby, R.A. Episodic, Transient Systemic Acidosis Delays Evolution of the Malignant Phenotype: Possible Mechanism for Cancer Prevention by Increased Physical Activity. Biol. Direct 2010, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, P.M.; Kaufman, S.A. Should Resistance Exercise Be Recommended during Breast Cancer Treatment? Med. Hypotheses 2010, 75, 192–195. [Google Scholar] [CrossRef]
- Wu, Y.; Sarkissyan, M.; Vadgama, J.v. Epigenetics in Breast and Prostate Cancer. Methods Mol. Biol. 2015, 1238, 425–466. [Google Scholar]
- Zeng, H.; Irwin, M.L.; Lu, L.; Risch, H.; Mayne, S.; Mu, L.; Deng, Q.; Scarampi, L.; Mitidieri, M.; Katsaros, D.; et al. Physical Activity and Breast Cancer Survival: An Epigenetic Link through Reduced Methylation of a Tumor Suppressor Gene L3MBTL1. Breast Cancer Res. Treat. 2012, 133, 127–135. [Google Scholar] [CrossRef]
- McCullough, L.E.; Chen, J.; Cho, Y.H.; Khankari, N.K.; Bradshaw, P.T.; White, A.J.; Teitelbaum, S.L.; Terry, M.B.; Neugut, A.I.; Hibshoosh, H.; et al. Modification of the Association between Recreational Physical Activity and Survival after Breast Cancer by Promoter Methylation in Breast Cancer-Related Genes. Breast Cancer Res. 2017, 19, 19. [Google Scholar] [CrossRef]
- Adraskela, K.; Veisaki, E.; Koutsilieris, M.; Philippou, A. Physical Exercise Positively Influences Breast Cancer Evolution. Clin. Breast Cancer 2017, 17, 408–417. [Google Scholar] [CrossRef]
- Hong, B.S.; Lee, K.P. A Systematic Review of the Biological Linking Physical activity and Breast Cancer. Phys. Act. Nutr. 2020, 24, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Ganguly, S.; Tollefsbol, T.O. Modulating Microbiota as a New Strategy for Breast Cancer Prevention and Treatment. Microorganisms 2022, 10, 1727. [Google Scholar] [CrossRef] [PubMed]
- Vitorino, M.; Alpuim Costa, D.; Vicente, R.; Caleça, T.; Santos, C. Local Breast Microbiota: A “New” Player on the Block. Cancers 2022, 14, 3811. [Google Scholar] [CrossRef] [PubMed]
- Bodai, B.I.; Nakata, T.E. Breast Cancer: Lifestyle, the Human Gut Microbiota/Microbiome, and Survivorship. Perm. J. 2020, 24, 19.129. [Google Scholar] [CrossRef]
- Sampsell, K.; Hao, D.; Reimer, R.A. The Gut Microbiota: A Potential Gateway to Improved Health Outcomes in Breast Cancer Treatment and Survivorship. Int. J. Mol. Sci. 2020, 21, 9239. [Google Scholar] [CrossRef]
- Mailing, L.J.; Allen, J.M.; Buford, T.W.; Fields, C.J.; Woods, J.A. Exercise and the Gut Microbiome: A Review of the Evidence, Potential Mechanisms, and Implications for Human Health. Exerc. Sports Sci. Rev. 2019, 47, 75–85. [Google Scholar] [CrossRef]
- Frugé, A.D.; Rogers, L.Q.; Morrow, C.D.; Tsuruta, Y.; Demark-Wahnefried, W. Fecal Akkermansia Muciniphila Is Associated with Body Composition and Microbiota Diversity in Overweight and Obese Women with Breast Cancer Participating in a Presurgical Weight Loss Trial. J. Acad. Nutr. Diet. 2020, 120, 650–659. [Google Scholar] [CrossRef]
- Helmink, B.A.; Khan, M.A.W.; Hermann, A.; Gopalakrishnan, V.; Wargo, J.A. The Microbiome, Cancer, and Cancer Therapy. Nat. Med. 2019, 25, 377–388. [Google Scholar] [CrossRef]
- Paulsen, J.A.; Ptacek, T.S.; Carter, S.J.; Liu, N.; Kumar, R.; Hyndman, L.K.; Lefkowitz, E.J.; Morrow, C.D.; Rogers, L.Q. Gut Microbiota Composition Associated with Alterations in Cardiorespiratory Fitness and Psychosocial Outcomes among Breast Cancer Survivors. Support. Care Cancer 2017, 25, 1563–1570. [Google Scholar] [CrossRef]
- Milczarek, M. The Premature Senescence in Breast Cancer Treatment Strategy. Cancers 2020, 12, 1815. [Google Scholar] [CrossRef]
- Garatachea, N.; Pareja-Galeano, H.; Sanchis-Gomar, F.; Santos-Lozano, A.; Fiuza-Luces, C.; Morán, M.; Emanuele, E.; Joyner, M.J.; Lucia, A. Exercise Attenuates the Major Hallmarks of Aging. Rejuvenation Res. 2015, 18, 57–89. [Google Scholar] [CrossRef] [PubMed]
- Colbert, L.H.; Westerlind, K.C.; Perkins, S.N.; Haines, D.C.; Berrigan, D.; Donehower, L.A.; Fuchs-Young, R.; Hursting, S.D. Exercise Effects on Tumorigenesis in a P53-Deficient Mouse Model of Breast Cancer. Med. Sci. Sports Exerc. 2009, 41, 1597–1605. [Google Scholar] [CrossRef] [PubMed]
- Nezamdoost, Z.; Saghebjoo, M.; Hoshyar, R.; Hedayati, M.; Keska, A. High-Intensity Training and Saffron: Effects on Breast Cancer-Related Gene Expression. Med. Sci. Sports Exerc. 2020, 52, 1470–1476. [Google Scholar] [CrossRef] [PubMed]
- González-Puertos, V.Y.; Maciel-Barón, L.A.; Barajas-Gómez, B.A.; López-Diazguerrero, N.E.; Königsberg, M. Senescence-Associated Secretory Phenotype (SASP) Involvement in the Development of Cancer, Aging and Age Related Diseases. Gac. Médica México 2015, 151, 460–468. [Google Scholar]
- Courneya, K.S.; Mckenzie, D.C.; Mackey, J.R.; Gelmon, K.; Friedenreich, C.M.; Yasui, Y.; Reid, R.D.; Vallerand, J.R.; Adams, S.C.; Proulx, C.; et al. Subgroup Effects in a Randomised Trial of Different Types and Doses of Exercise during Breast Cancer Chemotherapy. Br. J. Cancer 2014, 111, 1718–1725. [Google Scholar] [CrossRef] [PubMed]
- Friedenreich, C.M.; Neilson, H.K.; Farris, M.S.; Courneya, K.S.; Friedenreich, C. Physical Activity and Cancer Outcomes: A Precision Medicine Approach. Clinical Cancer Research. Clin. Cancer Res. 2016, 22, 4766–4775. [Google Scholar] [CrossRef]
- Woelfel, J.R.; Dudley-Javoroski, S.; Shields, R.K.; Dudley-Javoroski, S. Precision Physical Therapy: Exercise, the Epigenome, and the Heritability of Environmentally Modified Traits. Phys. Ther. 2018, 98, 946–952. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Chico, C.; López-Ortiz, S.; Peñín-Grandes, S.; Pinto-Fraga, J.; Valenzuela, P.L.; Emanuele, E.; Ceci, C.; Graziani, G.; Fiuza-Luces, C.; Lista, S.; et al. Physical Exercise and the Hallmarks of Breast Cancer: A Narrative Review. Cancers 2023, 15, 324. https://doi.org/10.3390/cancers15010324
García-Chico C, López-Ortiz S, Peñín-Grandes S, Pinto-Fraga J, Valenzuela PL, Emanuele E, Ceci C, Graziani G, Fiuza-Luces C, Lista S, et al. Physical Exercise and the Hallmarks of Breast Cancer: A Narrative Review. Cancers. 2023; 15(1):324. https://doi.org/10.3390/cancers15010324
Chicago/Turabian StyleGarcía-Chico, Celia, Susana López-Ortiz, Saúl Peñín-Grandes, José Pinto-Fraga, Pedro L. Valenzuela, Enzo Emanuele, Claudia Ceci, Grazia Graziani, Carmen Fiuza-Luces, Simone Lista, and et al. 2023. "Physical Exercise and the Hallmarks of Breast Cancer: A Narrative Review" Cancers 15, no. 1: 324. https://doi.org/10.3390/cancers15010324
APA StyleGarcía-Chico, C., López-Ortiz, S., Peñín-Grandes, S., Pinto-Fraga, J., Valenzuela, P. L., Emanuele, E., Ceci, C., Graziani, G., Fiuza-Luces, C., Lista, S., Lucia, A., & Santos-Lozano, A. (2023). Physical Exercise and the Hallmarks of Breast Cancer: A Narrative Review. Cancers, 15(1), 324. https://doi.org/10.3390/cancers15010324












