The Association between Baseline Proton Pump Inhibitors, Immune Checkpoint Inhibitors, and Chemotherapy: A Systematic Review with Network Meta-Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
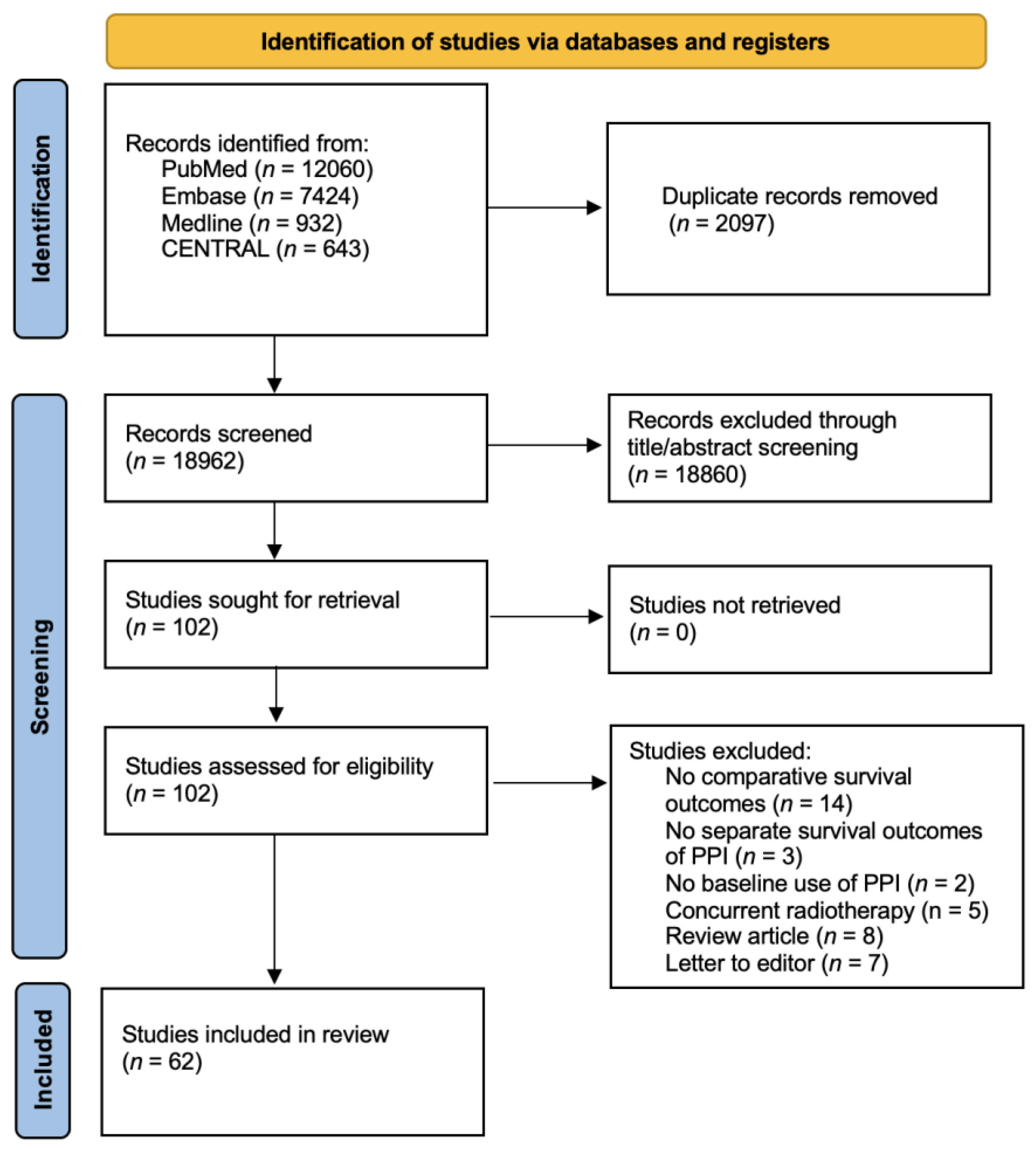
2.1. Study Selection
2.2. Eligibility Criteria
2.3. Data Extraction
2.4. Quality Assessment
2.5. Assessment of Transitivity Assumption
2.6. Main Outcomes and Statistical Analysis
3. Results
3.1. Characteristics of Included Studies
3.2. Network Meta-Analysis
3.3. Prespecified Sensitivity Analyses (Supplementary Results 5 and 6)
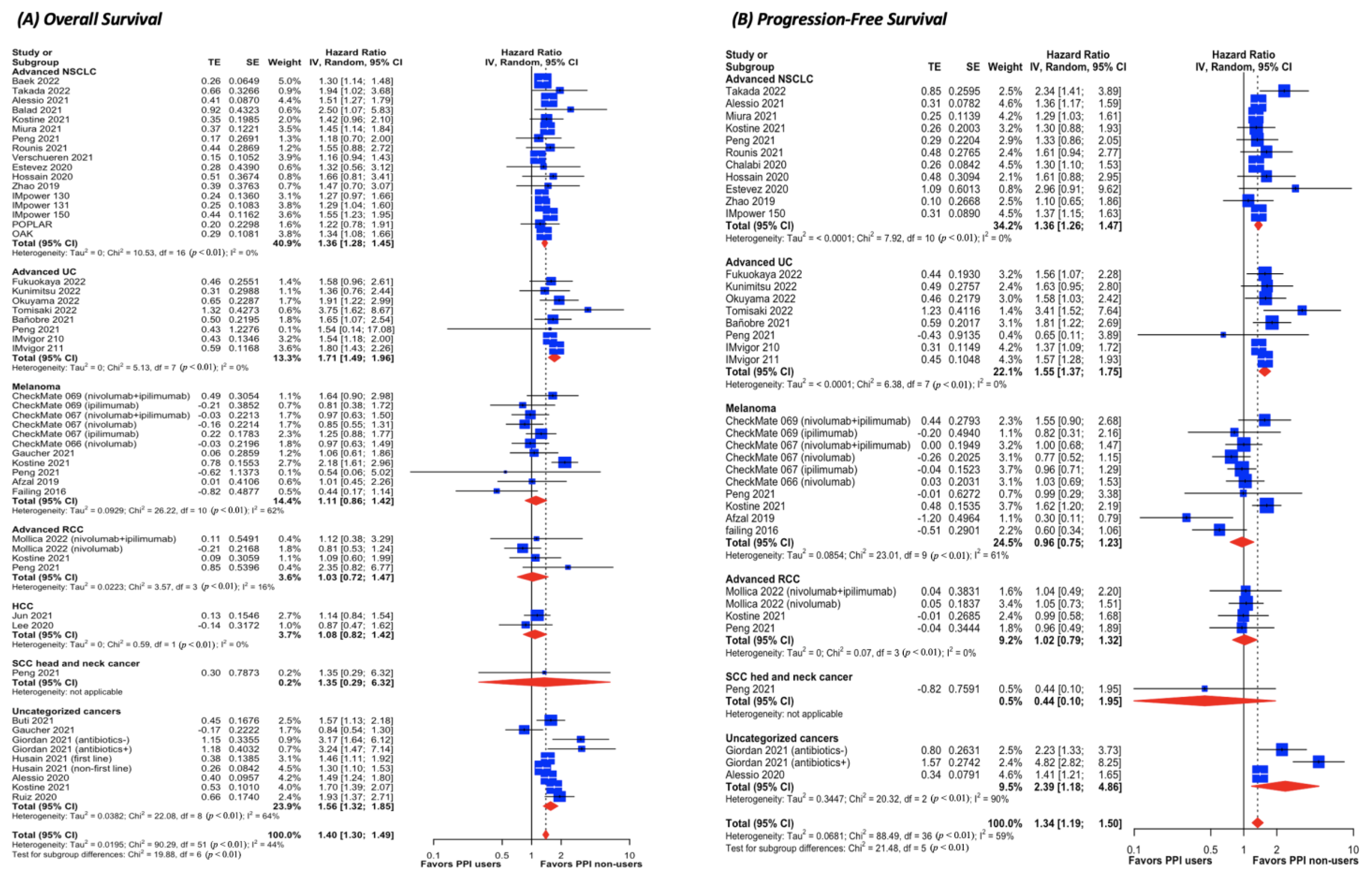
3.4. Pairwise Meta-Analysis for ICI Cohorts (Figure 3 and Supplementary Results 7)
3.5. Pairwise Meta-Analysis for Chemotherapy Cohorts (Supplementary Results 9)
3.6. Meta-Analysis Using Adjusted HR (Supplementary Results 10)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tang, J.; Yu, J.X.; Hubbard-Lucey, V.M.; Neftelinov, S.T.; Hodge, J.P.; Lin, Y. Trial watch: The clinical trial landscape for PD1/PDL1 immune checkpoint inhibitors. Nat. Rev. Drug. Discov. 2018, 17, 854–855. [Google Scholar] [CrossRef]
- Ribas, A.; Wolchok, J.D. Cancer immunotherapy using checkpoint blockade. Science 2018, 359, 1350–1355. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Tannir, N.M.; McDermott, D.F.; Aren Frontera, O.; Melichar, B.; Choueiri, T.K.; Plimack, E.R.; Barthélémy, P.; Porta, C.; George, S.; et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2018, 378, 1277–1290. [Google Scholar] [CrossRef] [PubMed]
- Feld, E.; Mitchell, T.C. Immunotherapy in melanoma. Immunotherapy 2018, 10, 987–998. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, A.; Mollica, V.; Cimadamore, A.; Santoni, M.; Scarpelli, M.; Giunchi, F.; Cheng, L.; Lopez-Beltran, A.; Fiorentino, M.; Montironi, R.; et al. Is There a Role for Immunotherapy in Prostate Cancer? Cells 2020, 9, 2051. [Google Scholar] [CrossRef]
- Rizzo, A.; Mollica, V.; Santoni, M.; Ricci, A.D.; Rosellini, M.; Marchetti, A.; Montironi, R.; Ardizzoni, A.; Massari, F. Impact of Clinicopathological Features on Survival in Patients Treated with First-line Immune Checkpoint Inhibitors Plus Tyrosine Kinase Inhibitors for Renal Cell Carcinoma: A Meta-analysis of Randomized Clinical Trials. Eur. Urol. Focus 2021, 8, 514–521. [Google Scholar] [CrossRef]
- Conforti, F.; Pala, L.; Bagnardi, V.; De Pas, T.; Martinetti, M.; Viale, G.; Gelber, R.D.; Goldhirsch, A. Cancer immunotherapy efficacy and patients’ sex: A systematic review and meta-analysis. Lancet Oncol. 2018, 19, 737–746. [Google Scholar] [CrossRef]
- Califano, R.; Gomes, F.; Ackermann, C.J.; Rafee, S.; Tsakonas, G.; Ekman, S. Immune checkpoint blockade for non-small cell lung cancer: What is the role in the special populations? Eur. J. Cancer 2020, 125, 1–11. [Google Scholar] [CrossRef]
- Buti, S.; Bersanelli, M.; Perrone, F.; Tiseo, M.; Tucci, M.; Adamo, V.; Stucci, L.S.; Russo, A.; Tanda, E.T.; Spagnolo, F.; et al. Effect of concomitant medications with immune-modulatory properties on the outcomes of patients with advanced cancer treated with immune checkpoint inhibitors: Development and validation of a novel prognostic index. Eur. J. Cancer 2020, 142, 18–28. [Google Scholar] [CrossRef]
- Cortellini, A.; Tucci, M.; Adamo, V.; Stucci, L.S.; Russo, A.; Tanda, E.T.; Spagnolo, F.; Rastelli, F.; Bisonni, R.; Santini, D.; et al. Integrated analysis of concomitant medications and oncological outcomes from PD-1/PD-L1 checkpoint inhibitors in clinical practice. J. Immunother. Cancer 2020, 8, 11. [Google Scholar] [CrossRef]
- Routy, B.; le Chatelier, E.; DeRosa, L.; Duong, C.P.M.; Alou, M.T.; Daillère, R.; Fluckiger, A.; Messaoudene, M.; Rauber, C.; Roberti, M.P.; et al. Gut microbiome influences efficacy of PD-1–based immunotherapy against epithelial tumors. Science 2018, 359, 91–97. [Google Scholar] [CrossRef]
- Iida, N.; Dzutsev, A.; Stewart, C.A.; Smith, L.; Bouladoux, N.; Weingarten, R.A.; Molina, D.A.; Salcedo, R.; Back, T.; Cramer, S.; et al. Commensal Bacteria Control Cancer Response to Therapy by Modulating the Tumor Microenvironment. Science 2019, 342, 967–970. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zeng, C.; Yao, J.; Ge, Y.; An, G. The association between proton pump inhibitors use and clinical outcome of patients receiving immune checkpoint inhibitors therapy. Int. Immunopharmacol. 2020, 88, 3052–3063. [Google Scholar] [CrossRef]
- Li, C.; Xia, Z.; Li, A.; Meng, J. The effect of proton pump inhibitor uses on outcomes for cancer patients treated with immune checkpoint inhibitors: A meta-analysis. Ann. Transl. Med. 2020, 8, 1655. [Google Scholar] [CrossRef] [PubMed]
- Qin, B.-D.; Jiao, X.-D.; Zhou, X.-C.; Shi, B.; Wang, J.; Liu, K.; Wu, Y.; Ling, Y.; Zang, Y.-S. Effects of concomitant proton pump inhibitor use on immune checkpoint inhibitor efficacy among patients with advanced cancer. Oncoimmunology 2021, 10, 1929727. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Guo, H.; Mao, H.; Tong, J.; Yang, M.; Yan, X. An Up-To-Date Investigation Into the Correlation Between Proton Pump Inhibitor Use and the Clinical Efficacy of Immune Checkpoint Inhibitors in Advanced Solid Cancers: A Systematic Review and Meta-Analysis. Front. Oncol. 2022, 12, 753234. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, A.; Cusmai, A.; Giovannelli, F.; Acquafredda, S.; Rinaldi, L.; Misino, A.; Montagna, E.S.; Ungaro, V.; Lorusso, M.; Palmiotti, G. Impact of Proton Pump Inhibitors and Histamine-2-Receptor Antagonists on Non-Small Cell Lung Cancer Immunotherapy: A Systematic Review and Meta-Analysis. Cancers 2022, 14, 1404. [Google Scholar] [CrossRef]
- Dar, S.; Merza, N.; Rahim, M.; Qatani, A.; Varughese, T.; Mohammad, A.; Masood, F.; Reza, F.; Shucenwan; Almas, T.; et al. Impact of proton-pump inhibitors on the efficacy of immune checkpoint inhibitors in non-small cell lung cancer: A systematic review and meta-analysis. Ann. Med. Surg. 2022, 78, 103752. [Google Scholar] [CrossRef]
- Powles, T.; Durán, I.; Van Der Heijden, M.S.; Loriot, Y.; Vogelzang, N.J.; De Giorgi, U.; Oudard, S.; Retz, M.M.; Castellano, D.; Bamias, A.; et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): A multicentre, open-label, phase 3 randomised controlled trial. Lancet 2018, 391, 748–757. [Google Scholar] [CrossRef]
- Socinski, M.A.; Jotte, R.M.; Cappuzzo, F.; Orlandi, F.; Stroyakovskiy, D.; Nogami, N.; Rodríguez-Abreu, D.; Moro-Sibilot, D.; Thomas, C.A.; Barlesi, F.; et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N. Engl. J. Med. 2018, 378, 2288–2301. [Google Scholar] [CrossRef]
- Rittmeyer, A.; Barlesi, F.; Waterkamp, D.; Park, K.; Ciardiello, F.; von Pawel, J.; Gadgeel, S.M.; Hida, T.; Kowalski, D.M.; Dols, M.C.; et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): A phase 3, open-label, multicentre randomised controlled trial. Lancet 2017, 389, 255–265, Erratum in Lancet 2017, 389, e5. [Google Scholar] [CrossRef] [PubMed]
- Fehrenbacher, L.; Spira, A.; Ballinger, M.; Kowanetz, M.; Vansteenkiste, J.; Mazieres, J.; Park, K.; Smith, D.; Artal-Cortes, A.; Lewanski, C.; et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): A multicentre, open-label, phase 2 randomised controlled trial. Lancet 2016, 387, 1837–1846. [Google Scholar] [CrossRef] [PubMed]
- Higgins JPT, T.J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (Updated February 2021); Cochrane: 2021. Available online: https://training.cochrane.org/handbook (accessed on 14 March 2021).
- Efthimiou, O.; Debray, T.P.A.; van Valkenhoef, G.; Trelle, S.; Panayidou, K.; Moons, K.G.M.; Reitsma, J.B.; Shang, A.; Salanti, G.; on behalf of GetReal Methods Review Group. GetReal in network meta-analysis: A review of the methodology. Res. Synth. Methods 2016, 7, 236–263. [Google Scholar] [CrossRef] [PubMed]
- Parmar, M.K.; Torri, V.; Stewart, L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat. Med. 1998, 17, 2815–2834. [Google Scholar] [CrossRef]
- Tierney, J.F.; Stewart, L.A.; Ghersi, D.; Burdett, S.; Sydes, M.R. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007, 8, 16. [Google Scholar] [CrossRef]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Afzal, M.Z.; Shirai, K. What impact do the proton pump inhibitors have on the efficacy of immune check point inhibitors in metastatic malignant melanoma? J. Clin. Oncol. 2019, 37 (Suppl. S15), e21040. [Google Scholar] [CrossRef]
- Araujo, H.A.; Moniz, C.M.V.; Braghiroli, O.F.M.; Mak, M.P.; Uratani, L.F.; Tiecher, R.D.; Moraes, P.M.; Barbosa, I.; De Camargo, V.P.; Braghiroli, M.I.F.M.; et al. Proton pump inhibitors and antibiotics impact on toxicities and clinical outcomes in cancer patients treated with immunotherapy. J. Clin. Oncol. 2021, 39, 2652. [Google Scholar] [CrossRef]
- Baek, Y.H.; Kang, E.J.; Hong, S.; Park, S.; Kim, J.H.; Shin, J.Y. Survival outcomes of patients with nonsmall cell lung cancer concomitantly receiving proton pump inhibitors and immune checkpoint inhibitors. Int. J. Cancer 2022, 150, 1291–1300. [Google Scholar] [CrossRef]
- Balar, A.V.; Galsky, M.D.; Rosenberg, J.E.; Powles, T.; Petrylak, D.P.; Bellmunt, J.; Loriot, Y.; Necchi, A.; Hoffman-Censits, J.; Perez-Gracia, J.L.; et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: A single-arm, multicentre, phase 2 trial. Lancet 2017, 389, 67–76, Erratum in Lancet 2017, 390, 848. [Google Scholar] [CrossRef] [PubMed]
- Carrato, A.; Swieboda-Sadlej, A.; Staszewska-Skurczynska, M. Fluorouracil, leucovorin, and irinotecan plus either sunitinib or placebo in metastatic colorectal cancer: A randomized, phase III trial. J. Clin. Oncol. 2013, 31, 1341–1347. [Google Scholar] [CrossRef]
- Balado, A.C.; Lores, M.T.; Sánchez, R.; Ferrán, B.B.; Montero, E.L.; Torre, A.M.; Del Molino, B.M.P.; Ferro, I.Z. 4CPS-301 Association of antibiotics and proton pump inhibitors on clinical activity of firstline pembrolizumab for non-small cell lung cancer: 2 years of real world data. BJM 2021, 28, A65. [Google Scholar] [CrossRef]
- Chalabi, M.; Cardona, A.; Nagarkar, D.; Scala, A.D.; Gandara, D.; Rittmeyer, A.; Albert, M.; Powles, T.; Kok, M.; Herrera, F. Efficacy of chemotherapy and atezolizumab in patients with non-small-cell lung cancer receiving antibiotics and proton pump inhibitors: Pooled post hoc analyses of the OAK and POPLAR trials. Ann. Oncol. 2020, 31, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Chu, M.P.; Hecht, J.R.; Slamon, D.; Wainberg, Z.A.; Bang, Y.J.; Hoff, P.M.; Sobrero, A.; Qin, S.; Afenjar, K.; Houe, V.; et al. Association of Proton Pump Inhibitors and Capecitabine Efficacy in Advanced Gastroesophageal Cancer: Secondary Analysis of the TRIO-013/LOGiC Randomized Clinical Trial. JAMA Oncol. 2017, 3, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Conde-Estévez, D.; Monge-Escartín, I.; Ríos-Hoyo, A.; Monzonis, X.; Echeverría-Esnal, D.; Moliner, L.; Duran-Jordà, X.; Taus, Á.; Arriola, E. Prognostic factors and effect on survival of immune-related adverse events in patients with non-small-cell lung cancer treated with immune checkpoint blockage. J. Chemother. 2020, 33, 32–39. [Google Scholar] [CrossRef]
- Cortellini, A.; Di Maio, M.; Nigro, O.; Leonetti, A.; Cortinovis, D.L.; Aerts, J.G.; Guaitoli, G.; Barbieri, F.; Giusti, R.; Ferrara, M.G.; et al. Differential influence of antibiotic therapy and other medications on oncological outcomes of patients with non-small cell lung cancer treated with first-line pembrolizumab versus cytotoxic chemotherapy. J. Immunother. Cancer 2021, 9, e002421. [Google Scholar] [CrossRef]
- Cutsem, E.V.; Tabernero, J.; Lakomy, R. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J. Clin. Oncol. 2012, 30, 3499–3506. [Google Scholar] [CrossRef]
- Failing, J.; Finnes, H.D.; Kottschade, L.A.; Allred, J.B.; Markovic, S. Effects of commonly used chronic medications on the outcomes of ipilimumab therapy in patients with metastatic melanoma. J. Clin. Oncol. 2016, 34, 609–615. [Google Scholar] [CrossRef]
- Gaucher, L.; Adda, L.; Séjourné, A.; Joachim, C.; Guillaume, C.; Poulet, C.; Liabeuf, S.; Gras-Champel, V.; Masmoudi, K.; Houessinon, A.; et al. Associations between dysbiosis-inducing drugs, overall survival and tumor response in patients treated with immune checkpoint inhibitors. Ther. Adv. Med Oncol. 2021, 13, 17588359211000591. [Google Scholar] [CrossRef]
- Giordan, Q.; Salleron, J.; Vallance, C.; Moriana, C.; Clement-Duchene, C. Impact of Antibiotics and Proton Pump Inhibitors on Efficacy and Tolerance of Anti-PD-1 Immune Checkpoint Inhibitors. Front. Immunol. 2021, 12, 716317. [Google Scholar] [CrossRef] [PubMed]
- Hecht, J.R.; Bang, Y.J.; Qin, S.K.; Chung, H.C.; Xu, J.M.; Park, J.O.; Jeziorski, K.; Shparyk, Y.; Hoff, P.M.; Sobrero, A.; et al. Lapatinib in Combination With Capecitabine Plus Oxaliplatin in Human Epidermal Growth Factor Receptor 2-Positive Advanced or Metastatic Gastric, Esophageal, or Gastroesophageal Adenocarcinoma: TRIO-013/LOGiC--A Randomized Phase III Trial. J. Clin. Oncol. 2016, 34, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, A.M.; Badaoui, S.; Kichenadasse, G.; Karapetis, C.S.; McKinnon, R.A.; Rowland, A.; Sorich, M.J. Efficacy of Atezolizumab in Patients With Advanced NSCLC Receiving Concomitant Antibiotic or Proton Pump Inhibitor Treatment: Pooled Analysis of Five Randomized Control Trials. J. Thorac. Oncol. 2022, 17, 758–767. [Google Scholar] [CrossRef]
- Hopkins, A.M.; Kichenadasse, G.; Karapetis, C.S.; Rowland, A.; Sorich, M.J. Concomitant proton pump inhibitor use and survival in urothelial carcinoma treated with atezolizumab. Clin. Cancer Res. 2020, 26, 5487–5493. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, A.M.; Kichenadasse, G.; McKinnon, R.A.; Abuhelwa, A.Y.; Logan, J.M.; Badaoui, S.; Karapetis, C.S.; Rowland, A.; Sorich, M.J. Efficacy of first-line atezolizumab combination therapy in patients with non-small cell lung cancer receiving proton pump inhibitors: Post hoc analysis of IMpower150. Br. J. Cancer 2021, 126, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Hossain, T.; Htut, S.; Pathmanathan, S.; Fletcher, J.; Pandey, R.; Lui, A.; Lyle, M.; Rainey, N. Immunotherapy efficacy and concomitant proton pump inhibitor use in non-small cell lung cancer. Asia-Pac. J. Clin. Oncol. 2020, 16 (Suppl. S2), 37. [Google Scholar]
- Hurwitz, H.; Fehrenbacher, L.; Novotny, W. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N. Engl. J. Med. 2004, 350, 2335–2342. [Google Scholar] [CrossRef]
- Husain, M.; Xu, M.; Patel, S.; Johns, A.; Grogan, M.; Li, M.; Lopez, G.; Miah, A.; Hoyd, R.; Liu, Y.; et al. Proton pump inhibitor use (PPI) in patients treated with immune checkpoint inhibitors (ICI) for advanced cancer: Survival and prior therapy. J. Clin. Oncol. 2021, 39, 2633. [Google Scholar] [CrossRef]
- Iglesias-Santamaría, A. Impact of antibiotic use and other concomitant medications on the efficacy of immune checkpoint inhibitors in patients with advanced cancer. Clin. Transl. Oncol. 2020, 22, 1481–1490. [Google Scholar] [CrossRef]
- Jotte, R.; Cappuzzo, F.; Vynnychenko, I.; Stroyakovskiy, D.; Rodríguez-Abreu, D.; Hussein, M.; Soo, R.; Conter, H.J.; Kozuki, T.; Huang, K.-C.; et al. Atezolizumab in Combination With Carboplatin and Nab-Paclitaxel in Advanced Squamous NSCLC (IMpower131): Results From a Randomized Phase III Trial. J. Thorac. Oncol. 2020, 15, 1351–1360. [Google Scholar] [CrossRef]
- Jun, T.; Dharmapuri, S.; Marron, T.U.; Sung, M.W.; Ang, C. Effect of baseline medications on response to immunotherapy in hepatocellular carcinoma. J. Clin. Oncol. 2020, 38, 484. [Google Scholar] [CrossRef]
- Kichenadasse, G.; Miners, J.O.; Mangoni, A.A.; Karapetis, C.S.; Hopkins, A.M.; Sorich, M.J. Proton Pump Inhibitors and Survival in Patients With Colorectal Cancer Receiving Fluoropyrimidine-Based Chemotherapy. J. Natl. Compr. Cancer Netw. JNCCN 2021, 19, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Lee, J.S.; Kang, J.; Morita, S.; Park, Y.S.; Sakamoto, J.; Muro, K.; Xu, R.-H.; Kim, T.W. Proton Pump Inhibitor Use and the Efficacy of Chemotherapy in Metastatic Colorectal Cancer: A Post Hoc Analysis of a Randomized Phase III Trial (AXEPT). Oncol. 2021, 26, e954–e962. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.C.; Chao, Y.; Lee, C.; Hou, M.C.; Huang, Y.H. Effect of antibiotics, proton pump inhibitors and steroids on survival and response to immune-checkpoint inhibitors in patients with hepatocellular carcinoma. J. Hepatol. 2020, 73, S912. [Google Scholar] [CrossRef]
- Miura, K.; Sano, Y.; Niho, S.; Kawasumi, K.; Mochizuki, N.; Yoh, K.; Matsumoto, S.; Zenke, Y.; Ikeda, T.; Nosaki, K.; et al. Impact of concomitant medication on clinical outcomes in patients with advanced non-small cell lung cancer treated with immune checkpoint inhibitors: A retrospective study. Thorac. Cancer 2021, 12, 1983–1994. [Google Scholar] [CrossRef]
- Mollica, V.; Santoni, M.; Matrana, M.R.; Basso, U.; De Giorgi, U.; Rizzo, A.; Maruzzo, M.; Marchetti, A.; Rosellini, M.; Bleve, S.; et al. Concomitant Proton Pump Inhibitors and Outcome of Patients Treated with Nivolumab Alone or Plus Ipilimumab for Advanced Renal Cell Carcinoma. Target. Oncol. 2021, 17, 61–68. [Google Scholar] [CrossRef]
- Okuyama, Y.; Hatakeyama, S.; Numakura, K.; Narita, T.; Tanaka, T.; Miura, Y.; Sasaki, D.; Noro, D.; Tokui, N.; Okamoto, T.; et al. Prognostic impact of proton pump inhibitors for immunotherapy in advanced urothelial carcinoma. BJUI Compass 2021, 3, 154–161. [Google Scholar] [CrossRef]
- Peng, K.; Chen, K.; Teply, B.A.; Yee, G.C.; Farazi, P.A.; Lyden, E.R. Impact of Proton Pump Inhibitor Use on the Effectiveness of Immune Checkpoint Inhibitors in Advanced Cancer Patients. Ann. Pharmacother. 2021, 56, 377–386. [Google Scholar] [CrossRef]
- Pérez-Ruiz, E.; Jiménez-Castro, J.; Berciano-Guerrero, M.-A.; Valdivia, J.; Estalella-Mendoza, S.; Toscano, F.; Artacho, M.R.D.L.B.; Garrido-Siles, M.; Martínez-Bautista, M.J.; Roldan, R.V.; et al. Impact of intestinal dysbiosis-related drugs on the efficacy of immune checkpoint inhibitors in clinical practice. Clin. Transl. Oncol. 2020, 22, 1778–1785. [Google Scholar] [CrossRef]
- Rounis, K.; Makrakis, D.; Papadaki, C.; Monastirioti, A.; Vamvakas, L.; Kalbakis, K.; Gourlia, K.; Xanthopoulos, I.; Tsamardinos, I.; Mavroudis, D.; et al. Prediction of outcome in patients with non-small cell lung cancer treated with second line PD-1/PDL-1 inhibitors based on clinical parameters: Results from a prospective, single institution study. PLoS One 2021, 16, e0252537. [Google Scholar] [CrossRef]
- Ruiz-Bañobre, J.; Molina-Díaz, A.; Fernández-Calvo, O.; Fernández-Núñez, N.; Medina-Colmenero, A.; Santomé, L.; Lázaro-Quintela, M.; Mateos-González, M.; García-Cid, N.; López-López, R.; et al. Rethinking prognostic factors in locally advanced or metastatic urothelial carcinoma in the immune checkpoint blockade era: A multicenter retrospective study. ESMO Open 2021, 6, 100090. [Google Scholar] [CrossRef] [PubMed]
- Saltz, L.B.; Clarke, S.; Díaz-Rubio, E. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: A randomized phase III study. J. Clin. Oncol. 2008, 26, 2013–2019. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Ilich, A.I.; Kim, C.A.; Chu, M.P.; Wong, G.G.; Ghosh, S.; Danilak, M.; Mulder, K.E.; Spratlin, J.L.; Chambers, C.R.; et al. Concomitant Administration of Proton Pump Inhibitors and Capecitabine is Associated With Increased Recurrence Risk in Early Stage Colorectal Cancer Patients. Clin. Color. Cancer 2015, 15, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Svaton, M.; Zemanova, M.; Zemanova, P.; Kultan, J.; Fischer, O.; Skrickova, J.; Jakubikova, L.; Cernovska, M.; Hrnciarik, M.; Jirousek, M.; et al. Impact of Concomitant Medication Administered at the Time of Initiation of Nivolumab Therapy on Outcome in Non-small Cell Lung Cancer. Anticancer. Res. 2020, 40, 2209–2217. [Google Scholar] [CrossRef]
- Tabernero, J.; Yoshino, T.; Cohn, A.L. Ramucirumab versus placebo in combination with second-line FOLFIRI in patients with metastatic colorectal carcinoma that progressed during or after first-line therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine (RAISE): A randomised, double-blind, multicentre, phase 3 study. Lancet Oncol. 2015, 16, 499–508. [Google Scholar]
- Takada, K.; Shimokawa, M.; Takamori, S.; Shimamatsu, S.; Hirai, F.; Ono, Y.; Tagawa, T.; Okamoto, T.; Hamatake, M.; Okamoto, I.; et al. The clinical impact of concomitant medication use on the outcome of postoperative recurrent non-small-cell lung cancer in patients receiving immune checkpoint inhibitors. PloS ONE 2022, 17, e0263247. [Google Scholar] [CrossRef] [PubMed]
- Tomisaki, I.; Harada, M.; Minato, A.; Nagata, Y.; Kimuro, R.; Higashijima, K.; Harada, K.; Fujimoto, N. Impact of the Use of Proton Pump Inhibitors on Pembrolizumab Effectiveness for Advanced Urothelial Carcinoma. Anticancer. Res. 2022, 42, 1629–1634. [Google Scholar] [CrossRef]
- Verschueren, M.V.; van der Welle, C.M.C.; Tonn, M.; Schramel, F.; Peters, B.J.M.; van de Garde, E.M.W. The association between gut microbiome affecting concomitant medication and the effectiveness of immunotherapy in patients with stage IV NSCLC. Sci. Rep. 2021, 11, 23331. [Google Scholar] [CrossRef]
- Wang, X.; Liu, C.; Wang, J.; Fan, Y.; Wang, Z.; Wang, Y. Proton pump inhibitors increase the chemosensitivity of patients with advanced colorectal cancer. Oncotarget 2017, 8, 58801–58808. [Google Scholar] [CrossRef][Green Version]
- West, H.; McCleod, M.; Hussein, M.; Morabito, A.; Rittmeyer, A.; Conter, H.J.; Kopp, H.-G.; Daniel, D.; McCune, S.; Mekhail, T.; et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019, 20, 924–937. [Google Scholar] [CrossRef]
- Wong, G.G.; Ha, V.; Chu, M.P.; Dersch-Mills, D.; Ghosh, S.; Chambers, C.R.; Sawyer, M.B. Effects of Proton Pump Inhibitors on FOLFOX and CapeOx Regimens in Colorectal Cancer. Clin. Color. Cancer 2018, 18, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.-H.; Muro, K.; Morita, S.; Iwasa, S.; Han, S.W.; Wang, W.; Kotaka, M.; Nakamura, M.; Ahn, J.B.; Deng, Y.-H.; et al. Modified XELIRI (capecitabine plus irinotecan) versus FOLFIRI (leucovorin, fluorouracil, and irinotecan), both either with or without bevacizumab, as second-line therapy for metastatic colorectal cancer (AXEPT): A multicentre, open-label, randomised, non-inferiority, phase 3 trial. Lancet Oncol. 2018, 19, 660–671. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Gao, G.; Li, W.; Li, X.; Zhao, C.; Jiang, T.; Jia, Y.; He, Y.; Li, A.; Su, C.; et al. Antibiotics are associated with attenuated efficacy of anti-PD-1/PD-L1 therapies in Chinese patients with advanced non-small cell lung cancer. Lung Cancer 2019, 130, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Kostine, M.; Mauric, E.; Tison, A.; Barnetche, T.; Barre, A.; Nikolski, M.; Rouxel, L.; Dutriaux, C.; Dousset, L.; Prey, S.; et al. Baseline co-medications may alter the anti-tumoural effect of checkpoint inhibitors as well as the risk of immune-related adverse events. Eur. J. Cancer 2021, 157, 474–484. [Google Scholar] [CrossRef] [PubMed]
- Kunimitsu, Y.; Morio, K.; Hirata, S.; Yamamoto, K.; Omura, T.; Hara, T.; Harada, K.; Fujisawa, M.; Yano, I. Effects of Proton Pump Inhibitors on Survival Outcomes in Patients with Metastatic or Unresectable Urothelial Carcinoma Treated with Pembrolizumab. Biol. Pharm. Bull. 2022, 45, 590–595. [Google Scholar] [CrossRef]
- Kitazume, Y.; Kawazoe, H.; Uozumi, R.; Yoshizawa, T.; Iihara, H.; Fujii, H.; Takahashi, M.; Arai, T.; Murachi, Y.; Sato, Y.; et al. Proton pump inhibitors affect capecitabine efficacy in patients with stage II–III colorectal cancer: A multicenter retrospective study. Sci. Rep. 2022, 12, 1–10. [Google Scholar] [CrossRef]
- Homicsko, K.; Dummer, R.; Hoeller, C.; Wolchok, J.D.; Hodi, F.S.; Larkin, J.; Ascierto, P.A.; Atkinson, V.; Robert, C.; Postow, M.A.; et al. Proton Pump Inhibitor Use and Efficacy of Nivolumab and Ipilimumab in Advanced Melanoma. Cancers 2022, 14, 2300. [Google Scholar] [CrossRef]
- Fukuokaya, W.; Kimura, T.; Komura, K.; Uchimoto, T.; Nishimura, K.; Oyama, Y.; Abe, H.; Azuma, H.; Miki, J.; Egawa, S. MP03-12 association of use of potassium competitive acid blockers and effectiveness of pembrolizumab in patients with metastatic urothelial carcinoma. J. Urol. 2022, 207, e24. [Google Scholar] [CrossRef]
- Iida, K.; Nagai, T.; Nozaki, S.; Etani, T.; Naiki, T.; Nakane, A.; Akita, H.; Kubota, H.; Kamiya, H.; Kawai, N.; et al. MP41-15 proton pump inhibitor use is a negative prognostic factor for metastatic urothelial carcinoma progression in pembrolizumab-treated patients. J. Urol. 2021, 206, e766–e767. [Google Scholar] [CrossRef]
- Ascierto, P.A.; Long, G.V.; Robert, C.; Brady, B.; Dutriaux, C.; Di Giacomo, A.M.; Mortier, L.; Hassel, J.C.; Rutkowski, P.; McNeil, C.; et al. Survival Outcomes in Patients With Previously Untreated BRAF Wild-Type Advanced Melanoma Treated With Nivolumab Therapy: Three-Year Follow-up of a Randomized Phase 3 Trial. JAMA Oncol. 2019, 5, 187–194. [Google Scholar] [CrossRef]
- Hodi, F.S.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.-J.; Rutkowski, P.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol. 2018, 19, 1480–1492. [Google Scholar] [CrossRef]
- Hodi, F.S.; Chesney, J.; Pavlick, A.C.; Robert, C.; Grossmann, K.F.; McDermott, D.F.; Linette, G.P.; Meyer, N.; Giguere, J.K.; Agarwala, S.S.; et al. Combined nivolumab and ipilimumab versus ipilimumab alone in patients with advanced melanoma: 2-year overall survival outcomes in a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol. 2016, 17, 1558–1568. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.A.; Goodrich, J.K.; Maxan, M.-E.; Freedberg, D.E.; Abrams, J.A.; Poole, A.C.; Sutter, J.L.; Welter, D.; Ley, R.E.; Bell, J.T.; et al. Proton pump inhibitors alter the composition of the gut microbiota. Gut 2016, 65, 749–756. Available online: http://www.ncbi.nlm.nih.gov/pubmed/26719299 (accessed on 19 January 2019). [CrossRef] [PubMed]
- Oster, P.; Vaillant, L.; Riva, E.; McMillan, B.; Begka, C.; Truntzer, C.; Richard, C.; Leblond, M.M.; Messaoudene, M.; Machremi, E.; et al. Helicobacter pylori infection has a detrimental impact on the efficacy of cancer immunotherapies. Gut 2021, 71, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Thorsson, V.; Gibbs, D.L.; Brown, S.D.; Wolf, D.; Bortone, D.S.; Ou Yang, T.-H.; Porta-Pardo, E.; Gao, G.F.; Plaisier, C.L.; Eddy, J.A.; et al. The Immune Landscape of Cancer. Immunity 2018, 48, 812–830.e14. [Google Scholar] [CrossRef] [PubMed]
- Matson, V.; Fessler, J.; Bao, R.; Chongsuwat, T.; Zha, Y.; Alegre, M.-L.; Luke, J.J.; Gajewski, T.F. The commensal microbiome is associated with anti–PD-1 efficacy in metastatic melanoma patients. Science 2018, 359, 104–108. [Google Scholar] [CrossRef]
- Gopalakrishnan, V.; Spencer, C.N.; Nezi, L.; Reuben, A.; Andrews, M.C.; Karpinets, T.V.; Prieto, P.A.; Vicente, D.; Hoffman, K.; Wei, S.C.; et al. Gut microbiome modulates response to anti–PD-1 immunotherapy in melanoma patients. Science 2018, 359, 97–103. [Google Scholar] [CrossRef]
- Ueda, K.; Yonekura, S.; Ogasawara, N.; Matsunaga, Y.; Hoshino, R.; Kurose, H.; Chikui, K.; Uemura, K.; Nakiri, M.; Nishihara, K.; et al. The Impact of Antibiotics on Prognosis of Metastatic Renal Cell Carcinoma in Japanese Patients Treated With Immune Checkpoint Inhibitors. Anticancer. Res. 2019, 39, 6265–6271. [Google Scholar] [CrossRef]
- Elkrief, A.; El Raichani, L.; Richard, C.; Messaoudene, M.; Belkaid, W.; Malo, J.; Belanger, K.; Miller, W.; Jamal, R.; Letarte, N.; et al. Antibiotics are associated with decreased progression-free survival of advanced melanoma patients treated with immune checkpoint inhibitors. Oncoimmunology 2019, 8, e1568812. [Google Scholar] [CrossRef] [PubMed]
- De Milito, A.; Fais, S. Tumor acidity, chemoresistance and proton pump inhibitors. Future Oncol. 2005, 1, 779–786. [Google Scholar] [CrossRef]
- Schmoll, H.-J.; Cunningham, D.; Sobrero, A.; Karapetis, C.S.; Rougier, P.; Koski, S.L.; Kocakova, I.; Bondarenko, I.; Bodoky, G.; Mainwaring, P.; et al. Cediranib With mFOLFOX6 Versus Bevacizumab With mFOLFOX6 As First-Line Treatment for Patients With Advanced Colorectal Cancer: A Double-Blind, Randomized Phase III Study (HORIZON III). J. Clin. Oncol. 2012, 30, 3588–3595. [Google Scholar] [CrossRef] [PubMed]
- Nishi, T.; Forgac, M. The vacuolar (H+)-ATPases--nature’s most versatile proton pumps. Nat. Rev. Mol. Cell Biol. 2002, 3, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Marino, M.L.; Fais, S.; Djavaheri-Mergny, M.; Villa, A.; Meschini, S.; Lozupone, F.; Venturi, G.; Della Mina, P.; Pattingre, S.; Rivoltini, L.; et al. Proton pump inhibition induces autophagy as a survival mechanism following oxidative stress in human melanoma cells. Cell Death Dis. 2010, 1, e87. [Google Scholar] [CrossRef]
- Lalani, A.A.; McKay, R.R.; Lin, X.; Simantov, R.; Kaymakcalan, M.D.; Choueiri, T.K. Proton Pump Inhibitors and Survival Outcomes in Patients With Metastatic Renal Cell Carcinoma. Clin. Genitourin. Cancer 2017, 15, 724–732. [Google Scholar] [CrossRef] [PubMed]
- Cheng, V.; Lemos, M.; Hunter, N.; Badry, N.; Lemos, J. Concomitant use of capecitabine and proton pump inhibitors—Is it safe? J. Oncol. Pharm. Pract. 2019, 25, 1705–1711. [Google Scholar] [CrossRef]
- Röhss, K.; Lind, T.; Wilder-Smith, C. Esomeprazole 40 mg provides more effective intragastric acid control than lansoprazole 30 mg, omeprazole 20 mg, pantoprazole 40 mg and rabeprazole 20 mg in patients with gastro-oesophageal reflux symptoms. Eur. J. Clin. Pharmacol. 2004, 60, 531–539. [Google Scholar] [CrossRef]
- Wishart, D.S.; Feunang, Y.D.; Guo, A.C.; Lo, E.J.; Marcu, A.; Grant, J.R.; Sajed, T.; Johnson, D.; Li, C.; Sayeeda, Z.; et al. DrugBank 5.0: A Major Update to the DrugBank Database for 2018. Nucleic Acids Res. 2018, 46, D1074–D1082. [Google Scholar] [CrossRef]
- Diaz, L.A.; Shiu, K.-K., Jr.; Kim, T.-W.; Jensen, B.V.; Jensen, L.H.; Punt, C.; Smith, D.; Garcia-Carbonero, R.; Benavides, M.; Gibbs, P.; et al. Pembrolizumab versus chemotherapy for microsatellite instability-high or mismatch repair-deficient metastatic colorectal cancer (KEYNOTE-177): Final analysis of a randomised, open-label, phase 3 study. Lancet Oncol. 2022, 23, 659–670. [Google Scholar] [CrossRef]
- Le, D.T.; Uram, J.N.; Wang, H.; Bartlett, B.R.; Kemberling, H.; Eyring, A.D.; Skora, A.D.; Luber, B.S.; Azad, N.S.; Laheru, D.; et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N. Engl. J. Med. 2015, 372, 2509–2520. [Google Scholar] [CrossRef]
- Bellmunt, J.; De Wit, R.; Vaughn, D.J.; Fradet, Y.; Lee, J.-L.; Fong, L.; Vogelzang, N.J.; Climent, M.A.; Petrylak, D.P.; Choueiri, T.K.; et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N. Engl. J. Med. 2017, 376, 1015–1026. [Google Scholar] [CrossRef]
- Fradet, Y.; Bellmunt, J.; Vaughn, D.J.; Lee, J.L.; Fong, L.; Vogelzang, N.J.; Climent, M.A.; Petrylak, D.P.; Choueiri, T.K.; Necchi, A.; et al. Randomized phase III KEYNOTE-045 trial of pembrolizumab versus paclitaxel, docetaxel, or vinflunine in recurrent advanced urothelial cancer: Results of >2 years of follow-up. Ann. Oncol. 2019, 30, 970–976. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Retz, M.; Siefker-Radtke, A.; Baron, A.; Necchi, A.; Bedke, J.; Plimack, E.R.; Vaena, D.; Grimm, M.-O.; Bracarda, S.; et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): A multicentre, single-arm, phase 2 trial. Lancet Oncol. 2017, 18, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; O’Donnell, P.H.; Massard, C.; Arkenau, H.T.; Friedlander, T.W.; Hoimes, C.J.; Lee, J.L.; Ong, M.; Sridhar, S.S.; Vogelzang, N.J.; et al. Efficacy and Safety of Durvalumab in Locally Advanced or Metastatic Urothelial Carcinoma: Updated Results From a Phase 1/2 Open-label Study. JAMA Oncol. 2017, 3, e172411. [Google Scholar] [CrossRef] [PubMed]
- Overman, M.J.; McDermott, R.; Leach, J.L.; Lonardi, S.; Lenz, H.-J.; Morse, M.A.; Desai, J.; Hill, A.; Axelson, M.; Moss, R.A.; et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): An open-label, multicentre, phase 2 study. Lancet Oncol. 2017, 18, 1182–1191. [Google Scholar] [CrossRef]

| Included Studies | Study Type | Country | Inclusion Period | Sample Size, n | Therapeutic Modality | Treatment-Naïve, n (%) | PPI *, n (%) | PPI Use Window | H2-Blocker, n (%) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Immune checkpoint inhibitors (n= 36) | CTLA-4, n (%) | PD-1, n (%) | PD-L1, n (%) | ||||||||
| Advanced NSCLC (n = 17) | |||||||||||
| IMpower130 [71] | RCT | 8 countries | 2015–2017 | 483 | 0 | 0 | 483 (100) | 483 (100) | N/A¶ | Following Rx | N/A |
| IMpower131 [51] | RCT | 26 countries | 2015–2017 | 681 | 0 | 0 | 681 (100) | 681 (100) | N/A¶ | Following Rx | N/A |
| IMpower150 [20] | RCT | 26 countries | 2015–2016 | 802 | 0 | 0 | 802 (100) | N/A | 290 (36.2) | 30 d before/after Rx | N/A |
| POPLAR [22] | RCT | USA/Europe | 2013–2014 | 144 | 0 | 0 | 144 (100) | 0 (0) | N/A | 30 d before/after Rx | N/A |
| OAK [21] | RCT | 31 countries | 2014–2015 | 613 | 0 | 0 | 613 (100) | 0 (0) | N/A | 30 d before/after Rx | N/A |
| Baek 2022 [31] | Retrospective | South Korea | 2017–2018 | 1646 | 0 | 1595 (97) | 51 (3) | 0 (0) | 823 (50) | 30 d before Rx | N/A |
| Takada 2022 [67] | Retrospective | Japan | 2016–2019 | 95 | 0 | 85 (89.5) | 10 (10.5) | N/A | 37 (39) | N/A | N/A |
| Alessio 2021 [38] | Retrospective | Italy | 2017–2020 | 950 | 0 | 950 (100) | 0 | 950 (100) | 474 (49.9) | N/A | N/A |
| Balado 2021 [34] | Retrospective | Spain | 2017–2020 | 49 | 0 | 49 (100) | 0 | 49 (100) | 26 (53.1) | 30 d before/after Rx | N/A |
| Kostine 2021 [75] | Retrospective | France | 2015–2017 | 150 | N/A | N/A | N/A | N/A | N/A | 30 d before/after Rx | N/A |
| Muira 2021 [56] | Retrospective | Japan | 2016–2018 | 300 | 0 | 300 (100) | 0 | 40 (13.3) | 163 (54.3) | N/A | N/A |
| Rounis 2021 [61] | Retrospective | Greece | 2017~2019 | 66 | 0 | 66 (1000) | 0 | 0 (0) | 23 (34.8) | 90 d before Rx | N/A |
| Verschueren 2021 [69] | Retrospective | Netherlands | 2015~2019 | 221 | 0 | 214 (97) | 7 (3) | 84 (38) | 96 (43.4) | 30 d before/after Rx | N/A |
| Estevez 2020 [37] | Retrospective | Spain | 2015~2018 | 70 | 0 | 64 (91.4) | 6 (8.6) | 0 (0) | 59 (84.3) | 90 d before Rx | N/A |
| Hossain 2020 [47] | Retrospective | Australia | 2015~2019 | 63 | N/A | N/A | N/A | N/A | 34 (54) | 28 d after Rx | N/A |
| Svaton 2020 [65] | Retrospective | Czech | 2015~2019 | 224 | 0 | 224 (100) | 0 | 9 (4.0) | 64 (28.6) | 30 d before/after Rx | N/A |
| Zhao 2019 [74] | Retrospective | China | 2016–2018 | 109 | 0 | 109 (100) | 0 | 28 (25.7) | 40 (36.7) | 30 d before/after Rx | N/A |
| Advanced UC (n = 8) | |||||||||||
| IMvigor 210 [32] | RCT | USA | 2014~2015 | 429 | 0 | 0 | 429 (100) | 119 (22.5) | 141 (32.9) | 30 d before/after Rx | N/A |
| IMvigor 211 [19] | RCT | USA | 2015~2017 | 467 | 0 | 0 | 467 (100) | 0 (0) | 145 (31.0) | 30 d before/after Rx | N/A |
| Fukuokaya 2022 [79] | Retrospective | Japan | N/A | 227 | N/A | N/A | N/A | N/A | 56 (24.7) | N/A | N/A |
| Kunimitsu 2022 [76] | Retrospective | Japan | 2017~2020 | 79 | 0 | 0 | 79 (100) | 0 (0) | 34 (43.0) | 30 d before/60 d after Rx | N/A |
| Okuyama 2022 [58] | Retrospective | Japan | 2015~2021 | 155 | 0 | 0 | 155 (100) | 0 (0) | 99 (63.9) | 30 d before Rx | N/A |
| Tomisaki 2022 [68] | Retrospective | Japan | 2018–2021 | 40 | 0 | 40 (100) | 0 | 0 (0) | 15 (37.5) | 60 d before/30 d after Rx | N/A |
| Bañobre 2021 [62] | Retrospective | Spain | 2016~2020 | 119 | 0 | 39 (32.7) | 80 (67.3) | 22 (18.5) | 54 (45.4) | N/A | N/A |
| Lida 2021 [80] | Retrospective | Japan | 2018~2021 | 115 | 0 | 0 | 115 (100) | 0 (0) | N/A | 30 d before/after Rx | N/A |
| Advanced melanoma (n = 8) | |||||||||||
| CheckMate 066 [81] | RCT | 80 centers | 2013~2021 | 210 | Nivolumab: 210 (100) | 210 (100) | 49 (23.3) | 30 d before Rx | N/A | ||
| CheckMate 067 [82] | RCT | 21 countries | 2013~now | 945 | Ipilimumab + Nivolumab: 314 (33.2); Ipilimumab: 315 (33.3); Nivolumab: 316 (33.4) | 945 (100) | 161 (17.0) | 30 d before Rx | N/A | ||
| CheckMate 069 [83] | RCT | France/ USA | 2013~2021 | 142 | Ipilimumab + Nivolumab: 95 (67.0); Ipilimumab: 47 (33.1) | 142 (100) | 33 (23.3) | 30 d before Rx | N/A | ||
| Gaucher 2021 [41] | Retrospective | Brazil | 2010~2019 | 110 | 15 (13.6) | 68 (61.8) | 27 (24.6) | 110 (100) | 39 (35.5) | 60 d after Rx | N/A |
| Kostine 2021 [75] | Retrospective | France | 2015~2017 | 293 | N/A | N/A | N/A | N/A | N/A | 30 d before/after Rx | N/A |
| Peng 2021 [59] | Retrospective | USA | 2014~2019 | 233 | 0 | 233 (100) | 0 | 95 (40.8) | 89 (38.2) | 30 d before/after Rx | N/A |
| Afzal 2019 [29] | Retrospective | Lebanon | N/A | 120 | Ipilimumab and/or Pembrolizumab | N/A | 29 (24.2) | N/A | N/A | ||
| Failing 2016 [40] | Retrospective | USA | 2011~2014 | 159 | Ipilimumab:159 (100) | 80 (50) § | 39 (24.5) | N/A | 9 (6) | ||
| Advanced RCC (n = 3) | |||||||||||
| Mollica 2022 [57] | Retrospective | USA | 2010~2021 | 63 | 63 (100) | 0 | 63 (100) | 63 (100) | 25 (39.7) | N/A | N/A |
| Mollica 2022 [57] | Retrospective | USA | 2010~2021 | 156 | 0 | 0 | 156 (100) | 110 (70.5) | 88 (56.4) | N/A | N/A |
| Kostine 2021 [75] | Retrospective | France | 2015~2017 | 83 | N/A | N/A | N/A | N/A | N/A | 30 d before/after Rx | N/A |
| Peng 2021 [59] | Retrospective | USA | 2014~2019 | 233 | 0 | 233 (100) | 0 | 95 (40.8) | 89 (38.2) | 30 d before/after Rx | N/A |
| HCC (n = 2) | |||||||||||
| Jun 2020 [52] | Retrospective | USA | 2017~2019 | 314 | 21 (7) | 293 (93) | 0 | 137 (43.6) | 110 (35.0) | 30 d before Rx | 45 (14.3) |
| Lee 2020 [55] | Retrospective | Taiwan | 2017~2019 | 94 | N/A | N/A | N/A | N/A | 30 (31.9) | 30 d before Rx | N/A |
| Uncategorized cancers † (n = 10) | |||||||||||
| Araujo 2021 [30] | Retrospective | Brazil | N/A | 216 | 35 (16.2) | 130 (60.2) | 27 (12.5) | 0 (0) | 114 (52.8) | 60 d before/after Rx | N/A |
| Buti 2021 [9] | Retrospective | Italy | 2014~2019 | 217 | 13 (6.0) | 186 (85.7) | 18 (8.3) | 45 (20.7) | 104 (47.9) | N/A | N/A |
| Gaucher 2021 [41] | Retrospective | Brazil | 2010~2019 | 370 | 25 (5.4) | 357 (94.6) | 0 | 87 (23.4) | 149 (40.1) | 60 d after Rx | N/A |
| Giordan 2021 [42] | Retrospective | France | 2018~2019 | 154 | 0 | 0 | 154 (100) | 64 (41.6) | 47 (30.5) | 30 d before Rx | N/A |
| Husain 2021 [49] | Retrospective | USA | 2011~2019 | 1091 | 274 (25.1) | 817 (74.9) | N/A | 415 (38.0) | N/A | N/A | |
| Peng 2021 [59] | Retrospective | USA | 2014~2019 | 233 | 0 | 233 (100) | 0 | 95 (40.8) | 89 (38.2) | 30 d before/after Rx | N/A |
| Alessio 2020 [10] | Retrospective | Italy | 2014~2020 | 1012 | 0 | 956 (94.5) | 56 (5.5) | 396 (39.1) | 491 (48.5) | N/A | 56 (5.5) |
| Santamaria 2020 [50] | Retrospective | Spain | 2015~2018 | 102 | 1 (1.0) | 86 (84.3) | 15 (14.7) | 73 (71.6) | 78 (77.2) | 30 d before/after Rx | N/A |
| Ruiz 2020 [60] | Retrospective | Spain | from 2015 | 253 | 31 (12.3) | 222 (87.7) | 0 (0) | 73 (28.9) | 135 (53.4) | 60 d before/30 d after Rx | N/A |
| Kostine 2021 [75] | Retrospective | France | 2015~2017 | 635 | 3 (0.5) | 435 (68.5) | 66 (10) | N/A | 293 (46.1) | 30 d before/after Rx | N/A |
| Chemotherapy (n = 19) | |||||||||||
| Advanced NSCLC (n = 6) | |||||||||||
| Verschueren 2021 [69] | Retrospective | Netherlands | 2015–2019 | 221 | Platinum-based agents | 84 (38) | 101 (45.7) | 30 d before/after Rx | N/A | ||
| Impower 130 [71] | RCT | 8 countries | 2015–2017 | 240 | Platinum-based agents | 240 (100) | N/A¶ | Following Rx | N/A | ||
| IMpower 131 [51] | RCT | 26 countries | 2015–2017 | 340 | Platinum-based agents | 340 (100) | N/A¶ | Following Rx | N/A | ||
| IMpower 150 [20] | RCT | 26 countries | 2015–2016 | 400 | Platinum-based agents | N/A | 151 (37.8) | 30 d before/after Rx | N/A | ||
| POPLAR [22] | RCT | USA/Europe | 2013~2014 | 143 | Docetaxel | 0 (0) | N/A | 30 d before/after Rx | N/A | ||
| OAK [21] | RCT | 31 countries | 2014~2015 | 612 | Docetaxel | 0 (0) | N/A | 30 d before/after Rx | N/A | ||
| Advanced UC (n = 1) | |||||||||||
| IMvigor 211 [19] | RCT | USA | 2015~2017 | 464 | Platinum-based agents | 0 (0) | 185 (39.9) | 30 d before/after Rx | N/A | ||
| Advanced melanoma (n = 1) | |||||||||||
| CheckMate 066 | RCT | 80 centers | 2013~2021 | 208 | Decarbazine | 0 (0) | 48 (23.1) | 30 d before Rx | N/A | ||
| Uncategorized cancers † (n = 1) | |||||||||||
| Alessio 2021 [38] | Retrospective | Italy | 2017~2020 | 595 | Platinum-based agents | 595 (100) | 321 (53.7) | N/A | N/A | ||
| FOLFOX, n (%) | FOLFIRI/ IFL, n (%) | Cape-based, n (%) | |||||||||
| Gastroesophageal carcinoma (n = 1) | |||||||||||
| TRIO013/ LOGiC [43] | RCT | 22 countries | 2008~2012 | 274 | 0 | 0 | 274 (100) | 274 (100) | 119 (43.4) | 20% overlapping Rx | N/A |
| Early-stage colorectal cancer (n = 3) | |||||||||||
| Kitazume 2022 [77] | Retrospective | Japan | 2009~2014 | 606 | 0 | 0 | 606 (100) | 606 (100) | 54 (8.9) | 20% overlapping Rx | N/A |
| Wong 2019 [72] | Retrospective | Canada | 2004~2013 | 389 | 175 (45) | 0 | 214 (55) | 389 (100) | 99 (25.4) | During Rx | N/A |
| Sun 2016 [64] | Retrospective | Canada | 2008~2012 | 298 | 0 | 0 | 298 (100) * | 298 (100) | 77 (26.0) | During Rx | N/A |
| Advanced colorectal cancer (n = 8) | |||||||||||
| Wang 2017 [70] | Retrospective | China | 2010~2014 | 671 | 307 (45.8) | 0 | 364 (54.2) | N/A | 474 (70.6) | During Rx | N/A |
| AXEPT [73] | RCT | Asia | 2013~2015 | 482 | 0 | 243 (50.4) | 239 (49.6) | 0 (0) | 49 (10.2) | 20% overlapping Rx | N/A |
| HORIZON III [92] | RCT | 28 countries | 2006~2009 | 666 | 666 (100) | 0 | 0 | 666 (100) | 87 (13.0) | During Rx | 15 (2.2) |
| N016966 [63] | RCT | N/A | 2004~2005 | 2035 | 1018 (50) | 0 | 1017 (50) | 2035 (100) | 327 (32.1) | During Rx | 115 (5.7) |
| AVF2107g [48] | RCT | 3 countries | 2000~2002 | 780 | 0 | 780 (100) | 0 | 780 (100) | 156 (20.0) | During Rx | 129 (16.5) |
| Carrato 2013 [33] | RCT | N/A | 2007~2010 | 348 | 0 | 348 (100) | 0 | 348 (100) | 39 (11.0) | During Rx | 15 (4.2) |
| VELOUR [39] | RCT | 28 countries | 2007~2010 | 584 | 0 | 584 (100) | 0 | 0 (0) | 105 (18.0) | During Rx | 16 (2.8) |
| RAISE [66] | RCT | 24 countries | 2010~2013 | 946 | 0 | 946 (100) | 0 | 0 (0) | 232 (24.5) | During Rx | 36 (3.8) |
| Post hoc analysis of RCTs (n = 8) | |||||||||||
| Hopkins 2022 [44] | Analysis of 5 trials [20,21,22,51,71] | 4458 | Advanced NSCLC | Immune checkpoint and chemotherapy | N/A | 1225 (27.5)¶ | 30 d before/after Rx | N/A | |||
| Hopkins 2021 [46] | Analysis of IMpower 150 [20] | 1202 | Advanced NSCLC | Immune checkpoint and chemotherapy | N/A | 441 (36.7) | 30 d before/after Rx | N/A | |||
| Homicsko 2022 [78] | Analysis of CheckMate 066 [81]/067 [82]/069 [83] | 1505 | Advanced Melanoma | Immune checkpoint and chemotherapy | 1505 (100) | 291 (19.3) | 30 d before Rx | N/A | |||
| Chalabi 2020 [35] | Analysis of OAK7 and POPLAR [21,22] | 757 | Advanced NSCLC | Immune checkpoint and chemotherapy | 0 (0) | 234 (30.9) | 30 d before/after Rx | N/A | |||
| Hopkins 2020 [45] | Analysis of IMvigor 210 [24] and 211 [19,32] | 1360 | Advanced UC | Immune checkpoint and chemotherapy | 119 (8.75) | 471 (34.6) | 30 d before/after Rx | N/A | |||
| Kim 2021 [54] | Analysis of AXEPT [73] | 482 | Advanced CRC | Chemotherapy | 0 (0) | 49 (10.2) | 20% overlapping Rx | N/A | |||
| Kichenadasse 2021 [53] | Analysis of 6 trials [33,39,48,63,66,92] | 5359 | Advanced CRC | Chemotherapy | 3829 (71.4) | 946 (17.7) | During Rx | N/A | |||
| Chu 2017 [36] | Analysis of TRIO013/LOGiC [43] | 274 | Advanced GEC | Chemotherapy | 274 (100) | 119 (43.4) | 20% overlapping Rx | N/A | |||
| (A) Overall Survival | |||
| Immune check point inhibitors (p-score, 1.0000) | 0.76 (0.67–0.85) | 0.70 (0.62–0.78) | 0.64 (0.53–0.78) |
| 0.79 (0.72–0.86) | Chemotherapy (p-score, 0.6664) | 0.85 (0.70–1.04) | 0.82 (0.76–0.89) |
| 0.67 (0.60–0.73) | 0.85 (0.76–0.94) | Immune check point inhibitors and PPI (p-score, 0.2016) | 1.07 (0.92–1.24) |
| 0.66 (0.60–0.72) | 0.84 (0.78–0.90) | 0.99 (0.89–1.09) | Chemotherapy and PPI (p-score, 0.1319) |
| (B) Progression-Free Survival | |||
| Immune check point inhibitors (p-score, 0.9706) | 0.84 (0.70–1.00) | 0.90 (0.71–1.13) | 0.72 (0.61–0.85) |
| 0.92 (0.81–1.04) | Chemotherapy (p-score, 0.6958) | 0.84 (0.77–0.92) | 0.81 (0.64–1.03) |
| 0.80 (0.70–0.91) | 0.87 (0.80–0.94) | Chemotherapy and PPI (p-score, 0.3217) | 0.83 (0.68–1.02) |
| 0.71 (0.62–0.80) | 0.77 (0.67–0.88) | 0.89 (0.78–1.01) | Immune check point inhibitors and PPI (p-score, 0.0119) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Y.; Lin, W.-Y.; Chang, Y.-C.; Huang, C.-H.; Tzeng, H.-E.; Abdul-Lattif, E.; Wang, T.-H.; Tseng, T.-H.; Kang, Y.-N.; Chi, K.-Y. The Association between Baseline Proton Pump Inhibitors, Immune Checkpoint Inhibitors, and Chemotherapy: A Systematic Review with Network Meta-Analysis. Cancers 2023, 15, 284. https://doi.org/10.3390/cancers15010284
Chang Y, Lin W-Y, Chang Y-C, Huang C-H, Tzeng H-E, Abdul-Lattif E, Wang T-H, Tseng T-H, Kang Y-N, Chi K-Y. The Association between Baseline Proton Pump Inhibitors, Immune Checkpoint Inhibitors, and Chemotherapy: A Systematic Review with Network Meta-Analysis. Cancers. 2023; 15(1):284. https://doi.org/10.3390/cancers15010284
Chicago/Turabian StyleChang, Yu, Wan-Ying Lin, Yu-Cheng Chang, Chin-Hsuan Huang, Huey-En Tzeng, Eahab Abdul-Lattif, Tsu-Hsien Wang, Tzu-Hsuan Tseng, Yi-No Kang, and Kuan-Yu Chi. 2023. "The Association between Baseline Proton Pump Inhibitors, Immune Checkpoint Inhibitors, and Chemotherapy: A Systematic Review with Network Meta-Analysis" Cancers 15, no. 1: 284. https://doi.org/10.3390/cancers15010284
APA StyleChang, Y., Lin, W.-Y., Chang, Y.-C., Huang, C.-H., Tzeng, H.-E., Abdul-Lattif, E., Wang, T.-H., Tseng, T.-H., Kang, Y.-N., & Chi, K.-Y. (2023). The Association between Baseline Proton Pump Inhibitors, Immune Checkpoint Inhibitors, and Chemotherapy: A Systematic Review with Network Meta-Analysis. Cancers, 15(1), 284. https://doi.org/10.3390/cancers15010284






