Radiotherapy Fraction in Limited-Stage Small Cell Lung Cancer in the Modern Era: A Systematic Review and Meta-Analysis of 8006 Reconstructed Individual Patient Data
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods and Materials
2.1. Search Strategy and Eligibility Criteria
2.2. Assessment of Study Quality
2.3. Data Extraction and IPD Reconstruction
2.4. Treatment Characteristics
2.5. Outcomes
2.6. Data Analysis
2.7. Subgroup Analysis
3. Results
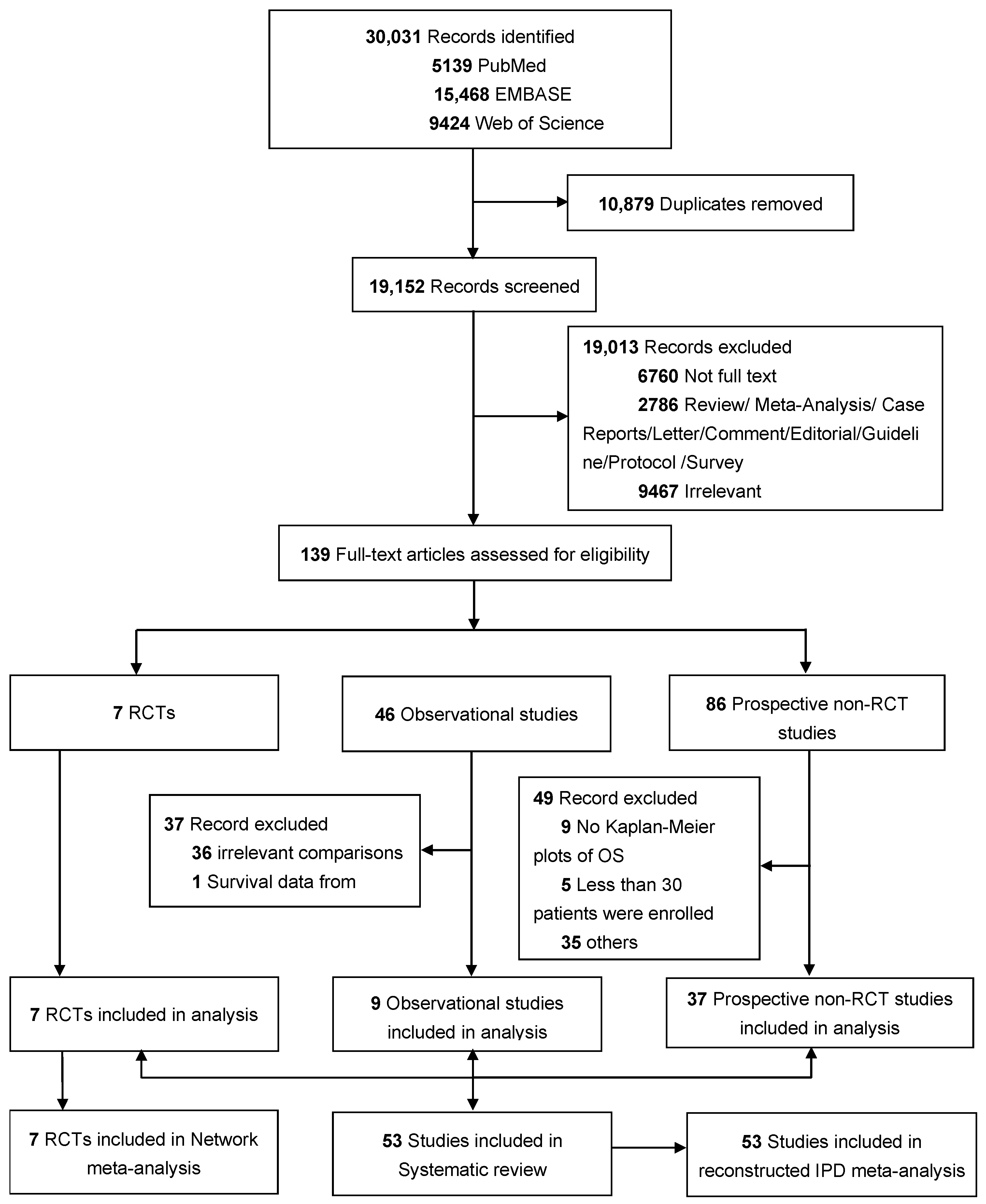
3.1. Study Selection and Characteristics
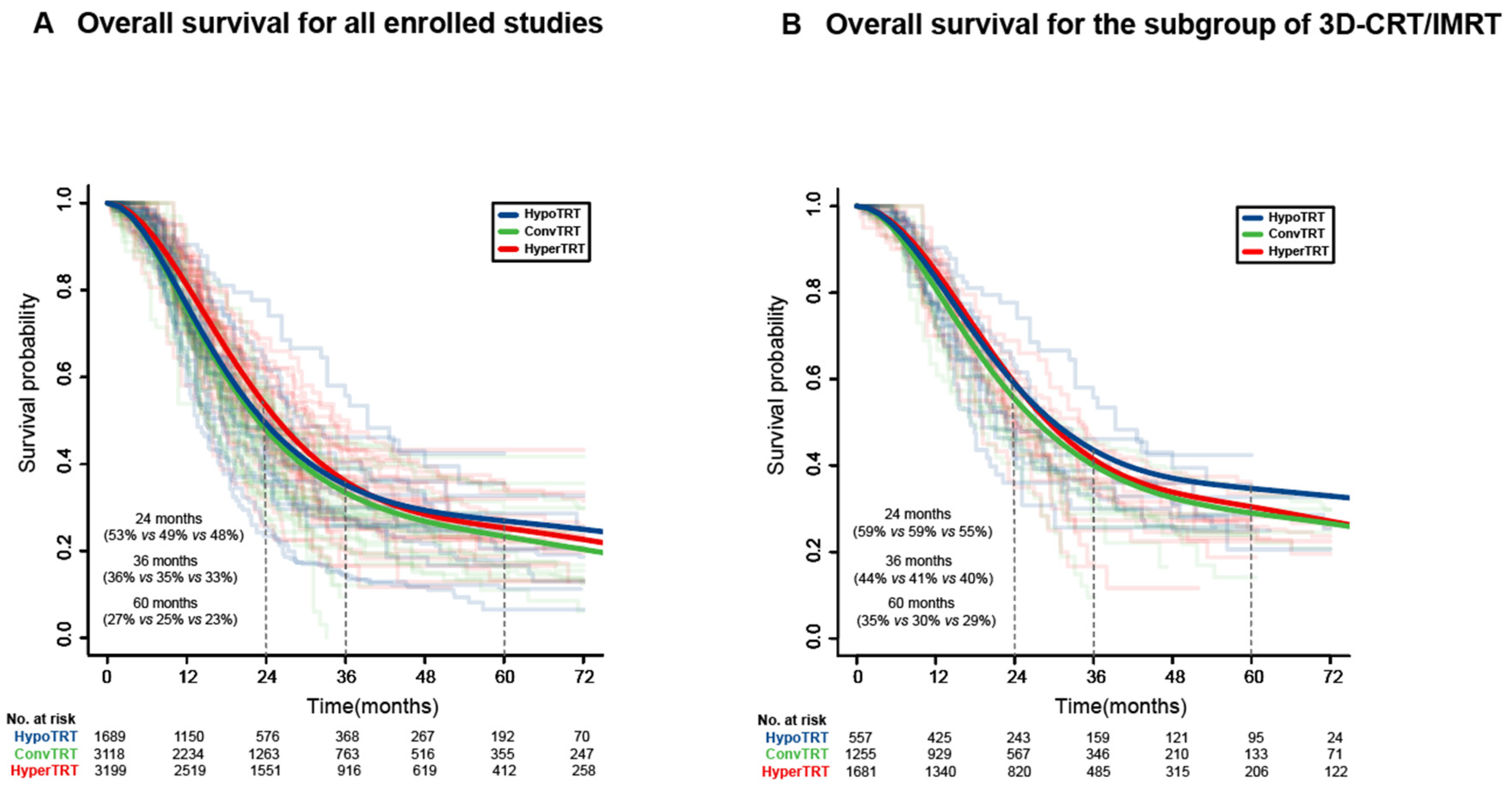
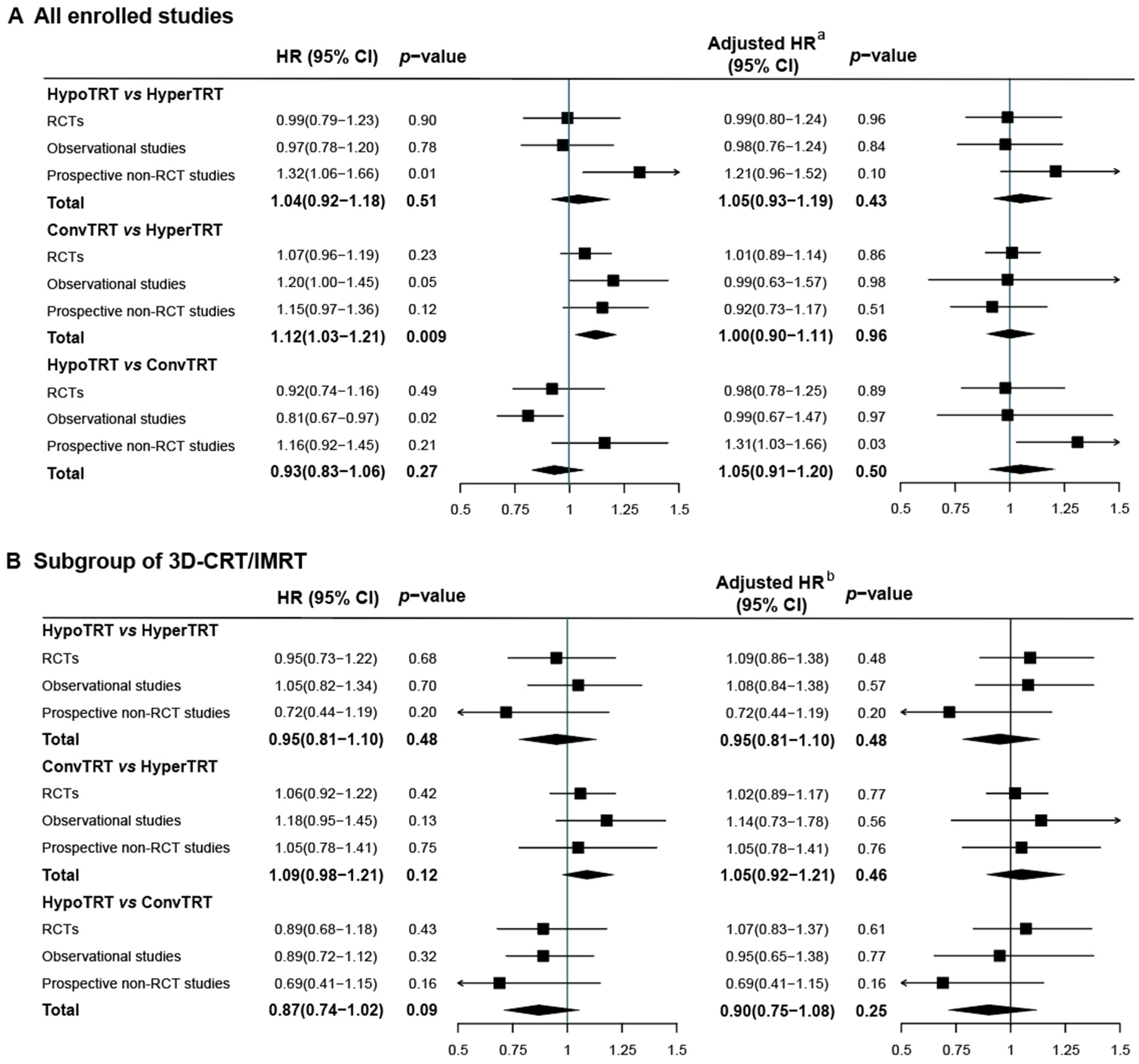
3.2. IPD Meta-Analysis
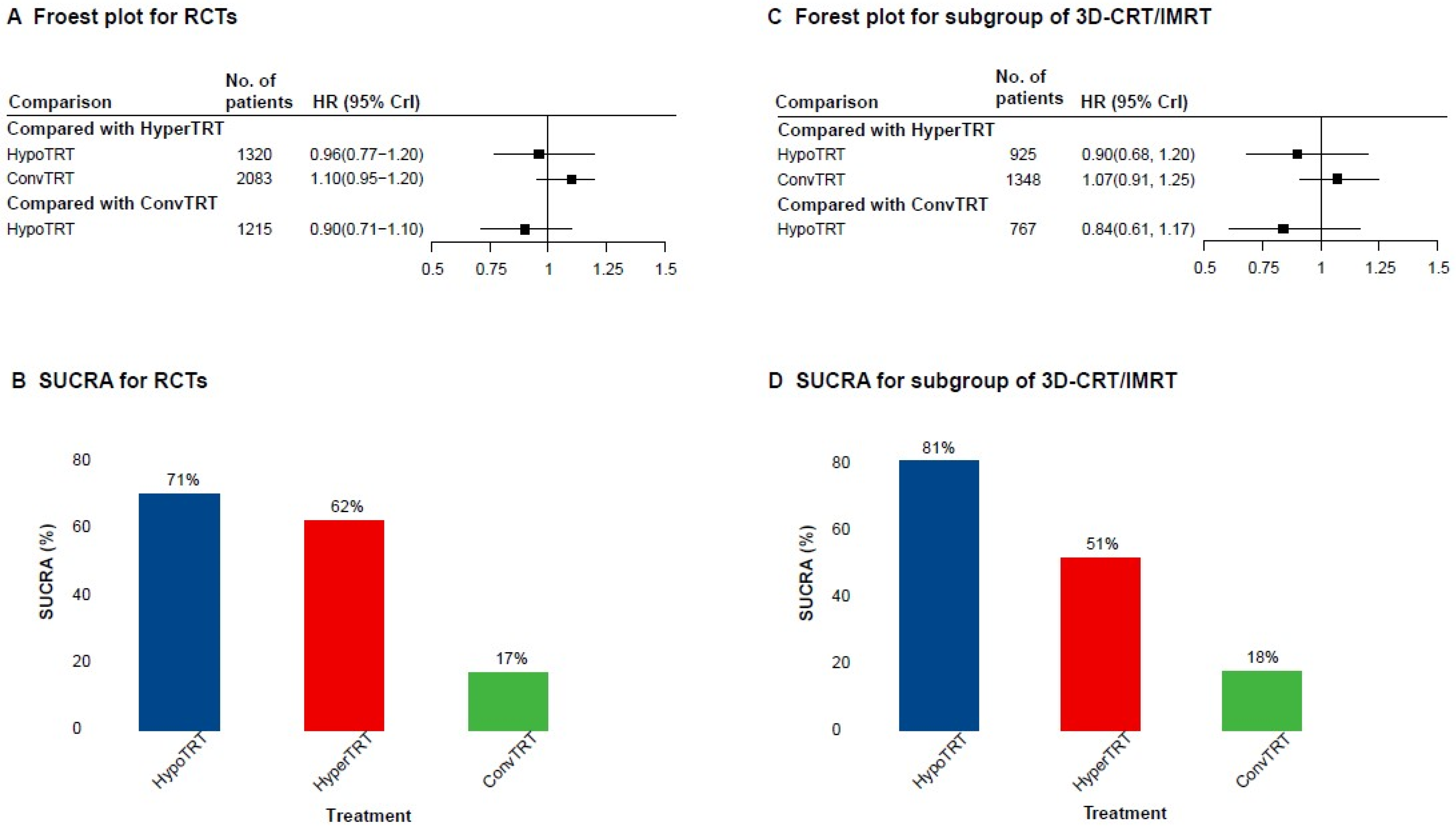
3.3. AD Network Meta-Analysis
3.4. Subgroup Analysis of 3D-CRT or IMRT
3.5. Incidences of RE and RP
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Pignon, J.P.; Arriagada, R.; Ihde, D.C.; Johnson, D.H.; Perry, M.C.; Souhami, R.L.; Brodin, O.; Joss, R.A.; Kies, M.S.; Lebeau, B.; et al. A meta-analysis of thoracic radiotherapy for small-cell lung cancer. N. Engl. J. Med. 1992, 327, 1618–1624. [Google Scholar] [CrossRef] [PubMed]
- Warde, P.; Payne, D. Does thoracic irradiation improve survival and local control in limited-stage small-cell carcinoma of the lung? A meta-analysis. J. Clin. Oncol. 1992, 10, 890–895. [Google Scholar] [CrossRef]
- Zeng, H.; De Ruysscher, D.K.M.; Hu, X.; Zheng, D.; Yang, L.; Ricardi, U.; Kong, F.-M.S.; Hendriks, L.E.L. Radiotherapy for small cell lung cancer in current clinical practice guidelines. J. Natl. Cancer Cent. 2022, 2, 113–125. [Google Scholar] [CrossRef]
- De Ruysscher, D.; Lueza, B.; Le Péchoux, C.; Johnson, D.H.; O’Brien, M.; Murray, N.; Spiro, S.; Wang, X.; Takada, M.; Lebeau, B.; et al. Impact of thoracic radiotherapy timing in limited-stage small-cell lung cancer: Usefulness of the individual patient data meta-analysis. Ann. Oncol. 2016, 27, 1818–1828. [Google Scholar] [CrossRef] [PubMed]
- De Ruysscher, D.; Pijls-Johannesma, M.; Bentzen, S.M.; Minken, A.; Wanders, R.; Lutgens, L.; Hochstenbag, M.; Boersma, L.; Wouters, B.; Lammering, G.; et al. Time between the first day of chemotherapy and the last day of chest radiation is the most important predictor of survival in limited-disease small-cell lung cancer. J. Clin. Oncol. 2006, 24, 1057–1063. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.J.; Tannock, I.F. Repopulation of cancer cells during therapy: An important cause of treatment failure. Nat. Rev. Cancer 2005, 5, 516–525. [Google Scholar] [CrossRef]
- Turrisi, A.T.; Kim, K.; Blum, R.; Sause, W.T.; Livingston, R.B.; Komaki, R.; Wagner, H.; Aisner, S.; Johnson, D.H. Twice-daily compared with once-daily thoracic radiotherapy in limited small-cell lung cancer treated concurrently with cisplatin and etoposide. N. Engl. J. Med. 1999, 340, 265–271. [Google Scholar] [CrossRef]
- Chun, S.G.; Hu, C.; Choy, H.; Komaki, R.U.; Timmerman, R.D.; Schild, S.E.; Bogart, J.A.; Dobelbower, M.C.; Bosch, W.; Galvin, J.M.; et al. Impact of Intensity-Modulated Radiation Therapy Technique for Locally Advanced Non-Small-Cell Lung Cancer: A Secondary Analysis of the NRG Oncology RTOG 0617 Randomized Clinical Trial. J. Clin. Oncol. 2017, 35, 56–62. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, Z.; Liang, J.; Feng, Q.; Xiao, Z.; Hui, Z.; Wang, X.; Lv, J.; Chen, D.; Zhang, H.; et al. Intensity-Modulated Radiation Therapy May Improve Local-Regional Tumor Control for Locally Advanced Non-Small Cell Lung Cancer Compared With Three-Dimensional Conformal Radiation Therapy. Oncologist 2016, 21, 1530–1537. [Google Scholar] [CrossRef]
- Faivre-Finn, C.; Snee, M.; Ashcroft, L.; Appel, W.; Barlesi, F.; Bhatnagar, A.; Bezjak, A.; Cardenal, F.; Fournel, P.; Harden, S.; et al. Concurrent once-daily versus twice-daily chemoradiotherapy in patients with limited-stage small-cell lung cancer (CONVERT): An open-label, phase 3, randomised, superiority trial. Lancet Oncol. 2017, 18, 1116–1125. [Google Scholar] [CrossRef]
- Bogart, J.A.; Wang, X.F.; Masters, G.A.; Gao, J.; Komaki, R.; Kuzma, C.S.; Heymach, J.; Petty, W.J.; Gaspar, L.E.; Waqar, S.N.; et al. Phase 3 comparison of high-dose once-daily (QD) thoracic radiotherapy (TRT) with standard twice-daily (BID) TRT in limited stage small cell lung cancer (LSCLC): CALGB 30610 (Alliance)/RTOG 0538. J. Clin. Oncol. 2021, 39, 8505. [Google Scholar] [CrossRef]
- Daly, M.E.; Ismaila, N.; Decker, R.H.; Higgins, K.; Owen, D.; Saxena, A.; Franklin, G.E.; Donaldson, D.; Schneider, B.J. Radiation Therapy for Small-Cell Lung Cancer: ASCO Guideline Endorsement of an ASTRO Guideline. J. Clin. Oncol. 2021, 39, 931–939. [Google Scholar] [CrossRef] [PubMed]
- Ganti, A.K.P.; Loo, B.W.; Bassetti, M.; Blakely, C.; Chiang, A.; D’Amico, T.A.; D’Avella, C.; Dowlati, A.; Downey, R.J.; Edelman, M.; et al. Small Cell Lung Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 1441–1464. [Google Scholar] [CrossRef]
- Zhang, J.; Fan, M.; Liu, D.; Zhao, K.-L.; Wu, K.-L.; Zhao, W.-X.; Zhu, Z.-F.; Fu, X.-L. Hypo- or conventionally fractionated radiotherapy combined with chemotherapy in patients with limited stage small cell lung cancer. Radiat. Oncol. 2017, 12, 51. [Google Scholar] [CrossRef] [PubMed]
- Zayed, S.; Chen, H.; Ali, E.; Rodrigues, G.B.; Warner, A.; Palma, D.A.; Louie, A.V. Is There a Role for Hypofractionated Thoracic Radiation Therapy in Limited-Stage Small Cell Lung Cancer? A Propensity Score Matched Analysis. Int. J. Radiat. Oncol. Biol. Phys. 2020, 108, 575–586. [Google Scholar] [CrossRef]
- Yan, M.; Sigurdson, S.; Greifer, N.; Kennedy, T.A.C.; Toh, T.S.; Lindsay, P.E.; Weiss, J.; Hueniken, K.; Yeung, C.; Sugumar, V.; et al. A Comparison of Hypofractionated and Twice-Daily Thoracic Irradiation in Limited-Stage Small-Cell Lung Cancer: An Overlap-Weighted Analysis. Cancers 2021, 13, 2895. [Google Scholar] [CrossRef]
- Socha, J.; Guzowska, A.; Tyc-Szczepaniak, D.; Wierzchowski, M.; Sprawka, A.; Szczesna, A.; Kepka, L. Accelerated hypofractionated thoracic radiotherapy in limited disease small cell lung cancer: Comparison with the results of conventionally fractionated radiotherapy. J. BUON 2015, 20, 146–157. [Google Scholar] [PubMed]
- Grønberg, B.H.; Halvorsen, T.O.; Fløtten, Ø.; Brustugun, O.T.; Brunsvig, P.F.; Aasebø, U.; Bremnes, R.M.; Tollåli, T.; Hornslien, K.; Aksnessæther, B.Y.; et al. Randomized phase II trial comparing twice daily hyperfractionated with once daily hypofractionated thoracic radiotherapy in limited disease small cell lung cancer. Acta Oncol. 2016, 55, 591–597. [Google Scholar] [CrossRef]
- Turgeon, G.A.; Souhami, L.; Kopek, N.; Hirsh, V.; Ofiara, L.; Faria, S.L. Thoracic irradiation in 3 weeks for limited-stage small cell lung cancer: Is twice a day fractionation really needed? Cancer Radiothér. 2017, 21, 89–98. [Google Scholar] [CrossRef]
- Qiu, B.; Li, Q.; Liu, J.; Huang, Y.; Pang, Q.; Zhu, Z.; Yang, X.; Wang, B.; Chen, L.; Fang, J.; et al. Moderately Hypofractionated Once-Daily Compared With Twice-Daily Thoracic Radiation Therapy Concurrently With Etoposide and Cisplatin in Limited-Stage Small Cell Lung Cancer: A Multicenter, Phase II, Randomized Trial. Int. J. Radiat. Oncol. Biol. Phys. 2021, 111, 424–435. [Google Scholar] [CrossRef]
- Mauguen, A.; Le Péchoux, C.; Saunders, M.I.; Schild, S.E.; Turrisi, A.T.; Baumann, M.; Sause, W.T.; Ball, D.; Belani, C.P.; Bonner, J.A.; et al. Hyperfractionated or accelerated radiotherapy in lung cancer: An individual patient data meta-analysis. J. Clin. Oncol. 2012, 30, 2788–2797. [Google Scholar] [CrossRef]
- Viani, G.A.; Gouveia, A.G.; Matsuura, F.K.; Jacinto, A.A.; Moraes, F.Y. Once daily (OD) versus twice-daily (BID) chemoradiation for limited stage small cell lung cancer (LS-SCLC): A meta-analysis of randomized clinical trials. Radiother. Oncol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Zhou, Y.; Lee, J.J. IPDfromKM: Reconstruct individual patient data from published Kaplan-Meier survival curves. BMC Med. Res. Methodol. 2021, 21, 111. [Google Scholar] [CrossRef]
- de Jong, V.M.T.; Moons, K.G.M.; Riley, R.D.; Tudur Smith, C.; Marson, A.G.; Eijkemans, M.J.C.; Debray, T.P.A. Individual participant data meta-analysis of intervention studies with time-to-event outcomes: A review of the methodology and an applied example. Res. Synth. Methods 2020, 11, 148–168. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.T.; Williamson, P.R.; Marson, A.G. Investigating heterogeneity in an individual patient data meta-analysis of time to event outcomes. Stat. Med. 2005, 24, 1307–1319. [Google Scholar] [CrossRef] [PubMed]
- Rücker, G.; Schwarzer, G.; Carpenter, J.; Olkin, I. Why add anything to nothing? The arcsine difference as a measure of treatment effect in meta-analysis with zero cells. Stat. Med. 2009, 28, 721–738. [Google Scholar] [CrossRef] [PubMed]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Blackstock, A.W.; Bogart, J.A.; Matthews, C.; Lovato, J.F.; McCoy, T.; Livengood, K.; Ho, C.; White, D.; Atkins, J.N.; Miller, A.A. Split-course versus continuous thoracic radiation therapy for limited-stage small-cell lung cancer: Final report of a randomized phase III trial. Clin. Lung Cancer 2005, 6, 287–292. [Google Scholar] [CrossRef]
- Bonner, J.A.; Sloan, J.A.; Shanahan, T.G.; Brooks, B.J.; Marks, R.S.; Krook, J.E.; Gerstner, J.B.; Maksymiuk, A.; Levitt, R.; Mailliard, J.A.; et al. Phase III comparison of twice-daily split-course irradiation versus once-daily irradiation for patients with limited stage small-cell lung carcinoma. J. Clin. Oncol. 1999, 17, 2681–2691. [Google Scholar] [CrossRef]
- Bettington, C.S.; Tripcony, L.; Bryant, G.; Hickey, B.; Pratt, G.; Fay, M. A retrospective analysis of survival outcomes for two different radiotherapy fractionation schedules given in the same overall time for limited stage small cell lung cancer. J. Med. Imaging Radiat. Oncol. 2013, 57, 105–112. [Google Scholar] [CrossRef]
- Gazula, A.; Baldini, E.H.; Chen, A.; Kozono, D. Comparison of once and twice daily radiotherapy for limited stage small-cell lung cancer. Lung 2014, 192, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Hao, S.; Tao, C.; Zhao, Q.; Wei, Y.; Song, Z.; Li, B. Comparison of once daily radiotherapy to 60 Gy and twice daily radiotherapy to 45 Gy for limited stage small-cell lung cancer. Thorac. Cancer 2015, 6, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Zhu, Q. Curative effect of hyperfractionated accelerated radiotherapy combined with EP chemotherapy regimen on limited-stage small cell lung cancer. J. BUON 2021, 26, 837–843. [Google Scholar] [PubMed]
- Tomita, N.; Kodaira, T.; Hida, T.; Tachibana, H.; Nakamura, T.; Nakahara, R.; Inokuchi, H. The impact of radiation dose and fractionation on outcomes for limited-stage small-cell lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, 1121–1126. [Google Scholar] [CrossRef] [PubMed]
- Baas, P.; Belderbos, J.S.; Senan, S.; Kwa, H.B.; van Bochove, A.; van Tinteren, H.; Burgers, J.A.; van Meerbeeck, J.P. Concurrent chemotherapy (carboplatin, paclitaxel, etoposide) and involved-field radiotherapy in limited stage small cell lung cancer: A Dutch multicenter phase II study. Br. J. Cancer 2006, 94, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Bogart, J.A.; Herndon, J.E., 2nd; Lyss, A.P.; Watson, D.; Miller, A.A.; Lee, M.E.; Turrisi, A.T.; Green, M.R. 70 Gy thoracic radiotherapy is feasible concurrent with chemotherapy for limited-stage small-cell lung cancer: Analysis of Cancer and Leukemia Group B study 39808. Int. J. Radiat. Oncol. Biol. Phys. 2004, 59, 460–468. [Google Scholar] [CrossRef]
- Bunn, P.A., Jr.; Crowley, J.; Kelly, K.; Hazuka, M.B.; Beasley, K.; Upchurch, C.; Livingston, R.; Weiss, G.R.; Hicks, W.J.; Gandara, D.R.; et al. Chemoradiotherapy with or without granulocyte-macrophage colony-stimulating factor in the treatment of limited-stage small-cell lung cancer: A prospective phase III randomized study of the Southwest Oncology Group. J. Clin. Oncol. 1995, 13, 1632–1641. [Google Scholar] [CrossRef]
- Chen, G.Y.; Jiang, G.L.; Wang, L.J.; Qian, H.; Fu, X.L.; Yang, H.; Wu, K.L.; Zhao, S. Cisplatin/etoposide chemotherapy combined with twice daily thoracic radiotherapy for limited small-cell lung cancer: A clinical phase II trial. Int. J. Radiat. Oncol. Biol. Phys. 2005, 61, 70–75. [Google Scholar] [CrossRef]
- Elias, A.; Ibrahim, J.; Skarin, A.T.; Wheeler, C.; McCauley, M.; Ayash, L.; Richardson, P.; Schnipper, L.; Antman, K.H.; Frei, E., 3rd. Dose-intensive therapy for limited-stage small-cell lung cancer: Long-term outcome. J. Clin. Oncol. 1999, 17, 1175. [Google Scholar] [CrossRef]
- Ettinger, D.S.; Berkey, B.A.; Abrams, R.A.; Fontanesi, J.; Machtay, M.; Duncan, P.J.; Curran, W.J., Jr.; Movsas, B.; Byhardt, R.W. Study of paclitaxel, etoposide, and cisplatin chemotherapy combined with twice-daily thoracic radiotherapy for patients with limited-stage small-cell lung cancer: A Radiation Therapy Oncology Group 9609 phase II study. J. Clin. Oncol. 2005, 23, 4991–4998. [Google Scholar] [CrossRef]
- Gregor, A.; Drings, P.; Burghouts, J.; Postmus, P.E.; Morgan, D.; Sahmoud, T.; Kirkpatrick, A.; Dalesio, O.; Giaccone, G. Randomized trial of alternating versus sequential radiotherapy/chemotherapy in limited-disease patients with small-cell lung cancer: A European Organization for Research and Treatment of Cancer Lung Cancer Cooperative Group Study. J. Clin. Oncol. 1997, 15, 2840–2849. [Google Scholar] [CrossRef] [PubMed]
- Grønberg, B.H.; Killingberg, K.T.; Fløtten, Ø.; Brustugun, O.T.; Hornslien, K.; Madebo, T.; Langer, S.W.; Schytte, T.; Nyman, J.; Risum, S.; et al. High-dose versus standard-dose twice-daily thoracic radiotherapy for patients with limited stage small-cell lung cancer: An open-label, randomised, phase 2 trial. Lancet Oncol. 2021, 22, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Han, J.Y.; Cho, K.H.; Lee, D.H.; Kim, H.Y.; Kim, E.A.; Lee, S.Y.; Lee, J.S. Phase II study of irinotecan plus cisplatin induction followed by concurrent twice-daily thoracic irradiation with etoposide plus cisplatin chemotherapy for limited-disease small-cell lung cancer. J. Clin. Oncol. 2005, 23, 3488–3494. [Google Scholar] [CrossRef]
- Han, J.Y.; Lee, D.H.; Song, J.E.; Lee, S.Y.; Kim, H.Y.; Kim, H.T.; Lee, J.S. Randomized phase 2 study of irinotecan plus cisplatin versus gemcitabine plus vinorelbine as first-line chemotherapy with second-line crossover in patients with advanced nonsmall cell lung cancer. Cancer 2008, 113, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Hanna, N.; Ansari, R.; Fisher, W.; Shen, J.; Jung, S.H.; Sandler, A. Etoposide, ifosfamide and cisplatin (VIP) plus concurrent radiation therapy for previously untreated limited small cell lung cancer (SCLC): A Hoosier Oncology Group (HOG) phase II study. Lung Cancer 2002, 35, 293–297. [Google Scholar] [CrossRef]
- Hu, X.; Bao, Y.; Xu, Y.J.; Zhu, H.N.; Liu, J.S.; Zhang, L.; Guo, Y.; Jin, Y.; Wang, J.; Ma, H.L.; et al. Final report of a prospective randomized study on thoracic radiotherapy target volume for limited-stage small cell lung cancer with radiation dosimetric analyses. Cancer 2020, 126, 840–849. [Google Scholar] [CrossRef] [PubMed]
- Hügli, A.; Moro, D.; Mermillod, B.; Bolla, M.; Alberto, P.; Bonnefoi, H.; Miralbell, R. Phase II trial of up-front accelerated thoracic radiotherapy combined with chemotherapy and optional up-front prophylactic cranial irradiation in limited small-cell lung cancer. Groupe d’Oncologie Thoracique des Régions Alpines. J. Clin. Oncol. 2000, 18, 1662–1667. [Google Scholar] [CrossRef] [PubMed]
- Jeremic, B.; Shibamoto, Y.; Acimovic, L.; Milisavljevic, S. Initial versus delayed accelerated hyperfractionated radiation therapy and concurrent chemotherapy in limited small-cell lung cancer: A randomized study. J. Clin. Oncol. 1997, 15, 893–900. [Google Scholar] [CrossRef]
- Jett, J.R.; Everson, L.; Therneau, T.M.; Krook, J.E.; Dalton, R.J.; Marschke, R.F., Jr.; Veeder, M.H.; Brunk, S.F.; Mailliard, J.A.; Twito, D.I.; et al. Treatment of limited-stage small-cell lung cancer with cyclophosphamide, doxorubicin, and vincristine with or without etoposide: A randomized trial of the North Central Cancer Treatment Group. J. Clin. Oncol. 1990, 8, 33–38. [Google Scholar] [CrossRef]
- Johnson, B.E.; Bridges, J.D.; Sobczeck, M.; Gray, J.; Linnoila, R.I.; Gazdar, A.F.; Hankins, L.; Steinberg, S.M.; Edison, M.; Frame, J.N.; et al. Patients with limited-stage small-cell lung cancer treated with concurrent twice-daily chest radiotherapy and etoposide/cisplatin followed by cyclophosphamide, doxorubicin, and vincristine. J. Clin. Oncol. 1996, 14, 806–813. [Google Scholar] [CrossRef]
- Kakolyris, S.; Agelidou, A.; Androulakis, N.; Tsaroucha, E.; Kouroussis, C.; Agelidou, M.; Karvounis, N.; Veslemes, M.; Christophylakis, C.; Argyraki, A.; et al. Cisplatin plus etoposide chemotherapy followed by thoracic irradiation and paclitaxel plus cisplatin consolidation therapy for patients with limited stage small cell lung carcinoma. Lung Cancer 2006, 53, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Kelley, M.J.; Bogart, J.A.; Hodgson, L.D.; Ansari, R.H.; Atkins, J.N.; Pang, H.; Green, M.R.; Vokes, E.E. Phase II study of induction cisplatin and irinotecan followed by concurrent carboplatin, etoposide, and thoracic radiotherapy for limited-stage small-cell lung cancer, CALGB 30206. J. Thorac. Oncol. 2013, 8, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Kubota, K.; Hida, T.; Ishikura, S.; Mizusawa, J.; Nishio, M.; Kawahara, M.; Yokoyama, A.; Imamura, F.; Takeda, K.; Negoro, S.; et al. Etoposide and cisplatin versus irinotecan and cisplatin in patients with limited-stage small-cell lung cancer treated with etoposide and cisplatin plus concurrent accelerated hyperfractionated thoracic radiotherapy (JCOG0202): A randomised phase 3 study. Lancet Oncol. 2014, 15, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Le, Q.T.; Moon, J.; Redman, M.; Williamson, S.K.; Lara, P.N., Jr.; Goldberg, Z.; Gaspar, L.E.; Crowley, J.J.; Moore, D.F., Jr.; Gandara, D.R. Phase II study of tirapazamine, cisplatin, and etoposide and concurrent thoracic radiotherapy for limited-stage small-cell lung cancer: SWOG 0222. J. Clin. Oncol. 2009, 27, 3014–3019. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Maurer, L.H.; Herndon, J.E., 2nd; Hollis, D.R.; Aisner, J.; Carey, R.W.; Skarin, A.T.; Perry, M.C.; Eaton, W.L.; Zacharski, L.L.; Hammond, S.; et al. Randomized trial of chemotherapy and radiation therapy with or without warfarin for limited-stage small-cell lung cancer: A Cancer and Leukemia Group B study. J. Clin. Oncol. 1997, 15, 3378–3387. [Google Scholar] [CrossRef] [PubMed]
- McClay, E.F.; Bogart, J.; Herndon, J.E., 2nd; Watson, D.; Evans, L.; Seagren, S.L.; Green, M.R. A phase III trial evaluating the combination of cisplatin, etoposide, and radiation therapy with or without tamoxifen in patients with limited-stage small cell lung cancer: Cancer and Leukemia Group B Study (9235). Am. J. Clin. Oncol. 2005, 28, 81–90. [Google Scholar] [CrossRef]
- Mennecier, B.; Jacoulet, P.; Dubiez, A.; Westeel, V.; Bosset, J.F.; Magnin, V.; Depierre, A. Concurrent cisplatin/etoposide chemotherapy plus twice daily thoracic radiotherapy in limited stage small cell lung cancer: A phase II study. Lung Cancer 2000, 27, 137–143. [Google Scholar] [CrossRef]
- Miller, A.A.; Wang, X.F.; Bogart, J.A.; Hodgson, L.D.; Rocha Lima, C.M.; Radford, J.E.; Vokes, E.E.; Green, M.R. Phase II trial of paclitaxel-topotecan-etoposide followed by consolidation chemoradiotherapy for limited-stage small cell lung cancer: CALGB 30002. J. Thorac. Oncol. 2007, 2, 645–651. [Google Scholar] [CrossRef]
- Murray, N.; Coy, P.; Pater, J.L.; Hodson, I.; Arnold, A.; Zee, B.C.; Payne, D.; Kostashuk, E.C.; Evans, W.K.; Dixon, P.; et al. Importance of timing for thoracic irradiation in the combined modality treatment of limited-stage small-cell lung cancer. The National Cancer Institute of Canada Clinical Trials Group. J. Clin. Oncol. 1993, 11, 336–344. [Google Scholar] [CrossRef]
- Saito, H.; Takada, Y.; Ichinose, Y.; Eguchi, K.; Kudoh, S.; Matsui, K.; Nakagawa, K.; Takada, M.; Negoro, S.; Tamura, K.; et al. Phase II study of etoposide and cisplatin with concurrent twice-daily thoracic radiotherapy followed by irinotecan and cisplatin in patients with limited-disease small-cell lung cancer: West Japan Thoracic Oncology Group 9902. J. Clin. Oncol. 2006, 24, 5247–5252. [Google Scholar] [CrossRef]
- Schild, S.E.; Bonner, J.A.; Hillman, S.; Kozelsky, T.F.; Vigliotti, A.P.; Marks, R.S.; Graham, D.L.; Soori, G.S.; Kugler, J.W.; Tenglin, R.C.; et al. Results of a phase II study of high-dose thoracic radiation therapy with concurrent cisplatin and etoposide in limited-stage small-cell lung cancer (NCCTG 95-20-53). J. Clin. Oncol. 2007, 25, 3124–3129. [Google Scholar] [CrossRef] [PubMed]
- Sculier, J.P.; Lafitte, J.J.; Efremidis, A.; Florin, M.C.; Lecomte, J.; Berchier, M.C.; Richez, M.; Berghmans, T.; Scherpereel, A.; Meert, A.P.; et al. A phase III randomised study of concomitant induction radiochemotherapy testing two modalities of radiosensitisation by cisplatin (standard versus daily) for limited small-cell lung cancer. Ann. Oncol. 2008, 19, 1691–1697. [Google Scholar] [CrossRef] [PubMed]
- Skarlos, D.V.; Samantas, E.; Briassoulis, E.; Panoussaki, E.; Pavlidis, N.; Kalofonos, H.P.; Kardamakis, D.; Tsiakopoulos, E.; Kosmidis, P.; Tsavdaridis, D.; et al. Randomized comparison of early versus late hyperfractionated thoracic irradiation concurrently with chemotherapy in limited disease small-cell lung cancer: A randomized phase II study of the Hellenic Cooperative Oncology Group (HeCOG). Ann. Oncol. 2001, 12, 1231–1238. [Google Scholar] [CrossRef]
- Sohn, J.H.; Moon, Y.W.; Lee, C.G.; Kim, G.E.; Chung, K.Y.; Chang, J.; Kim, S.K.; Kim, Y.S.; Choi, B.W.; Choi, H.J.; et al. Phase II trial of irinotecan and cisplatin with early concurrent radiotherapy in limited-disease small-cell lung cancer. Cancer 2007, 109, 1845–1950. [Google Scholar] [CrossRef]
- Sorensen, M.; Lassen, U.; Palshof, T.; Jensen, B.B.; Johansen, J.; Jensen, P.B.; Langer, S.W. Topotecan and cisplatin in combination with concurrent twice-daily chemoradiation in limited disease small cell lung cancer-a Danish Oncological Lung Cancer Group (DOLG) phase II trial. Lung Cancer 2008, 60, 252–258. [Google Scholar] [CrossRef]
- Sun, J.M.; Ahn, Y.C.; Choi, E.K.; Ahn, M.J.; Ahn, J.S.; Lee, S.H.; Lee, D.H.; Pyo, H.; Song, S.Y.; Jung, S.H.; et al. Phase III trial of concurrent thoracic radiotherapy with either first- or third-cycle chemotherapy for limited-disease small-cell lung cancer. Ann. Oncol. 2013, 24, 2088–2092. [Google Scholar] [CrossRef] [PubMed]
- Takada, M.; Fukuoka, M.; Kawahara, M.; Sugiura, T.; Yokoyama, A.; Yokota, S.; Nishiwaki, Y.; Watanabe, K.; Noda, K.; Tamura, T.; et al. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with cisplatin and etoposide for limited-stage small-cell lung cancer: Results of the Japan Clinical Oncology Group Study 9104. J. Clin. Oncol. 2002, 20, 3054–3060. [Google Scholar] [CrossRef]
- van Loon, J.; De Ruysscher, D.; Wanders, R.; Boersma, L.; Simons, J.; Oellers, M.; Dingemans, A.M.; Hochstenbag, M.; Bootsma, G.; Geraedts, W.; et al. Selective nodal irradiation on basis of (18)FDG-PET scans in limited-disease small-cell lung cancer: A prospective study. Int. J. Radiat. Oncol. Biol. Phys. 2010, 77, 329–336. [Google Scholar] [CrossRef]
- Wahba, H.A.; Halim, A.A.; El-Hadaad, H.A. Irinotecan plus cisplatin chemotherapy followed by concurrent thoracic irradiation with low-dose weekly cisplatin for limited-disease small-cell lung cancer. Med. Oncol. 2012, 29, 199–204. [Google Scholar] [CrossRef]
- Xenidis, N.; Kotsakis, A.; Kalykaki, A.; Christophyllakis, C.; Giassas, S.; Kentepozidis, N.; Polyzos, A.; Chelis, L.; Vardakis, N.; Vamvakas, L.; et al. Etoposide plus cisplatin followed by concurrent chemo-radiotherapy and irinotecan plus cisplatin for patients with limited-stage small cell lung cancer: A multicenter phase II study. Lung Cancer 2010, 68, 450–454. [Google Scholar] [CrossRef]
- Xia, B.; Hong, L.Z.; Cai, X.W.; Zhu, Z.F.; Liu, Q.; Zhao, K.L.; Fan, M.; Mao, J.F.; Yang, H.J.; Wu, K.L.; et al. Phase 2 study of accelerated hypofractionated thoracic radiation therapy and concurrent chemotherapy in patients with limited-stage small-cell lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 2015, 91, 517–523. [Google Scholar] [CrossRef]
- Fried, D.B.; Morris, D.E.; Poole, C.; Rosenman, J.G.; Halle, J.S.; Detterbeck, F.C.; Hensing, T.A.; Socinski, M.A. Systematic review evaluating the timing of thoracic radiation therapy in combined modality therapy for limited-stage small-cell lung cancer. J. Clin. Oncol. 2004, 22, 4837–4845. [Google Scholar] [CrossRef] [PubMed]
- Bourhis, J.; Overgaard, J.; Audry, H.; Ang, K.K.; Saunders, M.; Bernier, J.; Horiot, J.C.; Le Maître, A.; Pajak, T.F.; Poulsen, M.G.; et al. Hyperfractionated or accelerated radiotherapy in head and neck cancer: A meta-analysis. Lancet 2006, 368, 843–854. [Google Scholar] [CrossRef] [PubMed]
- Petit, C.; Lacas, B.; Pignon, J.P.; Le, Q.T.; Grégoire, V.; Grau, C.; Hackshaw, A.; Zackrisson, B.; Parmar, M.K.B.; Lee, J.W.; et al. Chemotherapy and radiotherapy in locally advanced head and neck cancer: An individual patient data network meta-analysis. Lancet Oncol. 2021, 22, 727–736. [Google Scholar] [CrossRef] [PubMed]
| HypoTRT | ConvTRT | HyperTRT | Total | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study Types | RCTs | Obs | P non-RCT | RCTs | Obs | P non-RCT | RCTs | Obs | P non-RCT | RCTs | Obs | P non-RCT | All Types |
| Studies | 3 | 5 | 5 | 5 | 7 | 15 | 6 | 6 | 17 | 7 | 9 | 37 | 53 |
| Participants | 226 | 326 | 1137 | 989 | 508 | 1621 | 1094 | 351 | 1754 | 2309 | 1185 | 4512 | 8006 |
| Sex, No. (%) | |||||||||||||
| Male | 159 (70) | 193 (59) | 767 (67) | 531 (54) | 357 (70) | 1044 (64) | 615 (56) | 216 (62) | 1296 (74) | 1305 (57) | 766 (65) | 3107 (69) | 5178 (65) |
| Female | 67 (30) | 133 (41) | 370 (33) | 458 (46) | 151 (30) | 577 (36) | 479 (44) | 135 (38) | 458 (26) | 1004 (43) | 419 (35) | 1405 (31) | 2828 (35) |
| Median age, years | 58–63 | 59–69 | 58–62 | 63 | 55–71 | 49–66 | 58–64 | 54–66 | 54–66 | 58–64 | 54–71 | 49–66 | 49–71 |
| TRT technique, No. (%) | |||||||||||||
| 2DRT | 54 (24) | 0 | 874 (77) | 394 (40) | 172 (34) | 651 (40) | 341 (31) | 37 (11) | 493 (28) | 789 (34) | 209 (18) | 2018 (45) | 3016 (38) |
| 3D-CRT/IMRT | 172 (76) | 326 (100) | 59 (5) | 595 (60) | 336 (66) | 324 (20) | 753 (69) | 314 (89) | 614 (35) | 1520 (66) | 976 (82) | 997 (22) | 3493 (44) |
| Unreported | 0 | 0 | 204 (18) | 0 | 0 | 646 (40) | 0 | 0 | 647 (37) | 0 | 0 | 1497 (33) | 1497 (19) |
| Corrected BED10, No. (%) | |||||||||||||
| High-dose group | 226 (100) | 270 (83) | 736 (65) | 651 (66) | 0 | 201 (12) | 964 (88) | 351 (100) | 1754 (100) | 1841 (80) | 621 (52) | 2691 (60) | 5153 (64) |
| Low-dose group | 0 | 56 (17) | 401 (35) | 338 (34) | 508 (100) | 1420 (88) | 130 (12) | 0 | 0 | 468 (20) | 564 (48) | 1821 (40) | 2853 (36) |
| CCRT, No. (%) | |||||||||||||
| Yes | 226 (100) | 226 (69) | 972 (85) | 989 (100) | 325 (64) | 1546 (95) | 1094 (100) | 351 (100) | 1583 (90) | 2309 (100) | 902 (76) | 4101 (91) | 7312 (91) |
| No/unreported | 0 | 100 (31) | 165 (15) | 0 | 183 (36) | 75 (5) | 0 | 0 | 171 (10) | 0 | 283 (24) | 411 (9) | 694 (9) |
| TRT timing, No. (%) | |||||||||||||
| Yes | 138 (61) | 111 (34) | 588 (52) | 857 (87) | 0 | 550 (34) | 870 (80) | 26 (7) | 997 (57) | 1865 (81) | 137 (12) | 2135 (47) | 4137 (52) |
| No/unreported | 88 (39) | 215 (66) | 549 (48) | 132 (13) | 508 (100) | 1071 (66) | 224 (20) | 325 (93) | 757 (43) | 444 (19) | 1048 (88) | 2377 (53) | 3869 (48) |
| PCI completion rates (%) | 51 | 52–67 | 64–100 | 60–85 | 21–67 | 12–81 | 81 | 54–65 | 32–90 | 51–85 | 21–67 | 12–100 | 12–100 |
| Median follow-up (months) | 14.7–59 | 20.4–162 | 19.5–60 | 14.7–96 | 22–67 | 15–69 | 24.3–96 | 20.4–34 | 16.3–75.6 | 14.7–96 | 20.4–162 | 15–75.6 | 14.7–162 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Wu, L.; Hu, C.; Bi, N.; Wang, L. Radiotherapy Fraction in Limited-Stage Small Cell Lung Cancer in the Modern Era: A Systematic Review and Meta-Analysis of 8006 Reconstructed Individual Patient Data. Cancers 2023, 15, 277. https://doi.org/10.3390/cancers15010277
Zhao J, Wu L, Hu C, Bi N, Wang L. Radiotherapy Fraction in Limited-Stage Small Cell Lung Cancer in the Modern Era: A Systematic Review and Meta-Analysis of 8006 Reconstructed Individual Patient Data. Cancers. 2023; 15(1):277. https://doi.org/10.3390/cancers15010277
Chicago/Turabian StyleZhao, Jingjing, Linfang Wu, Chen Hu, Nan Bi, and Luhua Wang. 2023. "Radiotherapy Fraction in Limited-Stage Small Cell Lung Cancer in the Modern Era: A Systematic Review and Meta-Analysis of 8006 Reconstructed Individual Patient Data" Cancers 15, no. 1: 277. https://doi.org/10.3390/cancers15010277
APA StyleZhao, J., Wu, L., Hu, C., Bi, N., & Wang, L. (2023). Radiotherapy Fraction in Limited-Stage Small Cell Lung Cancer in the Modern Era: A Systematic Review and Meta-Analysis of 8006 Reconstructed Individual Patient Data. Cancers, 15(1), 277. https://doi.org/10.3390/cancers15010277







