Atypical Response in Metastatic Non-Small Cell Lung Cancer Treated with PD-1/PD-L1 Inhibitors: Radiographic Patterns and Clinical Value of Local Therapy
Abstract
Simple Summary
Abstract
1. Introduction
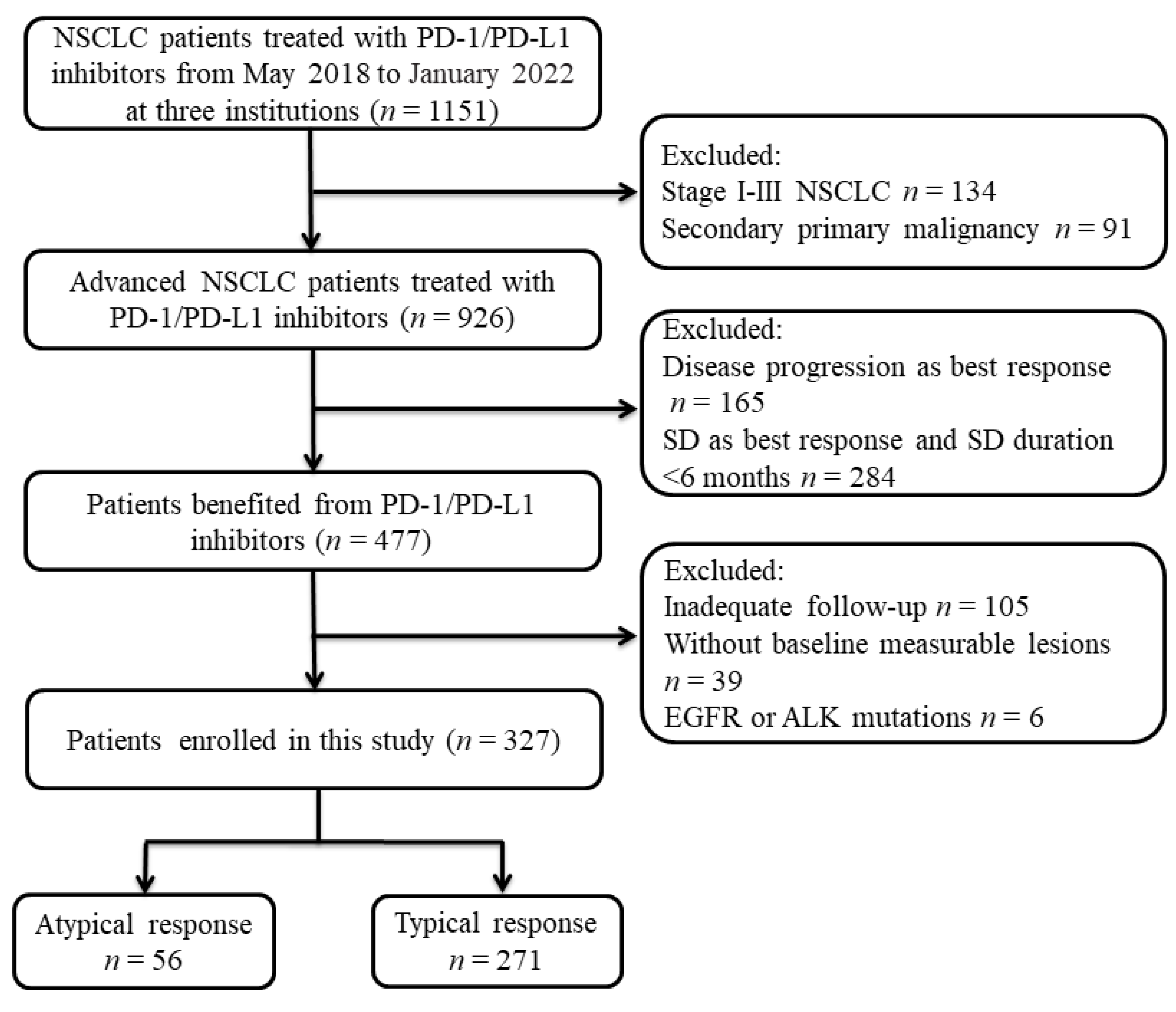
2. Methods
2.1. Patients
2.2. Follow-Up
2.3. Pattern of Response
2.4. Statistical Analysis
3. Results
3.1. Patient’s Characteristics
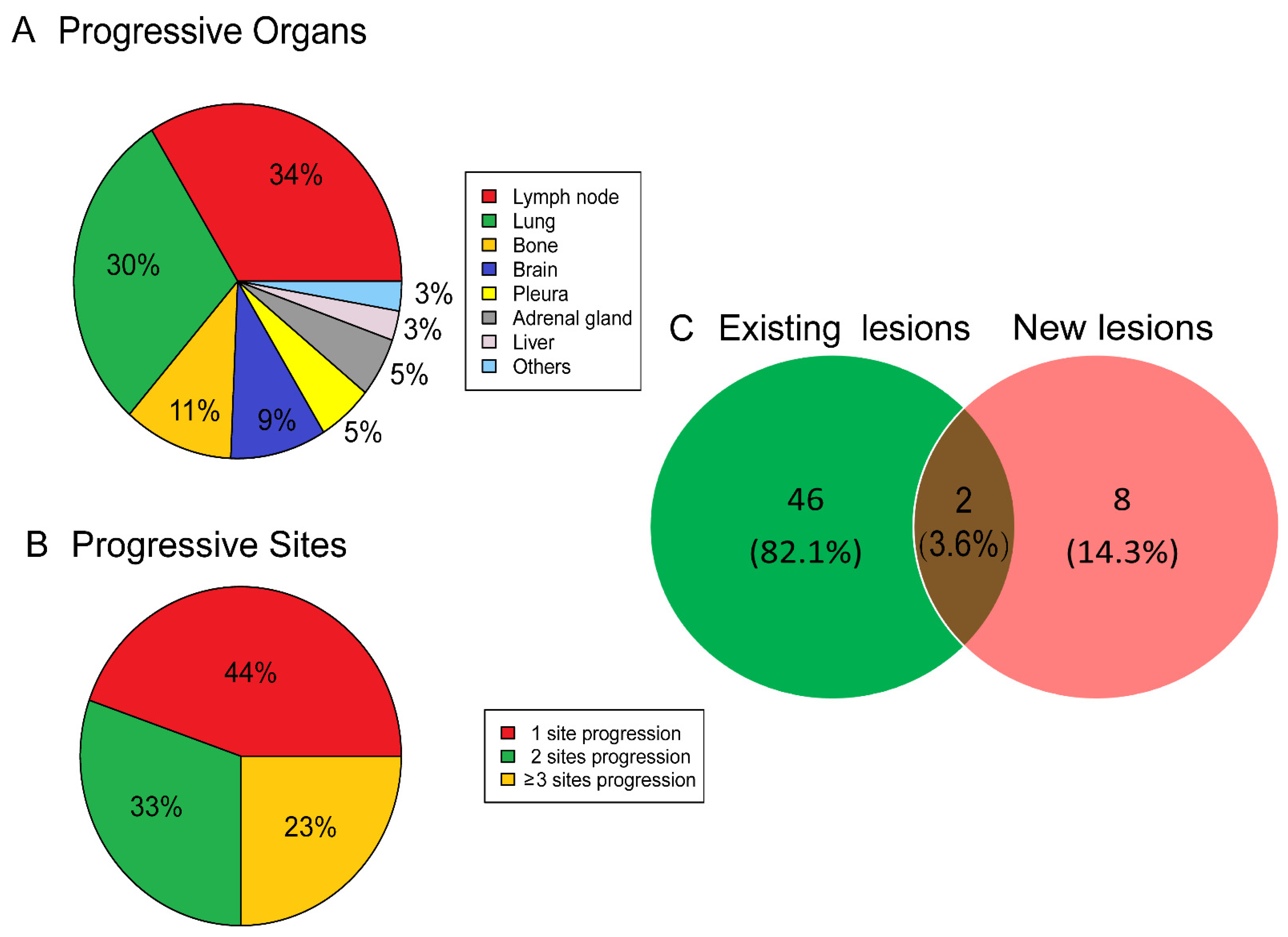
3.2. Clinical Features of Atypical Response
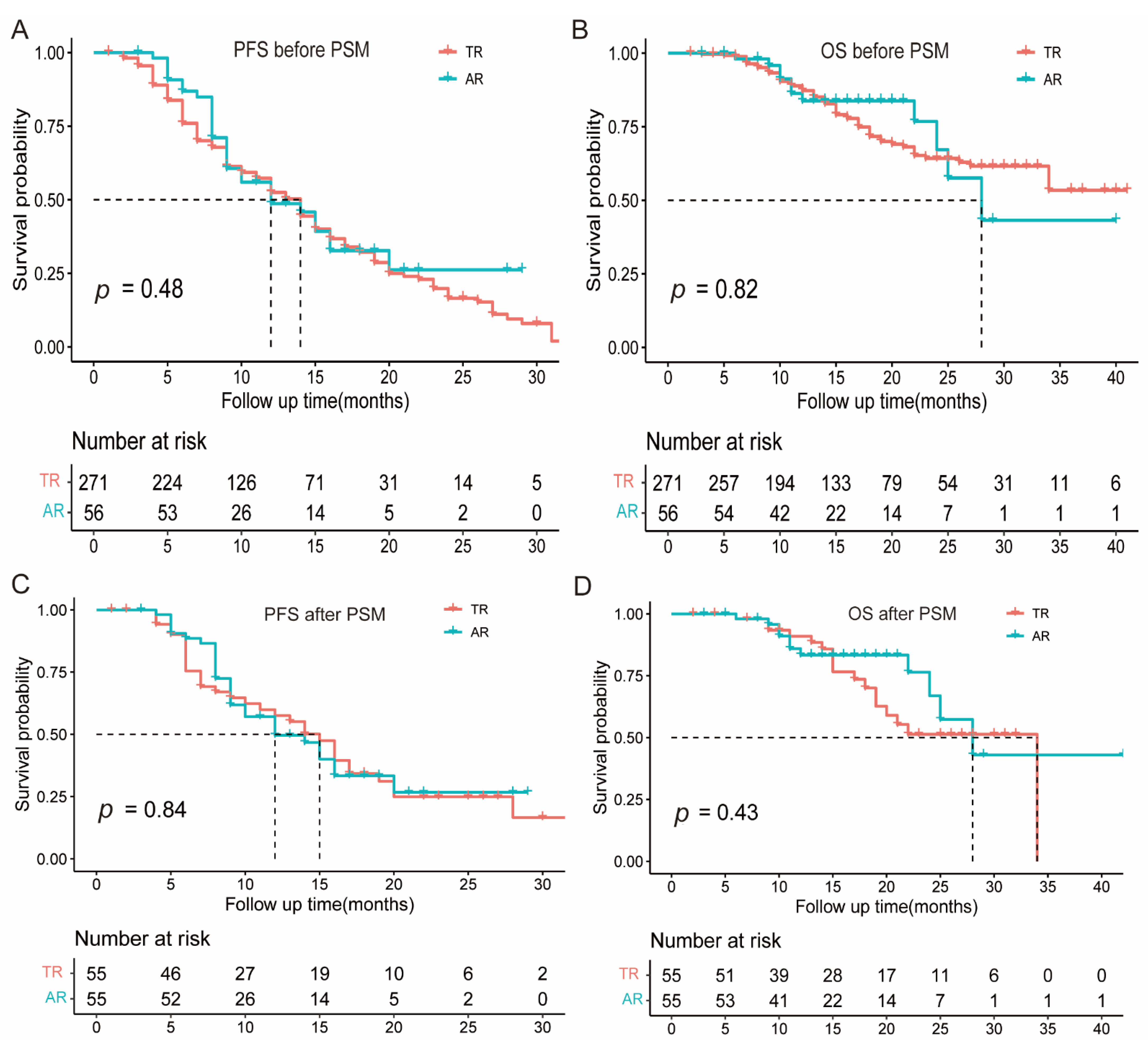
3.3. Prognostic Significance of Atypical Response
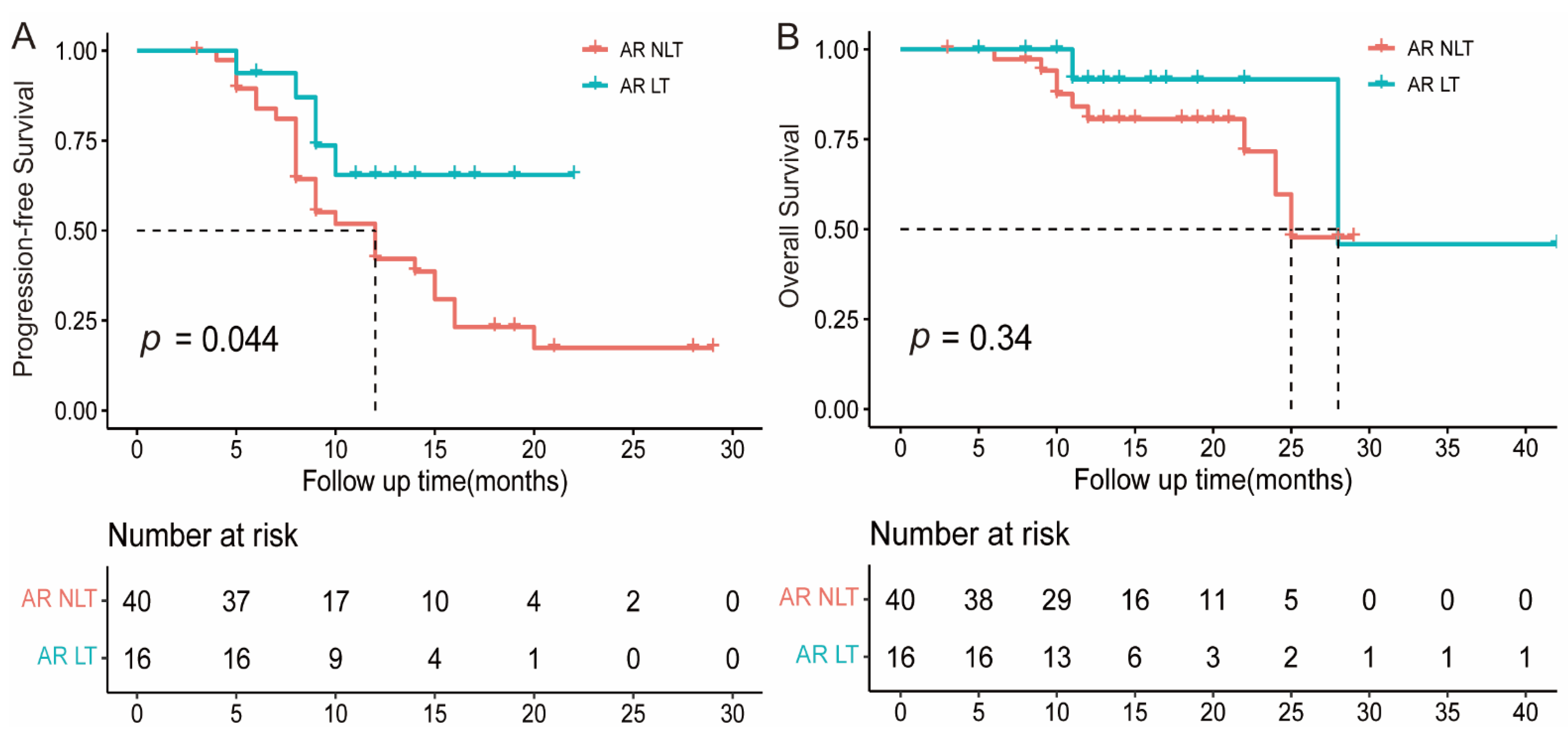
3.4. The Value of Local Treatment for Atypical Response
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brahmer, J.; Reckamp, K.; Baas, P.; Crino, L.; Eberhardt, W.; Poddubskaya, E.; Antonia, S.; Pluzanski, A.; Vokes, E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Borghaei, H.; Paz-Ares, L.; Horn, L.; Spigel, D.; Steins, M.; Ready, N.; Chow, L.; Vokes, E.; Felip, E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 1627–1639. [Google Scholar] [CrossRef] [PubMed]
- Herbst, R.; Baas, P.; Kim, D.; Felip, E.; Perez-Gracia, J.; Han, J.; Molina, J.; Kim, J.; Arvis, C.; Ahn, M.; et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): A randomised controlled trial. Lancet 2016, 387, 1540–1550. [Google Scholar] [CrossRef]
- Rittmeyer, A.; Barlesi, F.; Waterkamp, D.; Park, K.; Ciardiello, F.; von Pawel, J.; Gadgeel, S.; Hida, T.; Kowalski, D.; Dols, M.; et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): A phase 3, open-label, multicentre randomised controlled trial. Lancet 2017, 389, 255–265. [Google Scholar] [CrossRef]
- Tazdait, M.; Mezquita, L.; Lahmar, J.; Ferrara, R.; Bidault, F.; Ammari, S.; Balleyguier, C.; Planchard, D.; Gazzah, A.; Soria, J.; et al. Patterns of responses in metastatic NSCLC during PD-1 or PDL-1 inhibitor therapy: Comparison of RECIST 1.1, irRECIST and iRECIST criteria. Eur. J. Cancer 2018, 88, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Borcoman, E.; Kanjanapan, Y.; Champiat, S.; Kato, S.; Servois, V.; Kurzrock, R.; Goel, S.; Bedard, P.; Le Tourneau, C. Novel patterns of response under immunotherapy. Ann. Oncol. 2019, 30, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Borcoman, E.; Nandikolla, A.; Long, G.; Goel, S.; Le Tourneau, C. Patterns of Response and Progression to Immunotherapy. Am. Soc. Clin. Oncol. Educ. Book 2018, 38, 169–178. [Google Scholar] [CrossRef]
- Fujimoto, D.; Yoshioka, H.; Kataoka, Y.; Morimoto, T.; Hata, T.; Kim, Y.; Tomii, K.; Ishida, T.; Hirabayashi, M.; Hara, S.; et al. Pseudoprogression in Previously Treated Patients with Non-Small Cell Lung Cancer Who Received Nivolumab Monotherapy. J. Thorac. Oncol. 2019, 14, 468–474. [Google Scholar] [CrossRef]
- Won, S.; Park, H.; Byun, S.; Pyo, J.; Kim, J.; Choi, C.; Lee, J.; Lee, D.; Kim, S.; Yoon, S.; et al. Impact of pseudoprogression and treatment beyond progression on outcome in patients with non-small cell lung cancer treated with immune checkpoint inhibitors. Oncoimmunology 2020, 9, 1776058. [Google Scholar] [CrossRef] [PubMed]
- Tozuka, T.; Kitazono, S.; Sakamoto, H.; Yoshida, H.; Amino, Y.; Uematsu, S.; Yoshizawa, T.; Hasegawa, T.; Uchibori, K.; Yanagitani, N.; et al. Dissociated responses at initial computed tomography evaluation is a good prognostic factor in non-small cell lung cancer patients treated with anti-programmed cell death-1/ligand 1 inhibitors. BMC Cancer 2020, 20, 207. [Google Scholar] [CrossRef]
- Chicas-Sett, R.; Zafra, J.; Rodriguez-Abreu, D.; Castilla-Martinez, J.; Benitez, G.; Salas, B.; Hernandez, S.; Lloret, M.; Onieva, J.; Barragan, I.; et al. Combination of SABR With Anti-PD-1 in Oligoprogressive Non-Small Cell Lung Cancer and Melanoma: Results of a Prospective Multicenter Observational Study. Int. J. Radiat. Oncol. Biol. Phys. 2022, 114, 655–665. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, A.; Rizvi, H.; Memon, D.; Shaverdian, N.; Bott, M.; Sauter, J.; Tsai, C.; Lihm, J.; Hoyos, D.; Plodkowski, A.; et al. Systemic and Oligo-Acquired Resistance to PD-(L)1 Blockade in Lung Cancer. Clin. Cancer Res. 2022, 28, 3797–3803. [Google Scholar] [CrossRef] [PubMed]
- Gomez, D.; Tang, C.; Zhang, J.; Blumenschein, G.; Hernandez, M.; Lee, J.; Ye, R.; Palma, D.; Louie, A.; Camidge, D.; et al. Local Consolidative Therapy Vs. Maintenance Therapy or Observation for Patients with Oligometastatic Non-Small-Cell Lung Cancer: Long-Term Results of a Multi-Institutional, Phase II, Randomized Study. J. Clin. Oncol. 2019, 37, 1558–1565. [Google Scholar] [CrossRef] [PubMed]
- Weichselbaum, R.; Liang, H.; Deng, L.; Fu, Y. Radiotherapy and immunotherapy: A beneficial liaison? Nat. Rev. Clin. Oncol. 2017, 14, 365–379. [Google Scholar] [CrossRef]
- Theelen, W.; de Jong, M.; Baas, P. Synergizing systemic responses by combining immunotherapy with radiotherapy in metastatic non-small cell lung cancer: The potential of the abscopal effect. Lung Cancer 2020, 142, 106–113. [Google Scholar] [CrossRef]
- Goodman, A.; Sokol, E.; Frampton, G.; Lippman, S.; Kurzrock, R. Microsatellite-Stable Tumors with High Mutational Burden Benefit from Immunotherapy. Cancer Immunol. Res. 2019, 7, 1570–1573. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.; Albaqer, T.; Santiago, R.; Weiss, J.; Tanguay, J.; Cabanero, M.; Leung, Y.; Pal, P.; Khan, Z.; Lau, S.; et al. Prevalence and Heterogeneity of PD-L1 Expression by 22C3 Assay in Routine Population-Based and Reflexive Clinical Testing in Lung Cancer. J. Thorac. Oncol. 2021, 16, 1490–1500. [Google Scholar] [CrossRef]
- Austin, P. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm. Stat. 2011, 10, 150–161. [Google Scholar] [CrossRef]
- Rauwerdink, D.; Molina, G.; Frederick, D.; Sharova, T.; van der Hage, J.; Cohen, S.; Boland, G. Mixed Response to Immunotherapy in Patients with Metastatic Melanoma. Ann. Surg. Oncol. 2020, 27, 3488–3497. [Google Scholar] [CrossRef]
- Queirolo, P.; Spagnolo, F. Atypical responses in patients with advanced melanoma, lung cancer, renal-cell carcinoma and other solid tumors treated with anti-PD-1 drugs: A systematic review. Cancer Treat. Rev. 2017, 59, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Gettinger, S.; Choi, J.; Hastings, K.; Truini, A.; Datar, I.; Sowell, R.; Wurtz, A.; Dong, W.; Cai, G.; Melnick, M.; et al. Impaired HLA Class I Antigen Processing and Presentation as a Mechanism of Acquired Resistance to Immune Checkpoint Inhibitors in Lung Cancer. Cancer Discov. 2017, 7, 1420–1435. [Google Scholar] [CrossRef] [PubMed]
- Gettinger, S.; Wurtz, A.; Goldberg, S.; Rimm, D.; Schalper, K.; Kaech, S.; Kavathas, P.; Chiang, A.; Lilenbaum, R.; Zelterman, D.; et al. Clinical Features and Management of Acquired Resistance to PD-1 Axis Inhibitors in 26 Patients With Advanced Non-Small Cell Lung Cancer. J. Thorac. Oncol. 2018, 13, 831–839. [Google Scholar] [CrossRef]
- Xu, Y.; Li, H.; Fan, Y. Progression Patterns, Treatment, and Prognosis Beyond Resistance of Responders to Immunotherapy in Advanced Non-Small Cell Lung Cancer. Front. Oncol. 2021, 11, 642883. [Google Scholar] [CrossRef] [PubMed]
- Cheung, P.; Thibault, I.; Bjarnason, G. The emerging roles of stereotactic ablative radiotherapy for metastatic renal cell carcinoma. Curr. Opin. Support Palliat. Care 2014, 8, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.; Yoo, S.; Suh, K.; Kim, S.; Kim, Y.; Ock, C.; Kim, M.; Keam, B.; Kim, T.; Kim, D.; et al. Clinical pattern of failure after a durable response to immune check inhibitors in non-small cell lung cancer patients. Sci. Rep. 2021, 11, 2514. [Google Scholar] [CrossRef] [PubMed]
- Jia, Q.; Wu, W.; Wang, Y.; Alexander, P.; Sun, C.; Gong, Z.; Cheng, J.; Sun, H.; Guan, Y.; Xia, X.; et al. Local mutational diversity drives intratumoral immune heterogeneity in non-small cell lung cancer. Nat. Commun. 2018, 9, 5361. [Google Scholar] [CrossRef]
- Hong, L.; Negrao, M.; Dibaj, S.; Chen, R.; Reuben, A.; Bohac, J.; Liu, X.; Skoulidis, F.; Gay, C.; Cascone, T.; et al. Programmed Death-Ligand 1 Heterogeneity and Its Impact on Benefit From Immune Checkpoint Inhibitors in NSCLC. J. Thorac. Oncol. 2020, 15, 1449–1459. [Google Scholar] [CrossRef]
- Zhao, W.; Zhu, B.; Hutchinson, A.; Pesatori, A.; Consonni, D.; Caporaso, N.; Zhang, T.; Wang, D.; Shi, J.; Landi, M. Clinical Implications of Inter- and Intratumor Heterogeneity of Immune Cell Markers in Lung Cancer. J. Natl. Cancer. Inst. 2022, 114, 280–289. [Google Scholar] [CrossRef]
- Wang, H.; Agulnik, J.; Kasymjanova, G.; Fiset, P.; Camilleri-Broet, S.; Redpath, M.; Cohen, V.; Small, D.; Pepe, C.; Sakr, L.; et al. The metastatic site does not influence PD-L1 expression in advanced non-small cell lung carcinoma. Lung Cancer 2019, 132, 36–38. [Google Scholar] [CrossRef]
- McLaughlin, J.; Han, G.; Schalper, K.; Carvajal-Hausdorf, D.; Pelekanou, V.; Rehman, J.; Velcheti, V.; Herbst, R.; LoRusso, P.; Rimm, D. Quantitative Assessment of the Heterogeneity of PD-L1 Expression in Non-Small-Cell Lung Cancer. JAMA Oncol. 2016, 2, 46–54. [Google Scholar] [CrossRef]
- Topp, B.; Thiagarajan, K.; De Alwis, D.; Snyder, A.; Hellmann, M. Lesion-level heterogeneity of radiologic progression in patients treated with pembrolizumab. Ann. Oncol. 2021, 32, 1618–1625. [Google Scholar] [CrossRef] [PubMed]
- Oliver, A.; Lau, P.; Unsworth, A.; Loi, S.; Darcy, P.; Kershaw, M.; Slaney, C. Tissue-Dependent Tumor Microenvironments and Their Impact on Immunotherapy Responses. Front. Immunol. 2018, 9, 70. [Google Scholar] [CrossRef] [PubMed]
- Beaver, J.; Hazarika, M.; Mulkey, F.; Mushti, S.; Chen, H.; He, K.; Sridhara, R.; Goldberg, K.; Chuk, M.; Chi, D.; et al. Patients with melanoma treated with an anti-PD-1 antibody beyond RECIST progression: A US Food and Drug Administration pooled analysis. Lancet Oncol. 2018, 19, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Kazandjian, D.; Keegan, P.; Suzman, D.; Pazdur, R.; Blumenthal, G. Characterization of outcomes in patients with metastatic non-small cell lung cancer treated with programmed cell death protein 1 inhibitors past RECIST version 1.1-defined disease progression in clinical trials. Semin. Oncol. 2017, 44, 3–7. [Google Scholar] [CrossRef]
- Gandara, D.; von Pawel, J.; Mazieres, J.; Sullivan, R.; Helland, A.; Han, J.; Ponce, A.; Rittmeyer, A.; Barlesi, F.; Kubo, T.; et al. Atezolizumab Treatment Beyond Progression in Advanced NSCLC: Results From the Randomized, Phase III OAK Study. J. Thorac. Oncol. 2018, 13, 1906–1918. [Google Scholar] [CrossRef]
- Jasper, K.; Stiles, B.; McDonald, F.; Palma, D. Practical Management of Oligometastatic Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2022, 40, 635–641. [Google Scholar] [CrossRef]
- Gomez, D.; Blumenschein, G.; Lee, J.; Hernandez, M.; Ye, R.; Camidge, D.; Doebele, R.; Skoulidis, F.; Gaspar, L.; Gibbons, D.; et al. Local consolidative therapy versus maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer without progression after first-line systemic therapy: A multicentre, randomised, controlled, phase 2 study. Lancet Oncol. 2016, 17, 1672–1682. [Google Scholar] [CrossRef]
- Kagawa, Y.; Furuta, H.; Uemura, T.; Watanabe, N.; Shimizu, J.; Horio, Y.; Kuroda, H.; Inaba, Y.; Kodaira, T.; Masago, K.; et al. Efficacy of local therapy for oligoprogressive disease after programmed cell death 1 blockade in advanced non-small cell lung cancer. Cancer Sci. 2020, 111, 4442–4452. [Google Scholar] [CrossRef]
- Dovedi, S.; Adlard, A.; Lipowska-Bhalla, G.; McKenna, C.; Jones, S.; Cheadle, E.; Stratford, I.; Poon, E.; Morrow, M.; Stewart, R.; et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res. 2014, 74, 5458–5468. [Google Scholar] [CrossRef]
- Hendriks, L.; Menis, J.; De Ruysscher, D.; Reck, M. Combination of Immunotherapy and Radiotherapy-The Next Magic Step in the Management of Lung Cancer? J. Thorac. Oncol. 2020, 15, 166–169. [Google Scholar] [CrossRef]
| AR | TR | Univariate Analysis | Multivariate Analysis | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (N = 56) | (N = 271) | HR | 95% CI | p | HR | 95% CI | p | |||
| Age, years | ||||||||||
| ≤62 | 25 (44.6%) | 103 (38.0%) | 1 | |||||||
| >62 | 31 (55.4%) | 168 (62.0%) | 0.760 | 0.425 | 1.359 | 0.355 | ||||
| Gender | ||||||||||
| Male | 45 (80.4%) | 217 (80.1%) | 1 | |||||||
| Female | 11 (19.6%) | 54 (19.9%) | 0.982 | 0.476 | 2.025 | 0.961 | ||||
| ECOG PS Score | ||||||||||
| 0–1 | 52 (92.9%) | 252 (19.0%) | 1 | |||||||
| 2 | 4 (7.1%) | 19 (81.0%) | 1.020 | 0.333 | 3.123 | 0.972 | ||||
| Smoking status | ||||||||||
| Ever | 26 (46.4%) | 152 (56.1%) | 1 | |||||||
| Never | 30 (53.6%) | 119 (43.9%) | 0.903 | 0.507 | 1.609 | 0.73 | ||||
| Histology | ||||||||||
| Squamous-cell Carcinoma | 14 (25.0%) | 105 (38.7%) | 1 | 1 | ||||||
| Non-squamous-cell Carcinoma | 42 (75.0%) | 166 (61.3%) | 1.898 | 0.988 | 3.644 | 0.054 | 1.853 | 0.946 | 3.628 | 0.072 |
| NO. of metastatic organs | ||||||||||
| ≤3 | 45 (80.4%) | 255 (94.1%) | 1 | 1 | ||||||
| >3 | 11 (19.6%) | 16 (5.9%) | 3.896 | 1.698 | 8.939 | 0.001 | 2.708 | 1.056 | 6.943 | 0.038 |
| NO. of metastatic sites | ||||||||||
| ≤3 | 29 (51.8%) | 181 (66.8%) | 1 | 1 | ||||||
| >3 | 27 (48.2%) | 90 (33.2%) | 1.872 | 1.046 | 3.351 | 0.035 | 1.425 | 0.731 | 2.777 | 0.298 |
| Treatment regimens | ||||||||||
| ICI alone | 23 (41.1%) | 80 (29.5%) | 1 | 1 | ||||||
| ICI combination | 33 (58.9%) | 191 (70.5%) | 0.601 | 0.332 | 1.087 | 0.092 | 0.575 | 0.309 | 1.071 | 0.081 |
| Treatment lines | ||||||||||
| 1st | 27 (48.2%) | 114 (42.1%) | 1 | |||||||
| ≥2nd | 29 (51.8%) | 157 (57.9%) | 0.780 | 0.438 | 1.389 | 0.398 | ||||
| PD-L1 expression, % | ||||||||||
| <1 | 4 (7.1%) | 18 (6.6%) | 1 | |||||||
| 1–49 | 6 (10.7%) | 40 (14.8%) | 0.675 | 0.169 | 2.689 | 0.577 | ||||
| ≥50 | 13 (23.2%) | 48 (17.7%) | 1.219 | 0.351 | 4.231 | 0.755 | ||||
| Unknown | 33 (59.0%) | 165 (60.9%) | 0.900 | 0.286 | 2.831 | 0.857 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, S.; Zhang, J.; Chu, L.; Chu, X.; Yang, X.; Li, Y.; Guo, T.; Zhou, Y.; Xu, D.; Mao, J.; et al. Atypical Response in Metastatic Non-Small Cell Lung Cancer Treated with PD-1/PD-L1 Inhibitors: Radiographic Patterns and Clinical Value of Local Therapy. Cancers 2023, 15, 180. https://doi.org/10.3390/cancers15010180
Jiang S, Zhang J, Chu L, Chu X, Yang X, Li Y, Guo T, Zhou Y, Xu D, Mao J, et al. Atypical Response in Metastatic Non-Small Cell Lung Cancer Treated with PD-1/PD-L1 Inhibitors: Radiographic Patterns and Clinical Value of Local Therapy. Cancers. 2023; 15(1):180. https://doi.org/10.3390/cancers15010180
Chicago/Turabian StyleJiang, Shanshan, Jinmeng Zhang, Li Chu, Xiao Chu, Xi Yang, Yida Li, Tiantian Guo, Yue Zhou, Dayu Xu, Jiuang Mao, and et al. 2023. "Atypical Response in Metastatic Non-Small Cell Lung Cancer Treated with PD-1/PD-L1 Inhibitors: Radiographic Patterns and Clinical Value of Local Therapy" Cancers 15, no. 1: 180. https://doi.org/10.3390/cancers15010180
APA StyleJiang, S., Zhang, J., Chu, L., Chu, X., Yang, X., Li, Y., Guo, T., Zhou, Y., Xu, D., Mao, J., Zheng, Z., An, Y., Sun, H., Dong, H., Yu, S., Ye, R., Hu, J., Chu, Q., Ni, J., & Zhu, Z. (2023). Atypical Response in Metastatic Non-Small Cell Lung Cancer Treated with PD-1/PD-L1 Inhibitors: Radiographic Patterns and Clinical Value of Local Therapy. Cancers, 15(1), 180. https://doi.org/10.3390/cancers15010180





