The SAFFO Study: Sex-Related Prognostic Role and Cut-Off Definition of Monocyte-to-Lymphocyte Ratio (MLR) in Metastatic Colorectal Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Blood Sample Analysis
2.3. Statistical Analysis
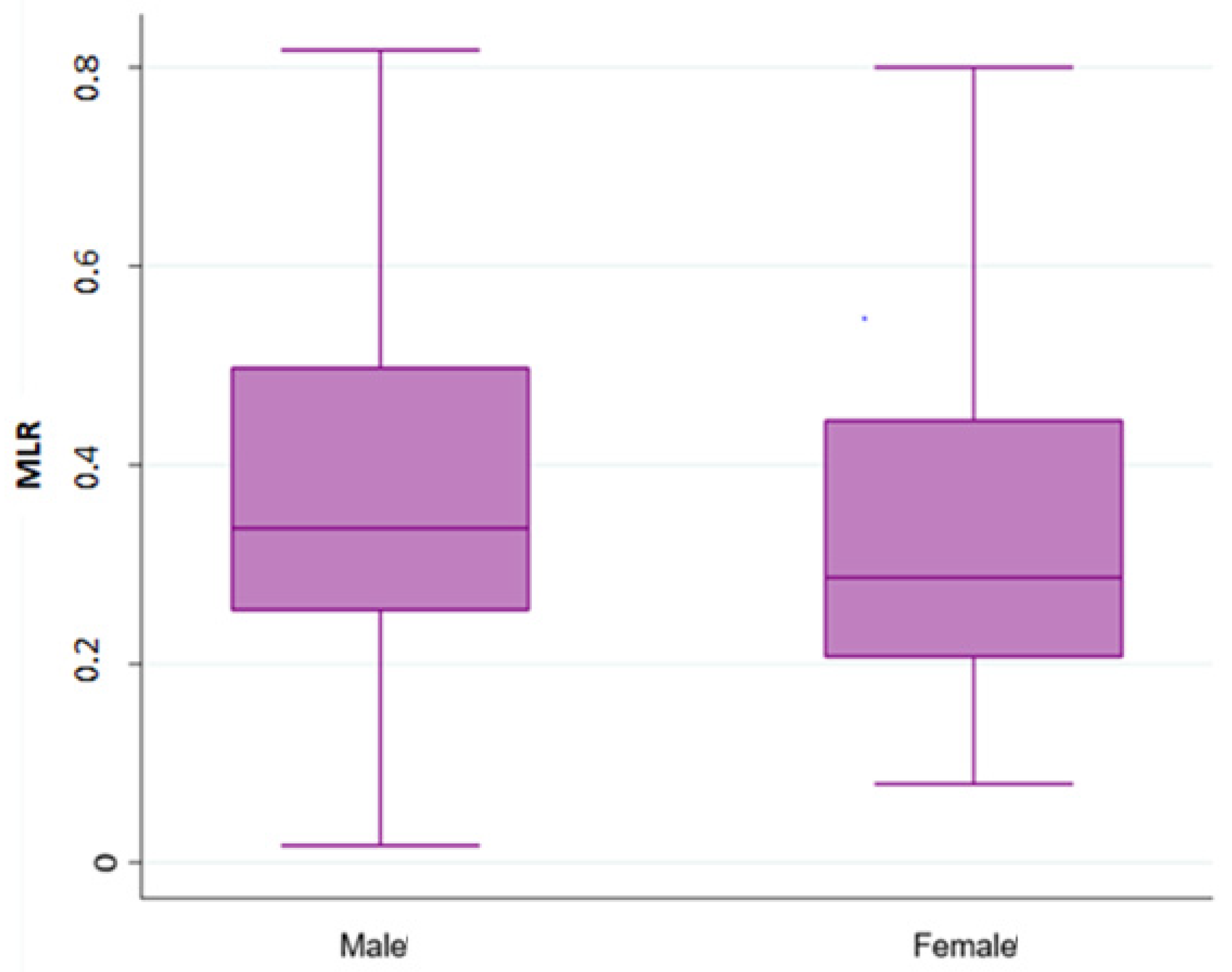
3. Results
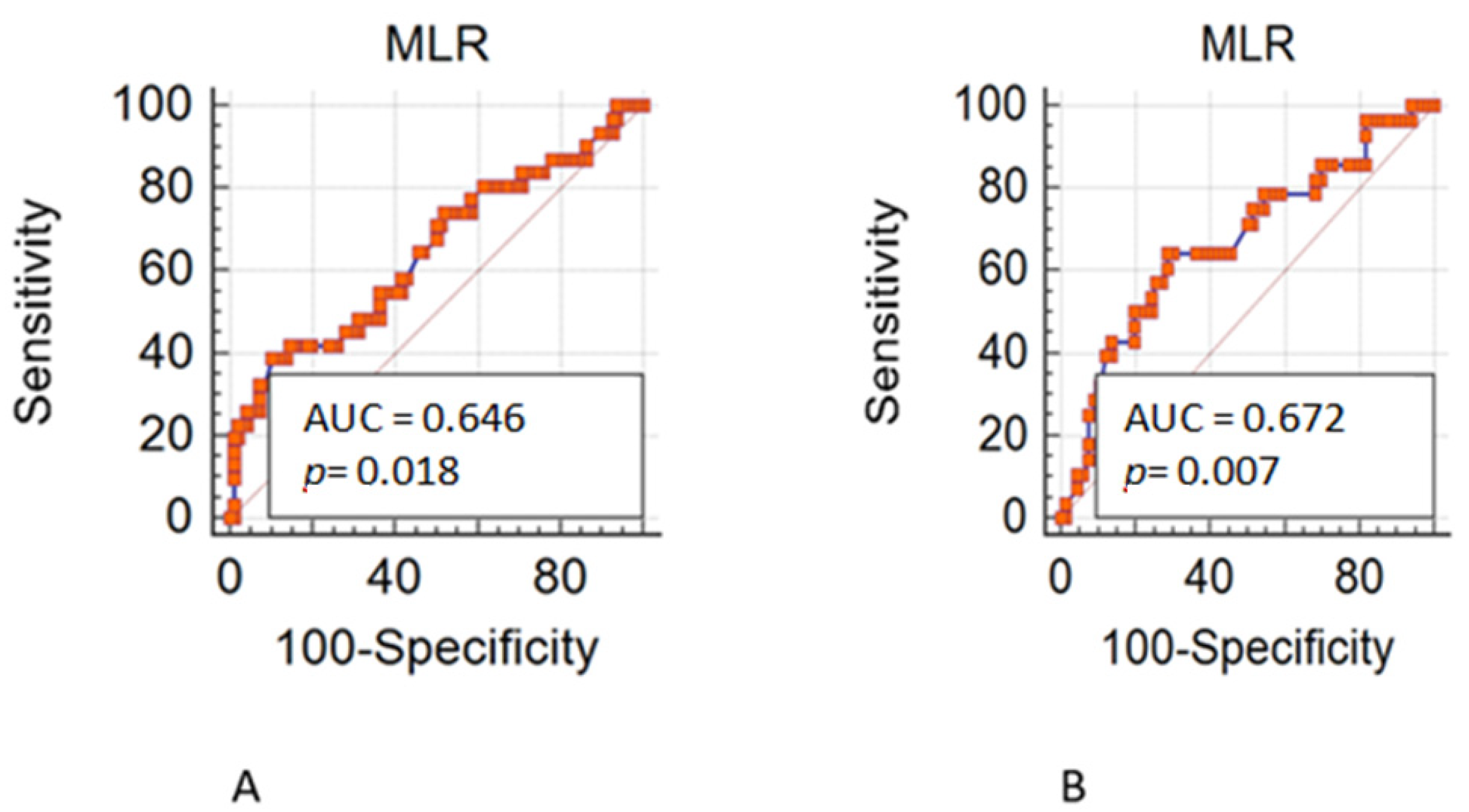
3.1. Training Set
3.2. Validation Set
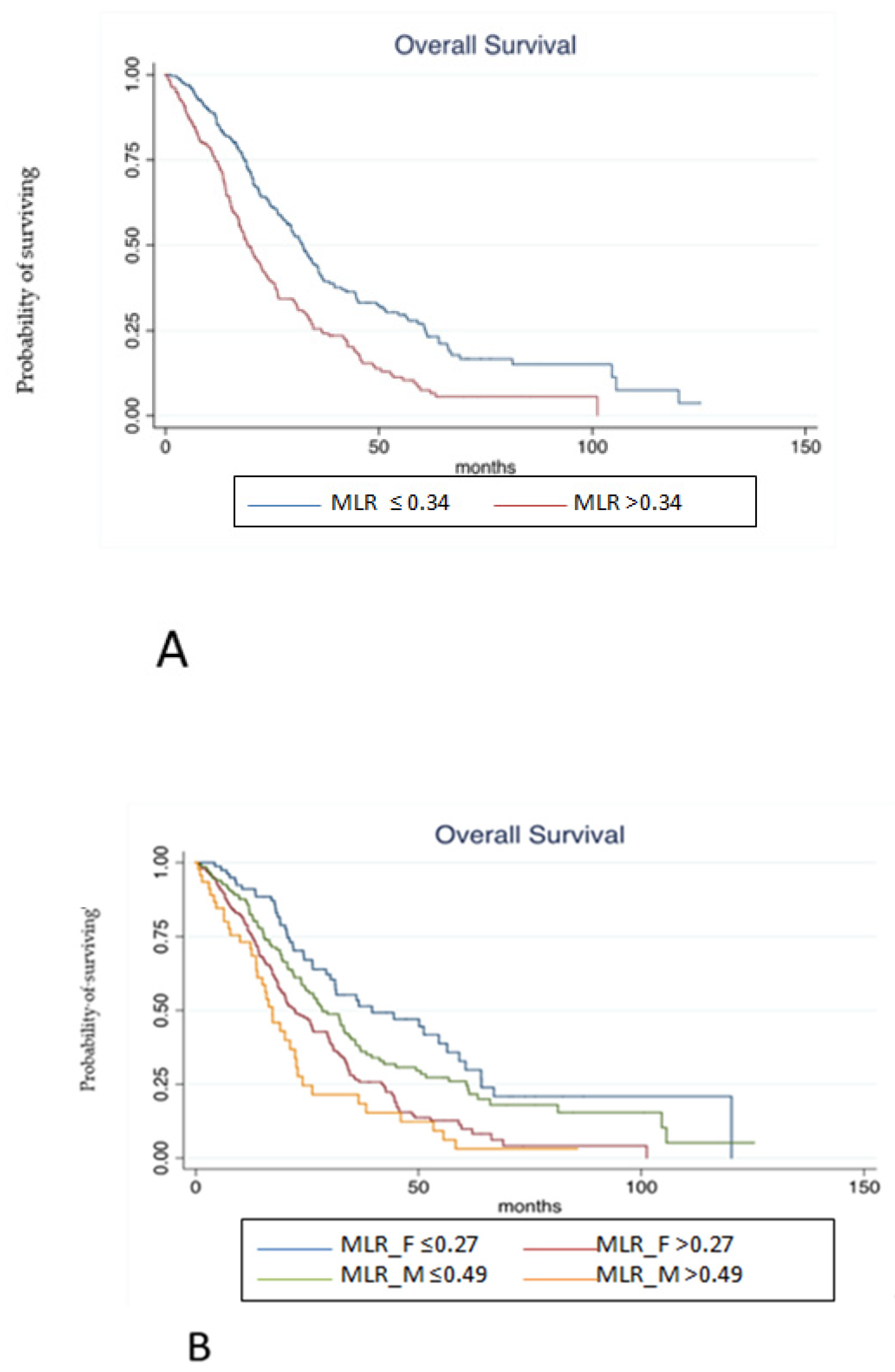
3.3. Pooled Population
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- André, T.; Shiu, K.-K.; Kim, T.W.; Jensen, B.V.; Jensen, L.H.; Punt, C.; Smith, D.; Garcia-Carbonero, R.; Benavides, M.; Gibbs, P.; et al. Pembrolizumab in Microsatellite-Instability–High Advanced Colorectal Cancer. N. Engl. J. Med. 2020, 383, 2207–2218. [Google Scholar] [CrossRef]
- Greten, F.R.; Grivennikov, S.I. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Shibutani, M.; Maeda, K.; Nagahara, H.; Ohtani, H.; Sakurai, K.; Yamazoe, S.; Kimura, K.; Toyokawa, T.; Amano, R.; Tanaka, H.; et al. Prognostic significance of the lymphocyte-to-monocyte ratio in patients with metastatic colorectal cancer. World J. Gastroenterol. WJG 2015, 21, 9966–9973. [Google Scholar] [CrossRef]
- Shibutani, M.; Maeda, K.; Nagahara, H.; Fukuoka, T.; Matsutani, S.; Kimura, K.; Amano, R.; Hirakawa, K.; Ohira, M. The prognostic value of the systemic inflammatory score in patients with unresectable metastatic colorectal cancer. Oncol. Lett. 2018, 16, 666–672. [Google Scholar] [CrossRef]
- Wu, Q.; Hu, T.; Zheng, E.; Deng, X.; Wang, Z. Prognostic role of the lymphocyte-to-monocyte ratio in colorectal cancer. Medicine 2017, 96, e7051. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Cowley, L.A.; Liu, X.-S. Sex Differences in Cancer Immunotherapy Efficacy, Biomarkers, and Therapeutic Strategy. Molecules 2019, 24, E3214. [Google Scholar] [CrossRef]
- Capone, I.; Marchetti, P.; Ascierto, P.A.; Malorni, W.; Gabriele, L. Sexual Dimorphism of Immune Responses: A New Perspective in Cancer Immunotherapy. Front. Immunol. 2018, 9, 552. [Google Scholar] [CrossRef]
- Ortona, E.; Pierdominici, M.; Rider, V. Editorial: Sex Hormones and Gender Differences in Immune Responses. Front. Immunol. 2019, 10, 1076. [Google Scholar] [CrossRef]
- Lotter, H.; Altfeld, M. Sex differences in immunity. Semin. Immunopathol. 2019, 41, 133–135. [Google Scholar] [CrossRef]
- Conforti, F.; Pala, L.; Bagnardi, V.; De Pas, T.; Martinetti, M.; Viale, G.; Gelber, R.D.; Goldhirsch, A. Cancer immunotherapy efficacy and patients’ sex: A systematic review and meta-analysis. Lancet Oncol. 2018, 19, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Wallis, C.J.D.; Butaney, M.; Satkunasivam, R.; Freedland, S.J.; Patel, S.P.; Hamid, O.; Pal, S.K.; Klaassen, Z. Association of Patient Sex with Efficacy of Immune Checkpoint Inhibitors and Overall Survival in Advanced Cancers: A Systematic Review and Meta-analysis. JAMA Oncol. 2019, 5, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Conforti, F.; Pala, L.; Bagnardi, V.; Viale, G.; De Pas, T.; Pagan, E.; Pennacchioli, E.; Cocorocchio, E.; Ferrucci, P.F.; De Marinis, F.; et al. Sex-Based Heterogeneity in Response to Lung Cancer Immunotherapy: A Systematic Review and Meta-Analysis. J. Natl. Cancer Inst. 2019, 111, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Carrera, C.; Potrony, M.; Puig, S. Sex as a predictor of response to cancer immunotherapy. Lancet Oncol. 2018, 19, e375. [Google Scholar] [CrossRef]
- Conforti, F.; Pala, L.; Goldhirsch, A. Different effectiveness of anticancer immunotherapy in men and women relies on sex-dimorphism of the immune system. Oncotarget 2018, 9, 31167–31168. [Google Scholar] [CrossRef]
- Shalapour, S.; Karin, M. Immunity, inflammation, and cancer: An eternal fight between good and evil. J. Clin. Investig. 2015, 125, 3347–3355. [Google Scholar] [CrossRef]
- Diakos, C.I.; Charles, K.A.; McMillan, D.C.; Clarke, S.J. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 2014, 15, e493–e503. [Google Scholar] [CrossRef]
- Ruffell, B.; Coussens, L.M. Macrophages and therapeutic resistance in cancer. Cancer Cell 2015, 27, 462–472. [Google Scholar] [CrossRef]
- Kong, J.C.; Guerra, G.R.; Pham, T.; Mitchell, C.; Lynch, A.C.; Warrier, S.K.; Ramsay, R.G.; Heriot, A.G. Prognostic Impact of Tumor-Infiltrating Lymphocytes in Primary and Metastatic Colorectal Cancer: A Systematic Review and Meta-analysis. Dis. Colon Rectum 2019, 62, 498–508. [Google Scholar] [CrossRef]
- Guthrie, G.J.K.; Charles, K.A.; Roxburgh, C.S.D.; Horgan, P.G.; McMillan, D.C.; Clarke, S.J. The systemic inflammation-based neutrophil-lymphocyte ratio: Experience in patients with cancer. Crit. Rev. Oncol. Hematol. 2013, 88, 218–230. [Google Scholar] [CrossRef]
- Stotz, M.; Pichler, M.; Absenger, G.; Szkandera, J.; Arminger, F.; Schaberl-Moser, R.; Samonigg, H.; Stojakovic, T.; Gerger, A. The preoperative lymphocyte to monocyte ratio predicts clinical outcome in patients with stage III colon cancer. Br. J. Cancer 2014, 110, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.-Y.; Raghav, K.; Lieu, C.H.; Jiang, Z.-Q.; Eng, C.; Vauthey, J.-N.; Chang, G.J.; Qiao, W.; Morris, J.; Hong, D.; et al. Cytokine profile and prognostic significance of high neutrophil-lymphocyte ratio in colorectal cancer. Br. J. Cancer 2015, 112, 1088–1097. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, M.; Nozawa, H.; Sasaki, K.; Hongo, K.; Hiyoshi, M.; Tada, N.; Murono, K.; Nirei, T.; Kawai, K.; Sunami, E.; et al. Elevated neutrophil to lymphocyte ratio predicts poor prognosis in advanced colorectal cancer patients receiving oxaliplatin-based chemotherapy. Oncology 2012, 82, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Basile, D.; Garattini, S.K.; Corvaja, C.; Montico, M.; Cortiula, F.; Pelizzari, G.; Gerratana, L.; Audisio, M.; Lisanti, C.; Fanotto, V.; et al. The MIMIC Study: Prognostic Role and Cutoff Definition of Monocyte-to-Lymphocyte Ratio and Lactate Dehydrogenase Levels in Metastatic Colorectal Cancer. Oncologist 2020, 25, 661–668. [Google Scholar] [CrossRef]
- Lisanti, C.; Basile, D.; Parnofiello, A.; Bertoli, E.; Andreotti, V.J.; Garattini, S.K.; Bartoletti, M.; Cattaneo, M.; Di Nardo, P.; Bonotto, M.; et al. The SENECA study: Prognostic role of serum biomarkers in older patients with metastatic colorectal cancer. J. Geriatr. Oncol. 2020, 11, 1268–1273. [Google Scholar] [CrossRef]
- Jakubowska, K.; Koda, M.; Grudzińska, M.; Kańczuga-Koda, L.; Famulski, W. Monocyte-to-Lymphocyte Ratio as a Prognostic Factor in Peripheral Whole Blood Samples of Colorectal Cancer Patients. World J. Gastroenterol. 2020, 26, 4639–4655. [Google Scholar] [CrossRef]
- Klein, S.L.; Flanagan, K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef]
- Spitzer, J.A. Gender differences in some host defense mechanisms. Lupus 1999, 8, 380–383. [Google Scholar] [CrossRef]
- Abdullah, M.; Chai, P.-S.; Chong, M.-Y.; Tohit, E.R.M.; Ramasamy, R.; Pei, C.P.; Vidyadaran, S. Gender effect on in vitro lymphocyte subset levels of healthy individuals. Cell Immunol. 2012, 272, 214–219. [Google Scholar] [CrossRef]
- Weinstein, Y.; Ran, S.; Segal, S. Sex-associated differences in the regulation of immune responses controlled by the MHC of the mouse. J. Immunol. Baltim. Md 1950 1984, 132, 656–661. [Google Scholar]
- Torcia, M.G.; Nencioni, L.; Clemente, A.M.; Civitelli, L.; Celestino, I.; Limongi, D.; Fadigati, G.; Perissi, E.; Cozzolino, F.; Garaci, E.; et al. Sex differences in the response to viral infections: TLR8 and TLR9 ligand stimulation induce higher IL10 production in males. PLoS ONE 2012, 7, e39853. [Google Scholar] [CrossRef] [PubMed]
- Berghöfer, B.; Frommer, T.; Haley, G.; Fink, L.; Bein, G.; Hackstein, H. TLR7 ligands induce higher IFN-alpha production in females. J. Immunol. Baltim. Md 1950 2006, 177, 2088–2096. [Google Scholar] [CrossRef]
- Roberts, C.W.; Walker, W.; Alexander, J. Sex-associated hormones and immunity to protozoan parasites. Clin. Microbiol. Rev. 2001, 14, 476–488. [Google Scholar] [CrossRef] [PubMed]
- Libert, C.; Dejager, L.; Pinheiro, I. The X chromosome in immune functions: When a chromosome makes the difference. Nat. Rev. Immunol. 2010, 10, 594–604. [Google Scholar] [CrossRef]
- Case, L.K.; Toussaint, L.; Moussawi, M.; Roberts, B.; Saligrama, N.; Brossay, L.; Huber, S.A.; Teuscher, C. Chromosome y regulates survival following murine coxsackievirus b3 infection. G3 Bethesda Md 2012, 2, 115–121. [Google Scholar] [CrossRef][Green Version]
| Variables | N = 490 | % |
|---|---|---|
| Sex | ||
| M | 288 | 58.78 |
| F | 202 | 41.22 |
| Age (years) | ||
| ≤70 | 317 | 64.69 |
| >70 | 170 | 34.69 |
| Missing | 3 | 0.6 |
| Sidedness | ||
| Right | 161 | 32.85 |
| Left-Rectum | 324 | 66.12 |
| Missing | 5 | 1.00 |
| Surgery | ||
| No | 92 | 18.87 |
| Yes | 339 | 69.18 |
| Missing | 59 | 11.20 |
| Number of sites | ||
| 1 | 268 | 54.69 |
| >1 | 221 | 45.10 |
| Missing | 1 | 0.2 |
| Sites of metastases | ||
| Liver | 178 | 36.32 |
| Lung | 91 | 18.57 |
| Lymph nodes | 70 | 14.28 |
| Peritoneum | 107 | 21.83 |
| Bone | 13 | 2.65 |
| CNS | 3 | 0.6 |
| Missing | 28 | 5.70 |
| KRAS | ||
| WT | 239 | 48.77 |
| Mut | 182 | 37.14 |
| Missing | 69 | 14.08 |
| BRAF | ||
| WT | 273 | 55.71 |
| Mut | 40 | 8.16 |
| Missing | 177 | 36.12 |
| Treatment received | ||
| Monotherapy +/− biologic | 51 | 10.41 |
| Doublet | 107 | 21.84 |
| Doublet plus biologic | 257 | 52.45 |
| Triple +/− biologic | 75 | 15.31 |
| MLR—Training set (N = 263) | ||
| F ≤ 0.27 | 61 | 23.28 |
| F > 0.27 | 87 | 33.20 |
| M ≤ 0.49 | 98 | 37.40 |
| M > 0.49 | 16 | 6.10 |
| Missing | 1 | 0.2 |
| MLR—Validation set (N = 227) | ||
| F ≤ 0.27 | 22 | 9.69 |
| F > 0.27 | 114 | 50.20 |
| M ≤ 0.49 | 57 | 25.11 |
| M > 0.49 | 30 | 13.21 |
| Missing | 4 | 1.76 |
| MLR—Overall cohort (N = 490) | ||
| F ≤ 0.27 | 83 | 16.93 |
| F > 0.27 | 201 | 41.02 |
| M ≤ 0.49 | 155 | 31.63 |
| M > 0.49 | 46 | 9.38 |
| Missing | 5 | 1.00 |
| Variables | Male N = 288 | Female N = 202 | p-Value |
|---|---|---|---|
| Age (years) ≤70 >70 Missing | 188 100 0 | 129 70 3 | 0.918 |
| Sidedness Right Left-Rectum Missing | 79 208 1 | 82 116 1 | 0.001 |
| Surgery No Yes Missing | 61 190 37 | 31 149 22 | 0.077 |
| Number of sites 1 >1 Missing | 154 134 0 | 114 87 1 | 0.478 |
| Sites of metastases Liver Lung Lymph nodes Peritoneum Bone CNS Missing | 111 56 42 55 9 3 12 | 67 35 28 52 4 0 16 | 0.284 |
| KRAS WT Mut Missing | 144 108 36 | 95 74 33 | 0.850 |
| BRAF WT Mut Missing | 167 19 102 | 106 21 75 | 0.100 |
| Treatment received Monotherapy +/− biologic Doublet Doublet plus biologic Triplet +/− biologic | 29 66 144 49 | 22 41 113 26 | 0.461 |
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| Variables | HR | p-Value | 95% C.I. | HR | p-Value | 95% C.I. |
| MLR—Overall cohort F > 0.27 M ≤ 0.49 M > 0.49 | 2.07 1.38 2.87 | ≤0.001 0.073 ≤0.001 | 1.48–2.91 0.97–1.97 1.85–4.45 | 2.77 1.85 5.39 | 0.002 0.068 ≤0.001 | 1.45–5.27 0.95–3.58 2.50–11.60 |
| KRAS Mut vs. wt | 1.37 | 0.008 | 1.08–1.75 | 1.19 | 0.392 | 0.79–1.82 |
| BRAF Mut vs. wt | 1.69 | 0.009 | 1.13–2.51 | 3.38 | ≤0.001 | 1.85–6.17 |
| Sidedness Right vs. Left | 1.59 | ≤0.001 | 1.45–1.76 | 1.20 | 0.373 | 0.80–1.79 |
| Number of sites >1 vs. ≤1 | 1.89 | ≤0.001 | 1.51–2.37 | 0.70 | 0.184 | 0.41–1.18 |
| Treatment received Monotherapy +/− biologic Doublet Doublet plus biologic Triplet +/− biologic | 1 1.16 0.77 0.73 | 0.471 0.174 0.169 | 0.77–1.74 0.53–1.12 0.47–1.14 | |||
| Sites of metastases Lung vs. liver Lymph nodes vs. liver Peritoneum vs. liver Bone vs. liver CNS vs. liver | 1.05 2.01 2.32 1.57 42.73 | 0.720 ≤0.001 ≤0.001 0.243 ≤0.001 | 0.77–1.45 1.43–2.83 1.73–3.10 0.73–3.39 12.74–143.32 | 0.98 1.12 2.50 1.10 30.98 | 0.966 0.738 0.003 0.832 ≤0.001 | 0.54–1.79 0.55–2.27 1.36–4.59 0.43–2.83 6.22–154.13 |
| Surgery Yes vs. No | 0.37 | ≤0.001 | 0.28-0.49 | 0.33 | ≤0.001 | 0.21–0.53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lisanti, C.; Basile, D.; Garattini, S.K.; Parnofiello, A.; Corvaja, C.; Cortiula, F.; Bertoli, E.; Ongaro, E.; Foltran, L.; Casagrande, M.; et al. The SAFFO Study: Sex-Related Prognostic Role and Cut-Off Definition of Monocyte-to-Lymphocyte Ratio (MLR) in Metastatic Colorectal Cancer. Cancers 2023, 15, 175. https://doi.org/10.3390/cancers15010175
Lisanti C, Basile D, Garattini SK, Parnofiello A, Corvaja C, Cortiula F, Bertoli E, Ongaro E, Foltran L, Casagrande M, et al. The SAFFO Study: Sex-Related Prognostic Role and Cut-Off Definition of Monocyte-to-Lymphocyte Ratio (MLR) in Metastatic Colorectal Cancer. Cancers. 2023; 15(1):175. https://doi.org/10.3390/cancers15010175
Chicago/Turabian StyleLisanti, Camilla, Debora Basile, Silvio Ken Garattini, Annamaria Parnofiello, Carla Corvaja, Francesco Cortiula, Elisa Bertoli, Elena Ongaro, Luisa Foltran, Mariaelena Casagrande, and et al. 2023. "The SAFFO Study: Sex-Related Prognostic Role and Cut-Off Definition of Monocyte-to-Lymphocyte Ratio (MLR) in Metastatic Colorectal Cancer" Cancers 15, no. 1: 175. https://doi.org/10.3390/cancers15010175
APA StyleLisanti, C., Basile, D., Garattini, S. K., Parnofiello, A., Corvaja, C., Cortiula, F., Bertoli, E., Ongaro, E., Foltran, L., Casagrande, M., Di Nardo, P., Cardellino, G. G., Fasola, G., Buonadonna, A., Pella, N., Aprile, G., & Puglisi, F. (2023). The SAFFO Study: Sex-Related Prognostic Role and Cut-Off Definition of Monocyte-to-Lymphocyte Ratio (MLR) in Metastatic Colorectal Cancer. Cancers, 15(1), 175. https://doi.org/10.3390/cancers15010175







