Gastric-Type Adenocarcinoma of the Uterine Cervix Associated with Poor Response to Definitive Radiotherapy
Abstract
Simple Summary
Abstract
1. Introduction
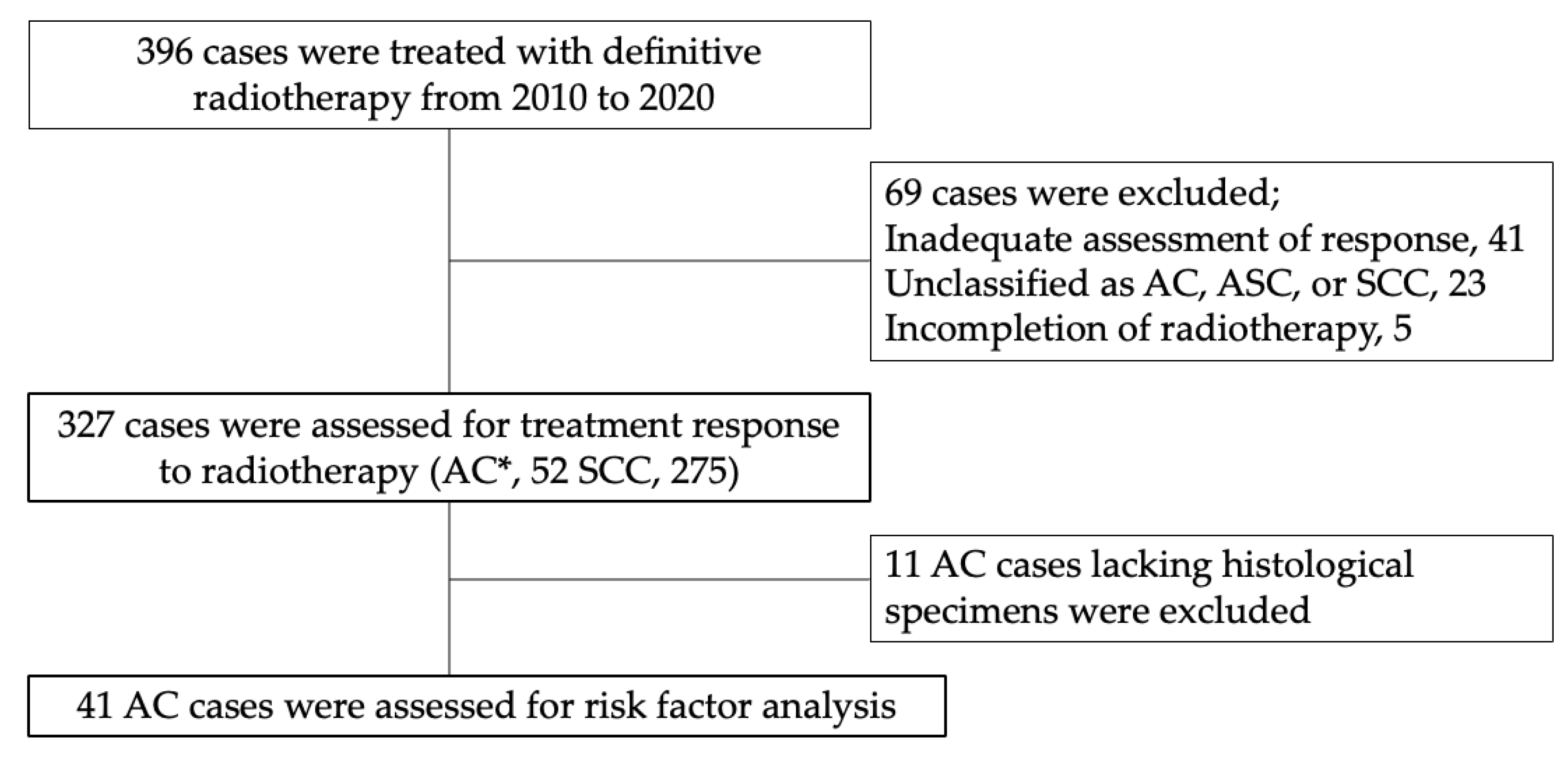
2. Materials and Methods
2.1. Study Sample
2.2. Study Data
2.3. Definitive RT
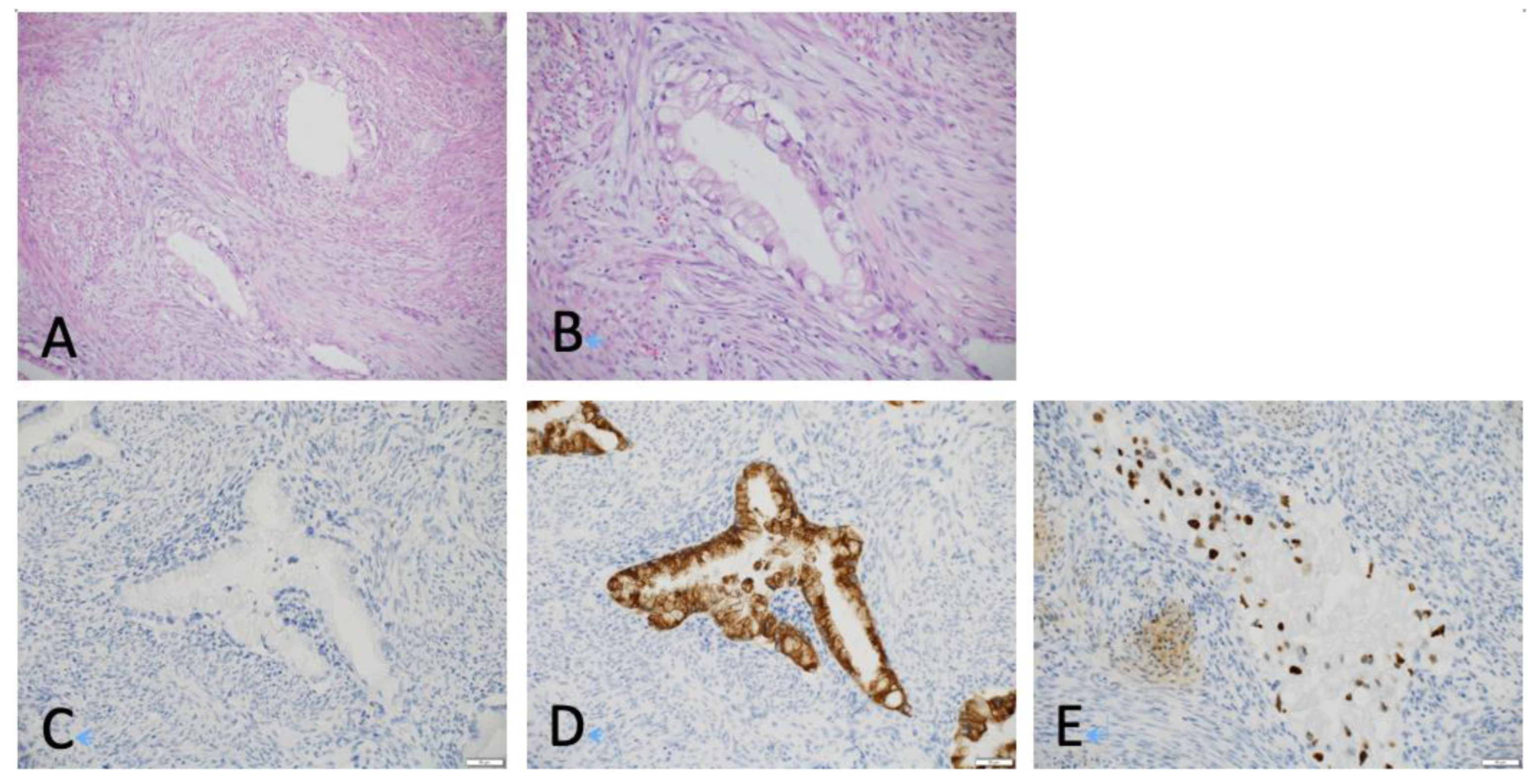
2.4. Histological Reevaluation
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Ploner, A.; Elfström, K.M.; Wang, J.; Roth, A.; Fang, F.; Sundström, K.; Dillner, J.; Sparén, P. Hpv vaccination and the risk of invasive cervical cancer. N. Engl. J. Med. 2020, 383, 1340–1348. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Cofie, L.E.; Berenson, A.B. Cervical cancer incidence in young u.S. Females after human papillomavirus vaccine introduction. Am. J. Prev. Med. 2018, 55, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Deng, Y.; Boakye, D.; Tin, M.S.; Lok, V.; Zhang, L.; Lucero-Prisno, D.E.; Xu, W.; Zheng, Z.J.; Elcarte, E.; et al. Global distribution, risk factors, and recent trends for cervical cancer: A worldwide country-level analysis. Gynecol. Oncol. 2022, 164, 85–92. [Google Scholar] [CrossRef]
- Yagi, A.; Ueda, Y.; Kakuda, M.; Tanaka, Y.; Ikeda, S.; Matsuzaki, S.; Kobayashi, E.; Morishima, T.; Miyashiro, I.; Fukui, K.; et al. Epidemiologic and clinical analysis of cervical cancer using data from the population-based osaka cancer registry. Cancer Res. 2019, 79, 1252–1259. [Google Scholar] [CrossRef]
- Tanaka, S.; Palmer, M.; Katanoda, K. Trends in cervical cancer incidence and mortality of young and middle adults in japan. Cancer Sci. 2022, 113, 1801–1807. [Google Scholar] [CrossRef]
- Wang, S.S.; Sherman, M.E.; Hildesheim, A.; Lacey, J.V.; Devesa, S. Cervical adenocarcinoma and squamous cell carcinoma incidence trends among white women and black women in the united states for 1976–2000. Cancer 2004, 100, 1035–1044. [Google Scholar] [CrossRef]
- National Comprehensive Cancer and Network. Cervical Cancer ver.1. 2022. Available online: https://www.nccn.org/professionals/physician_gls/pdf/cervical.pdf (accessed on 1 September 2022).
- Marth, C.; Landoni, F.; Mahner, S.; McCormack, M.; Gonzalez-Martin, A.; Colombo, N.; Committee, E.G. Cervical cancer: Esmo clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28, iv72–iv83. [Google Scholar] [CrossRef]
- Rose, P.G.; Java, J.J.; Whitney, C.W.; Stehman, F.B.; Lanciano, R.; Thomas, G.M. Locally advanced adenocarcinoma and adenosquamous carcinomas of the cervix compared to squamous cell carcinomas of the cervix in gynecologic oncology group trials of cisplatin-based chemoradiation. Gynecol. Oncol. 2014, 135, 208–212. [Google Scholar] [CrossRef]
- Katanyoo, K.; Sanguanrungsirikul, S.; Manusirivithaya, S. Comparison of treatment outcomes between squamous cell carcinoma and adenocarcinoma in locally advanced cervical cancer. Gynecol. Oncol. 2012, 125, 292–296. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, Y.T.; Kim, S.; Lee, B.; Lim, M.C.; Kim, J.W.; Won, Y.J. Prognosis of cervical cancer in the era of concurrent chemoradiation from national database in korea: A comparison between squamous cell carcinoma and adenocarcinoma. PLoS One 2015, 10, e0144887. [Google Scholar] [CrossRef] [PubMed]
- Yokoi, E.; Mabuchi, S.; Takahashi, R.; Matsumoto, Y.; Kuroda, H.; Kozasa, K.; Kimura, T. Impact of histological subtype on survival in patients with locally advanced cervical cancer that were treated with definitive radiotherapy: Adenocarcinoma/adenosquamous carcinoma versus squamous cell carcinoma. J. Gynecol. Oncol. 2017, 28, e19. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Tang, G.; Zhou, Q.; Kuang, W. Outcomes and prognostic factors in patients with locally advanced cervical cancer treated with concurrent chemoradiotherapy. Radiat. Oncol. 2022, 17, 142. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Carunchio, L.; Soveral, I.; Steenbergen, R.D.; Torné, A.; Martinez, S.; Fusté, P.; Pahisa, J.; Marimon, L.; Ordi, J.; del Pino, M. Hpv-negative carcinoma of the uterine cervix: A distinct type of cervical cancer with poor prognosis. BJOG 2015, 122, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.; Viveros-Carreño, D.; Hoegl, J.; Ávila, M.; Pareja, R. Human papillomavirus-independent cervical cancer. Int. J. Gynecol. Cancer 2022, 32, 1–7. [Google Scholar] [CrossRef] [PubMed]
- de Sanjose, S.; Quint, W.G.; Alemany, L.; Geraets, D.T.; Klaustermeier, J.E.; Lloveras, B.; Tous, S.; Felix, A.; Bravo, L.E.; Shin, H.R.; et al. Human papillomavirus genotype attribution in invasive cervical cancer: A retrospective cross-sectional worldwide study. Lancet Oncol. 2010, 11, 1048–1056. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, A.; Park, K.J.; Djordjevic, B.; Howitt, B.; Nucci, M.; Oliva, E.; Stolnicu, S.; Xu, B.; Soslow, R.; Parra-Herran, C. International endocervical adenocarcinoma criteria and classification: Validation and interobserver reproducibility. Am. J. Surg. Pathol. 2019, 43, 75–83. [Google Scholar] [CrossRef]
- Editorial Board; WHO. Who Classification of Tumours Female Genital Tumours International Agency for Research on Cancer, 5th ed.; International Agency for Research on Cancer: Lyon, France, 2020; Volume 4. [Google Scholar]
- Kusanagi, Y.; Kojima, A.; Mikami, Y.; Kiyokawa, T.; Sudo, T.; Yamaguchi, S.; Nishimura, R. Absence of high-risk human papillomavirus (hpv) detection in endocervical adenocarcinoma with gastric morphology and phenotype. Am. J. Pathol. 2010, 177, 2169–2175. [Google Scholar] [CrossRef]
- Kojima, A.; Mikami, Y.; Sudo, T.; Yamaguchi, S.; Kusanagi, Y.; Ito, M.; Nishimura, R. Gastric morphology and immunophenotype predict poor outcome in mucinous adenocarcinoma of the uterine cervix. Am. J. Surg. Pathol. 2007, 31, 664–672. [Google Scholar] [CrossRef]
- Carleton, C.; Hoang, L.; Sah, S.; Kiyokawa, T.; Karamurzin, Y.S.; Talia, K.L.; Park, K.J.; McCluggage, W.G. A detailed immunohistochemical analysis of a large series of cervical and vaginal gastric-type adenocarcinomas. Am. J. Surg. Pathol. 2016, 40, 636–644. [Google Scholar] [CrossRef]
- Yoshino, K.; Kurita, T.; Takahashi, F.; Nagase, S.; Board members of the 2021 Committee on Gynecologic Oncology of the Japan Society of Obstetrics and Gynecology. Annual report of the committee on gynecologic oncology, the japan society of obstetrics and gynecology: Annual patient report for 2019 and annual treatment report for 2014. J. Obstet. Gynaecol. Res. 2022, 48, 1570–1579. [Google Scholar] [CrossRef] [PubMed]
- Kojima, A.; Shimada, M.; Mikami, Y.; Nagao, S.; Takeshima, N.; Sugiyama, T.; Teramoto, N.; Kiyokawa, T.; Kigawa, J.; Nishimura, R.; et al. Chemoresistance of gastric-type mucinous carcinoma of the uterine cervix: A study of the sankai gynecology study group. Int. J. Gynecol. Cancer 2018, 28, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Holl, K.; Nowakowski, A.M.; Powell, N.; McCluggage, W.G.; Pirog, E.C.; De Souza, S.C.; Tjalma, W.A.; Rosenlund, M.; Fiander, A.; Sánchez, M.C.; et al. Human papillomavirus prevalence and type-distribution in cervical glandular neoplasias: Results from a european multinational epidemiological study. Int. J. Cancer 2015, 137, 2858–2868. [Google Scholar] [CrossRef] [PubMed]
- Nishio, S.; Mikami, Y.; Tokunaga, H.; Yaegashi, N.; Satoh, T.; Saito, M.; Okamoto, A.; Kasamatsu, T.; Miyamoto, T.; Shiozawa, T.; et al. Analysis of gastric-type mucinous carcinoma of the uterine cervix—An aggressive tumor with a poor prognosis: A multi-institutional study. Gynecol. Oncol. 2019, 153, 13–19. [Google Scholar] [CrossRef]
- Shimada, M.; Nagao, S.; Fujiwara, K.; Takeshima, N.; Takizawa, K.; Shoji, T.; Sugiyama, T.; Yamaguchi, S.; Nishimura, R.; Kigawa, J. Neoadjuvant chemotherapy with docetaxel and carboplatin followed by radical hysterectomy for stage ib2, iia2, and iib patients with non-squamous cell carcinoma of the uterine cervix. Int. J. Clin. Oncol. 2016, 21, 1128–1135. [Google Scholar] [CrossRef]
- Mabuchi, S.; Isohashi, F.; Maruoka, S.; Hisamatsu, T.; Takiuchi, T.; Yoshioka, Y.; Kimura, T. Post-treatment follow-up procedures in cervical cancer patients previously treated with radiotherapy. Arch. Gynecol. Obstet. 2012, 286, 179–185. [Google Scholar] [CrossRef]
- Zou, P.; Yang, E.; Li, Z. Neutrophil-to-lymphocyte ratio is an independent predictor for survival outcomes in cervical cancer: A systematic review and meta-analysis. Sci. Rep. 2020, 10, 21917. [Google Scholar] [CrossRef]
- Kawashima, A.; Isohashi, F.; Mabuchi, S.; Sawada, K.; Ueda, Y.; Kobayashi, E.; Matsumoto, Y.; Otani, K.; Tamari, K.; Seo, Y.; et al. A 3-year follow-up study of radiotherapy using computed tomography-based image-guided brachytherapy for cervical cancer. J. Radiat. Res. 2019, 60, 264–269. [Google Scholar] [CrossRef]
- Mabuchi, S.; Isohashi, F.; Okazawa, M.; Kitada, F.; Maruoka, S.; Ogawa, K.; Kimura, T. Chemoradiotherapy followed by consolidation chemotherapy involving paclitaxel and carboplatin and in figo stage iiib/iva cervical cancer patients. J. Gynecol. Oncol. 2017, 28, e15. [Google Scholar] [CrossRef]
- Yao, G.; Qiu, J.; Zhu, F.; Wang, X. Survival of patients with cervical cancer treated with definitive radiotherapy or concurrent chemoradiotherapy according to histological subtype: A systematic review and meta-analysis. Front. Med. (Lausanne) 2022, 9, 843262. [Google Scholar] [CrossRef]
- Karamurzin, Y.S.; Kiyokawa, T.; Parkash, V.; Jotwani, A.R.; Patel, P.; Pike, M.C.; Soslow, R.A.; Park, K.J. Gastric-type endocervical adenocarcinoma: An aggressive tumor with unusual metastatic patterns and poor prognosis. Am. J. Surg. Pathol. 2015, 39, 1449–1457. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas Research Network. Integrated genomic and molecular characterization of cervical cancer. Nature 2017, 543, 378–384. [Google Scholar] [CrossRef]
- Gordhandas, S.B.; Kahn, R.; Sassine, D.; Aviki, E.M.; Nelson, B.B.; Catchings, A.; Liu, Y.L.; Lakhman, Y.; Abu-Rustum, N.R.; Park, K.J.; et al. Gastric-type adenocarcinoma of the cervix in patients with peutz-jeghers syndrome: A systematic review of the literature with proposed screening guidelines. Int. J. Gynecol. Cancer 2022, 32, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Selenica, P.; Alemar, B.; Matrai, C.; Talia, K.L.; Veras, E.; Hussein, Y.; Oliva, E.; Beets-Tan, R.G.H.; Mikami, Y.; McCluggage, W.G.; et al. Massively parallel sequencing analysis of 68 gastric-type cervical adenocarcinomas reveals mutations in cell cycle-related genes and potentially targetable mutations. Mod. Pathol. 2021, 34, 1213–1225. [Google Scholar] [CrossRef] [PubMed]
- Park, E.; Kim, S.W.; Kim, S.; Kim, H.S.; Lee, J.Y.; Kim, Y.T.; Cho, N.H. Genetic characteristics of gastric-type mucinous carcinoma of the uterine cervix. Mod. Pathol. 2021, 34, 637–646. [Google Scholar] [CrossRef]
| Variables | Total (n = 327) | AC (n = 52) | SCC (n = 275) | p-Value | |||
|---|---|---|---|---|---|---|---|
| Follow-up time (months) | 52.2 | (3.3–144.7) | 47.3 | (3.5–100.3) | 53 | (3.3–144.7) | 0.25 |
| Patients’ characteristics | |||||||
| Age (year) | 59 | (26–93) | 57 | (30–78) | 61 | (26–93) | 0.12 |
| Age ≧ 60 | 163 | (49.8) | 21 | (40.4) | 142 | (51.6) | 0.14 |
| Non-parous * | 74 | (22.6) | 12 | (23.1) | 62 | (22.9) | 0.98 |
| BMI (kg/m2) | 21.4 | (13.3–40.6) | 21.7 | (16.6–40.6) | 21.2 | (13.3–40.1) | 0.28 |
| FIGO stage | |||||||
| I | 55 | (16.8) | 13 | (25.0) | 42 | (15.3) | 0.24 |
| II | 100 | (30.6) | 16 | (30.8) | 84 | (30.5) | |
| III | 163 | (49.8) | 23 | (44.2) | 140 | (50.9) | |
| IV | 9 | (2.8) | 0 | (0.0) | 9 | (3.3) | |
| FIGO 2018 stage ≧ IIB | 243 | (74.3) | 34 | (65.4) | 209 | (76.0) | 0.11 |
| Lymph node metastasis | 140 | (42.8) | 20 | (38.5) | 120 | (43.6) | 0.49 |
| Tumor size (cm) | 4.3 | (0.5–8.8) | 4.0 | (1.0–8.8) | 4.3 | (0.5–8.2) | 0.58 |
| Tumor size ≧ 5 cm ** | 102 | (32.4) | 14 | (28.6) | 88 | (33.1) | 0.54 |
| Pretreatment laboratory data | |||||||
| Hemoglobin level (g/dl) | 12.5 | (5.8–15.8) | 12.5 | (8.0–15.8) | 12.5 | (5.8–15.8) | 0.84 |
| NLR | 2.6 | (0.6–24.6) | 2.6 | (0.9–7.6) | 2.6 | (0.6–24.6) | 0.66 |
| NLR ≧ 2.5 | 157 | 53.0 | 27 | (56.3) | 130 | (43.9) | 0.63 |
| Type of treatment | |||||||
| RT alone | 74 | (22.6) | 11 | (21.2) | 63 | (22.9) | 0.78 |
| CCRT | 253 | (77.4) | 41 | (78.8) | 212 | (77.1) | |
| RT completion within 56 days | 317 | (96.9) | 49 | (94.2) | 268 | (97.5) | 0.20 |
| Treatment outcome | |||||||
| CR | 282 | (86.2) | 28 | (53.8) | 254 | (92.4) | <0.05 |
| PR | 24 | (7.3) | 12 | (23.1) | 12 | (4.4) | |
| SD | 11 | (3.4) | 8 | (15.4) | 3 | (1.1) | |
| PD | 10 | (3.1) | 4 | (7.7) | 6 | (2.2) | |
| Recurrence after CR | 57/282 | (20.2) | 5/28 | (17.9) | 52/254 | (20.5) | 1.00 |
| Variables | AC (n = 41) | ||||
|---|---|---|---|---|---|
| CR (n = 20) | Non-CR (n = 21) | p-Value | |||
| Patients’ characteristics | |||||
| Age (year) | 55 | (30–70) | 58 | (38–78) | 1.00 |
| Age ≧ 60 | 9 | (45.0) | 7 | (33.3) | 0.44 |
| Non-parous | 7 | (35.0) | 3 | (14.3) | 0.16 |
| BMI (kg/m2) | 21.0 | (16.6–37.0) | 22.0 | (17.2–29.6) | 0.38 |
| Histopathology | |||||
| Gastric type | 1 | (5.0) | 8 | (38.1) | <0.05 |
| Other type | 19 | (95.0) | 13 | (61.9) | |
| HPV status | |||||
| Associated | 14 | (70.0) | 5 | (23.8) | <0.05 |
| Independent | 4 | (20.0) | 11 | (52.4) | |
| Undetermined | 2 | (10.0) | 5 | (23.8) | |
| FIGO stage ≧ IIB | 8 | (40.0) | 18 | (85.7) | <0.05 |
| Lymph node metastasis | 4 | (20.0) | 9 | (42.9) | 0.18 |
| Tumor size (cm) | 3.8 | (1.0–6.6) | 4.3 | (1.7–8.8) | 0.08 |
| Tumor size ≧ 5.0 cm * | 4 | (23.5) | 9 | (42.9) | 0.30 |
| Pretreatment laboratory data | |||||
| Hemoglobin level (g/dl) | 13.0 | (8.5–15.8) | 12.3 | (8.0–14.2) | 0.09 |
| NLR | 2.2 | (1.5–4.6) | 3.5 | (0.9–7.6) | <0.05 |
| NLR ≧ 2.5 | 8 | (40.0) | 16 | (76.2) | <0.05 |
| Treatment | |||||
| CCRT | 14 | (70.0) | 18 | (85.7) | 0.28 |
| RT alone | 6 | (30.0) | 3 | (14.3) | |
| n | Univariate Analysis | Multivariate Analysis * | |||
|---|---|---|---|---|---|
| Variables | Non-CR/ Total (%) | cOR (95% CI) | p-Value | aOR (95% CI) | p-Value |
| Age | 0.45 | ||||
| <60 | 14/25 (56) | 1 | |||
| ≧60 | 7/16 (43.8) | 0.6 (0.2–2.2) | |||
| Parous | 0.13 | ||||
| nonparous | 3/10 (30) | 1 | |||
| multiparous | 18/31 (58.1) | 3.2 (0.7–14.9) | |||
| Histology | <0.05 | <0.05 | |||
| Other type | 13/32 (40.6) | 1 | 1 | ||
| Gastric type | 8/9 (88.9) | 11.7 (1.3–105.0) | 12.2 (1.0–145.6) | ||
| FIGO stage | <0.05 | 0.10 | |||
| <IIB | 3/15 (20.0) | 1 | 1 | ||
| ≧IIB | 18/26 (69.2) | 9 (2.0–40.9) | 4.8 (0.7–32.2) | ||
| Lymph node metastasis | 0.12 | ||||
| No | 12/28 (42.9) | 1 | |||
| Yes | 9/13 (69.2) | 3.0 (0.7–12.1) | |||
| Tumor size ** | 0.22 | 0.48 | |||
| <5 cm | 12/25 (48.0) | 1 | 1 | ||
| ≧5 cm | 9/13 (69.2) | 2.4 (0.6–10.0) | 1.9 (0.3–10.3) | ||
| NLR | <0.05 | 0.30 | |||
| <2.5 | 5/17 (29.4) | 1 | 1 | ||
| ≧2.5 | 16/24 (66.7) | 4.8 (1.3–18.4) | 2.4 (0.5–12.2) | ||
| Treatment | 0.23 | ||||
| RT alone | 3/9 (33.3) | 1 | |||
| CCRT | 18/32 (56.3) | 2.6 (0.5–12.1) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuruma, A.; Kodama, M.; Hori, Y.; Sato, K.; Fujii, M.; Isohashi, F.; Miyoshi, A.; Mabuchi, S.; Setoguchi, A.; Shimura, H.; et al. Gastric-Type Adenocarcinoma of the Uterine Cervix Associated with Poor Response to Definitive Radiotherapy. Cancers 2023, 15, 170. https://doi.org/10.3390/cancers15010170
Kuruma A, Kodama M, Hori Y, Sato K, Fujii M, Isohashi F, Miyoshi A, Mabuchi S, Setoguchi A, Shimura H, et al. Gastric-Type Adenocarcinoma of the Uterine Cervix Associated with Poor Response to Definitive Radiotherapy. Cancers. 2023; 15(1):170. https://doi.org/10.3390/cancers15010170
Chicago/Turabian StyleKuruma, Airi, Michiko Kodama, Yumiko Hori, Kazuaki Sato, Makoto Fujii, Fumiaki Isohashi, Ai Miyoshi, Seiji Mabuchi, Akira Setoguchi, Hiroko Shimura, and et al. 2023. "Gastric-Type Adenocarcinoma of the Uterine Cervix Associated with Poor Response to Definitive Radiotherapy" Cancers 15, no. 1: 170. https://doi.org/10.3390/cancers15010170
APA StyleKuruma, A., Kodama, M., Hori, Y., Sato, K., Fujii, M., Isohashi, F., Miyoshi, A., Mabuchi, S., Setoguchi, A., Shimura, H., Goto, T., Toda, A., Nakagawa, S., Kinose, Y., Takiuchi, T., Kobayashi, E., Hashimoto, K., Ueda, Y., Sawada, K., ... Kimura, T. (2023). Gastric-Type Adenocarcinoma of the Uterine Cervix Associated with Poor Response to Definitive Radiotherapy. Cancers, 15(1), 170. https://doi.org/10.3390/cancers15010170







