Identification of Cancerous Skin Lesions Using Vibrational Optical Coherence Tomography (VOCT): Use of VOCT in Conjunction with Machine Learning to Diagnose Skin Cancer Remotely Using Telemedicine
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Measurement of Resonant Frequency
2.3. Machine Learning Analysis
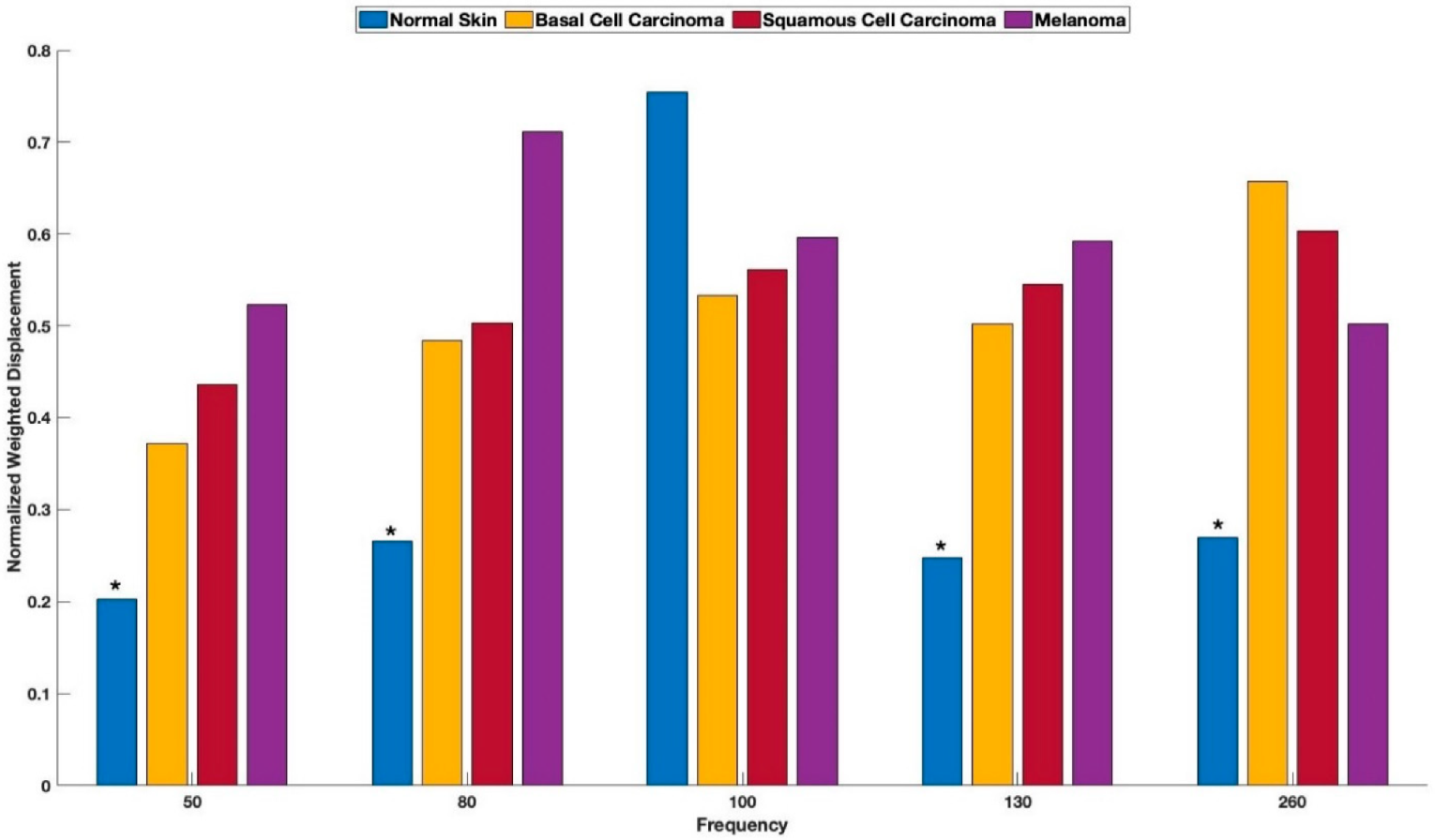
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Academy of Dermatology Association Website. Types of Skin Cancer. Available online: https://www.aad.org/public/diseases/skin-cancer/types/common (accessed on 7 September 2021).
- Leiter, U.; Keim, U.; Garbe, C. Epidemiology of Skin Cancer: Update 2019. Adv Exp Med Biol. 2020, 1268, 123–139. [Google Scholar]
- Warshaw, E.M.; Hillna, Y.J.; Greer, N.L.; Hagel, E.M.; MacDonald, R.; Rutks, I.R.; Wilt, T.J. Teledermatology for diagnosis and management of skin conditions. A systemic review. J. Am. Acad. Dermatol. 2011, 54, 759–772. [Google Scholar] [CrossRef] [PubMed]
- Trettel, A.; Eissing, L.; Augistin, M. Telemedicine in dermatology: Findings and experiences worldwide—A systematic literature review. JEADV 2018, 32, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Sud, E.; Anjankar, A. Applications of Telemedicine in Dermatology. Cureus 2022, 14, e27740. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, A.B.; Wells, A.F.; Joseph, F. Merola, J.F. Telemedicine and psoriatic arthritis: Best practices and considerations for dermatologists and rheumatologists. Clin. Rheumatol. 2022, 41, 1271–1283. [Google Scholar] [CrossRef] [PubMed]
- Emiroglu, N.; Cengiz, F.P.; Kemerliz, F. The relationship between dermoscopy and histopathology of basal cell carcinoma. An. Bras. Dermatol. 2015, 90, 351–356. [Google Scholar] [CrossRef]
- Weber, P.; Tschandl, P.; Sinz, C.; Kitler, H. Dermatoscopy of neoplastic skin lesions: Recent advances, updates, and revisions. Curr. Treat. Options Oncol. 2018, 19, 56. [Google Scholar] [CrossRef]
- Ungureanu, L.; Cosgarea, I.; Senila, S.; Vasilovici, A. Role of Dermoscopy in the Assessment of Basal Cell Carcinoma. Front. Med. 2021, 8, 718855. [Google Scholar] [CrossRef]
- Lupu, M.; Caruntu, C.; Popa, M.I.; Voiculescu, V.M.; Zurac, S.; Boda, D. Vascular patterns in basal cell carcinoma: Dermoscopic, confocal and histopathological perspectives. Oncol. Lett. 2019, 1, 4112–4125. [Google Scholar] [CrossRef]
- Wolner, Z.J.; Yelomas, O.; Liopyris, K.; Rodgers, T.; Marchetti, M.M.; Marghoob, A.A. Enhancing skin cancer diagnosis with dermoscopy. Dermatol. Clin. 2017, 35, 417–437. [Google Scholar] [CrossRef]
- Russo, T.; Piccolo, V.; Lallas, A.; Jacomel, J.; Moscarella, E.; Alfano, R.; Argenziano, G. Dermoscopy of Malignant Skin Tumours: What’s New? Dermatology 2017, 233, 64–73. [Google Scholar] [CrossRef]
- Kato, J.; Horimoto, K.; Sato, S.; Minowa, T.; Uhara, H. Dermoscopy of Melanoma and Non-melanoma Skin Cancers. Front. Med. 2019, 6, 180. [Google Scholar] [CrossRef] [PubMed]
- Silver, F.H.; Deshmukh, T.; Benedetto, D.; Kelkar, N. Mechano-vibrational spectroscopy of skin: Are changes in collagen and vascular tissue components early signs of basal cell carcinoma formation? Skin Res. Technol. 2020, 27, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Silver, F.H.; Kelkar, N.; Deshmukh, T.; Ritter, N.; Ryan, N.; Nadiminiti, H. Characterization of the biomechanical properties of skin using vibrational optical coherence tomography: Do changes in the biomechanical properties of skin stroma reflect structural changes in the extracellular matrix of cancerous lesions? Biomolecules 2021, 11, 1712. [Google Scholar] [CrossRef] [PubMed]
- Silver, F.H.; Deshmukh, T.; Kelkar, N.; Ritter, N.; Ryan, N.; Nadiminti, H. The “Virtual Biopsy” of Cancerous Lesions in 3D: Non-Invasive Differentiation between Melanoma and Other Lesions Using Vibrational Optical Coherence Tomography. Dermatopathology 2021, 8, 539–551. [Google Scholar] [CrossRef]
- Silver, F.H.; Shah, R.G.; Richard, M.; Benedetto, D. Comparative “virtual biopsies” of normal skin and skin lesions using vibrational optical coherence tomography. Skin Res. Technol. 2019, 25, 743–749. [Google Scholar] [CrossRef]
- Silver, F.H.; Shah, R.G.; Richard, M.; Benedetto, D. Use of Vibrational Optical Coherence Tomography to Image and Characterize a Squamous Cell Carcinoma. J. Dermatol. Res. Ther. 2019, 5, 067. [Google Scholar] [CrossRef]
- Shah, R.G.; Devore, D.; Pierce, M.C.; Silver, F.H. Vibrational analysis of implants and tissues: Calibration and mechanical spectroscopy of multi-component materials. J. Biomed. Mater. Res. Part A 2017, 105, 1666–1671. [Google Scholar] [CrossRef]
- Silver, F.H.; Kelkar, N.; Desmukh, T.; Horvath, I.; Shah, R.G. Mechano-Vibrational Spectroscopy of Tissues and Materials Using Vibrational Optical Coherence Tomography: A New Non-Invasive and Non-Destructive Technique. Recent Prog. Mater. 2020, 2, 1. [Google Scholar] [CrossRef]
- Silver, F.H.; Deshmukh, T.; Ryan, N.; Romm, A.; Nadiminti, H. “Fingerprinting” Benign and Cancerous Skin Lesions Using Vibrational Optical Coherence Tomography: Differentiation among Cancerous Lesion Types Based on the Presence of New Cells, Blood Vessels, and Fibrosis. Biomolecules 2022, 12, 1332. [Google Scholar] [CrossRef]
- Dinnes, J.; Deeks, J.J.; Chuchu, N.; Ferrante di Ruano, L.; Matin, R.N.; Thomson, D.R.; Wong, K.Y.; Aldridge, R.B.; Abbott, R.; Fawzy, M.; et al. Dermoscopy, with and without visual inspection, for diagnosing melanoma in adults. Cochrane Database Syst. Rev. 2018, 12, CD011902. [Google Scholar] [CrossRef] [PubMed]
- Trevethan, R. Sensitivity, Specificity, and Predictive Values: Foundations, Pliabilities, and Pitfalls in Research and Practice. Front. Public Health 2017, 5, 307. [Google Scholar] [CrossRef] [PubMed]
- Privalle, A.; Havighurst, T.; Kim, K.M.; Bennett, D.D.; Xu, Y.G. Number of skin biopsies needed per malignancy: Comparing the use of skin biopsies among dermatologists and nondermatologist clinicians. J. Am. Acad. Dermatol. 2020, 82, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Wernli, K.J.; Henrikson, N.B.; Morrison, C.C.; Nguyen, M.; Pocobelli, G.; Blasi, P.R. Screening for skin cancer in adults updated evidence report and systematic review for the US preventive services task force. JAMA 2016, 316, 436–447. [Google Scholar] [CrossRef]




| BCC | SCC | Melanoma | |
|---|---|---|---|
| Sensitivity | 90.9% | 91.6% | 83.3% |
| Specificity | 87.5% | 87.5% | 77.8% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silver, F.H.; Mesica, A.; Gonzalez-Mercedes, M.; Deshmukh, T. Identification of Cancerous Skin Lesions Using Vibrational Optical Coherence Tomography (VOCT): Use of VOCT in Conjunction with Machine Learning to Diagnose Skin Cancer Remotely Using Telemedicine. Cancers 2023, 15, 156. https://doi.org/10.3390/cancers15010156
Silver FH, Mesica A, Gonzalez-Mercedes M, Deshmukh T. Identification of Cancerous Skin Lesions Using Vibrational Optical Coherence Tomography (VOCT): Use of VOCT in Conjunction with Machine Learning to Diagnose Skin Cancer Remotely Using Telemedicine. Cancers. 2023; 15(1):156. https://doi.org/10.3390/cancers15010156
Chicago/Turabian StyleSilver, Frederick H., Arielle Mesica, Michael Gonzalez-Mercedes, and Tanmay Deshmukh. 2023. "Identification of Cancerous Skin Lesions Using Vibrational Optical Coherence Tomography (VOCT): Use of VOCT in Conjunction with Machine Learning to Diagnose Skin Cancer Remotely Using Telemedicine" Cancers 15, no. 1: 156. https://doi.org/10.3390/cancers15010156
APA StyleSilver, F. H., Mesica, A., Gonzalez-Mercedes, M., & Deshmukh, T. (2023). Identification of Cancerous Skin Lesions Using Vibrational Optical Coherence Tomography (VOCT): Use of VOCT in Conjunction with Machine Learning to Diagnose Skin Cancer Remotely Using Telemedicine. Cancers, 15(1), 156. https://doi.org/10.3390/cancers15010156






