Clinicopathological and Dermoscopic Baselines in Patients with Lynch Syndrome
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
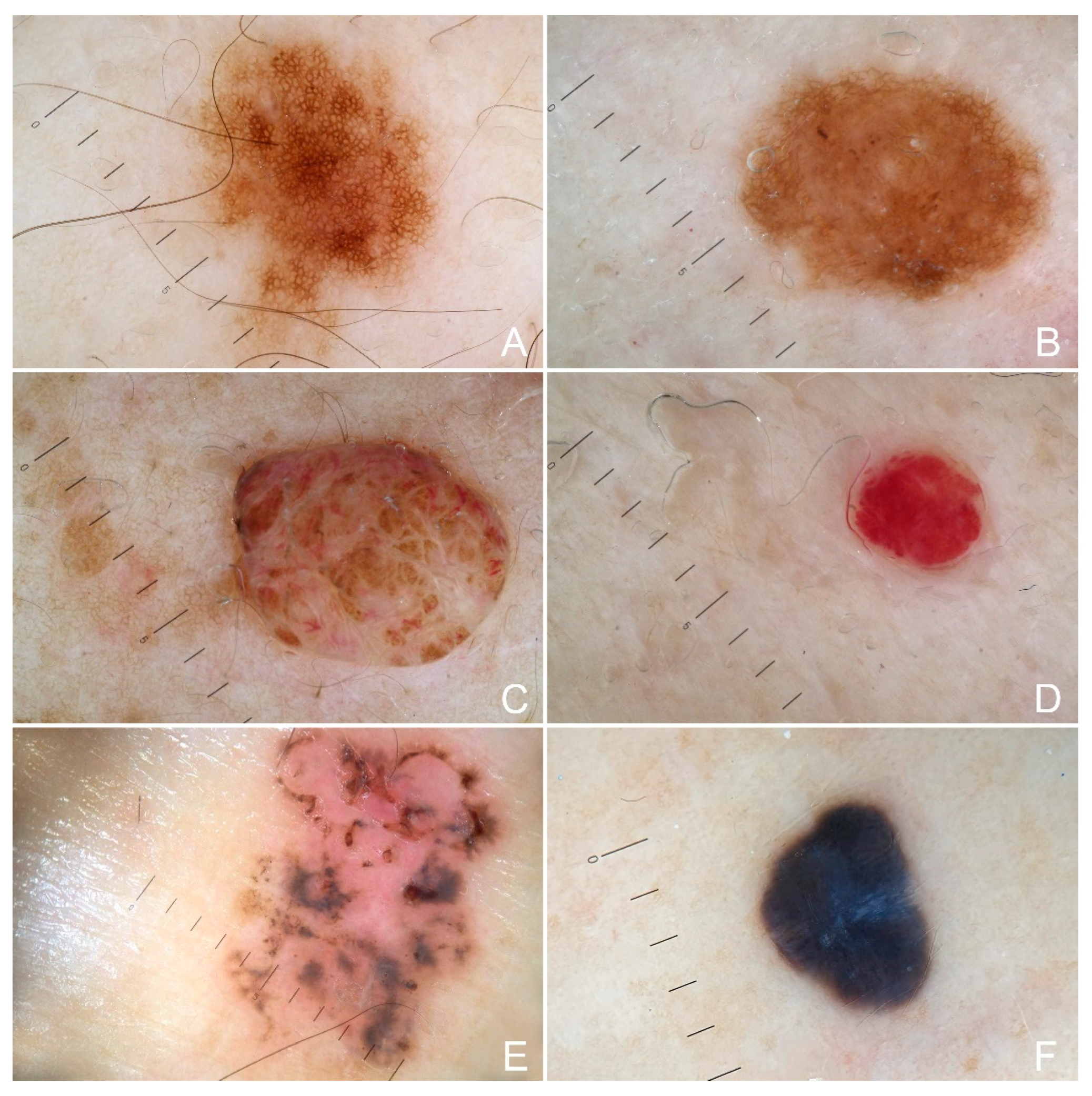
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- da Silva, F.C.; de Oliveira, L.P.; Santos, E.M.; Nakagawa, W.T.; Aguiar Junior, S.; Valentin, M.D.; Rossi, B.M.; de Oliveira Ferreira, F. Frequency of extracolonic tumors in Brazilian families with Lynch syndrome: Analysis of a hereditary colorectal cancer institutional registry. Fam. Cancer 2010, 9, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Møller, P.; Seppälä, T.; Bernstein, I.; Holinski-Feder, E.; Sala, P.; Evans, D.G.; Lindblom, A.; Macrae, F.; Blanco, I.; Sijmons, R.; et al. Cancer incidence and survival in Lynch syndrome patients receiving colonoscopic and gynaecological surveillance: First report from the prospective Lynch syndrome database. Gut 2017, 66, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Lynch, H.T.; Smyrk, T. Hereditary nonpolyposis colorectal cancer (Lynch syndrome). An updated review. Cancer 1996, 78, 1149–1167. [Google Scholar] [CrossRef]
- Lindor, N.M. Familial colorectal cancer type X: The other half of hereditary nonpolyposis colon cancer syndrome. Surg. Oncol. Clin. N. Am. 2009, 18, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Samadder, N.J.; Smith, K.R.; Wong, J.; Thomas, A.; Hanson, H.; Boucher, K.; Kopituch, C.; Cannon-Albright, L.A.; Burt, R.W.; Curtin, K. Cancer Risk in Families Fulfilling the Amsterdam Criteria for Lynch Syndrome. JAMA Oncol. 2017, 3, 1697–1701. [Google Scholar] [CrossRef] [PubMed]
- Ojha, S.K.; Laslett, N. Hereditary Nonpolyposis Colon Cancer; StatPearls: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK564511/ (accessed on 5 December 2022).
- Zalaudek, I.; Schmid, K.; Marghoob, A.A.; Scope, A.; Manzo, M.; Moscarella, E.; Malvehy, J.; Puig, S.; Pellacani, G.; Thomas, L.; et al. Frequency of dermoscopic nevus subtypes by age and body site: A cross-sectional study. Arch. Dermatol. 2011, 147, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Paolino, G.; Pampena, R.; Di Nicola, M.R.; Longo, C.; Rognone, A.; Zambelli, S.; Bianchini, G.; Mercuri, S.R. Dermatological and Dermoscopic Baselines in BRCA Mutation Carriers. Front. Med. 2022, 29, 863468. [Google Scholar] [CrossRef] [PubMed]
- Zalaudek, I.; Grinschgl, S.; Argenziano, G.; Marghoob, A.A.; Blum, A.; Richtig, E.; Wolf, I.H.; Fink-Puches, R.; Kerl, H.; Soyer, H.P.; et al. Age-related prevalence of dermoscopy patterns in acquired melanocytic naevi. Br. J. Dermatol. 2006, 154, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Gandini, S.; Sera, F.; Cattaruzza, M.S.; Pasquini, P.; Abeni, D.; Boyle, P.; Melchi, C.F. Metaanalysis of risk factors for cutaneous melanoma: I. Common and atypical naevi. Eur. J. Cancer 2005, 41, 28–44. [Google Scholar] [CrossRef] [PubMed]
- Murphy, H.R.; Armstrong, R.; Cairns, D.; Greenhalgh, K.L. Muir-Torre syndrome: Expanding the genotype and phenotype—A further family with a MSH6 mutation. Fam. Cancer 2008, 7, 255–257. [Google Scholar] [CrossRef] [PubMed]
- Goecke, T.; Schulmann, K.; Engel, C.; Holinski-Feder, E.; Pagenstecher, C.; Kloor, H.K.S.; Kunstmann, E.; Vogelsang, H.; Keller, G.; Dietmaier, W.; et al. German HNPCC Consortium. Genotype-phenotype comparison of German MLH1 and MSH2 mutation carriers clinically affected with Lynch syndrome: A report by the German HNPCC Consortium. J. Clin. Oncol. 2006, 24, 4285–4292. [Google Scholar] [CrossRef] [PubMed]
- Lynch, H.T.; Lynch, P.M.; Pester, J.; Fusaro, R.M. The cancer family syndrome. Rare cutaneous phenotypic linkage of Torre’s syndrome. Arch. Intern. Med. 1981, 141, 607–611. [Google Scholar] [CrossRef] [PubMed]
- Barrow, E.; Robinson, L.; Alduaij, W.; Shenton, A.; Clancy, T.; Lalloo, F.; Hill, J.; Evans, D.E. Cumulative lifetime incidence of extracolonic cancers in Lynch syndrome: A report of 121 families with proven mutations. Clin. Genet. 2009, 75, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Hitchins, M.P.; Ward, R.L. Constitutional (germline) MLH1 epimutation as an aetiological mechanism for hereditary non-polyposis colorectal cancer. J. Med. Genet. 2009, 46, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Donati, P.; Paolino, G.; Donati, M.; Panetta, C. Adenocarcinoma of the cervix associated with a neuroendocrine small cell carcinoma of the cervix in the spectrum of Muir-Torre syndrome. Eur. J. Gynaecol. Oncol. 2015, 36, 213–215. [Google Scholar]
- Donati, M.; Paolino, G.; Muscardin, L.; Panetta, C.; Donati, P. Resolution of Benign and Malignant Sebaceous Neoplasms, in a Renal Transplant Patient Treated with Everolimus. Exp. Clin. Transplant 2017, 15, 100–102. [Google Scholar]
- Czakó, L.; Tiszlavicz, L.; Takács, R.; Baradnay, G.; Lonovics, J.; Cserni, G.; Závodná, K.; Bartosova, Z. The first molecular analysis of a Hungarian HNPCC family: A novel MSH2 germline mutation. Orv. Hetil. 2005, 146, 1009–1016. [Google Scholar]
- Bosetti, C.; Traini, E.; Alam, T.; Allen, C.A.; Carreras, G.; Compton, K.; Fitzmaurice, C.; Force, L.M.; Gallus, S.; Gorini, G.; et al. National burden of cancer in Italy, 1990–2017: A systematic analysis for the global burden of disease study 2017. Sci. Rep. 2020, 10, 22099. [Google Scholar] [CrossRef] [PubMed]
| n | % | cn | c% | p * | |
|---|---|---|---|---|---|
| Gender | NS | ||||
| Male | 15 | 35.7 | 28 | 40 | |
| Female | 27 | 64.3 | 42 | 60 | |
| Hairs | NS | ||||
| Blond | 3 | 7.1 | 6 | 8.5 | |
| Brown | 35 | 83.3 | 57 | 82 | |
| Black | 4 | 9.5 | 7 | 10 | |
| Eyes | NS | ||||
| Blue | 6 | 14.3 | 9 | 13 | |
| Brown | 31 | 73.8 | 52 | 74 | |
| Black | 3 | 7.1 | 5 | 7 | |
| Green | 2 | 4.8 | 4 | 6 | |
| FP | NS | ||||
| I | 1 | 2.4 | 3 | 4 | |
| II | 9 | 21.4 | 15 | 21 | |
| III | 30 | 71.4 | 49 | 70 | |
| IV | 2 | 4.8 | 3 | 4 | |
| PCH | <0.001 | ||||
| Positive * | 15 | 36 | 5 | 7 | |
| Negative | 27 | 64 | 65 | 93 | |
| CL | NS | ||||
| None | 4 | 9.5 | 12 | 17 | |
| Positive | 38 | 90.5 | 58 | 83 | |
| TN | NS | ||||
| >10 | 19 | 47 | 34 | 49 | |
| ≤10 | 23 | 53 | 36 | 51 | |
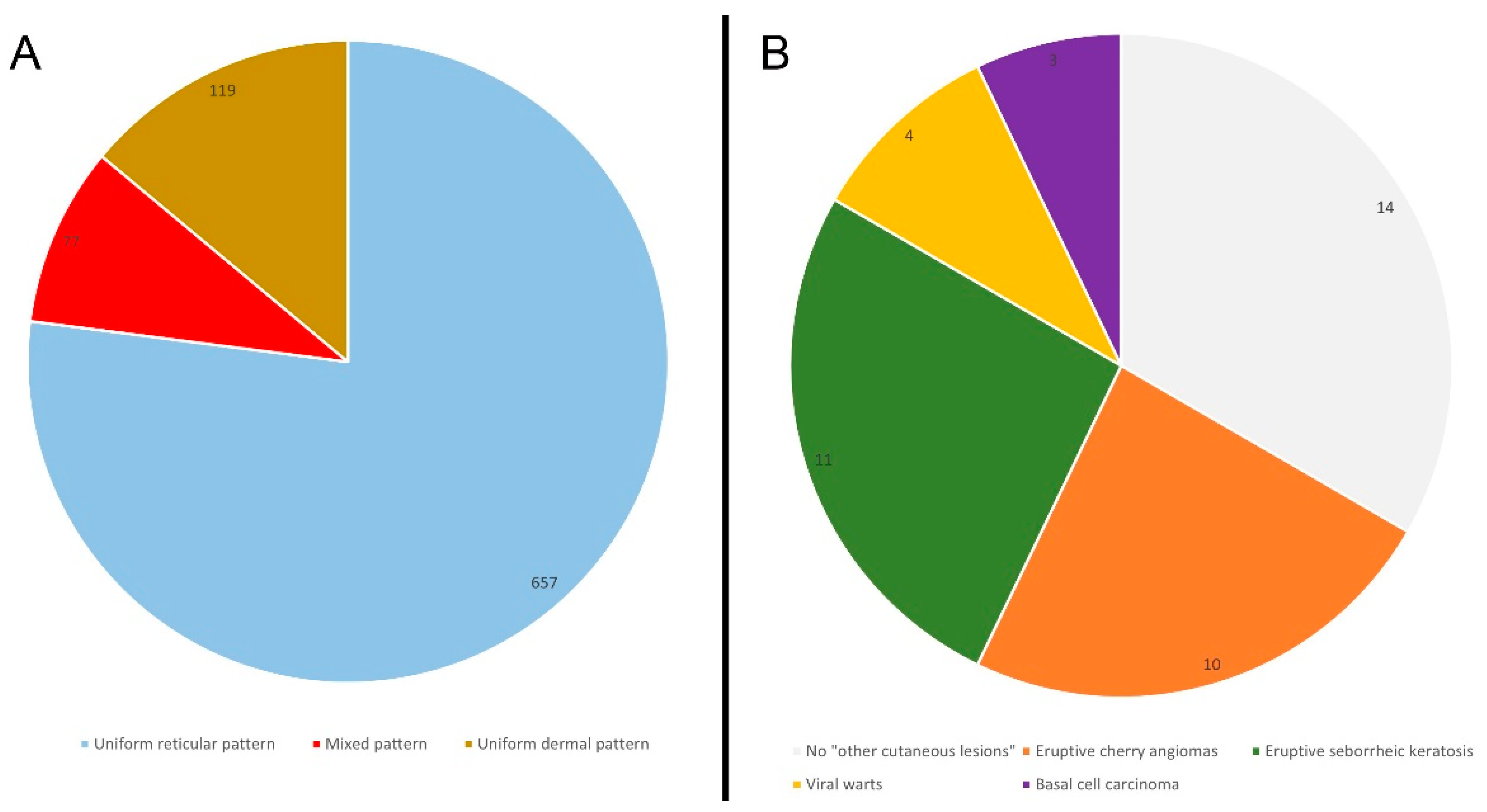
| Nevi pattern | NS | ||||
| R | 657 | 77 | 1.118 | 74 | |
| MC | 25 | 2.9 | 61 | 4 | |
| MP | 32 | 3.7 | 45 | 3 | |
| G | 20 | 2.3 | 61 | 4 | |
| U | 119 | 14 | 227 | 15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paolino, G.; Pampena, R.; Di Nicola, M.R.; Mercuri, S.R. Clinicopathological and Dermoscopic Baselines in Patients with Lynch Syndrome. Cancers 2023, 15, 114. https://doi.org/10.3390/cancers15010114
Paolino G, Pampena R, Di Nicola MR, Mercuri SR. Clinicopathological and Dermoscopic Baselines in Patients with Lynch Syndrome. Cancers. 2023; 15(1):114. https://doi.org/10.3390/cancers15010114
Chicago/Turabian StylePaolino, Giovanni, Riccardo Pampena, Matteo Riccardo Di Nicola, and Santo Raffaele Mercuri. 2023. "Clinicopathological and Dermoscopic Baselines in Patients with Lynch Syndrome" Cancers 15, no. 1: 114. https://doi.org/10.3390/cancers15010114
APA StylePaolino, G., Pampena, R., Di Nicola, M. R., & Mercuri, S. R. (2023). Clinicopathological and Dermoscopic Baselines in Patients with Lynch Syndrome. Cancers, 15(1), 114. https://doi.org/10.3390/cancers15010114






