Merkel Cell Carcinoma—Update on Diagnosis, Management and Future Perspectives
Abstract
Simple Summary
Abstract
1. Introduction
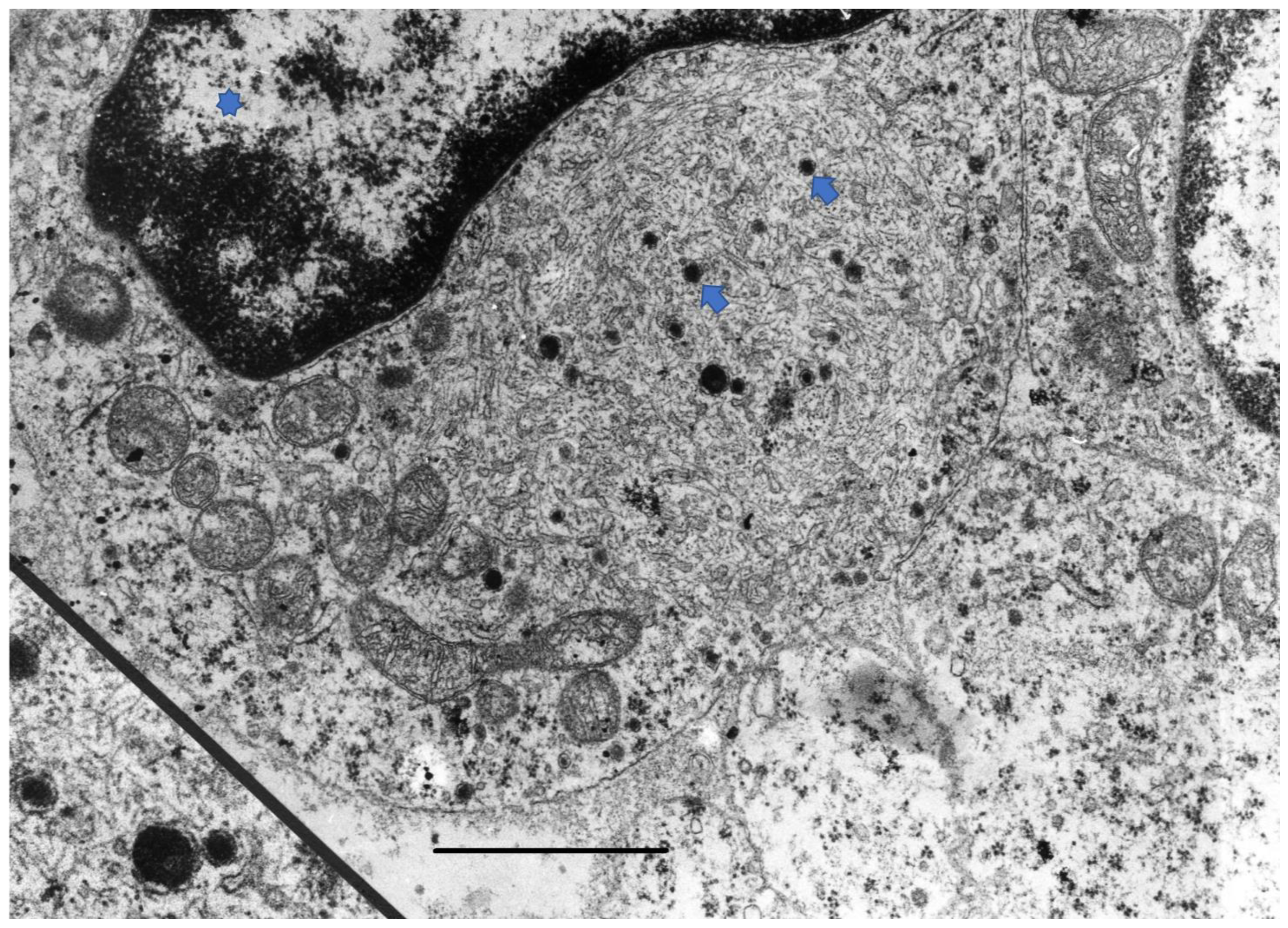
2. Disease Pathogenesis
3. Diagnosis
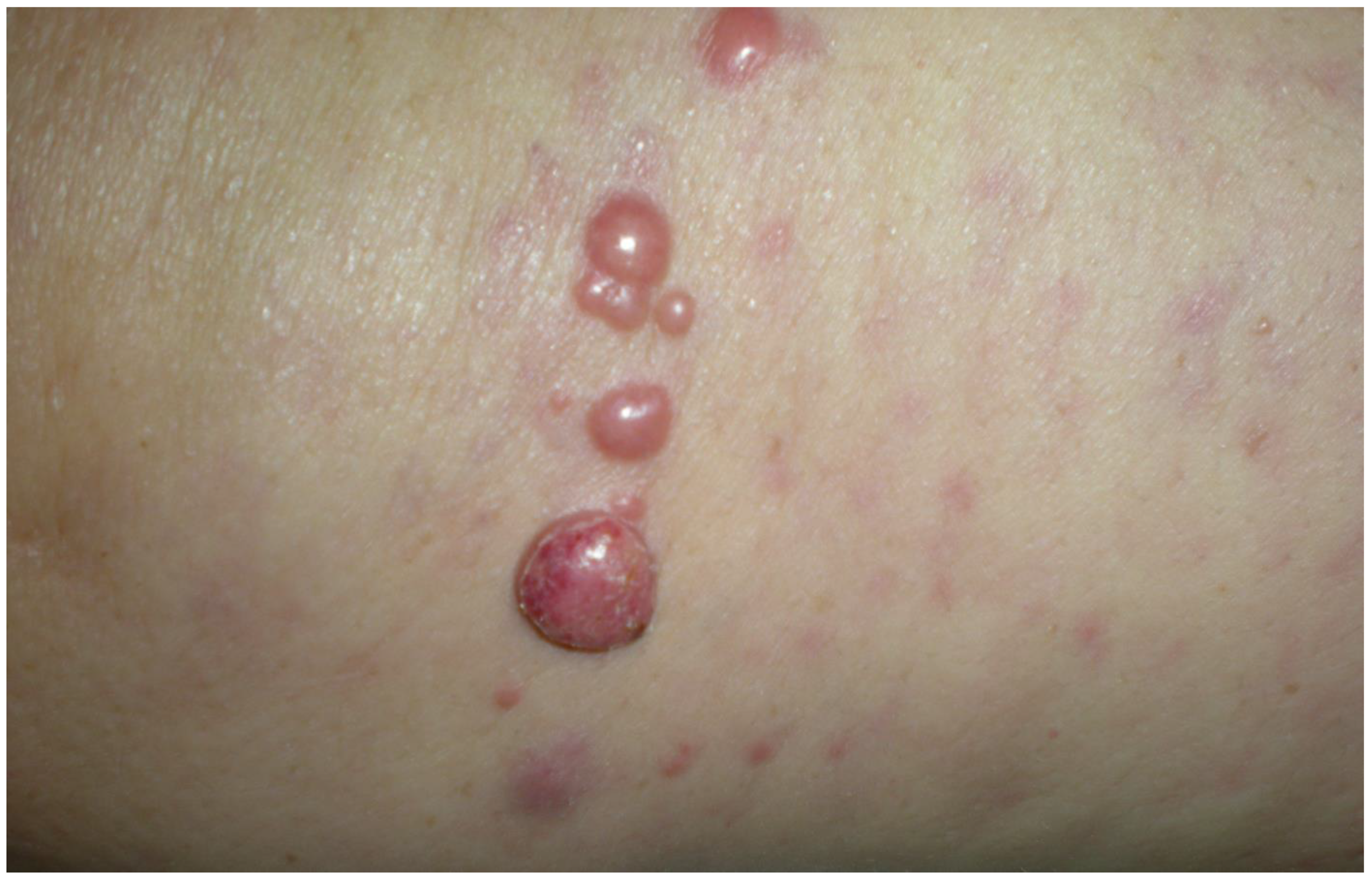
3.1. Clinical Examination
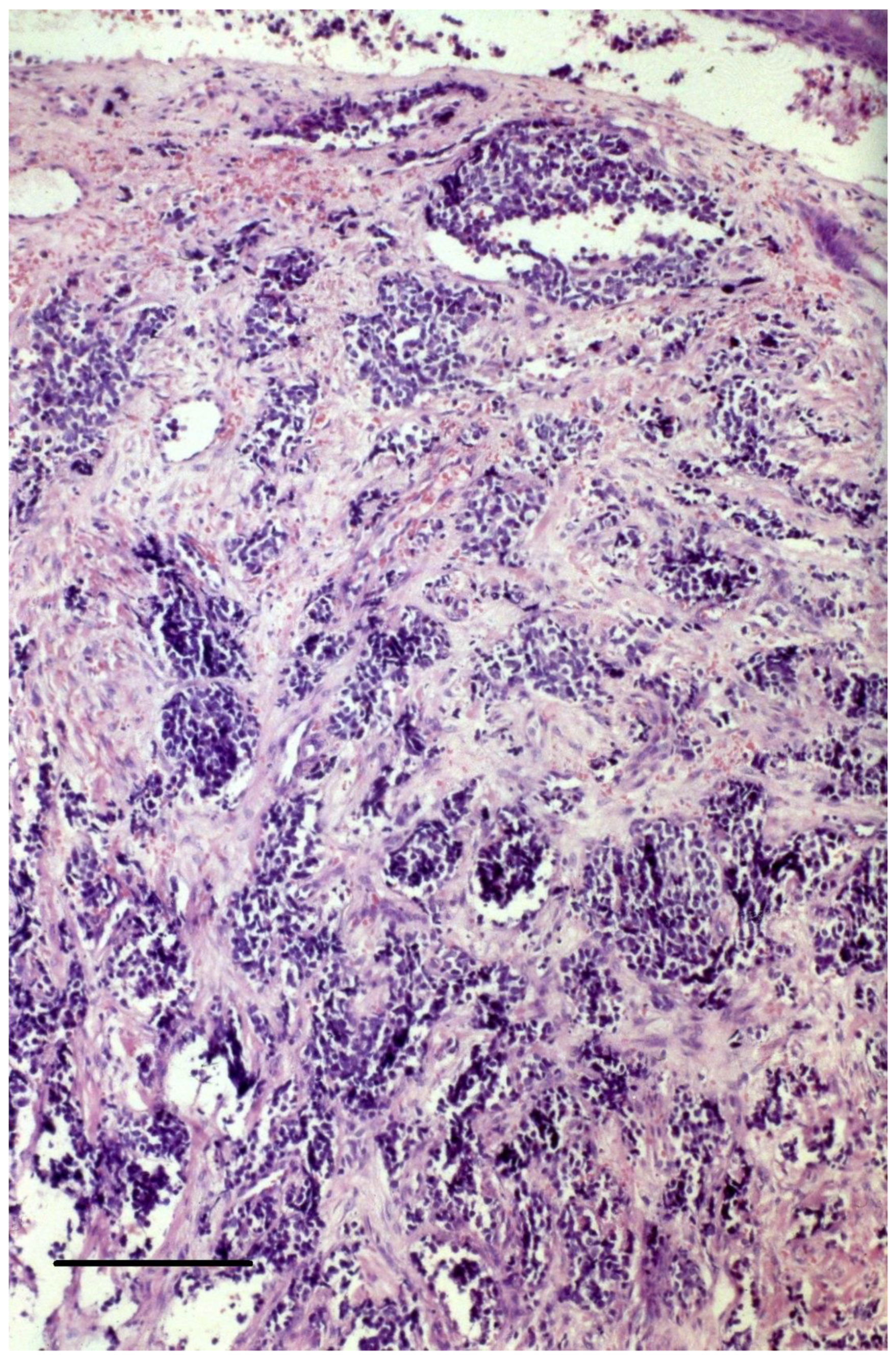
3.2. Histopathology of the Primary Tumor
4. Disease Workup and Staging
4.1. Baseline Imaging
4.2. Evaluation of the Lymph Node Status
4.3. Eighth Edition American Joint Committee on Cancer (AJCC) Staging for MCC and Prognostic Factors
5. Treatment of the Primary Tumor
5.1. Surgical Management of the Primary Tumor and the Draining Lymphatic Basin
5.2. Radiation Therapy
5.3. Patient Follow-Up after Excision of the Primary Tumor and the Draining Lymphatic Basin
6. Treatment of Locally Advanced and Metastatic Disease
6.1. In-Transit and Local Recurrences
6.2. Distant Metastases
6.2.1. Conventional Chemotherapy
- -
- Platinum agents (carboplatin or cisplatinum agents (carboplatin or cisplatin) with etoposide and topotecan.
- -
- Cyclophosphamide, often with doxorubicin/epirubicin and vincristine, or with methotrexate and 5-fluorouracil.
- -
- Paclitaxel and a variety of other agents.
6.2.2. Immune Checkpoint Inhibitors (ICI)
- Association with radiotherapy: Radiotherapy could induce an immunogenic cell death, potentiating the effect of ICI. In two patients with progressive metastatic MCC on anti-PD-1 inhibitors, single-fraction palliative radiotherapy induced durable in-field and abscopal responses [75]. Two ongoing, phase II trials evaluate the association of either pembrolizumab or nivolumab and ipilimumab, respectively, with stereotactic body radiation therapy in metastatic MCC patients (NCT03304639, NCT03071406) [73].
- Switching between different anti-PD-1/PD-L1 inhibitors or adding ipilimumab could overcome resistance to ICI [72,76]. In a case series of 13 ICI refractory patients, sequentially administered salvage therapy with anti-CTLA4 alone or in combination with an anti-PD1/PD-L1 inhibitor allowed objective responses in 31% of cases [76].
- Combination of ICI with targeted or other immune-based therapies (see Table S3 in the Supplementary Materials).
6.2.3. Promising Novel Therapies
Targeted Therapies
Vascular Endothelial Growth Factor (VEGF) Receptor Inhibitors
KIT Inhibitors
Somatostatin Analogs
PI3K/mTOR Inhibitors
Domatinostat
Immune-Based Strategies
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Toker, C. Trabecular carcinoma of the skin. Arch. Dermatol. 1972, 105, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Kervarrec, T.; Samimi, M.; Guyétant, S.; Sarma, B.; Chéret, J.; Blanchard, E.; Berthon, P.; Schrama, D.; Houben, R.; Touze, A. Histogenesis of Merkel Cell Carcinoma: A Comprehensive Review. Front. Oncol. 2019, 9, 451. [Google Scholar] [CrossRef] [PubMed]
- Sunshine, J.C.; Jahchan, N.S.; Sage, J.; Choi, J. Are there multiple cells of origin of Merkel cell carcinoma? Oncogene 2018, 37, 1409–1416. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Yang, R.; Payne, A.S.; Schowalter, R.M.; Spurgeon, M.E.; Lambert, P.F.; Xu, X.; Buck, C.B.; You, J. Identifying the target cells and mechanisms of Merkel cell polyomavirus infection. Cell Host Microbe 2016, 19, 775–787. [Google Scholar] [CrossRef]
- Paulson, K.G.; Park, S.Y.; Vandeven, N.A.; Lachance, K.; Thomas, H.; Chapuis, A.G.; Harms, K.L.; Thompson, J.A.; Bhatia, S.; Stang, A.; et al. Merkel cell carcinoma: Current US incidence and projected increases based on changing demographics. J. Am. Acad. Dermatol. 2018, 78, 457–463. [Google Scholar] [CrossRef]
- Kuwamoto, S. Recent advances in the biology of Merkel cell carcinoma. Hum. Pathol. 2011, 42, 1063–1077. [Google Scholar] [CrossRef]
- Schadendorf, D.; Lebbe, C.; Zur Hausen, A.; Avril, M.F.; Hariharan, S.; Bharmal, M.; Becker, J.C. Merkel cell carcinoma: Epidemiology, prognosis, therapy and unmet medical needs. Eur. J. Cancer 2017, 71, 53–69. [Google Scholar] [CrossRef]
- Trofymenko, O.; Zeitouni, N.C.; Kurtzman, D.J.B. Factors associated with advanced-stage Merkel cell carcinoma at initial diagnosis and the use of radiation therapy: Results from the National Cancer Database. J. Am. Acad. Dermatol. 2018, 79, 680–688. [Google Scholar] [CrossRef]
- Heath, M.; Jaimes, N.; Lemos, B.; Mostaghimi, A.; Wang, L.C.; Peñas, P.F.; Nghiem, P. Clinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: The AEIOU features. J. Am. Acad. Dermatol. 2008, 58, 375–381. [Google Scholar] [CrossRef]
- Garneski, K.M.; Nghiem, P. Merkel cell carcinoma adjuvant therapy: Current data support radiation but not chemotherapy. J. Am. Acad. Dermatol. 2007, 57, 166–169. [Google Scholar] [CrossRef]
- Engels, E.A.; Frisch, M.; Goedert, J.J.; Biggar, R.J.; Miller, R.W. Merkel cell carcinoma and HIV infection. Lancet 2002, 359, 497–498. [Google Scholar] [CrossRef] [PubMed]
- Penn, I.; First, M.R. Merkel’s cell carcinoma in organ recipients: Report of 41 cases. Transplantation 1999, 68, 1717–1721. [Google Scholar] [CrossRef] [PubMed]
- Girschik, J.; Thorn, K.; Beer, T.W.; Heenan, P.J.; Fritschi, L. Merkel cell carcinoma in Western Australia: A population-based study of incidence and survival. Br. J. Dermatol. 2011, 165, 1051–1057. [Google Scholar] [CrossRef] [PubMed]
- van der Zwan, J.M.; Trama, A.; Otter, R.; Larrañaga, N.; Tavilla, A.; Marcos-Gragera, R.; Dei Tos, A.P.; Baudin, E.; Poston, G.; Links, T. Rare neuroendocrine tumors: Results of the surveillance of rare cancers in Europe project. Eur. J. Cancer 2013, 49, 2565–2578. [Google Scholar] [CrossRef]
- Grabowski, J.; Saltzstein, S.L.; Sadler, G.R.; Tahir, Z.; Blair, S. A Comparison of Merkel Cell Carcinoma and Melanoma: Results from the California Cancer Registry. Clin. Med. Oncol. 2008, 2, 327–333. [Google Scholar] [CrossRef]
- Sridharan, V.; Muralidhar, V.; Margalit, D.N.; Tishler, R.B.; DeCaprio, J.A.; Thakuria, M.; Rabinowits, G.; Schoenfeld, J.D. Merkel cell carcinoma: A population analysis on survival. J. Natl. Compr. Cancer Netw. 2016, 14, 1247–1257. [Google Scholar] [CrossRef]
- Allen, P.J.; Bowne, W.B.; Jaques, D.P.; Brennan, M.F.; Busam, K.; Coit, D.G. Merkel cell carcinoma: Prognosis and treatment of patients from a single institution. J. Clin. Oncol. 2005, 23, 2300–2309. [Google Scholar] [CrossRef]
- Feng, H.; Shuda, M.; Chang, Y.; Moore, P.S. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science 2008, 319, 1096–1100. [Google Scholar] [CrossRef]
- Tolstov, Y.L.; Knauer, A.; Chen, J.G.; Kensler, T.W.; Kingsley, L.A.; Moore, P.S.; Chang, Y. Asymptomatic primary Merkel cell polyomavirus infection among adults. Emerg. Infect. Dis. 2011, 17, 1371–1380. [Google Scholar] [CrossRef]
- Moshiri, A.S.; Doumani, R.; Yelistratova, L.; Shinohara, M.M.; Delaney, M.; Chang, O.; McArdle, S.; Thomas, H.; Asgari, M.M.; Huang, M.L.; et al. Polyomavirus-Negative Merkel Cell Carcinoma: A More Aggressive Subtype Based on Analysis of 282 Cases Using Multimodal Tumor Virus Detection. J. Investig. Dermatol. 2017, 137, 819–827. [Google Scholar] [CrossRef]
- van der Meijden, E.; Feltkamp, M. The human polyomavirus middle and alternative T-antigens; thoughts on roles and relevance to cancer. Front. Microbiol. 2018, 9, 398. [Google Scholar] [CrossRef] [PubMed]
- Houben, R.; Adam, C.; Baeurle, A.; Hesbacher, S.; Grimm, J.; Angermeyer, S.; Henzel, K.; Hauser, S.; Elling, R.; Brocker, E.B.; et al. An intact retinoblastoma protein-binding site in Merkel cell polyomavirus large T antigen is required for promoting growth of Merkel cell carcinoma cells. Int. J. Cancer 2012, 130, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Houben, R.; Dreher, C.; Angermeyer, S.; Borst, A.; Utikal, J.; Haferkamp, S.; Peitsch, W.; Schrama, D.; Hesbacher, S. Mechanisms of p53 restriction in Merkel cell carcinoma cells are independent of the Merkel cell polyoma virus T antigens. J. Investig. Dermatol. 2013, 133, 2453–2460. [Google Scholar] [CrossRef] [PubMed]
- Kwun, H.J.; Shuda, M.; Feng, H.; Camacho, C.J.; Moore, P.S.; Chang, Y. Merkel cell polyomavirus small T antigen controls viral replication and oncoprotein expression by targeting the cellular ubiquitin ligase SCFFbw7. Cell Host Microbe 2013, 14, 125–135. [Google Scholar] [CrossRef]
- Goh, G.; Walradt, T.; Markarov, V.; Blom, A.; Riaz, N.; Doumani, R.; Stafstrom, K.; Moshiri, A.; Yelistratova, L.; Levinsohn, J.; et al. Mutational landscape of MCPyV-positive and MCPyV-negative Merkel cell carcinomas with implications for immunotherapy. Oncotarget 2016, 7, 3403–3415. [Google Scholar] [CrossRef] [PubMed]
- Houben, R.; Grimm, J.; Willmes, C.; Weinkam, R.; Becker, J.C.; Schrama, D. Merkel cell carcinoma and Merkel cell polyomavirus: Evidence for hit-and-run oncogenesis. J. Investig. Dermatol. 2012, 132, 254–256. [Google Scholar] [CrossRef]
- Paulson, K.G.; Iyer, J.G.; Simonson, W.T.; Blom, A.; Thibodeau, R.M.; Schmidt, M.; Pietromonaco, S.; Sokil, M.; Warton, M.; Asgari, M.M.; et al. CD8+ lymphocyte intratumoral infiltration as a stage-independent predictor of Merkel cell carcinoma survival: A population-based study. Am. J. Clin. Pathol. 2014, 142, 452–458. [Google Scholar] [CrossRef]
- Miller, N.J.; Church, C.D.; Dong, L.; Crispin, D.; Fitzgibbon, M.P.; Lachance, K.; Jing, L.; Shinohara, M.; Gavvovidis, I.; Willimsky, G.; et al. Tumor-Infiltrating Merkel Cell Polyomavirus-Specific T Cells Are Diverse and Associated with Improved Patient Survival. Cancer Immunol. Res. 2017, 5, 137–147. [Google Scholar] [CrossRef]
- Jing, L.; Ott, M.; Church, C.D.; Kulikauskas, R.M.; Ibrani, D.; Iyer, J.G.; Afanasiev, O.K.; Colunga, A.; Cook, M.M.; Xie, H.; et al. Prevalent and diverse intratumoral oncoprotein-specific CD8+ T cells within polyoma virus–driven Merkel cell carcinomas. Cancer Immunol. Res. 2020, 8, 648–659. [Google Scholar] [CrossRef]
- Paulson, K.G.; Lewis, C.W.; Redman, M.W.; Simonson, W.T.; Lisberg, A.; Ritter, D.; Morishima, C.; Hutchinson, K.; Mudgistratova, L.; Blom, A.; et al. Viral oncoprotein antibodies as a marker for recurrence of Merkel cell carcinoma: A prospective validation study. Cancer 2017, 123, 1464–1474. [Google Scholar] [CrossRef]
- Dowlatshahi, M.; Huang, V.; Gehad, A.; Jiang, Y.; Calarese, A.; Teague, J.E.; Dorosario, A.A.; Cheng, J.; Nghiem, P.; Schanbacher, C.F.; et al. Tumor-specific T cells in human Merkel cell carcinomas: A possible role for Tregs and T cell exhaustion in reducing T cell responses. J. Investig. Dermatol. 2013, 133, 1879–1889. [Google Scholar] [CrossRef] [PubMed]
- Ferringer, T.; Rogers, H.C.; Metcalf, J.S. Merkel cell carcinoma in situ. J. Cutan. Pathol. 2004, 32, 162–165. [Google Scholar] [CrossRef] [PubMed]
- Ratner, D.; Nelson, B.R.; Brown, M.D.; Johnson, T. Merkel Cell Carcinoma. J. Am. Acad. Dermatol. 1993, 29, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Allen, P.J.; Busam, K.; Hill, A.D.; Stojadinovic, A.; Coit, D.G. Immunohistochemical analysis of sentinel lymph nodes from patients with Merkel cell carcinoma. Cancer 2001, 92, 1650–1655. [Google Scholar] [CrossRef]
- Chan, J.K.; Suster, S.; Wenig, B.M.; Tsang, W.Y.; Chan, J.B.; Lau, A.L. Cytokeratin 20 immunoreactivity distinguishes Merkel cell (primary cutaneous neuroendocrine) carcinomas and salivary gland small cell carcinomas from small cell. Am. J. Surg. Pathol. 1997, 21, 226–234. [Google Scholar] [CrossRef]
- Kontochristopoulos, G.J.; Stavropoulos, P.G.; Krasagakis, K.; Goerdt, S.; Zouboulis, C.C. Differentiation between merkel cell carcinoma and malignant melanoma: An immunohistochemical study. Dermatology 2000, 201, 123–126. [Google Scholar] [CrossRef]
- Ordóñez, N.G. Value of thyroid transcription factor-1 immunostaining in distinguishing small cell lung carcinomas from other small cell carcinomas. Am. J. Surg. Pathol. 2000, 24, 1217–1223. [Google Scholar] [CrossRef]
- Busam, K.J.; Jungbluth, A.A.; Rekthman, N.; Coit, D.; Pulitzer, M.; Bini, J.; Arora, R.; Hanson, N.; Tassello, J.A.; Frosina, D.; et al. Merkel cell polyomavirus expression in merkel cell carcinomas and its absence in combined tumors and pulmonary neuroendocrine carcinomas. Am. J. Surg. Pathol. 2009, 33, 1378–1385. [Google Scholar] [CrossRef]
- Singh, N.; Alexandre, N.A.; Lachance, K.; Lewis, C.W.; McEvoy, A.M.; Akaike, G.; Byrd, D.; Behnia, S.; Bhatia, S.; Paulson, K.G.; et al. Clinical benefit of baseline imaging in Merkel cell carcinoma: Analysis of 584 patients. J. Am. Acad. Dermatol. 2021, 84, 330–339. [Google Scholar] [CrossRef]
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Merkel Cell Carcinoma. Version 1.2022—November 17. 2021. Available online: https://www.nccn.org/professionals/physician_gls/pdf/mcc.pdf (accessed on 15 March 2022).
- Becker, J.C.; Eigentler, T.; Frerich, B.; Gambichler, T.; Grabbe, S.; Holler, U.; Klumpp, B.; Loquai, C.; Krause-Bergmann, A.; Muller-Richter, U.; et al. S2k guidelines for Merkel cell carcinoma (MCC, neuroendocrine carcinoma of the skin)—Update 2018. J. Dtsch. Dermatol. Ges. 2019, 17, 562–576. [Google Scholar] [CrossRef]
- Lebbé, C.; Becker, J.C.; Grob, J.J.; Malvehy, J.; Del Marmol, V.; Pehamberger, H.; Perris, K.; Saiag, P.; Middleton, M.R.; Bastholt, L.; et al. Diagnosis and treatment of Merkel Cell Carcinoma. European consensus-based interdisciplinary guideline. Eur. J. Cancer 2015, 51, 2396–2403. [Google Scholar] [CrossRef] [PubMed]
- Rastrelli, M.; Del Fiore, P.; Buja, A.; Vecchiato, A.; Rossi, C.R.; Sileni, V.C.; Tropea, S.; Russano, F.; Zorzi, M.; Spina, R.; et al. A Therapeutic and Diagnostic Multidisciplinary Pathway for Merkel Cell Carcinoma Patients. Front. Oncol. 2020, 10, 529. [Google Scholar] [CrossRef] [PubMed]
- Hawryluk, E.B.; O’Regan, K.N.; Sheehy, N.; Guo, Y.; Dorosario, A.; Sakellis, C.G.; Jacene, H.A.; Wang, L.C. Positron emission tomography/computed tomography imaging in Merkel cell carcinoma: A study of 270 scans in 97 patients at the Dana-Farber/Brigham and Women’s Cancer Center. J. Am. Acad. Dermatol. 2013, 68, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Iyer, J.G.; Storer, B.E.; Paulson, K.G.; Lemos, B.; Phillips, J.L.; Bichakjian, C.; Zeitouni, N.; Gershenwald, J.E.; Sondak, V.; Otley, C.C.; et al. Relationships between primary tumor size, number of involved nodes and survival among 8,044 cases of Merkel cell carcinoma. J. Am. Acad. Dermatol. 2014, 70, 637–643. [Google Scholar] [CrossRef]
- Gupta, S.G.; Wang, L.C.; Penas, P.F.; Gellenthin, M.; Lee, S.J.; Nghiem, P. Sentinel lymph node biopsy for evaluation and treatment of patients with Merkel cell carcinoma: The Dana-Farber experience and meta-analysis of the literature. Arch. Dermatol. 2006, 142, 685–690. [Google Scholar] [CrossRef]
- Harms, K.L.; Healy, M.A.; Nghiem, P.; Sober, A.J.; Johnson, T.M.; Bichkjian, C.K.; Wong, S. Analysis of Prognostic Factors from 9387 Merkel Cell Carcinoma Cases Forms the Basis for the New 8th Edition AJCC Staging System. Ann. Surg. Oncol. 2016, 23, 3564–3571. [Google Scholar] [CrossRef]
- Gunaratne, D.A.; Howle, J.R.; Veness, M.J. Sentinel lymph node biopsy in Merkel cell carcinoma: A 15-year institutional experience and statistical analysis of 721 reported cases. Br. J. Dermatol. 2016, 174, 273–281. [Google Scholar] [CrossRef]
- Foote, M.; Veness, M.; Zarate, D.; Poulsen, M. Merkel cell carcinoma: The prognostic implications of an occult primary in stage IIIB (nodal) disease. J. Am. Acad. Dermatol. 2012, 67, 395–399. [Google Scholar] [CrossRef]
- Trinidad, C.M.; Torres-Cabala, C.A.; Prieto, V.G.; Aung, P.P. Update on eighth edition American Joint Committee on Cancer classification for Merkel cell carcinoma and histopathological parameters that determine prognosis. J. Clin. Pathol. 2019, 72, 337–340. [Google Scholar] [CrossRef]
- Krasagakis, K.; Toska, A. Overview of Merkel cell carcinoma and recent advances in research. Int. J. Dermatol. 2003, 42, 669–676. [Google Scholar] [CrossRef]
- Andruska, N.; Fischer-Valuck, B.W.; Mahapatra, L.; Brenneman, R.J.; Gay, H.A.; Thorstad, W.L.; Fields, R.C.; MacArthur, K.M.; Baumann, B.C. Association Between Surgical Margins Larger Than 1 cm and Overall Survival in Patients With Merkel Cell Carcinoma. JAMA Dermatol. 2021, 157, 540. [Google Scholar] [CrossRef]
- Tarabadkar, E.S.; Fu, T.; Lachance, K.; Hippe, D.S.; Pulliam, T.; Thomas, H.; Li, J.Y.; Lewis, C.W.; Doolittle-Amieva, C.; Byrd, D.; et al. Narrow excision margins are appropriate for Merkel cell carcinoma when combined with adjuvant radiation: Analysis of 188 cases of localized disease and proposed management algorithm. J. Am. Acad. Dermatol. 2021, 84, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Carrasquillo, O.Y.; Cancel-Artau, K.J.; Ramos-Rodriguez, A.J.; Cruzval-O’Reilly, E.; Merritt, B.G. Mohs Micrographic Surgery Versus Wide Local Excision in the Treatment of Merkel Cell Carcinoma: A Systematic Review. Dermatol. Surg. 2022, 48, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Qureshi, M.M.; Truong, M.T.; Sahni, D. Demographics and outcomes of stage I and II Merkel cell carcinoma treated with Mohs micrographic surgery compared with wide local excision in the National Cancer Database. J. Am. Acad. Dermatol. 2018, 79, 126–134.e3. [Google Scholar] [CrossRef]
- Lewis, K.G.; Weinstock, M.A.; Weaver, A.L.; Otley, C.C. Adjuvant local irradiation for Merkel cell carcinoma. Arch. Dermatol. 2006, 142, 693–700. [Google Scholar] [CrossRef]
- Jouary, T.; Levral, C.; Dreno, B.; Doussau, A.; Sassolas, B.; Beylot-Barry, M.; Renaud-Vilmer, C.; Guillot, B.; Bernard, P.; Lok, C.; et al. Adjuvant prophylactic regional radiotherapy versus observation in stage I Merkel cell carcinoma: A multicentric prospective randomized study. Ann. Oncol. 2012, 23, 1074–1080. [Google Scholar] [CrossRef]
- Santamaria-Barria, J.A.; Boland, G.M.; Yeap, B.Y.; Nardi, V.; Diaz-Santagata, D.; Cusac, J.C., Jr. Merkel cell carcinoma: 30-year experience from a single institution. Ann. Surg. Oncol. 2013, 20, 1365–1373. [Google Scholar] [CrossRef]
- Grotz, T.E.; Tarantola, T.I.; Otley, C.C.; Weaver, A.L.; McGree, M.; Jacub, J.W. Natural history of merkel cell carcinoma following locoregional recurrence. Ann. Surg. Oncol. 2012, 19, 2556–2562. [Google Scholar] [CrossRef]
- Nghiem, P.; Kaufman, H.L.; Bharmal, M.; Mahnke, L.; Phatak, H.; Becker, J. Systemic literature review of efficacy, safety and tolerability outcomes of chemotherapy regimens in patients with metastatic Merkel cell carcinoma. Future Oncol. 2017, 13, 1263–1279. [Google Scholar] [CrossRef]
- Becker, J.C.; Lorenz, E.; Ugurel, S.; Eigentler, T.K.; Kiecker, F.; Pfohler, C.; Kellner, I.; Meier, F.; Kahler, K.; Mohr, P.; et al. Evaluation of real-world treatment outcomes in patients with distant metastatic Merkel cell carcinoma following second-line chemotherapy in Europe. Oncotarget 2017, 8, 79731–79741. [Google Scholar] [CrossRef]
- Iyer, J.G.; Afanasiev, O.K.; McClurkan, C.; Paulson, K.; Nagase, K.; Jing, L.; O Marshak, J.; Dong, L.; Carter, J.; Lai, I.; et al. Merkel cell polyomavirus-specific CD8(+) and CD4(+) T-cell responses identified in Merkel cell carcinomas and blood. Clin. Cancer Res. 2011, 17, 6671–6680. [Google Scholar] [CrossRef] [PubMed]
- Giraldo, N.A.; Nguyen, P.; Engle, E.L.; Kaunitz, G.J.; Cottrell, T.R.; Berry, S.; Green, B.; Soni, A.; Cuda, J.D.; Stein, J.E.; et al. Multidimensional, quantitative assessment of PD-1/PD-L1 expression in patients with Merkel cell carcinoma and association with response to pembrolizumab. J. Immunother. Cancer 2018, 6, 99. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, H.L.; Russell, J.; Hamid, O.; Bhatia, S.; Terheyden, P.; D’Angelo, S.P.; Shih, K.C.; Lebbe, C.; Linette, G.P.; Milella, M.; et al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: A multicentre, single-group, open-label, phase 2 trial. Lancet Oncol. 2016, 17, 1374–1385. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, S.P.; Bhatia, S.; Brohl, A.S.; Hamoid, O.; Mehnert, J.M.; Terheyden, P.; Shih, K.C.; Brownell, I.; Lebbe, C.; Lewis, K.C.; et al. Avelumab in patients with previously treated metastatic Merkel cell carcinoma: Long-term data and biomarker analyses from the single-arm phase 2 JAVELIN Merkel 200 trial. J. Immunother. Cancer 2020, 8, e000674. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, S.P.; Lebbé, C.; Mortier, L.; Brohl, A.S.; Fazio, N.; Grob, J.J.; Prinzi, N.; Hanna, G.J.; Hassel, J.C.; Kiecker, F.; et al. First-line avelumab in a cohort of 116 patients with metastatic Merkel cell carcinoma (JAVELIN Merkel 200): Primary and biomarker analyses of a phase II study. J. Immunother. Cancer 2021, 9, e002646. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.W.; Lebbé, C.; Grignani, G.; Nathan, P.; Dirix, L.; Fenig, E.; Ascierto, P.A.; Sandhu, S.; Munhoz, R.; Benincasa, E.; et al. Efficacy and safety of avelumab treatment in patients with metastatic Merkel cell carcinoma: Experience from a global expanded access program. J. Immunother. Cancer 2020, 8, e000313. [Google Scholar] [CrossRef]
- Nghiem, P.; Bhatia, S.; Lipson, E.J.; Sharfman, W.H.; Kudchadkar, R.R.; Brohl, A.S.; Friedlander, P.A.; Daud, A.; Kluger, H.M.; Reddy, S.A.; et al. Durable Tumor Regression and Overall Survival in Patients with Advanced Merkel Cell Carcinoma Receiving Pembrolizumab as First-Line Therapy. Clin. Oncol. 2019, 37, 693–702. [Google Scholar] [CrossRef]
- Nghiem, P.; Bhatia, S.; Lipson, E.J.; Kudchadkar, R.R.; Miller, N.J.; Annamalai, L.; Berry, S.; Chartash, E.; Daud, A.; Fling, S.P.; et al. PD-1 Blockade with Pembrolizumab in Advanced Merkel-Cell Carcinoma. N. Engl. J. Med. 2016, 374, 2542–2552. [Google Scholar] [CrossRef]
- Topalian, S.L.; Bhatia, S.; Hollebecque, A.; Awada, A.; De Boer, J.; Kud-Chadkar, R. Non-comparative, open label, multiple cohort, phase 1/2 study to evaluate nivolumab in patients with virus-associated tumors (CheckMate 358): Efficacy and safety in Merkel cell carcinoma [abstract]. Cancer Res. 2017, 77, CT074. [Google Scholar] [CrossRef]
- Topalian, S.L.; Bhatia, S.; Amin, A.; Kudchadkar, R.R.; Sharfman, W.H.; Lebbé, C.; Delord, J.P.; Dunn, L.A.; Shinohara, M.M.; Kulikauskas, R.; et al. Neoadjuvant Nivolumab for Patients With Resectable Merkel Cell Carcinoma in the CheckMate 358 Trial. J. Clin. Oncol. 2020, 38, 2476–2487. [Google Scholar] [CrossRef]
- Glutsch, V.; Kneitz, H.; Gesueruch, A.; Goebeler, M.; Haferkamp, S.; Becker, J.C.; Ugurel, S.; Schilling, B. Activity of ipilimumab plus nivolumab in avelumab-refractory Merkel cell carcinoma. Cancer Immunol. Immunother. 2021, 70, 2087–2093. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Khushalani, N.I.; Eroglu, Z.; Russell, J.; Wuthrick, E.; Caudell, J.; Harrison, L.; Aoki, M.; Shah, H.; Blakaj, D.; et al. A phase II, randomized study of nivolumab (NIVO) and Ipilimumab (IPI) versus NIVO, IPI and stereotactic body radiation therapy (SBRT) for metastatic Merkel cell carcinoma (MCC, NCT03071406): A preliminary report. Ann. Oncol. 2019, 30, v538–v539. [Google Scholar] [CrossRef]
- Becker, J.C.; Hassel, J.C.; Menzer, C.; Kahler, K.C.; Eigentler, T.K.; Meier, F.E.; Berking, C.; Gutzmer, R.; Mohr, P.; Kiecker, F.; et al. Adjuvant ipilimumab compared with observation in completely resected Merkel cell carcinoma (ADMEC): A randomized, multicenter DeCOG/ADO study. J. Clin. Oncol. 2018, 36, 9527. [Google Scholar] [CrossRef]
- Xu, M.J.; Wu, S.; Daud, A.I.; Yu, S.S.; Yom, S.S. In-field and abscopal response after short-course radiation therapy in patients with metastatic Merkel cell carcinoma progressing on PD-1 checkpoint blockade: A case series. J. Immunother. Cancer 2018, 6, 43. [Google Scholar] [CrossRef] [PubMed]
- LoPiccolo, J.; Schollenberger, M.D.; Dakhil, S.; Rosner, S.; Ali, O.; Sharfman, W.H.; Silk, A.W.; Bhatia, S.; Lipson, E.J. Rescue therapy for patients with anti-PD-1-refractory Merkel cell carcinoma: A multicenter, retrospective case series. J. Immunother. Cancer 2019, 7, 170. [Google Scholar] [CrossRef]
- Garza-Davila, V.F.; Valdespino-Valdes, J.; Barrera, F.J.; Ocampo-Candiani, J.; Garza-Rodriguez, V. Clinical impact of immunotherapy in Merkel cell carcinoma patients: A systematic review and meta-analysis. J. Am. Acad. Dermatol. 2022, 87, 121–130. [Google Scholar] [CrossRef]
- Zelin, E.; Maronese, C.A.; Cri, A.; Toffoli, L.; Di Meo, N.; Nazzaro, G.; Zalaudek, I. Identifying Candidates for Immunotherapy among Patients with Non-Melanoma Skin Cancer: A Review of the Potential Predictors of Response. J. Clin Med. 2022, 11, 3364. [Google Scholar] [CrossRef]
- Kacew, A.J.; Dharaneeswaran, H.; Starrett, G.J.; Thakuria, M.; LeBoeuf, N.R.; Silk, A.W.; DeCaprio, J.A.; Hanna, G.J. Predictors of immunotherapy benefit in Merkel cell carcinoma. Oncotarget 2020, 11, 4401–4410. [Google Scholar] [CrossRef]
- Spassova, I.; Ugurel, S.; Terheyden, P.; Sucker, A.; Hassel, J.C.; Ritter, C.; Kubat, L.; Habermann, D.; Farahpour, F.; Saeedghalati, M.; et al. Predominance of Central Memory T Cells with High T-Cell Receptor Repertoire Diversity is Associated with Response to PD-1/PD-L1 Inhibition in Merkel Cell Carcinoma. Clin. Cancer Res. 2020, 26, 2257–2267. [Google Scholar] [CrossRef]
- Liu, L.; Bai, X.; Wang, J.; Tang, X.R.; Wu, D.H.; Du, S.S.; Du, X.J.; Zhang, Y.W.; Zhu, H.B.; Fang, Y.; et al. Combinationof TMB and CNA Stratifies Prognostic and Predictive Responses to Immunotherapy Across Metastatic Cancer. Clin. Cancer Res. 2019, 25, 7413–7423. [Google Scholar] [CrossRef]
- Hu, C.; Jiang, X. The effect of anti-angiogenic drugs on regulatory T cells in the tumor microenvironment. Biomed. Pharmacother. 2017, 88, 134–137. [Google Scholar] [CrossRef] [PubMed]
- Brunner, M.; Thurnher, D.; Pammer, J.; Geleff, S.; Heiduschka, G.; Reinich, C.M.; Petzelbauer, P.; Erovic, B.M. Expression of VEGF-A/C, VEGF-R2, PDGF-alpha/beta, c-kit, EGFR, Her-2/Neu, Mcl-1 and Bmi-1 in Merkel cell carcinoma. Mod. Pathol. 2008, 21, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Tarabadkar, E.S.; Thomas, H.; Blom, A.; Parvathaneni, U.; Olencki, T.; Nghiem, P.; Bhatia, S. Clinical benefit from tyrosine kinase inhibitors in metastatic Merkel cell carcinoma: A case series of 5 patients. Am. J. Case Rep. 2018, 19, 505–511. [Google Scholar] [CrossRef]
- Nathan, P.D.; Gaunt, P.; Wheatley, K.; Bowden, S.J.; Savage, J.; Faust, G.; Nobes, J.; Goodman, A.; Ritchie, D.; Kumar, S.; et al. UKMCC-01: A Phase II study of pazopanib (PAZ) in metastatic Merkel cell carcinoma. J. Clin. Oncol. 2016, 34, 9542. [Google Scholar] [CrossRef]
- Rabinowits, G.; Lezcano, C.; Catalano, P.J.; McHugh, P.; Becker, H.; Reilly, M.M.; Huang, J.; Tyagi, A.; Thakuria, M.; Bresler, S.C.; et al. Cabozantinib in Patients with Advanced Merkel Cell Carcinoma. Oncologist 2018, 23, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Swick, B.L.; Ravdel, L.; Fitzpatrick, J.E.; Robinson, W.A. Merkel cell carcinoma: Evaluation of KIT (CD117) expression and failure to demonstrate activating mutations in the C-KIT proto-oncogene: Implications for treatment with imatinib mesylate. J. Cutan. Pathol. 2007, 34, 324–329. [Google Scholar] [CrossRef]
- Krasagakis, K.; Kruger-Krasagakis, S.; Eberle, J.; Tsatsakis, A.; Tosca, A.D.; Stathopoulos, E.N. Co-expression of KIT receptor and its ligand stem cell factor in Merkel cell carcinoma. Dermatology 2009, 218, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Krasagakis, K.; Fragiadaki, I.; Metaxari, M.; Kruger-Krasagakis, S.; Tzanakakis, G.N.; Stathopoulos, E.N.; Eberle, J.; Tavernakis, N.; Tosca, A.D. KIT receptor activation by autocrine and paracrine stem cell factor stimulates growth of merkel cell carcinoma in vitro. J. Cell. Physiol. 2010, 226, 1099–1109. [Google Scholar] [CrossRef]
- Samlowski, W.E.; Moon, J.; Tuthill, R.J.; Heinrich, M.C.; Balzer-Haas, N.S.; Merl, S.A.; DeConti, R.C.; Thompson, J.A.; Witter, M.T.; Flaherty, L.E.; et al. A phase II trial of imatinib mesylate in merkel cell carcinoma (neuroendocrine carcinoma of the skin): A Southwest Oncology Group study (S0331). Am. J. Clin. Oncol. 2010, 33, 495–499. [Google Scholar] [CrossRef]
- Akaike, T.; Qazi, J.; Anderson, A.D.; Behnia, F.S.; Shinohara, M.M.; Akaike, G.; Hippe, D.S.; Thomas, H.; Takagishi, S.R.; Lachance, K.; et al. High somatostatin receptor expression and efficacy of somatostatin analogues in patients with metastatic Merkel cell carcinoma. Br. J. Dermatol. 2021, 184, 319–327. [Google Scholar] [CrossRef]
- Leccia, M.-T.; Mouret, S.; Dalle, S.; Desquatrebarbes, J.; Dreno, B.; Dupuy, A.; Dutriaux, C.; Grange, F.; Grob, J.J.; Guillot, B.; et al. Treatment of inoperable and/or metastatic Merkel carcinomas with a somatostatin analogue. National multicenter single-arm phase II study. Ann. Dermatol. Venereol. 2018, 145, S126. [Google Scholar] [CrossRef]
- Nardi, V.; Song, Y.C.; Santamaria-Barria, J.A.; Cosper, A.K.; Lam, Q.; Faber, A.C.; Boland, G.M.; Yeap, B.Y.; Bergethon, K.; Scialabba, V.L.; et al. Activation of PI3K signaling in Merkel cell carcinoma. Clin. Cancer Res. 2012, 18, 1227–1236. [Google Scholar] [CrossRef] [PubMed]
- Shiver, M.B.; Mahmoud, F.; Gao, L. Response to Idelalisib in a Patient with Stage IV Merkel-Cell Carcinoma. N. Engl. J. Med. 2015, 373, 1580–1582. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Bretz, A.C.; Gravemeyer, J.; Spassova, I.; Muminova, S.; Gambichler, T.; Sriram, A.; Ferrone, S.; Becker, J.C. The HDAC Inhibitor Domatinostat Promotes Cell-Cycle Arrest, Induces Apoptosis, and Increases Immunogenicity of Merkel Cell Carcinoma Cells. J. Investig. Dermatol. 2021, 141, 903–912.e4. [Google Scholar] [CrossRef]
- Paulson, K.G.; Tegeder, A.; Willmes, C.; Iyer, J.G.; Afanasiev, O.K.; Schrama, D.; Koba, S.; Thibodeau, R.; Nagase, K.; Simonson, W.T.; et al. Downregulation of MHC-I expression is prevalent but reversible in Merkel cell carcinoma. Cancer Immunol. Res. 2014, 2, 1071–1079. [Google Scholar] [CrossRef]
- Willmes, C.; Adam, C.; Alb, M.; Voőlkert, L.; Houben, R.; Becker, J.C.; Schrama, D. Type I and II IFNs inhibit Merkel cell carcinoma via modulation of the Merkel cell polyomavirus T antigens. Cancer Res. 2012, 72, 2120–2128. [Google Scholar] [CrossRef]
- Lasek, W.; Zagożdżon, R.; Jakobisiak, M. Interleukin 12: Still a promising candidate for tumor immunotherapy? Cancer Immunol. Immunother. 2014, 63, 419–435. [Google Scholar] [CrossRef]
- Bhatia, S.; Miller, N.J.; Lu, H.; Longino, N.V.; Ibrani, D.; Shinohara, M.M.; Byrd, D.R.; Parvathaneni, U.; Kulikauskas, R.; Meulen, J.T.; et al. Intratumoral G100, a TLR4 Agonist, Induces Antitumor Immune Responses and Tumor Regression in Patients with Merkel Cell Carcinoma. Clin. Cancer Res. 2019, 25, 1185–1195. [Google Scholar] [CrossRef]
- Westbrook, B.C.; Norwood, T.G.; Terry, N.L.J.; McKee, S.; Conry, R.M. Talimogene laherparepvec induces durable response of regionally advanced Merkel cell carcinoma in 4 consecutive patients. JAAD Case Rep. 2019, 5, 782–786. [Google Scholar] [CrossRef]
- Hansen, U.K.; Lyngaa, R.; Ibrani, D.; Church, C.; Verhaegen, M.; Dlugosz, A.A.; Becker, J.C.; Straten, P.T.; Nghiem, P.; Hadrup, S.R. Extended T-Cell Epitope Landscape in Merkel Cell Polyomavirus Large T and Small T Oncoproteins Identified Uniquely in Patients with Cancer. J. Investig. Dermatol. 2022, 142, 239–243.e13. [Google Scholar] [CrossRef]
- Xu, D.; Jiang, S.; He, Y.; Jin, X.; Zhao, G.; Wang, D. Development of a therapeutic vaccine targeting Merkel cell polyomavirus capsid protein VP1 against Merkel cell carcinoma. NPJ Vaccines 2021, 6, 119. [Google Scholar] [CrossRef] [PubMed]
- Rosean, C.B.; Sinha, P.; Koelle, D.; Nghiem, P.; Heiland, T. LAMP1 targeting of the large T antigen of merkel cell polyomavirus elicits potent CD4+ T cell responses and prevents tumor growth. J ImmunoTher. Cancer. 2020, 8, A510–A511. [Google Scholar]
- Verma, V.; Shrimali, R.K.; Ahmad, S.; Dai, W.; Wang, H.; Lu, S.; Nandre, R.; Gaur, P.; Lopez, J.; Sade-Feldman, M.; et al. PD-1 blockade in subprimed CD8 cells induce dysfunctional PD-1 + CD38hi cells and anti-PD-1 resistance. Nat. Immunol. 2019, 20, 1231–1243. [Google Scholar] [CrossRef]
- Available online: https://www.clinicaltrials.gov/ct2/show/results/NCT02584829?term=NCT02584829&draw=2&rank=1 (accessed on 23 March 2022).
- Davies, S.I.; Barrett, J.; Wong, S.; Chang, M.J.; Muranski, P.J.; Brownell, I. Robust Production of Merkel Cell Polyomavirus Oncogene Specific T Cells From Healthy Donors for Adoptive Transfer. Front. Immunol. 2020, 11, 592721. [Google Scholar] [CrossRef]
- Angeles, C.V.; Sabel, M.S. Immunotherapy for Merkel cell carcinoma. J. Surg. Oncol. 2021, 123, 775–781. [Google Scholar] [CrossRef] [PubMed]
| Drug | Avelumab | Pembrolizumab | Nivolumab | ||
|---|---|---|---|---|---|
| Study | JAVELIN part A | JAVELIN part B | EAP | KEYNOTE-017 | CHECKMATE358 |
| Phase | 2 | 2 | 2 | 1/2 | |
| Regimen | 10 mg/kg every 2 weeks | 2 mg/kg every 3 weeks | 240 mg/kg every 2 weeks | ||
| N | 88 | 116 | 240 | 50 | 22 |
| Study Duration (m) | 20 | 36 | 39 | 12 | 8 |
| Line | ≥2 | 1 | ≥1 | 1 | ≤3 |
| ORR | 33% | 39.7% | 46.7% | 56% | 68% |
| CR | 11.4% | 16.4% | 22.9% | 24% | 14% |
| Median PFS (m) | 2.7 | 4.1 | NA | 16.8 | 3-month PFS 82% |
| Median OS (m) | 12.6 | 20.3 | NA | Not reached at 24 months | 3-month OS 92% |
| Median FU (m) | 40.8 | 21.2 | NA | 14.9 | 6.5 |
| TRAEs | 70% | 81% | NA | 98% | 68% |
| Grade 3–4 | 11.4% | 18.1% | NA | 30% | 20% |
| References | 64, 65 | 66 | 67 | 68, 69 | 70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaggana, E.; Konstantinou, M.P.; Krasagakis, G.H.; de Bree, E.; Kalpakis, K.; Mavroudis, D.; Krasagakis, K. Merkel Cell Carcinoma—Update on Diagnosis, Management and Future Perspectives. Cancers 2023, 15, 103. https://doi.org/10.3390/cancers15010103
Zaggana E, Konstantinou MP, Krasagakis GH, de Bree E, Kalpakis K, Mavroudis D, Krasagakis K. Merkel Cell Carcinoma—Update on Diagnosis, Management and Future Perspectives. Cancers. 2023; 15(1):103. https://doi.org/10.3390/cancers15010103
Chicago/Turabian StyleZaggana, Eleni, Maria Polina Konstantinou, Gregor Herrmann Krasagakis, Eelco de Bree, Konstantinos Kalpakis, Dimitrios Mavroudis, and Konstantinos Krasagakis. 2023. "Merkel Cell Carcinoma—Update on Diagnosis, Management and Future Perspectives" Cancers 15, no. 1: 103. https://doi.org/10.3390/cancers15010103
APA StyleZaggana, E., Konstantinou, M. P., Krasagakis, G. H., de Bree, E., Kalpakis, K., Mavroudis, D., & Krasagakis, K. (2023). Merkel Cell Carcinoma—Update on Diagnosis, Management and Future Perspectives. Cancers, 15(1), 103. https://doi.org/10.3390/cancers15010103









