Microbiota in the Natural History of Pancreatic Cancer: From Predisposition to Therapy
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
3. Microbiota Pancreatic Diseases and Pancreatic Oncogenesis
3.1. Microbiota and Chronic Pancreatitis (CP)
3.2. Microbiota and Autoimmune Pancreatitis (AIP)
3.3. Microbiota and Pancreatic Cystic Neoplasms (PCNs)
4. Microbiota and Pancreatic Cancer (PC)
- α-diversity reduction, which leads to lower variability in human microbiota core and interactions.
- Bacteroides–Firmicutes ratio imbalance with an abundance of pathogenic bacteria and loss of physiological ones.
- Pro-inflammatory and anti-inflammatory metabolite production imbalance with a decrease in SCFA production instead of an increase in TMAO and its processing of its derivatives.
| Disease | Ref. | Main Microbiota Alterations | Main Modifications Linked to Microbiota Alterations |
|---|---|---|---|
| Autoimmune pancreatitis (AIP) | [63,64] | ↑ Escherichia coli | connections with typical alterations of AIP and increase in serum IgG |
| [11,65] | ↑ Bifidobacterium, Fusobacterium, and Klebsiella spp. | antibiotics can prevent AIP development by reducing the accumulation of the APC in pancreatic tissue | |
| [66,67] | ↑ Helicobacter pylori | ↑ AIP development through the induction of autoimmunity and apoptosis through molecular mimicry pathways: strong homology between CA-II and HpCA | |
| Pancreatic cystic neoplasms (PCNs) | [72] | ↑ Bacteroides spp., Escherichia-Shigella spp., Acidaminococcus spp., Staphylococcus spp., Fusobacterium spp., Helicobacter pylori | |
| [73,74,75] | ↑ levels of intracystic bacterial DNA ↑ Fusobacterium nucleatum, Parvimonas micra, Eikenella corrodens, Hemophilus parahaemolyticus, Actinomyces odontolyticus, Prevotella melaninogenica, and Campylobacter spp. | ↑ pro-inflammatory cytokine IL-1β in IPMN with HGD and IPMN; in contrast, non-IPMN cysts were low in bacterial DNA and IL-1β | |
| [77] | ↑ Firmicutes with related taxa and ↓ Proteobacteria with related taxa in patients with IPMN and PDAC compared to healthy controls | ||
| Pancreatic ductal adenocarcinoma (PDAC) | [87] | ↑ 31 bacterial species/clusters and ↓ 25 ones belong to Firmicutes, Proteobacteria, Actinobacteria, and CFB group bacteria philia in patients with PDAC compared to healthy controls | innate and acquired immunity gene upregulation through ↑ TLR-signaling, ↑ NF-Kb activation, ↑ chronic flogosis, and cancerogenesis |
| [80] | ↑ Porphyramonas gingivalis and Aggregatibacter actinomycetecomitans ↓ Fusobacteria and Leptotrichi | connections between periodontal pathogens and increased risk of pancreatic cancer; associations unlikely due to smoking or other potential confounders | |
| [88] | ↑ Fusobacteria | ||
| [81] | ↑ Porphyramonas gingivalis antibodies (>200 ng/mL) linked to a higher risk of PC | ||
| [42,43,44] | ↓ α-diversity in microbiota profile ↑ LPS-producing bacteria (Prevotella, Hallella, Enterobacter, Veillonella, Klebsiella, and Selenomonas) ↓ SCFA-producing bacteria |
| |
| [15,97] | ↑ SCFA-producing bacteria |
| |
| [20,91] | effects of Helicobacter pylori infection in PDAC cell lines ↑ Helicobacter pylori antibodies in patients with PDAC and GAC compared to healthy controls |
| |
| [93] | ↑ Candida, Malassezia spp., and Trichosporon |
|
5. Microbiota among the Different Phases of Locoregional Treatment
5.1. Biliopancreatic Endoscopy and Surgery
5.2. Radiation
6. How the Microbiome Could Guide Systemic Therapy
6.1. Chemotherapy
6.2. Immunotherapy
6.3. Faecal Microbiota Transplantation (FMT)
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rahib, L.; Smith, B.D.; Aizenberg, R.; Rosenzweig, A.B.; Fleshman, J.M.; Matrisian, L.M. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014, 74, 2913–2921. [Google Scholar] [CrossRef] [PubMed]
- Ciernikova, S.; Novisedlakova, M.; Cholujova, D.; Stevurkova, V.; Mego, M. The Emerging Role of Microbiota and Microbiome in Pancreatic Ductal Adenocarcinoma. Biomedicines 2020, 8, 565. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Alkassis, S.; Yazdanpanah, O.; Philip, P.A. BRCA mutations in pancreatic cancer and progress in their targeting. Expert Opin. Ther. Targets 2021, 25, 547–557. [Google Scholar] [CrossRef]
- Verdu, E.F.; Galipeau, H.J.; Jabri, B. Novel players in coeliac disease pathogenesis: Role of the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 497–506. [Google Scholar] [CrossRef]
- Distrutti, E.; Monaldi, L.; Ricci, P.; Fiorucci, S. Gut microbiota role in irritable bowel syndrome: New therapeutic strategies. World J. Gastroenterol. 2016, 22, 2219–2241. [Google Scholar] [CrossRef]
- Zhao, Y.; Jaber, V.; Lukiw, W.J. Gastrointestinal Tract Microbiome-Derived Pro-inflammatory Neurotoxins in Alzheimer’s Disease. J. Aging Sci. 2021, 9, 002. [Google Scholar]
- Kowalewska, B.; Zorena, K.; Szmigiero-Kawko, M.; Wąż, P.; Myśliwiec, M. Higher diversity in fungal species discriminates children with type 1 diabetes mellitus from healthy control. Patient Prefer. Adherence 2016, 10, 591–599. [Google Scholar] [CrossRef][Green Version]
- Brandi, G.; Turroni, S.; McAllister, F.; Frega, G. The Human Microbiomes in Pancreatic Cancer: Towards Evidence-Based Manipulation Strategies? Int. J. Mol. Sci. 2021, 22, 9914. [Google Scholar] [CrossRef]
- Schepis, T.; De Lucia, S.S.; Nista, E.C.; Manilla, V.; Pignataro, G.; Ojetti, V.; Piccioni, A.; Gasbarrini, A.; Franceschi, F.; Candelli, M. Microbiota in Pancreatic Diseases: A Review of the Literature. J. Clin. Med. 2021, 10, 5920. [Google Scholar] [CrossRef]
- Gibiino, G.; De Siena, M.; Sbrancia, M.; Binda, C.; Sambri, V.; Gasbarrini, A.; Fabbri, C. Dietary Habits and Gut Microbiota in Healthy Adults: Focusing on the Right Diet. A Systematic Review. Int. J. Mol. Sci. 2021, 22, 6728. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, J.Y.; Zhou, S.Y.; Yang, S.J.; Zhong, S.L. Circular RNA expression in pancreatic ductal adenocarcinoma. Oncol. Lett. 2019, 18, 2923–2930. [Google Scholar] [CrossRef]
- Casero, R.A.; Murray Stewart, T.; Pegg, A.E. Polyamine metabolism and cancer: Treatments, challenges and opportunities. Nat. Rev. Cancer 2018, 18, 681–695. [Google Scholar] [CrossRef]
- Pushalkar, S.; Hundeyin, M.; Daley, D.; Zambirinis, C.P.; Kurz, E.; Mishra, A.; Mohan, N.; Aykut, B.; Usyk, M.; Torres, L.E.; et al. The Pancreatic Cancer Microbiome Promotes Oncogenesis by Induction of Innate and Adaptive Immune Suppression. Cancer Discov. 2018, 8, 403–416. [Google Scholar] [CrossRef]
- Diehl, G.E.; Longman, R.S.; Zhang, J.-X.; Breart, B.; Galan, C.; Cuesta, A.; Schwab, S.R.; Littman, D.R. Microbiota restricts trafficking of bacteria to mesenteric lymph nodes by CX(3)CR1(hi) cells. Nature 2013, 494, 116–120. [Google Scholar] [CrossRef]
- Sammallahti, H.; Kokkola, A.; Rezasoltani, S.; Ghanbari, R.; Asadzadeh Aghdaei, H.; Knuutila, S.; Puolakkainen, P.; Sarhadi, V.K. Microbiota Alterations and Their Association with Oncogenomic Changes in Pancreatic Cancer Patients. Int. J. Mol. Sci. 2021, 22, 12978. [Google Scholar] [CrossRef]
- Tofalo, R.; Cocchi, S.; Suzzi, G. Polyamines and Gut Microbiota. Front. Nutr. 2019, 6, 16. [Google Scholar] [CrossRef]
- Mendez, R.; Kesh, K.; Arora, N.; Di Martino, L.; McAllister, F.; Merchant, N.; Banerjee, S.; Banerjee, S. Microbial dysbiosis and polyamine metabolism as predictive markers for early detection of pancreatic cancer. Carcinogenesis 2020, 41, 561–570. [Google Scholar] [CrossRef]
- Thomas, R.M.; Jobin, C. Microbiota in pancreatic health and disease: The next frontier in microbiome research. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 53–64. [Google Scholar] [CrossRef]
- Takayama, S.; Takahashi, H.; Matsuo, Y.; Okada, Y.; Manabe, T. Effects of Helicobacter pylori infection on human pancreatic cancer cell line. Hepatogastroenterology 2007, 54, 2387–2391. [Google Scholar] [PubMed]
- Arzmi, M.H.; Dashper, S.; McCullough, M. Polymicrobial interactions of Candida albicans and its role in oral carcinogenesis. J. Oral Pathol. Med. 2019, 48, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Luan, C.; Xie, L.; Yang, X.; Miao, H.; Lv, N.; Zhang, R.; Xiao, X.; Hu, Y.; Liu, Y.; Wu, N.; et al. Dysbiosis of fungal microbiota in the intestinal mucosa of patients with colorectal adenomas. Sci. Rep. 2015, 5, 7980. [Google Scholar] [CrossRef] [PubMed]
- Rustgi, S.D.; Amin, S.; Yang, A.; Kim, M.K.; Nagula, S.; Kumta, N.A.; DiMaio, C.J.; Boffetta, P.; Lucas, A.L. Preoperative Endoscopic Retrograde Cholangiopancreatography Is Not Associated with Increased Pancreatic Cancer Mortality. Clin. Gastroenterol. Hepatol. 2019, 17, 1580–1586.e4. [Google Scholar] [CrossRef] [PubMed]
- Scheufele, F.; Aichinger, L.; Jäger, C.; Demir, I.E.; Schorn, S.; Sargut, M.; Erkan, M.; Kleeff, J.; Friess, H.; Ceyhan, G.O. Effect of preoperative biliary drainage on bacterial flora in bile of patients with periampullary cancer. Br. J. Surg. 2017, 104, e182–e188. [Google Scholar] [CrossRef] [PubMed]
- Riquelme, E.; Zhang, Y.; Zhang, L.; Montiel, M.; Zoltan, M.; Dong, W.; Quesada, P.; Sahin, I.; Chandra, V.; San Lucas, A.; et al. Tumor Microbiome Diversity and Composition Influence Pancreatic Cancer Outcomes. Cell 2019, 178, 795–806.e12. [Google Scholar] [CrossRef]
- Kita, A.; Fujiya, M.; Konishi, H.; Tanaka, H.; Kashima, S.; Iwama, T.; Ijiri, M.; Murakami, Y.; Takauji, S.; Goto, T.; et al. Probiotic-derived ferrichrome inhibits the growth of refractory pancreatic cancer cells. Int. J. Oncol. 2020, 57, 721–732. [Google Scholar] [CrossRef]
- Signoretti, M.; Roggiolani, R.; Stornello, C.; Delle Fave, G.; Capurso, G. Gut microbiota and pancreatic diseases. Minerva Gastroenterol. Dietol. 2017, 63, 399–410. [Google Scholar] [CrossRef]
- Forbes, J.D.; Van Domselaar, G.; Bernstein, C.N. The Gut Microbiota in Immune-Mediated Inflammatory Diseases. Front. Microbiol. 2016, 7, 1081. [Google Scholar] [CrossRef]
- Camilleri, M. Leaky gut: Mechanisms, measurement and clinical implications in humans. Gut 2019, 68, 1516–1526. [Google Scholar] [CrossRef]
- Li, Q.; Jin, M.; Liu, Y.; Jin, L. Gut Microbiota: Its Potential Roles in Pancreatic Cancer. Front. Cell. Infect. Microbiol. 2020, 10, 572492. [Google Scholar] [CrossRef]
- Binda, C.; Gibiino, G.; Coluccio, C.; Sbrancia, M.; Dajti, E.; Sinagra, E.; Capurso, G.; Sambri, V.; Cucchetti, A.; Ercolani, G.; et al. Biliary Diseases from the Microbiome Perspective: How Microorganisms Could Change the Approach to Benign and Malignant Diseases. Microorganisms 2022, 10, 312. [Google Scholar] [CrossRef]
- Mowat, A.M.; Agace, W.W. Regional specialization within the intestinal immune system. Nat. Rev. Immunol. 2014, 14, 667–685. [Google Scholar] [CrossRef]
- Ijssennagger, N.; Belzer, C.; Hooiveld, G.J.; Dekker, J.; van Mil, S.W.C.; Müller, M.; Kleerebezem, M.; van der Meer, R. Gut microbiota facilitates dietary heme-induced epithelial hyperproliferation by opening the mucus barrier in colon. Proc. Natl. Acad. Sci. USA 2015, 112, 10038–10043. [Google Scholar] [CrossRef]
- Reinhardt, C.; Bergentall, M.; Greiner, T.U.; Schaffner, F.; Ostergren-Lundén, G.; Petersen, L.C.; Ruf, W.; Bäckhed, F. Tissue factor and PAR1 promote microbiota-induced intestinal vascular remodelling. Nature 2012, 483, 627–631. [Google Scholar] [CrossRef]
- Michaud, D.S.; Izard, J.; Wilhelm-Benartzi, C.S.; You, D.-H.; Grote, V.A.; Tjønneland, A.; Dahm, C.C.; Overvad, K.; Jenab, M.; Fedirko, V.; et al. Plasma antibodies to oral bacteria and risk of pancreatic cancer in a large European prospective cohort study. Gut 2013, 62, 1764–1770. [Google Scholar] [CrossRef]
- Xu, F.; Yang, C.; Tang, M.; Wang, M.; Cheng, Z.; Chen, D.; Chen, X.; Liu, K. The Role of Gut Microbiota and Genetic Susceptibility in the Pathogenesis of Pancreatitis. Gut Liver 2022, 16, 686–696. [Google Scholar] [CrossRef]
- Chu, L.C.; Goggins, M.G.; Fishman, E.K. Diagnosis and Detection of Pancreatic Cancer. Cancer J. 2017, 23, 333–342. [Google Scholar] [CrossRef]
- Yang, Q.; Zhang, J.; Zhu, Y. Potential Roles of the Gut Microbiota in Pancreatic Carcinogenesis and Therapeutics. Front. Cell. Infect. Microbiol. 2022, 12, 872019. [Google Scholar] [CrossRef]
- Akshintala, V.S.; Talukdar, R.; Singh, V.K.; Goggins, M. The Gut Microbiome in Pancreatic Disease. Clin. Gastroenterol. Hepatol. 2019, 17, 290–295. [Google Scholar] [CrossRef]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef]
- Ren, Z.; Jiang, J.; Xie, H.; Li, A.; Lu, H.; Xu, S.; Zhou, L.; Zhang, H.; Cui, G.; Chen, X.; et al. Gut microbial profile analysis by MiSeq sequencing of pancreatic carcinoma patients in China. Oncotarget 2017, 8, 95176–95191. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.-Y.; Shi, S.; Liang, C.; Meng, Q.-C.; Hua, J.; Zhang, Y.-Y.; Liu, J.; Zhang, B.; Xu, J.; Yu, X.-J. The microbiota and microbiome in pancreatic cancer: More influential than expected. Mol. Cancer 2019, 18, 97. [Google Scholar] [CrossRef] [PubMed]
- Daniluk, J.; Liu, Y.; Deng, D.; Chu, J.; Huang, H.; Gaiser, S.; Cruz-Monserrate, Z.; Wang, H.; Ji, B.; Logsdon, C.D. An NF-κB pathway-mediated positive feedback loop amplifies Ras activity to pathological levels in mice. J. Clin. Investig. 2012, 122, 1519–1528. [Google Scholar] [CrossRef] [PubMed]
- Ochi, A.; Graffeo, C.S.; Zambirinis, C.P.; Rehman, A.; Hackman, M.; Fallon, N.; Barilla, R.M.; Henning, J.R.; Jamal, M.; Rao, R.; et al. Toll-like receptor 7 regulates pancreatic carcinogenesis in mice and humans. J. Clin. Investig. 2012, 122, 4118–4129. [Google Scholar] [CrossRef]
- Pagliari, D.; Saviano, A.; Newton, E.E.; Serricchio, M.L.; Dal Lago, A.A.; Gasbarrini, A.; Cianci, R. Gut Microbiota-Immune System Crosstalk and Pancreatic Disorders. Mediat. Inflamm. 2018, 2018, 7946431. [Google Scholar] [CrossRef]
- Eibl, G.; Rozengurt, E. KRAS, YAP, and obesity in pancreatic cancer: A signaling network with multiple loops. Semin. Cancer Biol. 2019, 54, 50–62. [Google Scholar] [CrossRef]
- Beyer, G.; Habtezion, A.; Werner, J.; Lerch, M.M.; Mayerle, J. Chronic pancreatitis. Lancet 2020, 396, 499–512. [Google Scholar] [CrossRef]
- Frost, F.; Weiss, F.U.; Sendler, M.; Kacprowski, T.; Rühlemann, M.; Bang, C.; Franke, A.; Völker, U.; Völzke, H.; Lamprecht, G.; et al. The Gut Microbiome in Patients with Chronic Pancreatitis Is Characterized by Significant Dysbiosis and Overgrowth by Opportunistic Pathogens. Clin. Transl. Gastroenterol. 2020, 11, e00232. [Google Scholar] [CrossRef]
- El Kurdi, B.; Babar, S.; El Iskandarani, M.; Bataineh, A.; Lerch, M.M.; Young, M.; Singh, V.P. Factors That Affect Prevalence of Small Intestinal Bacterial Overgrowth in Chronic Pancreatitis: A Systematic Review, Meta-Analysis, and Meta-Regression. Clin. Transl. Gastroenterol. 2019, 10, e00072. [Google Scholar] [CrossRef]
- Capurso, G.; Signoretti, M.; Archibugi, L.; Stigliano, S.; Delle Fave, G. Systematic review and meta-analysis: Small intestinal bacterial overgrowth in chronic pancreatitis. United Eur. Gastroenterol. J. 2016, 4, 697–705. [Google Scholar] [CrossRef]
- Jandhyala, S.M.; Madhulika, A.; Deepika, G.; Rao, G.V.; Reddy, D.N.; Subramanyam, C.; Sasikala, M.; Talukdar, R. Altered intestinal microbiota in patients with chronic pancreatitis: Implications in diabetes and metabolic abnormalities. Sci. Rep. 2017, 7, 43640. [Google Scholar] [CrossRef]
- Huang, H.; Daniluk, J.; Liu, Y.; Chu, J.; Li, Z.; Ji, B.; Logsdon, C.D. Oncogenic K-Ras requires activation for enhanced activity. Oncogene 2014, 33, 532–535. [Google Scholar] [CrossRef]
- Polansky, O.; Sekelova, Z.; Faldynova, M.; Sebkova, A.; Sisak, F.; Rychlik, I. Important Metabolic Pathways and Biological Processes Expressed by Chicken Cecal Microbiota. Appl. Environ. Microbiol. 2015, 82, 1569–1576. [Google Scholar] [CrossRef]
- Zhou, C.-H.; Meng, Y.-T.; Xu, J.-J.; Fang, X.; Zhao, J.-L.; Zhou, W.; Zhao, J.; Han, J.-C.; Zhang, L.; Wang, K.-X.; et al. Altered diversity and composition of gut microbiota in Chinese patients with chronic pancreatitis. Pancreatology 2020, 20, 16–24. [Google Scholar] [CrossRef]
- Maekawa, T.; Fukaya, R.; Takamatsu, S.; Itoyama, S.; Fukuoka, T.; Yamada, M.; Hata, T.; Nagaoka, S.; Kawamoto, K.; Eguchi, H.; et al. Possible involvement of Enterococcus infection in the pathogenesis of chronic pancreatitis and cancer. Biochem. Biophys. Res. Commun. 2018, 506, 962–969. [Google Scholar] [CrossRef]
- Wu, C.; Li, M.; Chen, W. Characteristics of Gut Microbiota in Cerulein-Induced Chronic Pancreatitis. Diabetes Metab. Syndr. Obes. 2021, 14, 285–294. [Google Scholar] [CrossRef]
- Nishiyama, H.; Nagai, T.; Kudo, M.; Okazaki, Y.; Azuma, Y.; Watanabe, T.; Goto, S.; Ogata, H.; Sakurai, T. Supplementation of pancreatic digestive enzymes alters the composition of intestinal microbiota in mice. Biochem. Biophys. Res. Commun. 2018, 495, 273–279. [Google Scholar] [CrossRef]
- Shimosegawa, T.; Chari, S.T.; Frulloni, L.; Kamisawa, T.; Kawa, S.; Mino-Kenudson, M.; Kim, M.-H.; Klöppel, G.; Lerch, M.M.; Löhr, M.; et al. International consensus diagnostic criteria for autoimmune pancreatitis: Guidelines of the International Association of Pancreatology. Pancreas 2011, 40, 352–358. [Google Scholar] [CrossRef]
- Fukui, Y.; Uchida, K.; Sakaguchi, Y.; Fukui, T.; Nishio, A.; Shikata, N.; Sakaida, N.; Uemura, Y.; Satoi, S.; Okazaki, K. Possible involvement of Toll-like receptor 7 in the development of type 1 autoimmune pancreatitis. J. Gastroenterol. 2015, 50, 435–444. [Google Scholar] [CrossRef]
- Watanabe, T.; Yamashita, K.; Fujikawa, S.; Sakurai, T.; Kudo, M.; Shiokawa, M.; Kodama, Y.; Uchida, K.; Okazaki, K.; Chiba, T. Involvement of activation of toll-like receptors and nucleotide-binding oligomerization domain-like receptors in enhanced IgG4 responses in autoimmune pancreatitis. Arthritis Rheum. 2012, 64, 914–924. [Google Scholar] [CrossRef] [PubMed]
- Soga, Y.; Komori, H.; Miyazaki, T.; Arita, N.; Terada, M.; Kamada, K.; Tanaka, Y.; Fujino, T.; Hiasa, Y.; Matsuura, B.; et al. Toll-like receptor 3 signaling induces chronic pancreatitis through the Fas/Fas ligand-mediated cytotoxicity. Tohoku J. Exp. Med. 2009, 217, 175–184. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Haruta, I.; Yanagisawa, N.; Kawamura, S.; Furukawa, T.; Shimizu, K.; Kato, H.; Kobayashi, M.; Shiratori, K.; Yagi, J. A mouse model of autoimmune pancreatitis with salivary gland involvement triggered by innate immunity via persistent exposure to avirulent bacteria. Lab. Investig. 2010, 90, 1757–1769. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, N.; Haruta, I.; Shimizu, K.; Furukawa, T.; Higuchi, T.; Shibata, N.; Shiratori, K.; Yagi, J. Identification of commensal flora-associated antigen as a pathogenetic factor of autoimmune pancreatitis. Pancreatology 2014, 14, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Kamata, K.; Watanabe, T.; Minaga, K.; Hara, A.; Yoshikawa, T.; Okamoto, A.; Yamao, K.; Takenaka, M.; Park, A.-M.; Kudo, M. Intestinal dysbiosis mediates experimental autoimmune pancreatitis via activation of plasmacytoid dendritic cells. Int. Immunol. 2019, 31, 795–809. [Google Scholar] [CrossRef]
- Kountouras, J.; Zavos, C.; Gavalas, E.; Tzilves, D. Challenge in the pathogenesis of autoimmune pancreatitis: Potential role of helicobacter pylori infection via molecular mimicry. Gastroenterology 2007, 133, 368–369. [Google Scholar] [CrossRef]
- Guarneri, F.; Guarneri, C.; Benvenga, S. Helicobacter pylori and autoimmune pancreatitis: Role of carbonic anhydrase via molecular mimicry? J. Cell. Mol. Med. 2005, 9, 741–744. [Google Scholar] [CrossRef]
- Elta, G.H.; Enestvedt, B.K.; Sauer, B.G.; Lennon, A.M. ACG Clinical Guideline: Diagnosis and Management of Pancreatic Cysts. Am. J. Gastroenterol. 2018, 113, 464–479. [Google Scholar] [CrossRef]
- Aronsson, L.; Andersson, R.; Ansari, D. Intraductal papillary mucinous neoplasm of the pancreas—Epidemiology, risk factors, diagnosis, and management. Scand. J. Gastroenterol. 2017, 52, 803–815. [Google Scholar] [CrossRef]
- Machado, N.O.; Al Qadhi, H.; Al Wahibi, K. Intraductal Papillary Mucinous Neoplasm of Pancreas. N. Am. J. Med. Sci. 2015, 7, 160–175. [Google Scholar] [CrossRef]
- European Study Group on Cystic Tumours of the Pancreas European evidence-based guidelines on pancreatic cystic neoplasms. Gut 2018, 67, 789–804. [CrossRef]
- Li, S.; Fuhler, G.M.; Bn, N.; Jose, T.; Bruno, M.J.; Peppelenbosch, M.P.; Konstantinov, S.R. Pancreatic cyst fluid harbors a unique microbiome. Microbiome 2017, 5, 147. [Google Scholar] [CrossRef]
- Gaiser, R.A.; Halimi, A.; Alkharaan, H.; Lu, L.; Davanian, H.; Healy, K.; Hugerth, L.W.; Ateeb, Z.; Valente, R.; Fernández Moro, C.; et al. Enrichment of oral microbiota in early cystic precursors to invasive pancreatic cancer. Gut 2019, 68, 2186–2194. [Google Scholar] [CrossRef]
- Zaura, E.; Keijser, B.J.F.; Huse, S.M.; Crielaard, W. Defining the healthy “core microbiome” of oral microbial communities. BMC Microbiol. 2009, 9, 259. [Google Scholar] [CrossRef]
- Brennan, C.A.; Garrett, W.S. Fusobacterium nucleatum—Symbiont, opportunist and oncobacterium. Nat. Rev. Microbiol. 2019, 17, 156–166. [Google Scholar] [CrossRef]
- Alkharaan, H.; Lu, L.; Gabarrini, G.; Halimi, A.; Ateeb, Z.; Sobkowiak, M.J.; Davanian, H.; Fernández Moro, C.; Jansson, L.; Del Chiaro, M.; et al. Circulating and Salivary Antibodies to Fusobacterium nucleatum Are Associated with Cystic Pancreatic Neoplasm Malignancy. Front. Immunol. 2020, 11, 2003. [Google Scholar] [CrossRef]
- Olson, S.H.; Satagopan, J.; Xu, Y.; Ling, L.; Leong, S.; Orlow, I.; Saldia, A.; Li, P.; Nunes, P.; Madonia, V.; et al. The oral microbiota in patients with pancreatic cancer, patients with IPMNs, and controls: A pilot study. Cancer Causes Control 2017, 28, 959–969. [Google Scholar] [CrossRef]
- Carding, S.; Verbeke, K.; Vipond, D.T.; Corfe, B.M.; Owen, L.J. Dysbiosis of the gut microbiota in disease. Microb. Ecol. Health Dis. 2015, 26, 26191. [Google Scholar] [CrossRef]
- Belkaid, Y.; Hand, T.W. Role of the microbiota in immunity and inflammation. Cell 2014, 157, 121–141. [Google Scholar] [CrossRef]
- Fan, X.; Alekseyenko, A.V.; Wu, J.; Peters, B.A.; Jacobs, E.J.; Gapstur, S.M.; Purdue, M.P.; Abnet, C.C.; Stolzenberg-Solomon, R.; Miller, G.; et al. Human oral microbiome and prospective risk for pancreatic cancer: A population-based nested case-control study. Gut 2018, 67, 120–127. [Google Scholar] [CrossRef]
- Michaud, D.S.; Izard, J. Microbiota, oral microbiome, and pancreatic cancer. Cancer J. 2014, 20, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Segers, S.; Hayes, R.B. Periodontal disease, Porphyromonas gingivalis serum antibody levels and orodigestive cancer mortality. Carcinogenesis 2012, 33, 1055–1058. [Google Scholar] [CrossRef] [PubMed]
- Michaud, D.S.; Joshipura, K.; Giovannucci, E.; Fuchs, C.S. A prospective study of periodontal disease and pancreatic cancer in US male health professionals. J. Natl. Cancer Inst. 2007, 99, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Hiraki, A.; Matsuo, K.; Suzuki, T.; Kawase, T.; Tajima, K. Teeth loss and risk of cancer at 14 common sites in Japanese. Cancer Epidemiol. Biomark. Prev. 2008, 17, 1222–1227. [Google Scholar] [CrossRef] [PubMed]
- Stolzenberg-Solomon, R.Z.; Dodd, K.W.; Blaser, M.J.; Virtamo, J.; Taylor, P.R.; Albanes, D. Tooth loss, pancreatic cancer, and Helicobacter pylori. Am. J. Clin. Nutr. 2003, 78, 176–181. [Google Scholar] [CrossRef]
- Hujoel, P.P.; Drangsholt, M.; Spiekerman, C.; Weiss, N.S. An exploration of the periodontitis-cancer association. Ann. Epidemiol. 2003, 13, 312–316. [Google Scholar] [CrossRef]
- Farrell, J.J.; Zhang, L.; Zhou, H.; Chia, D.; Elashoff, D.; Akin, D.; Paster, B.J.; Joshipura, K.; Wong, D.T.W. Variations of oral microbiota are associated with pancreatic diseases including pancreatic cancer. Gut 2012, 61, 582–588. [Google Scholar] [CrossRef]
- Nejman, D.; Livyatan, I.; Fuks, G.; Gavert, N.; Zwang, Y.; Geller, L.T.; Rotter-Maskowitz, A.; Weiser, R.; Mallel, G.; Gigi, E.; et al. The human tumor microbiome is composed of tumor type-specific intracellular bacteria. Science 2020, 368, 973–980. [Google Scholar] [CrossRef]
- Ro, C.; Chai, W.; Yu, V.E.; Yu, R. Pancreatic neuroendocrine tumors: Biology, diagnosis, and treatment. Chin. J. Cancer 2013, 32, 312–324. [Google Scholar] [CrossRef]
- Chen, S.-M.; Chieng, W.-W.; Huang, S.-W.; Hsu, L.-J.; Jan, M.-S. The synergistic tumor growth-inhibitory effect of probiotic Lactobacillus on transgenic mouse model of pancreatic cancer treated with gemcitabine. Sci. Rep. 2020, 10, 20319. [Google Scholar] [CrossRef]
- Raderer, M.; Wrba, F.; Kornek, G.; Maca, T.; Koller, D.Y.; Weinlaender, G.; Hejna, M.; Scheithauer, W. Association between Helicobacter pylori infection and pancreatic cancer. Oncology 1998, 55, 16–19. [Google Scholar] [CrossRef]
- Greiner, T.U.; Hyötyläinen, T.; Knip, M.; Bäckhed, F.; Orešič, M. The gut microbiota modulates glycaemic control and serum metabolite profiles in non-obese diabetic mice. PLoS ONE 2014, 9, e110359. [Google Scholar] [CrossRef]
- Kaźmierczak-Siedlecka, K.; Dvořák, A.; Folwarski, M.; Daca, A.; Przewłócka, K.; Makarewicz, W. Fungal Gut Microbiota Dysbiosis and Its Role in Colorectal, Oral, and Pancreatic Carcinogenesis. Cancers 2020, 12, 1326. [Google Scholar] [CrossRef]
- Arafa, A.; Eshak, E.S.; Abdel Rahman, T.A.; Anwar, M.M. Hepatitis C virus infection and risk of pancreatic cancer: A meta-analysis. Cancer Epidemiol. 2020, 65, 101691. [Google Scholar] [CrossRef]
- Li, L.; Wu, B.; Yang, L.-B.; Yin, G.-C.; Liu, J.-Y. Chronic hepatitis B virus infection and risk of pancreatic cancer: A meta-analysis. Asian Pac. J. Cancer Prev. 2013, 14, 275–279. [Google Scholar] [CrossRef][Green Version]
- Kohi, S.; Macgregor-Das, A.; Dbouk, M.; Yoshida, T.; Chuidian, M.; Abe, T.; Borges, M.; Lennon, A.M.; Shin, E.J.; Canto, M.I.; et al. Alterations in the Duodenal Fluid Microbiome of Patients with Pancreatic Cancer. Clin. Gastroenterol. Hepatol. 2022, 20, e196–e227. [Google Scholar] [CrossRef]
- Thomas, R.M.; Gharaibeh, R.Z.; Gauthier, J.; Beveridge, M.; Pope, J.L.; Guijarro, M.V.; Yu, Q.; He, Z.; Ohland, C.; Newsome, R.; et al. Intestinal microbiota enhances pancreatic carcinogenesis in preclinical models. Carcinogenesis 2018, 39, 1068–1078. [Google Scholar] [CrossRef]
- Amini, N.; Spolverato, G.; Kim, Y.; Pawlik, T.M. Trends in Hospital Volume and Failure to Rescue for Pancreatic Surgery. J. Gastrointest. Surg. Off. J. Soc. Surg. Aliment. Tract 2015, 19, 1581–1592. [Google Scholar] [CrossRef]
- Major Postoperative Complications Are Associated with Impaired Long-Term Survival after Gastro-Esophageal and Pancreatic Cancer Surgery: A Complete National Cohort Study. Available online: https://pubmed.ncbi.nlm.nih.gov/27193578/ (accessed on 29 August 2022).
- Current Trends in Preoperative Biliary Stenting in Patients with Pancreatic Cancer. Available online: https://pubmed.ncbi.nlm.nih.gov/23889947/ (accessed on 29 August 2022).
- Poruk, K.E.; Lin, J.A.; Cooper, M.A.; He, J.; Makary, M.A.; Hirose, K.; Cameron, J.L.; Pawlik, T.M.; Wolfgang, C.L.; Eckhauser, F.; et al. A novel, validated risk score to predict surgical site infection after pancreaticoduodenectomy. HPB Off. J. Int. Hepato Pancreato Biliary Assoc. 2016, 18, 893–899. [Google Scholar] [CrossRef]
- Barreto, S.G.; Singh, M.K.; Sharma, S.; Chaudhary, A. Determinants of Surgical Site Infections Following Pancreatoduodenectomy. World J. Surg. 2015, 39, 2557–2563. [Google Scholar] [CrossRef]
- Morris-Stiff, G.; Tamijmarane, A.; Tan, Y.-M.; Shapey, I.; Bhati, C.; Mayer, A.D.; Buckels, J.A.C.; Bramhall, S.R.; Mirza, D.F. Pre-operative stenting is associated with a higher prevalence of post-operative complications following pancreatoduodenectomy. Int. J. Surg. 2011, 9, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Müssle, B.; Hempel, S.; Kahlert, C.; Distler, M.; Weitz, J.; Welsch, T. Prognostic Impact of Bacterobilia on Morbidity and Postoperative Management After Pancreatoduodenectomy: A Systematic Review and Meta-analysis. World J. Surg. 2018, 42, 2951–2962. [Google Scholar] [CrossRef] [PubMed]
- Stecca, T.; Nistri, C.; Pauletti, B.; Greco, A.; Di Giacomo, A.; Caratozzolo, E.; Bonariol, L.; Massani, M. Bacteriobilia resistance to antibiotic prophylaxis increases morbidity after pancreaticoduodenectomy: A monocentric retrospective study of 128 patients. Updates Surg. 2020, 72, 1073–1080. [Google Scholar] [CrossRef] [PubMed]
- Cortes, A.; Sauvanet, A.; Bert, F.; Janny, S.; Sockeel, P.; Kianmanesh, R.; Ponsot, P.; Ruszniewski, P.; Belghiti, J. Effect of bile contamination on immediate outcomes after pancreaticoduodenectomy for tumor. J. Am. Coll. Surg. 2006, 202, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Howard, T.J.; Yu, J.; Greene, R.B.; George, V.; Wairiuko, G.M.; Moore, S.A.; Madura, J.A. Influence of bactibilia after preoperative biliary stenting on postoperative infectious complications. J. Gastrointest. Surg. 2006, 10, 523–531. [Google Scholar] [CrossRef]
- Sudo, T.; Murakami, Y.; Uemura, K.; Hayashidani, Y.; Hashimoto, Y.; Ohge, H.; Sueda, T. Specific antibiotic prophylaxis based on bile cultures is required to prevent postoperative infectious complications in pancreatoduodenectomy patients who have undergone preoperative biliary drainage. World J. Surg. 2007, 31, 2230–2235. [Google Scholar] [CrossRef]
- Nomura, T.; Shirai, Y.; Hatakeyama, K. Enterococcal bactibilia in patients with malignant biliary obstruction. Dig. Dis. Sci. 2000, 45, 2183–2186. [Google Scholar] [CrossRef]
- Fong, Z.V.; McMillan, M.T.; Marchegiani, G.; Sahora, K.; Malleo, G.; De Pastena, M.; Loehrer, A.P.; Lee, G.C.; Ferrone, C.R.; Chang, D.C.; et al. Discordance between Perioperative Antibiotic Prophylaxis and Wound Infection Cultures in Patients Undergoing Pancreaticoduodenectomy. JAMA Surg. 2016, 151, 432–439. [Google Scholar] [CrossRef]
- Goel, N.; Nadler, A.; Reddy, S.; Hoffman, J.P.; Pitt, H.A. Biliary microbiome in pancreatic cancer: Alterations with neoadjuvant therapy. HPB 2019, 21, 1753–1760. [Google Scholar] [CrossRef]
- Nalluri, H.; Jensen, E.; Staley, C. Role of biliary stent and neoadjuvant chemotherapy in the pancreatic tumor microbiome. BMC Microbiol. 2021, 21, 280. [Google Scholar] [CrossRef]
- Soderlund, C.; Linder, S. Covered metal versus plastic stents for malignant common bile duct stenosis: A prospective, randomized, controlled trial. Gastrointest. Endosc. 2006, 63, 986–995. [Google Scholar] [CrossRef]
- Moses, P.L.; Alnaamani, K.M.; Barkun, A.N.; Gordon, S.R.; Mitty, R.D.; Branch, M.S.; Kowalski, T.E.; Martel, M.; Adam, V. Randomized trial in malignant biliary obstruction: Plastic vs partially covered metal stents. World J. Gastroenterol. 2013, 19, 8638–8646. [Google Scholar] [CrossRef]
- Bilgiç, Ç.; Keske, Ş.; Sobutay, E.; Can, U.; Zenger, S.; Gürbüz, B.; Ergönül, Ö.; Bilge, O. Surgical site infections after pancreaticoduodenectomy: Preoperative biliary system interventions and antimicrobial prophylaxis. Int. J. Infect. Dis. 2020, 95, 148–152. [Google Scholar] [CrossRef]
- Shrader, H.R.; Miller, A.M.; Tomanek-Chalkley, A.; McCarthy, A.; Coleman, K.L.; Ear, P.H.; Mangalam, A.K.; Salem, A.K.; Chan, C.H.F. Effect of bacterial contamination in bile on pancreatic cancer cell survival. Surgery 2021, 169, 617–622. [Google Scholar] [CrossRef]
- Veillette, G.; Dominguez, I.; Ferrone, C.; Thayer, S.P.; McGrath, D.; Warshaw, A.L.; Fernández-del Castillo, C. Implications and management of pancreatic fistulas following pancreaticoduodenectomy: The Massachusetts General Hospital experience. Arch. Surg. 2008, 143, 476–481. [Google Scholar] [CrossRef]
- Ohgi, K.; Sugiura, T.; Yamamoto, Y.; Okamura, Y.; Ito, T.; Uesaka, K. Bacterobilia may trigger the development and severity of pancreatic fistula after pancreatoduodenectomy. Surgery 2016, 160, 725–730. [Google Scholar] [CrossRef]
- Liou, A.P.; Paziuk, M.; Luevano, J.-M.; Machineni, S.; Turnbaugh, P.J.; Kaplan, L.M. Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. Sci. Transl. Med. 2013, 5, 178ra41. [Google Scholar] [CrossRef]
- Vernuccio, F.; Messina, C.; Merz, V.; Cannella, R.; Midiri, M. Resectable and Borderline Resectable Pancreatic Ductal Adenocarcinoma: Role of the Radiologist and Oncologist in the Era of Precision Medicine. Diagnostics 2021, 11, 2166. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kim, J.; Park, S.-J. High-throughput 16S rRNA gene sequencing reveals alterations of mouse intestinal microbiota after radiotherapy. Anaerobe 2015, 33, 1–7. [Google Scholar] [CrossRef]
- Parker, B.J.; Wearsch, P.A.; Veloo, A.C.; Rodriguez-Palacios, A. The Genus Alistipes: Gut Bacteria with Emerging Implications to Inflammation, Cancer, and Mental Health. Front. Immunol. 2020, 11, 906. [Google Scholar] [CrossRef]
- Vande Voorde, J.; Sabuncuoğlu, S.; Noppen, S.; Hofer, A.; Ranjbarian, F.; Fieuws, S.; Balzarini, J.; Liekens, S. Nucleoside-catabolizing enzymes in mycoplasma-infected tumor cell cultures compromise the cytostatic activity of the anticancer drug gemcitabine. J. Biol. Chem. 2014, 289, 13054–13065. [Google Scholar] [CrossRef] [PubMed]
- Geller, L.T.; Barzily-Rokni, M.; Danino, T.; Jonas, O.H.; Shental, N.; Nejman, D.; Gavert, N.; Zwang, Y.; Cooper, Z.A.; Shee, K.; et al. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science 2017, 357, 1156–1160. [Google Scholar] [CrossRef] [PubMed]
- Kesh, K.; Mendez, R.; Abdelrahman, L.; Banerjee, S.; Banerjee, S. Type 2 diabetes induced microbiome dysbiosis is associated with therapy resistance in pancreatic adenocarcinoma. Microb. Cell Fact. 2020, 19, 75. [Google Scholar] [CrossRef] [PubMed]
- Yeung, C.-Y.; Chiang Chiau, J.-S.; Cheng, M.-L.; Chan, W.-T.; Chang, S.-W.; Chang, Y.-H.; Jiang, C.-B.; Lee, H.-C. Modulations of probiotics on gut microbiota in a 5-fluorouracil-induced mouse model of mucositis. J. Gastroenterol. Hepatol. 2020, 35, 806–814. [Google Scholar] [CrossRef] [PubMed]
- García-González, A.P.; Ritter, A.D.; Shrestha, S.; Andersen, E.C.; Yilmaz, L.S.; Walhout, A.J.M. Bacterial Metabolism Affects the C. elegans Response to Cancer Chemotherapeutics. Cell 2017, 169, 431–441.e8. [Google Scholar] [CrossRef]
- Yuan, L.; Zhang, S.; Li, H.; Yang, F.; Mushtaq, N.; Ullah, S.; Shi, Y.; An, C.; Xu, J. The influence of gut microbiota dysbiosis to the efficacy of 5-Fluorouracil treatment on colorectal cancer. Biomed. Pharmacother. 2018, 108, 184–193. [Google Scholar] [CrossRef]
- Yu, T.; Guo, F.; Yu, Y.; Sun, T.; Ma, D.; Han, J.; Qian, Y.; Kryczek, I.; Sun, D.; Nagarsheth, N.; et al. Fusobacterium nucleatum Promotes Chemoresistance to Colorectal Cancer by Modulating Autophagy. Cell 2017, 170, 548–563.e16. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, Y.; Weng, W.; Guo, B.; Cai, G.; Ma, Y.; Cai, S. Fusobacterium nucleatum promotes chemoresistance to 5-fluorouracil by upregulation of BIRC3 expression in colorectal cancer. J. Exp. Clin. Cancer Res. 2019, 38, 14. [Google Scholar] [CrossRef]
- Iida, N.; Dzutsev, A.; Stewart, C.A.; Smith, L.; Bouladoux, N.; Weingarten, R.A.; Molina, D.A.; Salcedo, R.; Back, T.; Cramer, S.; et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science 2013, 342, 967–970. [Google Scholar] [CrossRef]
- Chandra, V.; McAllister, F. Therapeutic potential of microbial modulation in pancreatic cancer. Gut 2021, 70, 1419–1425. [Google Scholar] [CrossRef]
- Sethi, V.; Kurtom, S.; Tarique, M.; Lavania, S.; Malchiodi, Z.; Hellmund, L.; Zhang, L.; Sharma, U.; Giri, B.; Garg, B.; et al. Gut Microbiota Promotes Tumor Growth in Mice by Modulating Immune Response. Gastroenterology 2018, 155, 33–37.e6. [Google Scholar] [CrossRef]
- Mima, K.; Nakagawa, S.; Sawayama, H.; Ishimoto, T.; Imai, K.; Iwatsuki, M.; Hashimoto, D.; Baba, Y.; Yamashita, Y.-I.; Yoshida, N.; et al. The microbiome and hepatobiliary-pancreatic cancers. Cancer Lett. 2017, 402, 9–15. [Google Scholar] [CrossRef]
- Carpenter, E.; Nelson, S.; Bednar, F.; Cho, C.; Nathan, H.; Sahai, V.; di Magliano, M.P.; Frankel, T.L. Immunotherapy for pancreatic ductal adenocarcinoma. J. Surg. Oncol. 2021, 123, 751–759. [Google Scholar] [CrossRef]
- Schizas, D.; Charalampakis, N.; Kole, C.; Economopoulou, P.; Koustas, E.; Gkotsis, E.; Ziogas, D.; Psyrri, A.; Karamouzis, M.V. Immunotherapy for pancreatic cancer: A 2020 update. Cancer Treat. Rev. 2020, 86, 102016. [Google Scholar] [CrossRef]
- Balachandran, V.P.; Beatty, G.L.; Dougan, S.K. Broadening the Impact of Immunotherapy to Pancreatic Cancer: Challenges and Opportunities. Gastroenterology 2019, 156, 2056–2072. [Google Scholar] [CrossRef]
- Tan, W.; Duong, M.T.-Q.; Zuo, C.; Qin, Y.; Zhang, Y.; Guo, Y.; Hong, Y.; Zheng, J.H.; Min, J.-J. Targeting of pancreatic cancer cells and stromal cells using engineered oncolytic Salmonella typhimurium. Mol. Ther. 2022, 30, 662–671. [Google Scholar] [CrossRef]
- Ebelt, N.D.; Zamloot, V.; Zuniga, E.; Passi, K.B.; Sobocinski, L.J.; Young, C.A.; Blazar, B.R.; Manuel, E.R. Collagenase-Expressing Salmonella Targets Major Collagens in Pancreatic Cancer Leading to Reductions in Immunosuppressive Subsets and Tumor Growth. Cancers 2021, 13, 3565. [Google Scholar] [CrossRef]
- Ebelt, N.D.; Zuniga, E.; Passi, K.B.; Sobocinski, L.J.; Manuel, E.R. Hyaluronidase-Expressing Salmonella Effectively Targets Tumor-Associated Hyaluronic Acid in Pancreatic Ductal Adenocarcinoma. Mol. Cancer Ther. 2020, 19, 706–716. [Google Scholar] [CrossRef]
- Kim, V.M.; Blair, A.B.; Lauer, P.; Foley, K.; Che, X.; Soares, K.; Xia, T.; Muth, S.T.; Kleponis, J.; Armstrong, T.D.; et al. Anti-pancreatic tumor efficacy of a Listeria-based, Annexin A2-targeting immunotherapy in combination with anti-PD-1 antibodies. J. Immunother. Cancer 2019, 7, 132. [Google Scholar] [CrossRef]
- Deng, W.; Lira, V.; Hudson, T.E.; Lemmens, E.E.; Hanson, W.G.; Flores, R.; Barajas, G.; Katibah, G.E.; Desbien, A.L.; Lauer, P.; et al. Recombinant Listeria promotes tumor rejection by CD8+ T cell-dependent remodeling of the tumor microenvironment. Proc. Natl. Acad. Sci. USA 2018, 115, 8179–8184. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Q.; Liao, Q.; Zhao, Y. Pancreatic Cancer, Gut Microbiota, and Therapeutic Efficacy. J. Cancer 2020, 11, 2749–2758. [Google Scholar] [CrossRef] [PubMed]
- Rezasoltani, S.; Yadegar, A.; Asadzadeh Aghdaei, H.; Reza Zali, M. Modulatory effects of gut microbiome in cancer immunotherapy: A novel paradigm for blockade of immune checkpoint inhibitors. Cancer Med. 2021, 10, 1141–1154. [Google Scholar] [CrossRef] [PubMed]
- Sethi, V.; Vitiello, G.A.; Saxena, D.; Miller, G.; Dudeja, V. The Role of the Microbiome in Immunologic Development and its Implication For Pancreatic Cancer Immunotherapy. Gastroenterology 2019, 156, 2097–2115.e2. [Google Scholar] [CrossRef] [PubMed]
- Heshiki, Y.; Vazquez-Uribe, R.; Li, J.; Ni, Y.; Quainoo, S.; Imamovic, L.; Li, J.; Sørensen, M.; Chow, B.K.C.; Weiss, G.J.; et al. Predictable modulation of cancer treatment outcomes by the gut microbiota. Microbiome 2020, 8, 28. [Google Scholar] [CrossRef]
- Viaud, S.; Saccheri, F.; Mignot, G.; Yamazaki, T.; Daillère, R.; Hannani, D.; Enot, D.P.; Pfirschke, C.; Engblom, C.; Pittet, M.J.; et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science 2013, 342, 971–976. [Google Scholar] [CrossRef]
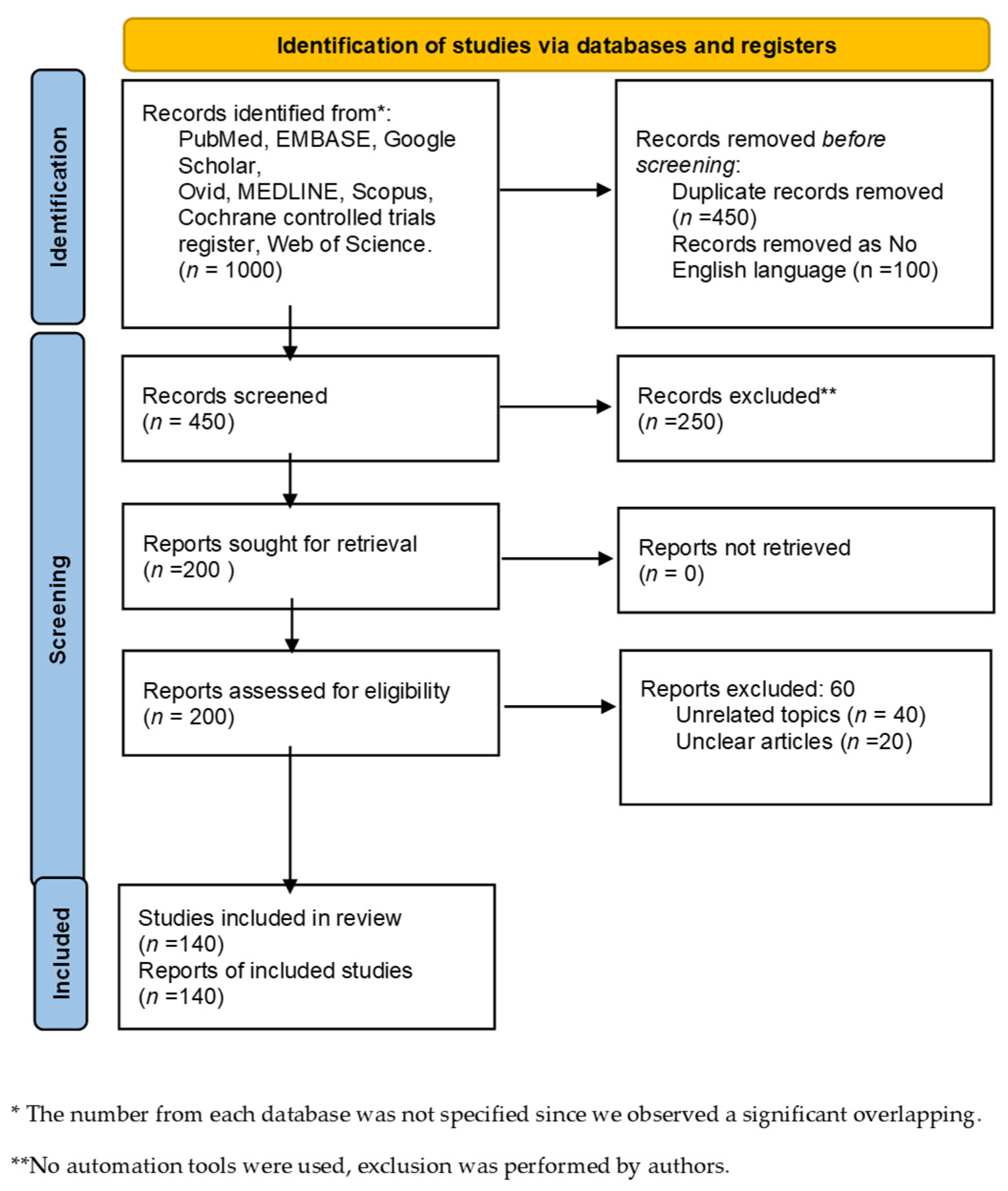
| Treatment | Ref. | Microbiota in Locoregional and Systemic Treatment |
|---|---|---|
| Biliary drainage and surgery | [25,102,103,106,108,109,116,118] |
|
| Radiation | [121,122] |
|
| Chemotherapy | [15,97,124,131,132,133] |
|
| Immunotherapy | [138,139,141,142,143,144,145] |
|
| Fecal microbiota transplantation (FMT) | [144] |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Binda, C.; Gibiino, G.; Sbrancia, M.; Coluccio, C.; Cazzato, M.; Carloni, L.; Cucchetti, A.; Ercolani, G.; Sambri, V.; Fabbri, C. Microbiota in the Natural History of Pancreatic Cancer: From Predisposition to Therapy. Cancers 2023, 15, 1. https://doi.org/10.3390/cancers15010001
Binda C, Gibiino G, Sbrancia M, Coluccio C, Cazzato M, Carloni L, Cucchetti A, Ercolani G, Sambri V, Fabbri C. Microbiota in the Natural History of Pancreatic Cancer: From Predisposition to Therapy. Cancers. 2023; 15(1):1. https://doi.org/10.3390/cancers15010001
Chicago/Turabian StyleBinda, Cecilia, Giulia Gibiino, Monica Sbrancia, Chiara Coluccio, Maria Cazzato, Lorenzo Carloni, Alessandro Cucchetti, Giorgio Ercolani, Vittorio Sambri, and Carlo Fabbri. 2023. "Microbiota in the Natural History of Pancreatic Cancer: From Predisposition to Therapy" Cancers 15, no. 1: 1. https://doi.org/10.3390/cancers15010001
APA StyleBinda, C., Gibiino, G., Sbrancia, M., Coluccio, C., Cazzato, M., Carloni, L., Cucchetti, A., Ercolani, G., Sambri, V., & Fabbri, C. (2023). Microbiota in the Natural History of Pancreatic Cancer: From Predisposition to Therapy. Cancers, 15(1), 1. https://doi.org/10.3390/cancers15010001






