Impaired Global Longitudinal Strain Is Associated with Cardiovascular Events in Hodgkin Lymphoma Survivors
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
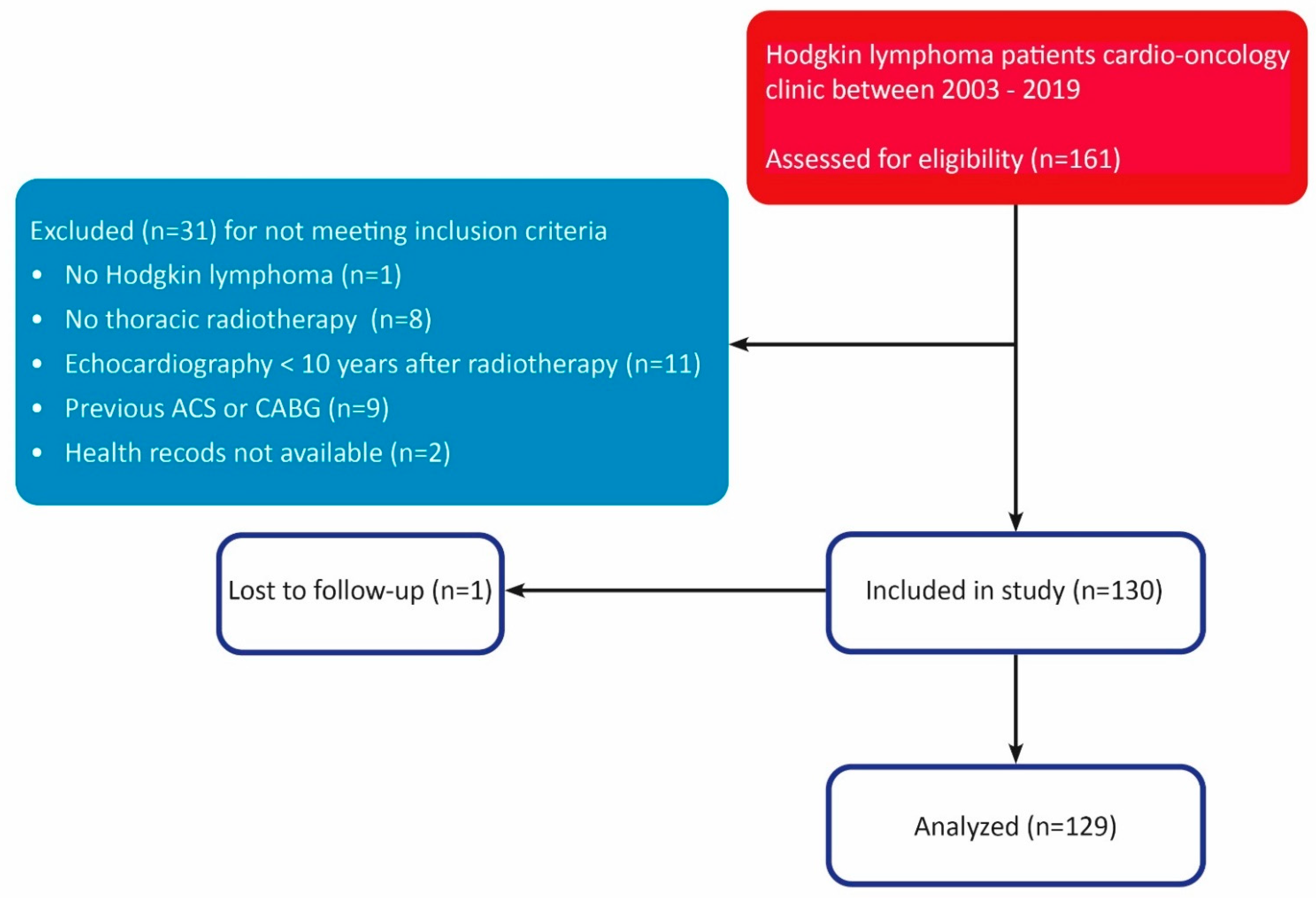
2.1. Study Population
2.2. Data Collection
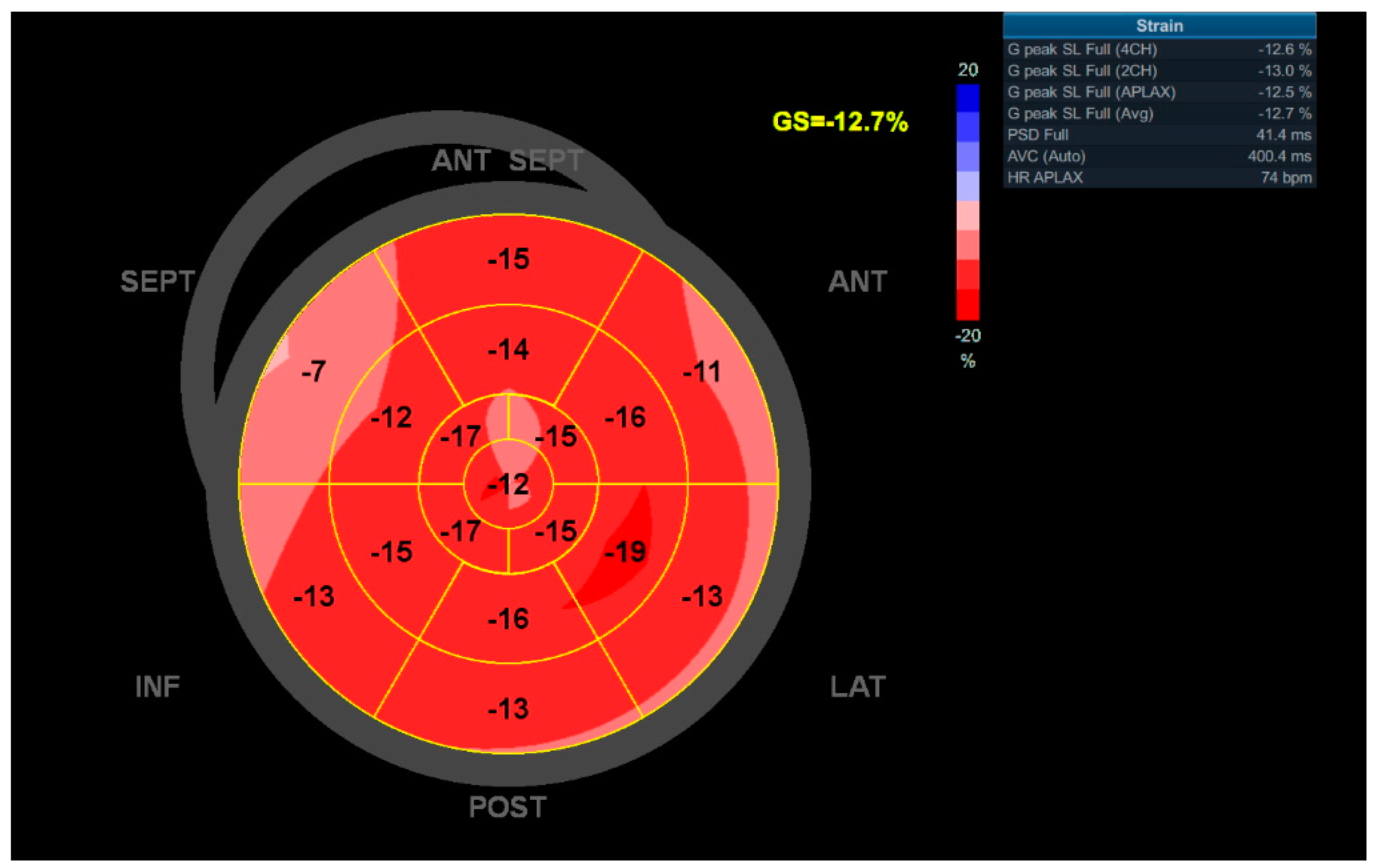
2.3. Echocardiography
2.4. Study Endpoint
2.5. Statistical Analysis
3. Results
3.1. Study Population
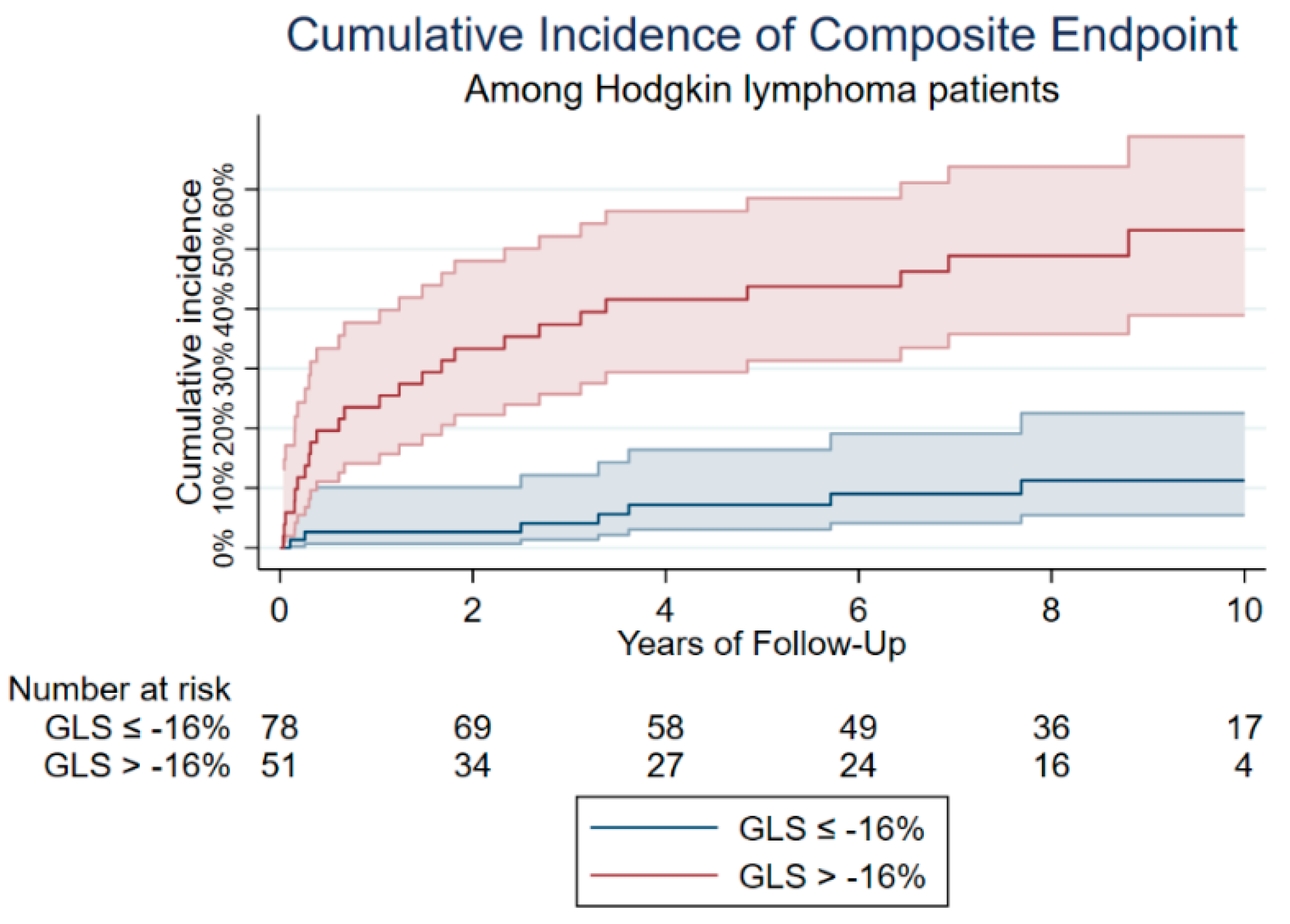
3.2. Cardiovascular Outcome
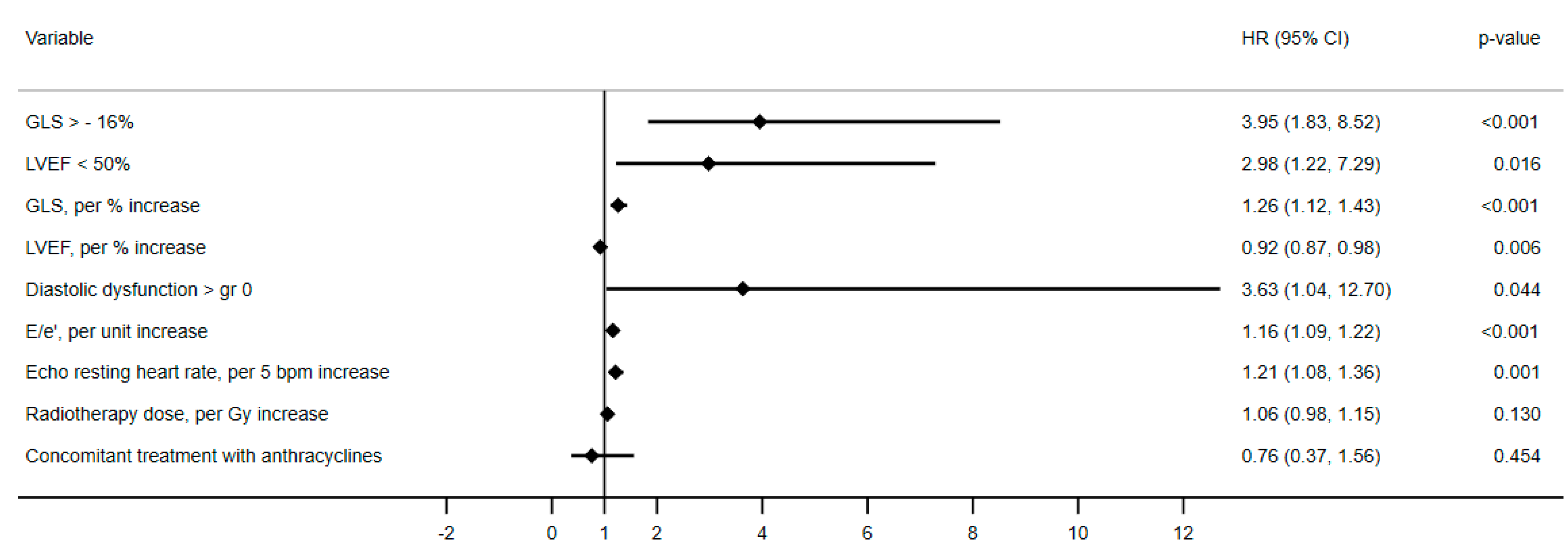
3.3. Echocardiographic Parameters Associated to the Cardiovascular Outcome
3.4. Performance of GLS Compared to LVEF to Predict Cardiac Events at Follow-Up
4. Discussion
4.1. Echocardiographic Follow-Up in Cancer Patients
4.2. LV Dysfunction in CHL Survivors
4.3. LV GLS in Relation to Cardiovascular Outcome
4.4. Clinical Implications of LV Dysfunction in CHL Survivors
4.5. Sex Differences
4.6. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aleman, B.M.; van den Belt-Dusebout, A.W.; De Bruin, M.L.; van’t Veer, M.B.; Baaijens, M.H.; de Boer, J.P.; Hart, A.A.; Klokman, W.J.; Kuenen, M.A.; Ouwens, G.M.; et al. Late cardiotoxicity after treatment for Hodgkin lymphoma. Blood 2007, 109, 1878–1886. [Google Scholar] [CrossRef] [PubMed]
- Diehl, V.; Thomas, R.K.; Re, D. Part II: Hodgkin’s lymphoma--diagnosis and treatment. Lancet Oncol. 2004, 5, 19–26. [Google Scholar] [CrossRef]
- Myint, Z.W.; Shrestha, R.; Siddiqui, S.; Slone, S.; Huang, B.; Ramlal, R.; Monohan, G.P.; Hildebrandt, G.C.; Saeed, H. Ten-year survival outcomes for patients with early stage classical Hodgkin lymphoma: An analysis from Kentucky Cancer Registry. Hematol. Oncol. Stem Cell Ther. 2020, 13, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Jacob, A.; Thyagarajan, B.; Kumar, M.P.; Shaikh, N.; Sharon, D. Cardiovascular effects of Hodgkin’s lymphoma: A review of literature. J. Cancer Res. Clin. Oncol. 2018, 144, 99–107. [Google Scholar] [CrossRef]
- Nederlandse Kanker Registratie (NKR). Intergraal Kankercentrum Nederland (IKNL). Available online: www.iknl.nl (accessed on 1 April 2022).
- Galper, S.L.; Yu, J.B.; Mauch, P.M.; Strasser, J.F.; Silver, B.; Lacasce, A.; Marcus, K.J.; Stevenson, M.A.; Chen, M.H.; Ng, A.K. Clinically significant cardiac disease in patients with Hodgkin lymphoma treated with mediastinal irradiation. Blood 2011, 117, 412–418. [Google Scholar] [CrossRef]
- Mulrooney, D.A.; Yeazel, M.W.; Kawashima, T.; Mertens, A.C.; Mitby, P.; Stovall, M.; Donaldson, S.S.; Green, D.M.; Sklar, C.A.; Robison, L.L.; et al. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: Retrospective analysis of the Childhood Cancer Survivor Study cohort. BMJ 2009, 339, b4606. [Google Scholar] [CrossRef]
- van der Pal, H.J.; van Dalen, E.C.; van Delden, E.; van Dijk, I.W.; Kok, W.E.; Geskus, R.B.; Sieswerda, E.; Oldenburger, F.; Koning, C.C.; van Leeuwen, F.E.; et al. High risk of symptomatic cardiac events in childhood cancer survivors. J. Clin. Oncol. 2012, 30, 1429–1437. [Google Scholar] [CrossRef]
- van Nimwegen, F.A.; Schaapveld, M.; Janus, C.P.; Krol, A.D.; Petersen, E.J.; Raemaekers, J.M.; Kok, W.E.; Aleman, B.M.; van Leeuwen, F.E. Cardiovascular disease after Hodgkin lymphoma treatment: 40-year disease risk. JAMA Intern. Med. 2015, 175, 1007–1017. [Google Scholar] [CrossRef]
- Schaapveld, M.; Aleman, B.M.; van Eggermond, A.M.; Janus, C.P.; Krol, A.D.; van der Maazen, R.W.; Roesink, J.; Raemaekers, J.M.; de Boer, J.P.; Zijlstra, J.M.; et al. Second Cancer Risk Up to 40 Years after Treatment for Hodgkin’s Lymphoma. N. Engl. J. Med. 2015, 373, 2499–2511. [Google Scholar] [CrossRef]
- Bates, J.E.; Howell, R.M.; Liu, Q.; Yasui, Y.; Mulrooney, D.A.; Dhakal, S.; Smith, S.A.; Leisenring, W.M.; Indelicato, D.J.; Gibson, T.M.; et al. Therapy-Related Cardiac Risk in Childhood Cancer Survivors: An Analysis of the Childhood Cancer Survivor Study. J. Clin. Oncol. 2019, 37, 1090–1101. [Google Scholar] [CrossRef]
- van Nimwegen, F.A.; Schaapveld, M.; Cutter, D.J.; Janus, C.P.; Krol, A.D.; Hauptmann, M.; Kooijman, K.; Roesink, J.; van der Maazen, R.; Darby, S.C.; et al. Radiation Dose-Response Relationship for Risk of Coronary Heart Disease in Survivors of Hodgkin Lymphoma. J. Clin. Oncol. 2016, 34, 235–243. [Google Scholar] [CrossRef] [PubMed]
- van Nimwegen, F.A.; Ntentas, G.; Darby, S.C.; Schaapveld, M.; Hauptmann, M.; Lugtenburg, P.J.; Janus, C.P.M.; Daniels, L.; van Leeuwen, F.E.; Cutter, D.J.; et al. Risk of heart failure in survivors of Hodgkin lymphoma: Effects of cardiac exposure to radiation and anthracyclines. Blood 2017, 129, 2257–2265. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.J.; Lipsitz, S.R.; Colan, S.D.; Tarbell, N.J.; Treves, S.T.; Diller, L.; Greenbaum, N.; Mauch, P.; Lipshultz, S.E. Cardiovascular status in long-term survivors of Hodgkin’s disease treated with chest radiotherapy. J. Clin. Oncol. 2004, 22, 3139–3148. [Google Scholar] [CrossRef] [PubMed]
- Boivin, J.F.; Hutchison, G.B.; Lubin, J.H.; Mauch, P. Coronary artery disease mortality in patients treated for Hodgkin’s disease. Cancer 1992, 69, 1241–1247. [Google Scholar] [CrossRef]
- Hancock, S.L.; Donaldson, S.S.; Hoppe, R.T. Cardiac disease following treatment of Hodgkin’s disease in children and adolescents. J. Clin. Oncol. 1993, 11, 1208–1215. [Google Scholar] [CrossRef]
- Piovaccari, G.; Ferretti, R.M.; Prati, F.; Traini, A.M.; Gobbi, M.; Caravita, L.; Branzi, A.; Magnani, B. Cardiac disease after chest irradiation for Hodgkin’s disease: Incidence in 108 patients with long follow-up. Int. J. Cardiol. 1995, 49, 39–43. [Google Scholar] [CrossRef]
- Reinders, J.G.; Heijmen, B.J.; Olofsen-van Acht, M.J.; van Putten, W.L.; Levendag, P.C. Ischemic heart disease after mantlefield irradiation for Hodgkin’s disease in long-term follow-up. Radiother. Oncol. 1999, 51, 35–42. [Google Scholar] [CrossRef]
- Kremer, L.C.; van Dalen, E.C.; Offringa, M.; Ottenkamp, J.; Voute, P.A. Anthracycline-induced clinical heart failure in a cohort of 607 children: Long-term follow-up study. J. Clin. Oncol. 2001, 19, 191–196. [Google Scholar] [CrossRef]
- Hequet, O.; Le, Q.H.; Moullet, I.; Pauli, E.; Salles, G.; Espinouse, D.; Dumontet, C.; Thieblemont, C.; Arnaud, P.; Antal, D.; et al. Subclinical late cardiomyopathy after doxorubicin therapy for lymphoma in adults. J. Clin. Oncol. 2004, 22, 1864–1871. [Google Scholar] [CrossRef]
- Myrehaug, S.; Pintilie, M.; Tsang, R.; Mackenzie, R.; Crump, M.; Chen, Z.; Sun, A.; Hodgson, D.C. Cardiac morbidity following modern treatment for Hodgkin lymphoma: Supra-additive cardiotoxicity of doxorubicin and radiation therapy. Leuk. Lymphoma 2008, 49, 1486–1493. [Google Scholar] [CrossRef]
- Ng, A.K.; Bernardo, M.P.; Weller, E.; Backstrand, K.H.; Silver, B.; Marcus, K.C.; Tarbell, N.J.; Friedberg, J.; Canellos, G.P.; Mauch, P.M. Long-term survival and competing causes of death in patients with early-stage Hodgkin’s disease treated at age 50 or younger. J. Clin. Oncol. 2002, 20, 2101–2108. [Google Scholar] [CrossRef] [PubMed]
- Aleman, B.M.; van den Belt-Dusebout, A.W.; Klokman, W.J.; Van’t Veer, M.B.; Bartelink, H.; van Leeuwen, F.E. Long-term cause-specific mortality of patients treated for Hodgkin’s disease. J. Clin. Oncol. 2003, 21, 3431–3439. [Google Scholar] [CrossRef] [PubMed]
- Zamorano, J.L.; Lancellotti, P.; Munoz, D.R.; Aboyans, V.; Asteggiano, R.; Galderisi, M.; Habib, G.; Lenihan, D.J.; Lip, G.Y.; Lyon, A.R.; et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines. Kardiol. Pol. 2016, 74, 1193–1233. [Google Scholar] [CrossRef] [PubMed]
- Heemelaar, J.C.; Krol, A.D.G.; Louwerens, M.; Holman, E.R.; Schalij, M.J.; Louisa Antoni, M. Elevated resting heart rate is a marker of subclinical left ventricular dysfunction in hodgkin lymphoma survivors. Int. J. Cardiol. Heart Vasc. 2021, 35, 100830. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Hancock, S.L.; Vagelos, R.H.; Lee, B.K.; Schnittger, I. Diastolic dysfunction after mediastinal irradiation. Am. Heart J. 2005, 150, 977–982. [Google Scholar] [CrossRef]
- Liu, J.E.; Barac, A.; Thavendiranathan, P.; Scherrer-Crosbie, M. Strain Imaging in Cardio-Oncology. JACC CardioOncol. 2020, 2, 677–689. [Google Scholar] [CrossRef]
- Oikonomou, E.K.; Kokkinidis, D.G.; Kampaktsis, P.N.; Amir, E.A.; Marwick, T.H.; Gupta, D.; Thavendiranathan, P. Assessment of Prognostic Value of Left Ventricular Global Longitudinal Strain for Early Prediction of Chemotherapy-Induced Cardiotoxicity: A Systematic Review and Meta-analysis. JAMA Cardiol. 2019, 4, 1007–1018. [Google Scholar] [CrossRef]
- Chang, H.Y.; Lee, C.H.; Su, P.L.; Li, S.S.; Chen, M.Y.; Chen, Y.P.; Hsu, Y.T.; Tsai, W.C.; Liu, P.Y.; Chen, T.Y.; et al. Subtle cardiac dysfunction in lymphoma patients receiving low to moderate dose chemotherapy. Sci. Rep. 2021, 11, 7100. [Google Scholar] [CrossRef]
- van der Velde, N.; Janus, C.P.M.; Bowen, D.J.; Hassing, H.C.; Kardys, I.; van Leeuwen, F.E.; So-Osman, C.; Nout, R.A.; Manintveld, O.C.; Hirsch, A. Detection of Subclinical Cardiovascular Disease by Cardiovascular Magnetic Resonance in Lymphoma Survivors. JACC CardioOncol. 2021, 3, 695–706. [Google Scholar] [CrossRef]
- Kosaraju, A.; Goyal, A.; Grigorova, Y.; Makaryus, A.N. Left Ventricular Ejection Fraction; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Nagueh, S.F.; Appleton, C.P.; Gillebert, T.C.; Marino, P.N.; Oh, J.K.; Smiseth, O.A.; Waggoner, A.D.; Flachskampf, F.A.; Pellikka, P.A.; Evangelista, A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J. Am. Soc. Echocardiogr. 2009, 22, 107–133. [Google Scholar] [CrossRef]
- Yang, H.; Wright, L.; Negishi, T.; Negishi, K.; Liu, J.; Marwick, T.H. Research to Practice: Assessment of Left Ventricular Global Longitudinal Strain for Surveillance of Cancer Chemotherapeutic-Related Cardiac Dysfunction. JACC Cardiovasc. Imaging 2018, 11, 1196–1201. [Google Scholar] [CrossRef] [PubMed]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.; Coats, A.J.; Falk, V.; Gonzalez-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2016, 18, 891–975. [Google Scholar] [CrossRef] [PubMed]
- Charbonnel, C.; Convers-Domart, R.; Rigaudeau, S.; Taksin, A.L.; Baron, N.; Lambert, J.; Ghez, S.; Georges, J.L.; Farhat, H.; Lambert, J.; et al. Assessment of global longitudinal strain at low-dose anthracycline-based chemotherapy, for the prediction of subsequent cardiotoxicity. Eur. Heart J.-Cardiovasc. Imaging 2017, 18, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Gripp, E.A.; Oliveira, G.E.; Feijo, L.A.; Garcia, M.I.; Xavier, S.S.; Sousa, A.S. Global Longitudinal Strain Accuracy for Cardiotoxicity Prediction in a Cohort of Breast Cancer Patients During Anthracycline and/or Trastuzumab Treatment. Arq. Bras. Cardiol. 2018, 110, 140–150. [Google Scholar] [CrossRef]
- Guerra, F.; Marchesini, M.; Contadini, D.; Menditto, A.; Morelli, M.; Piccolo, E.; Battelli, N.; Pistelli, M.; Berardi, R.; Cascinu, S.; et al. Speckle-tracking global longitudinal strain as an early predictor of cardiotoxicity in breast carcinoma. Support. Care Cancer 2016, 24, 3139–3145. [Google Scholar] [CrossRef]
- Milks, M.W.; Velez, M.R.; Mehta, N.; Ishola, A.; Van Houten, T.; Yildiz, V.O.; Reinbolt, R.; Lustberg, M.; Smith, S.A.; Orsinelli, D.A. Usefulness of Integrating Heart Failure Risk Factors Into Impairment of Global Longitudinal Strain to Predict Anthracycline-Related Cardiac Dysfunction. Am. J. Cardiol. 2018, 121, 867–873. [Google Scholar] [CrossRef]
- Portugal, G.; Branco, L.M.; Galrinho, A.; Carmo, M.M.; Timoteo, A.T.; Feliciano, J.; Abreu, J.; Oliveira, S.D.; Batarda, L.; Ferreira, R.C. Global and regional patterns of longitudinal strain in screening for chemotherapy-induced cardiotoxicity. Rev. Port. Cardiol. 2017, 36, 9–15. [Google Scholar] [CrossRef][Green Version]
- Tang, Q.; Jiang, Y.; Xu, Y.L.; Xia, H.M. Speckle tracking echocardiography predicts early subclinical anthracycline cardiotoxicity in patients with breast cancer. J. Clin. Ultrasound 2017, 45, 222–230, (Retracted in J. Clin. Ultrasound 2019, 47, 59). [Google Scholar] [CrossRef]
- Plana, J.C.; Galderisi, M.; Barac, A.; Ewer, M.S.; Ky, B.; Scherrer-Crosbie, M.; Ganame, J.; Sebag, I.A.; Agler, D.A.; Badano, L.P.; et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: A report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2014, 15, 1063–1093. [Google Scholar] [CrossRef]
- Mitchell, J.D.; Cehic, D.A.; Morgia, M.; Bergrom, C.; Toohey, J.; Guerrero, P.A.; Ferencik, M.; Kikuchi, R.; Carver, J.R.; Zaha, V.G.; et al. Cardiovascular Manifestations From Therapeutic Radiation A Multidisciplinary Expert Consensus Statement From the International Cardio-Oncology Society. Jacc-Cardiooncol. 2021, 3, 360–380. [Google Scholar] [CrossRef]
- Biering-Sorensen, T.; Biering-Sorensen, S.R.; Olsen, F.J.; Sengelov, M.; Jorgensen, P.G.; Mogelvang, R.; Shah, A.M.; Jensen, J.S. Global Longitudinal Strain by Echocardiography Predicts Long-Term Risk of Cardiovascular Morbidity and Mortality in a Low-Risk General Population: The Copenhagen City Heart Study. Circ. Cardiovasc. Imaging 2017, 10, e005521. [Google Scholar] [CrossRef] [PubMed]
- Hatazawa, K.; Tanaka, H.; Nonaka, A.; Takada, H.; Soga, F.; Hatani, Y.; Matsuzoe, H.; Shimoura, H.; Ooka, J.; Sano, H.; et al. Baseline Global Longitudinal Strain as a Predictor of Left Ventricular Dysfunction and Hospitalization for Heart Failure of Patients With Malignant Lymphoma After Anthracycline Therapy. Circ. J. 2018, 82, 2566–2574. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.R.; Gjesdal, O.; Wethal, T.; Haugaa, K.H.; Fossa, A.; Fossa, S.D.; Edvardsen, T. Left ventricular function assessed by two-dimensional speckle tracking echocardiography in long-term survivors of Hodgkin’s lymphoma treated by mediastinal radiotherapy with or without anthracycline therapy. Am. J. Cardiol. 2011, 107, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Tuohinen, S.S.; Skytta, T.; Huhtala, H.; Virtanen, V.; Kellokumpu-Lehtinen, P.L.; Raatikainen, P. Changes in left ventricular systolic strain and rotation in speckle tracking echocardiography among early-stage breast cancer patients three years after radiotherapy. Eur. Heart J. 2018, 39, 1042–1043. [Google Scholar] [CrossRef]
- Trivedi, S.J.; Choudhary, P.; Lo, Q.; Sritharan, H.P.; Iyer, A.; Batumalai, V.; Delaney, G.P.; Thomas, L. Persistent reduction in global longitudinal strain in the longer term after radiation therapy in patients with breast cancer. Radiother. Oncol. 2019, 132, 148–154. [Google Scholar] [CrossRef]
- Skytta, T.; Tuohinen, S.; Luukkaala, T.; Virtanen, V.; Raatikainen, P.; Kellokumpu-Lehtinen, P.L. Adjuvant radiotherapy-induced cardiac changes among patients with early breast cancer: A three-year follow-up study*. Acta Oncologica 2019, 58, 1250–1258. [Google Scholar] [CrossRef]
- Negishi, K.; Negishi, T.; Hare, J.L.; Haluska, B.A.; Plana, J.C.; Marwick, T.H. Independent and incremental value of deformation indices for prediction of trastuzumab-induced cardiotoxicity. J. Am. Soc. Echocardiogr. 2013, 26, 493–498. [Google Scholar] [CrossRef]
- Sawaya, H.; Sebag, I.A.; Plana, J.C.; Januzzi, J.L.; Ky, B.; Tan, T.C.; Cohen, V.; Banchs, J.; Carver, J.R.; Wiegers, S.E.; et al. Assessment of Echocardiography and Biomarkers for the Extended Prediction of Cardiotoxicity in Patients Treated With Anthracyclines, Taxanes, and Trastuzumab. Circ.-Cardiovasc. Imaging 2012, 5, 596–603. [Google Scholar] [CrossRef]
- Krishnasamy, R.; Isbel, N.M.; Hawley, C.M.; Pascoe, E.M.; Burrage, M.; Leano, R.; Haluska, B.A.; Marwick, T.H.; Stanton, T. Left Ventricular Global Longitudinal Strain (GLS) Is a Superior Predictor of All-Cause and Cardiovascular Mortality When Compared to Ejection Fraction in Advanced Chronic Kidney Disease. PLoS ONE 2015, 10, e0127044. [Google Scholar] [CrossRef]
- Ternacle, J.; Berry, M.; Alonso, E.; Kloeckner, M.; Couetil, J.P.; Rande, J.L.; Gueret, P.; Monin, J.L.; Lim, P. Incremental value of global longitudinal strain for predicting early outcome after cardiac surgery. Eur. Heart J. Cardiovasc. Imaging 2013, 14, 77–84. [Google Scholar] [CrossRef]
- Raafs, A.G.; Boscutti, A.; Henkens, M.; van den Broek, W.W.A.; Verdonschot, J.A.J.; Weerts, J.; Stolfo, D.; Nuzzi, V.; Manca, P.; Hazebroek, M.R.; et al. Global Longitudinal Strain is Incremental to Left Ventricular Ejection Fraction for the Prediction of Outcome in Optimally Treated Dilated Cardiomyopathy Patients. J. Am. Heart Assoc. 2022, 11, e024505. [Google Scholar] [CrossRef] [PubMed]
- Cho, G.Y.; Marwick, T.H.; Kim, H.S.; Kim, M.K.; Hong, K.S.; Oh, D.J. Global 2-Dimensional Strain as a New Prognosticator in Patients With Heart Failure. J. Am. Coll. Cardiol. 2009, 54, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Rhea, I.B.; Uppuluri, S.; Sawada, S.; Schneider, B.P.; Feigenbaum, H. Incremental prognostic value of echocardiographic strain and its association with mortality in cancer patients. J. Am. Soc. Echocardiogr. 2015, 28, 667–673. [Google Scholar] [CrossRef]
- Negishi, K.; Negishi, T.; Haluska, B.A.; Hare, J.L.; Plana, J.C.; Marwick, T.H. Use of speckle strain to assess left ventricular responses to cardiotoxic chemotherapy and cardioprotection. Eur. Heart J.-Cardiovasc. Imaging 2014, 15, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Silber, J.H.; Cnaan, A.; Clark, B.J.; Paridon, S.M.; Chin, A.J.; Rychik, J.; Hogarty, A.N.; Cohen, M.I.; Barber, G.; Rutkowski, M.; et al. Enalapril to prevent cardiac function decline in long-term survivors of pediatric cancer exposed to anthracyclines. J. Clin. Oncol. 2004, 22, 820–828. [Google Scholar] [CrossRef] [PubMed]
- Lipshultz, S.E.; Lipsitz, S.R.; Sallan, S.E.; Simbre, V.C., 2nd; Shaikh, S.L.; Mone, S.M.; Gelber, R.D.; Colan, S.D. Long-term enalapril therapy for left ventricular dysfunction in doxorubicin-treated survivors of childhood cancer. J. Clin. Oncol. 2002, 20, 4517–4522. [Google Scholar] [CrossRef]
- Cardinale, D.; Colombo, A.; Sandri, M.T.; Lamantia, G.; Colombo, N.; Civelli, M.; Martinelli, G.; Veglia, F.; Fiorentini, C.; Cipolla, C.M. Prevention of high-dose chemotherapy-induced cardiotoxicity in high-risk patients by angiotensin-converting enzyme inhibition. Circulation 2006, 114, 2474–2481. [Google Scholar] [CrossRef]
- Cardinale, D.; Ciceri, F.; Latini, R.; Franzosi, M.G.; Sandri, M.T.; Civelli, M.; Cucchi, G.; Menatti, E.; Mangiavacchi, M.; Cavina, R.; et al. Anthracycline-induced cardiotoxicity: A multicenter randomised trial comparing two strategies for guiding prevention with enalapril: The International CardioOncology Society-one trial. Eur. J. Cancer 2018, 94, 126–137. [Google Scholar] [CrossRef]
- Armstrong, G.T.; Oeffinger, K.C.; Chen, Y.; Kawashima, T.; Yasui, Y.; Leisenring, W.; Stovall, M.; Chow, E.J.; Sklar, C.A.; Mulrooney, D.A.; et al. Modifiable Risk Factors and Major Cardiac Events Among Adult Survivors of Childhood Cancer. J. Clin. Oncol. 2013, 31, 3673–3680. [Google Scholar] [CrossRef]
- Nathan, P.C.; Ford, J.S.; Henderson, T.O.; Hudson, M.M.; Emmons, K.M.; Casillas, J.N.; Lown, E.A.; Ness, K.K.; Oeffinger, K.C. Health behaviors, medical care, and interventions to promote healthy living in the Childhood Cancer Survivor Study cohort. J. Clin. Oncol. 2009, 27, 2363–2373. [Google Scholar] [CrossRef]
| Total (N = 129) | No Events (N = 94) | Events (N = 35) | p-Value | |
|---|---|---|---|---|
| Female | 79 (61.2%) | 65 (69.1%) | 14 (40.0%) | 0.003 |
| BMI, kg/m2 | 24.8 ± 4.8 | 24.5 ± 4.4 | 25.5 ± 5.7 | 0.31 |
| Age at diagnosis, yrs | 24.4 [18.8;29.0] | 23.1 [18.7;28.2] | 26.0 [20.2;30.5] | 0.19 |
| Cardiovascular risk factors | ||||
| Hypertension | 26 (20.2%) | 15 (16.0%) | 11 (31.4%) | 0.051 |
| Diabetes Mellitus | 4 (3.1%) | 2 (2.1%) | 2 (5.7%) | 0.30 |
| Hypercholesterolemia | 20 (15.5%) | 12 (12.8%) | 8 (22.9%) | 0.16 |
| Positive family history of cardiovascular disease | 25 (19.4%) | 19 (20.2%) | 6 (17.1%) | 0.69 |
| Smoking | 6 (4.7%) | 3 (3.2%) | 3 (8.6%) | 0.20 |
| Congestive heart failure | 1 (0.8%) | 0 (0.0%) | 1 (2.9%) | 0.100 |
| Ischemic heart disease | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Renal insufficiency | 4 (3.1%) | 3 (3.2%) | 1 (2.9%) | 0.92 |
| Laboratory results | ||||
| Hb, mmol/L | 8.7 ± 0.9 | 8.5 ± 0.8 | 9.0 ± 0.9 | 0.006 |
| Leukocytes, × 109/L | 7.4 ± 2.9 | 7.0 ± 1.8 | 8.4 ± 4.6 | 0.019 |
| LDL, mmol/l | 3.1 ± 1.0 | 2.9 ± 0.8 | 3.8 ± 1.2 | <0.001 |
| Total cholesterol, mmol/L | 5.3 ± 1.2 | 5.1 ± 1.1 | 5.8 ± 1.3 | 0.007 |
| Creatinine, µmol/L | 73.1 ± 15.0 | 71.1 ± 14.9 | 78.9 ± 13.8 | 0.012 |
| Stage Hodgkin Lymphoma | 0.67 | |||
| I–II | 96 (74.4%) | 69 (73.4%) | 27 (77.1%) | |
| III–IV | 33 (25.6%) | 25 (26.6%) | 8 (22.9%) | |
| Location of radiotherapy | 0.032 | |||
| Mantle field | 46 (35.7%) | 27 (28.7%) | 19 (54.3%) | |
| Mediastinal | 54 (41.9%) | 46 (48.9%) | 8 (22.9%) | |
| Subtotal | 24 (18.6%) | 17 (18.1%) | 7 (20.0%) | |
| Other | 5 (3.9%) | 4 (4.3%) | 1 (2.9%) | |
| Radiotherapeutic dose, Gy | 36.0 (35.0;40.0) | 36.0 (35.0;40.0) | 36.0 (35.0;40.0) | 0.47 |
| Treated with chemotherapy | 101 (78.3%) | 74 (78.7%) | 27 (77.1%) | 0.85 |
| Chemotherapeutic regimen (N = 101) | 0.51 | |||
| ABVD | 29 (22.5%) | 21 (22.3%) | 8 (22.9%) | |
| MOPP/ABV | 28 (21.7%) | 22 (23.4%) | 6 (17.1%) | |
| EBVP | 9 (7.0%) | 7 (7.4%) | 2 (5.7%) | |
| BEAUCOPP | 5 (3.9%) | 5 (5.3%) | 0 (0.0%) | |
| MOPP | 20 (15.5%) | 12 (12.8%) | 8 (22.9%) | |
| Other | 9 (7.0%) | 6 (6.4%) | 3 (8.6%) | |
| Treated with anthracyclines | 79 (61.2%) | 61 (64.9%) | 18 (51.4%) | 0.16 |
| Type of anthracycline (N = 79) | 0.87 | |||
| Doxorubicine | 71 (55.0%) | 55 (58.5%) | 16 (45.7%) | |
| Epirubicine | 8 (6.2%) | 6 (6.4%) | 2 (5.7%) | |
| Cumulative dose anthracycline, mg/m2 | 210.0 [150.0;300.0] | 210.0 [140.0;280.0] | 210.0 [200.0;300.0] | 0.36 |
| Total (N = 129) | No Events (N = 94) | Events (N = 35) | p-Value | |
|---|---|---|---|---|
| Body surface area during echocardiogram | 1.9 [1.7;2.0] | 1.9 [1.7;2.0] | 2.0 [1.8;2.1] | 0.026 |
| LVESV, mL | 38.0 [31.0;47.0] | 36.0 [28.0;46.0] | 42.0 [35.0;56.0] | 0.014 |
| LVESVi, mL/m2 | 20.1 [16.2;25.0] | 19.6 [15.5;24.7] | 22.0 [17.3;26.9] | 0.054 |
| LVEDV, mL | 83.0 [68.0;102.0] | 80.0 [64.0;97.0] | 93.0 [74.0;111.0] | 0.044 |
| LVEDVi, mL/m2 | 45.9 [36.0;52.6] | 44.3 [35.6;51.2] | 46.1 [36.6;56.3] | 0.17 |
| Mitral regurgitation | 0.54 | |||
| None | 90 (69.8%) | 68 (72.3%) | 22 (62.9%) | |
| Mild | 25 (19.4%) | 18 (19.1%) | 7 (20.0%) | |
| Moderate | 12 (9.3%) | 7 (7.4%) | 5 (14.3%) | |
| Severe | 2 (1.6%) | 1 (1.1%) | 1 (2.9%) | |
| E, m/s | 77.0 [66.0;89.0] | 76.5 [67.0;88.0] | 79.0 [59.0;102.0] | 0.75 |
| A, m/s | 75.0 [59.0;93.0] | 71.0 [55.0;90.0] | 83.5 [69.0;110.0] | 0.026 |
| Deceleration time, ms | 196.0 [168.0;251.0] | 194.0 [168.0;245.0] | 200.0 [167.0;277.0] | 0.42 |
| e’ mean, cm/s | 8.0 [6.5;10.0] | 9.0 [7.0;10.5] | 6.5 [5.0;7.5] | <0.001 |
| E/e’ mean | 9.1 [7.2;12.7] | 8.7 [6.8;11.1] | 13.6 [8.6;18.9] | <0.001 |
| Diastolic dysfunction | <0.001 | |||
| Grade 0 | 46 (35.7%) | 43 (45.7%) | 3 (8.6%) | |
| Grade 1 | 49 (38.0%) | 31 (33.0%) | 18 (51.4%) | |
| Grade 2 | 24 (18.6%) | 16 (17.0%) | 8 (22.9%) | |
| Grade 3 | 8 (6.2%) | 3 (3.2%) | 5 (14.3%) | |
| Diastolic dysfunction > grade 0 | 81 (63.8%) | 50 (53.8%) | 31 (91.2%) | <0.001 |
| LAVi, mL/m2 | 18.4 [15.5;22.8] | 18.8 [16.1;22.8] | 16.6 [13.9;22.9] | 0.38 |
| LVEF, % | 55.0 [52.0;58.0] | 56.0 [53.0;59.0] | 53.0 [50.0;56.0] | 0.004 |
| LVEF < 50% | 14 (10.9%) | 7 (7.4%) | 7 (20.0%) | 0.042 |
| GLS, % | −17.1 [−19.0;−15.2] | −17.5 [−19.3;−15.9] | −14.9 [−16.2;−13.3] | <0.001 |
| GLS > −16% | 51 [39.2%] | 26 [27.4%] | 25 [71.4%] | <0.001 |
| RHR during echocardiography | 77.5 [70.0;88.5] | 76.0 [70.0;86.0] | 85.0 [74.0;94.0] | 0.009 |
| Cardiovascular Events | N = 129 |
|---|---|
| ACS | 3 (2.3%) |
| Cardiac surgery | 27 (20.9%) |
| Type of cardiac surgery | |
| CABG | 5 (3.9%) |
| CABG + Valve surgery | 5 (3.9%) |
| Valve surgery | 11 (8.5%) |
| TAVI | 4 (3.1%) |
| Mitraclip | 1 (0.8%) |
| Ostiumplasty of LM coronary artery | 1 (0.8%) |
| Admission for HF | 7 (5.4%) |
| Univariable | Multivariable | |||
|---|---|---|---|---|
| Variable | HR (95% CI) | p-Value | HR (95% CI) | p-Value |
| GLS > −16% | 4.92 (2.36–10.27) | <0.001 | 3.95 (1.83–8.52) | <0.001 |
| LVEF < 50% | 2.32 (1.01–5.40) | 0.048 | 2.98 (1.22–7.29) | 0.016 |
| E/e’, per 1 unit increase | 1.16 (1.10–1.21) | <0.001 | 1.16 (1.09–1.22) | <0.001 |
| GLS, per % increase | 1.29 (1.16–1.44) | <0.001 | 1.26 (1.12–1.43) | <0.001 |
| LVEF, per % increase | 0.93 (0.88–0.99) | 0.014 | 0.92 (0.87–0.98) | 0.006 |
| Diastolic dysfunction > gr 0 | 6.01 (1.84–19.68) | 0.003 | 3.63 (1.04–12.70) | 0.044 |
| Echo RHR, per 5 bpm increase | 1.15 (1.03–1.29) | 0.015 | 1.21 (1.08–1.36) | 0.001 |
| Radiotherapy dose, per Gy increase | 1.07 (0.98–1.16) | 0.112 | 1.06 (0.98–1.15) | 0.130 |
| Concomitant treatment with anthracyclines | 0.60 (0.30–1.17) | 0.132 | 0.76 (0.37–1.56) | 0.454 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polomski, E.A.S.; Heemelaar, J.C.; Krol, A.D.G.; Louwerens, M.; Beeres, S.L.M.A.; Holman, E.R.; Jukema, J.W.; Schalij, M.J.; Antoni, M.L. Impaired Global Longitudinal Strain Is Associated with Cardiovascular Events in Hodgkin Lymphoma Survivors. Cancers 2022, 14, 2329. https://doi.org/10.3390/cancers14092329
Polomski EAS, Heemelaar JC, Krol ADG, Louwerens M, Beeres SLMA, Holman ER, Jukema JW, Schalij MJ, Antoni ML. Impaired Global Longitudinal Strain Is Associated with Cardiovascular Events in Hodgkin Lymphoma Survivors. Cancers. 2022; 14(9):2329. https://doi.org/10.3390/cancers14092329
Chicago/Turabian StylePolomski, Elissa A. S., Julius C. Heemelaar, Augustinus D. G. Krol, Marloes Louwerens, Saskia L. M. A. Beeres, Eduard R. Holman, J. Wouter Jukema, Martin J. Schalij, and M. Louisa Antoni. 2022. "Impaired Global Longitudinal Strain Is Associated with Cardiovascular Events in Hodgkin Lymphoma Survivors" Cancers 14, no. 9: 2329. https://doi.org/10.3390/cancers14092329
APA StylePolomski, E. A. S., Heemelaar, J. C., Krol, A. D. G., Louwerens, M., Beeres, S. L. M. A., Holman, E. R., Jukema, J. W., Schalij, M. J., & Antoni, M. L. (2022). Impaired Global Longitudinal Strain Is Associated with Cardiovascular Events in Hodgkin Lymphoma Survivors. Cancers, 14(9), 2329. https://doi.org/10.3390/cancers14092329






