Optimal Clinical Target Volume of Radiotherapy Based on Microscopic Extension around the Primary Gross Tumor in Non-Small-Cell Lung Cancer: A Systematic Review
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
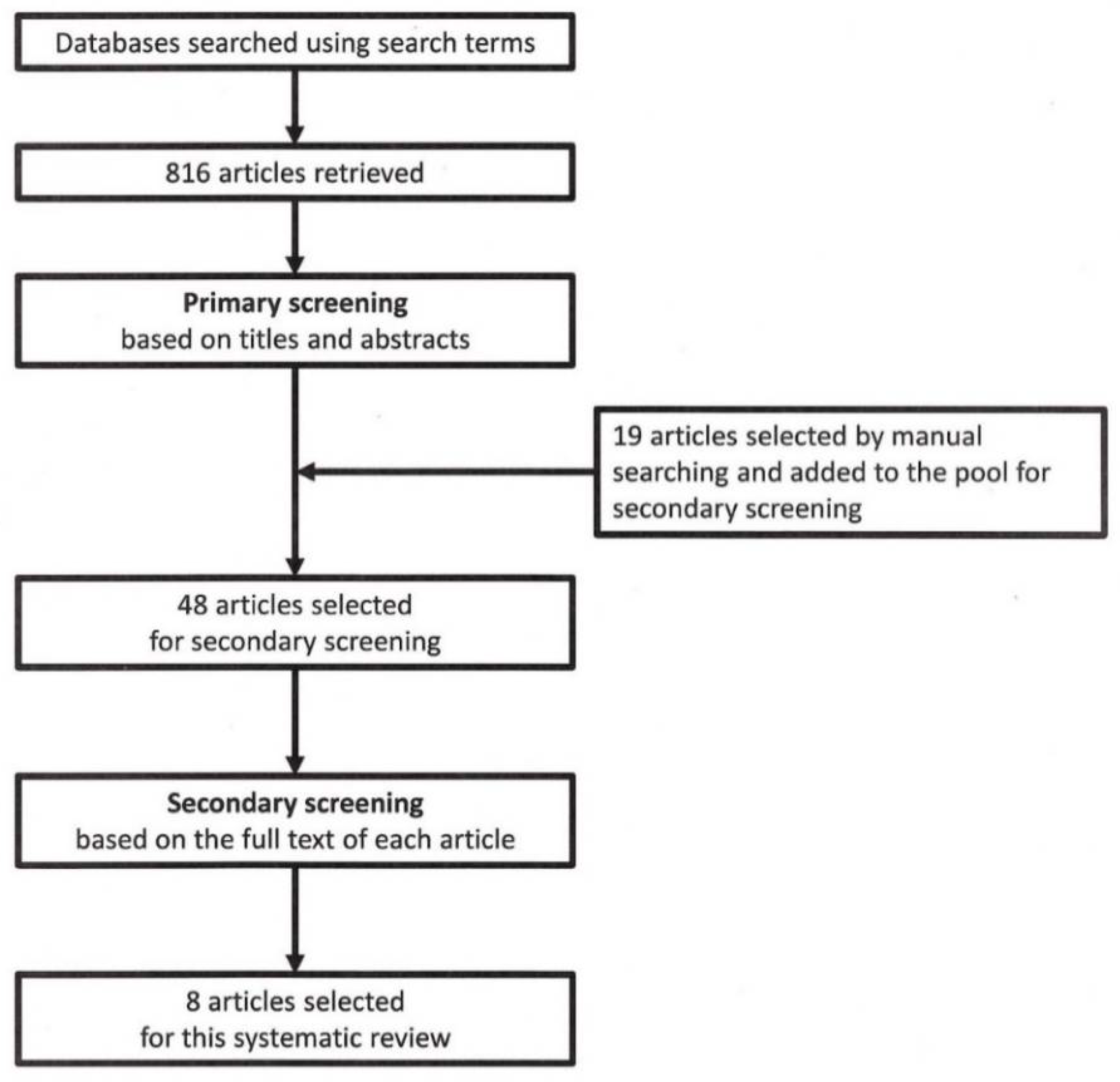
3.1. Search Results
3.2. Microscopic Extension from the Gross Primary Tumor into the Lung Parenchyma
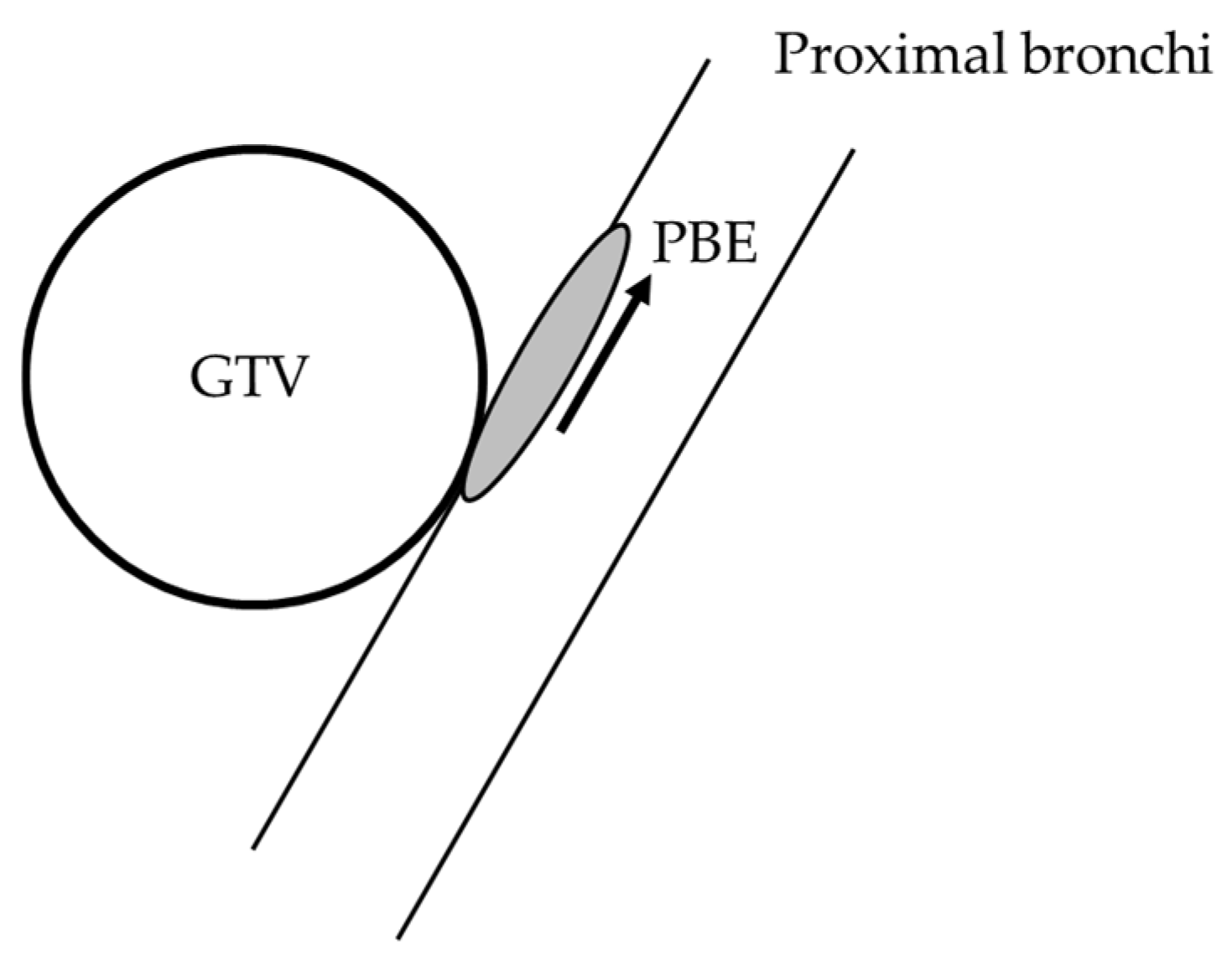
3.3. Microscopic Proximal Bronchial Extension from the Gross Primary Tumor
4. Discussion
4.1. ME from the Gross Primary Tumor into the Lung Parenchyma
4.2. Microscopic Proximal Bronchial Extension from the Gross Primary Tumor
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- National Comprehensive Cancer Network. NCCN Guidelines Version 3. 2019. Non-Small Cell Lung Cancer. Available online: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf (accessed on 17 March 2019).
- Giraud, P.; Antoine, M.; Larrouy, A.; Milleron, B.; Callard, P.; De Rycke, Y.; Carette, M.F.; Rosenwald, J.C.; Cosset, J.M.; Housset, M.; et al. Evaluation of microscopic tumor extension in non-small-cell lung cancer for three-dimensional conformal radiotherapy planning. Int. J. Radiat. Oncol. Biol. Phys. 2000, 48, 1015–1024. [Google Scholar] [CrossRef]
- Goldstein, N.S.; Ferkowicz, M.; Kestin, L.; Chmielewski, G.W.; Welsh, R.J. Wedge resection margin distances and residual adenocarcinoma in lobectomy specimens. Am. J. Clin. Pathol. 2003, 120, 720–724. [Google Scholar] [CrossRef] [PubMed]
- Grills, I.S.; Fitch, D.L.; Goldstein, N.S.; Yan, D.; Chmielewski, G.W.; Welsh, R.J.; Kestin, L.L. Clinicopathologic analysis of microscopic extension in lung adenocarcinoma: Defining clinical target volume for radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2007, 69, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Stroom, J.; Blaauwgeers, H.; van Baardwijk, A.; Boersma, L.; Lebesque, J.; Theuws, J.; van Suylen, R.J.; Klomp, H.; Liesker, K.; van Pel, R.; et al. Feasibility of pathology-correlated lung imaging for accurate target definition of lung tumors. Int. J. Radiat. Oncol. Biol. Phys. 2007, 69, 267–275. [Google Scholar] [CrossRef] [PubMed]
- van Loon, J.; Siedschlag, C.; Stroom, J.; Blauwgeers, H.; van Suylen, R.J.; Knegjens, J.; Rossi, M.; van Baardwijk, A.; Boersma, L.; Klomp, H.; et al. Microscopic disease extension in three dimensions for non-small-cell lung cancer: Development of a prediction model using pathology-validated positron emission tomography and computed tomography features. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Sun, X.; Mu, D.; Xing, L.; Ma, L.; Zhang, B.; Zhao, S.; Yang, G.; Kong, F.M.; Yu, J. Noninvasive evaluation of microscopic tumor extensions using standardized uptake value and metabolic tumor volume in non-small-cell lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 960–966. [Google Scholar] [CrossRef] [PubMed]
- Kara, M.; Sak, S.D.; Orhan, D.; Yavuzer, S. Changing patterns of lung cancer; (3/4, in.) 1.9 cm; still a safe length for bronchial resection margin? Lung Cancer 2000, 30, 161–168. [Google Scholar] [CrossRef]
- Kara, M.; Dizbay Sak, S.; Orhan, D.; Kavukçu, S. Proximal bronchial extension with special reference to tumor localization in non-small cell lung cancer. Eur. J. Cardiothorac. Surg. 2001, 20, 350–355. [Google Scholar] [CrossRef] [Green Version]
- Goldstraw, P.; Crowley, J.; Chansky, K.; Giroux, D.J.; Groome, P.A.; Rami-Porta, R.; Postmus, P.E.; Rusch, V.; Sobin, L.; International Association for the Study of Lung Cancer International Staging Committee; et al. The IASLC Lung Cancer Staging Project: Proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J. Thorac. Oncol. 2007, 2, 706–714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics 2016. CA Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bosmans, G.; van Baardwijk, A.; Dekker, A.; Ollers, M.; Boersma, L.; Minken, A.; Lambin, P.; De Ruysscher, D. Intra-patient variability of tumor volume and tumor motion during conventionally fractionated radiotherapy for locally advanced non-small-cell lung cancer: A prospective clinical study. Int. J. Radiat. Oncol. Biol. Phys. 2006, 66, 748–753. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.; Ford, E.; Redmond, K.; Zhou, J.; Wong, J.; Song, D.Y. Quantification of tumor volume changes during radiotherapy for non-small-cell lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 2009, 74, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Nestle, U.; De Ruysscher, D.; Ricardi, U.; Geets, X.; Belderbos, J.; Pöttgen, C.; Dziadiuszko, R.; Peeters, S.; Lievens, Y.; Hurkmans, C.; et al. ESTRO ACROP guidelines for target volume definition in the treatment of locally advanced non-small cell lung cancer. Radiother. Oncol. 2018, 127, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.Y.; Ajlouni, M.; Chen, Q.; Kong, F.M.; Ryu, S.; Movsas, B. Quantification of incidental dose to potential clinical target volume (CTV) under different stereotactic body radiation therapy (SBRT) techniques for non-small cell lung cancer—tumor motion and using internal target volume (ITV) could improve dose distribution in CTV. Radiother. Oncol. 2007, 85, 267–276. [Google Scholar] [PubMed]
- Timmerman, R.; Paulus, R.; Galvin, J.; Michalski, J.; Straube, W.; Bradley, J.; Fakiris, A.; Bezjak, A.; Videtic, G.; Johnstone, D.; et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 2010, 303, 1070–1076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Timmerman, R.D.; Paulus, R.; Pass, H.I.; Gore, E.M.; Edelman, M.J.; Galvin, J.; Straube, W.L.; Nedzi, L.A.; McGarry, R.C.; Robinson, C.G.; et al. Stereotactic body radiation therapy for operable early-stage lung cancer: Findings from the NRG Oncology RTOG 0618 Trial. JAMA Oncol. 2018, 4, 1263–1266. [Google Scholar] [CrossRef] [PubMed]

| Author (Year Published) | Number of Patients | Patient Characteristics | Analysis Results for ME Measured from Pathological GTV |
|---|---|---|---|
| Giraud (2000) [2] | 42 * | AC 23/SCC 19 Stage 1–4 | Mean (SD): AC 2.69 mm (2.76 mm), SCC 1.48 mm (2.37 mm) Range: AC 0–12 mm, SCC 0–13 mm 5-mm margin coverage: AC 80%, SCC 91% 95% coverage margin: AC 8 mm, SCC 6 mm |
| Goldstein (2003) [3] | 31 | AC 31 Stage 1 (T1N0M0) G1/G2/G3 = 12/12/7 | Mean (SD): 7.4 mm (2.9 mm) Median: 7 mm (range: 3.0–14.0 mm) |
| Grills (2007) [4] | 36 | AC 36 Stage 1 (T1N0M0) G1/G2/G3 = 11/15/10 | Mean (SD): 7.2 mm (3.1 mm) Range: 2–16 mm Pathological GTV-CTV margin (90% of cases): all grades, 12.5 mm Pathological GTV-CTV margin: G1 13 mm, G2 9.7 mm, G3 4.4 mm Radiologic GTV-CTV margin (90% confidence): all grades, 9 mm Radiologic GTV-CTV margin (80% confidence): G1 9 mm, G2 7 mm, G3 4 mm |
| Stroom (2007) [5] | 5 | SCC 3/SCC + AC 1/sacromatoid + AC 1 Stage 1–3 | Median: 5 mm (range, 0–9 mm) Mean: 5 mm (without deformation correction) Mean: 9 mm (with deformation correction) |
| van Loon (2012) [6] | 34 | AC 18/SCC 6/LCC 4/Others 6 | ME observed in 50% of patients (17/34) 90% coverage margin: 14 mm (without deformation correction) 90% coverage margin: 26 mm (with deformation correction) |
| Meng (2012) [7] | 39 | SCC 17/AC 22 Stage 1–3 G1/G2/G3 = 10/16/13 | Mean (SD): 3.38 mm (2.80 mm) Range: 0–13 mm 95% coverage margin: 1.93mm (SUVmax ≤ 5), 3.90 mm (SUVmax 5–10), 9.60 mm (SUVmax > 10) |
| Author (Year Published) | Number of Patients | Patient Characteristics | Results from Analysis of PBE |
|---|---|---|---|
| Kara (2000) [8] | 70 | SCC 38/AC 23 ASC 6/LCC 3 Central tumors: 33 Peripheral tumors: 37 | PBE observed in 17/70 patients (24.2%) PBE positive rate: central 30.3%, peripheral 18.9% Mean (SD) [overall]: 10.94 mm (7.07 mm) Mean (SD) [central]: 7.60 mm (3.47 mm) Mean (SD) [peripheral]: 15.71 mm (8.38 mm) |
| Kara (2001) [9] | 70 | SCC 38/AC 15 /BAC 8/ASC 6/LCC 3 | PBE observed in 17/70 patients (24.2%) Range: 0–3 cm 1.0 cm margin coverage: 86% 1.5 cm margin coverage: 93% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamaki, Y.; Aibe, N.; Komiyama, T.; Nagasaka, S.; Imagumbai, T.; Itazawa, T.; Onishi, H.; Akimoto, T.; Nagata, Y.; Nakayama, Y. Optimal Clinical Target Volume of Radiotherapy Based on Microscopic Extension around the Primary Gross Tumor in Non-Small-Cell Lung Cancer: A Systematic Review. Cancers 2022, 14, 2318. https://doi.org/10.3390/cancers14092318
Tamaki Y, Aibe N, Komiyama T, Nagasaka S, Imagumbai T, Itazawa T, Onishi H, Akimoto T, Nagata Y, Nakayama Y. Optimal Clinical Target Volume of Radiotherapy Based on Microscopic Extension around the Primary Gross Tumor in Non-Small-Cell Lung Cancer: A Systematic Review. Cancers. 2022; 14(9):2318. https://doi.org/10.3390/cancers14092318
Chicago/Turabian StyleTamaki, Yukihisa, Norihiro Aibe, Takafumi Komiyama, Satoshi Nagasaka, Toshiyuki Imagumbai, Tomoko Itazawa, Hiroshi Onishi, Tetsuo Akimoto, Yasushi Nagata, and Yuko Nakayama. 2022. "Optimal Clinical Target Volume of Radiotherapy Based on Microscopic Extension around the Primary Gross Tumor in Non-Small-Cell Lung Cancer: A Systematic Review" Cancers 14, no. 9: 2318. https://doi.org/10.3390/cancers14092318
APA StyleTamaki, Y., Aibe, N., Komiyama, T., Nagasaka, S., Imagumbai, T., Itazawa, T., Onishi, H., Akimoto, T., Nagata, Y., & Nakayama, Y. (2022). Optimal Clinical Target Volume of Radiotherapy Based on Microscopic Extension around the Primary Gross Tumor in Non-Small-Cell Lung Cancer: A Systematic Review. Cancers, 14(9), 2318. https://doi.org/10.3390/cancers14092318






