Setting Up an Ultra-Fast Next-Generation Sequencing Approach as Reflex Testing at Diagnosis of Non-Squamous Non-Small Cell Lung Cancer; Experience of a Single Center (LPCE, Nice, France)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
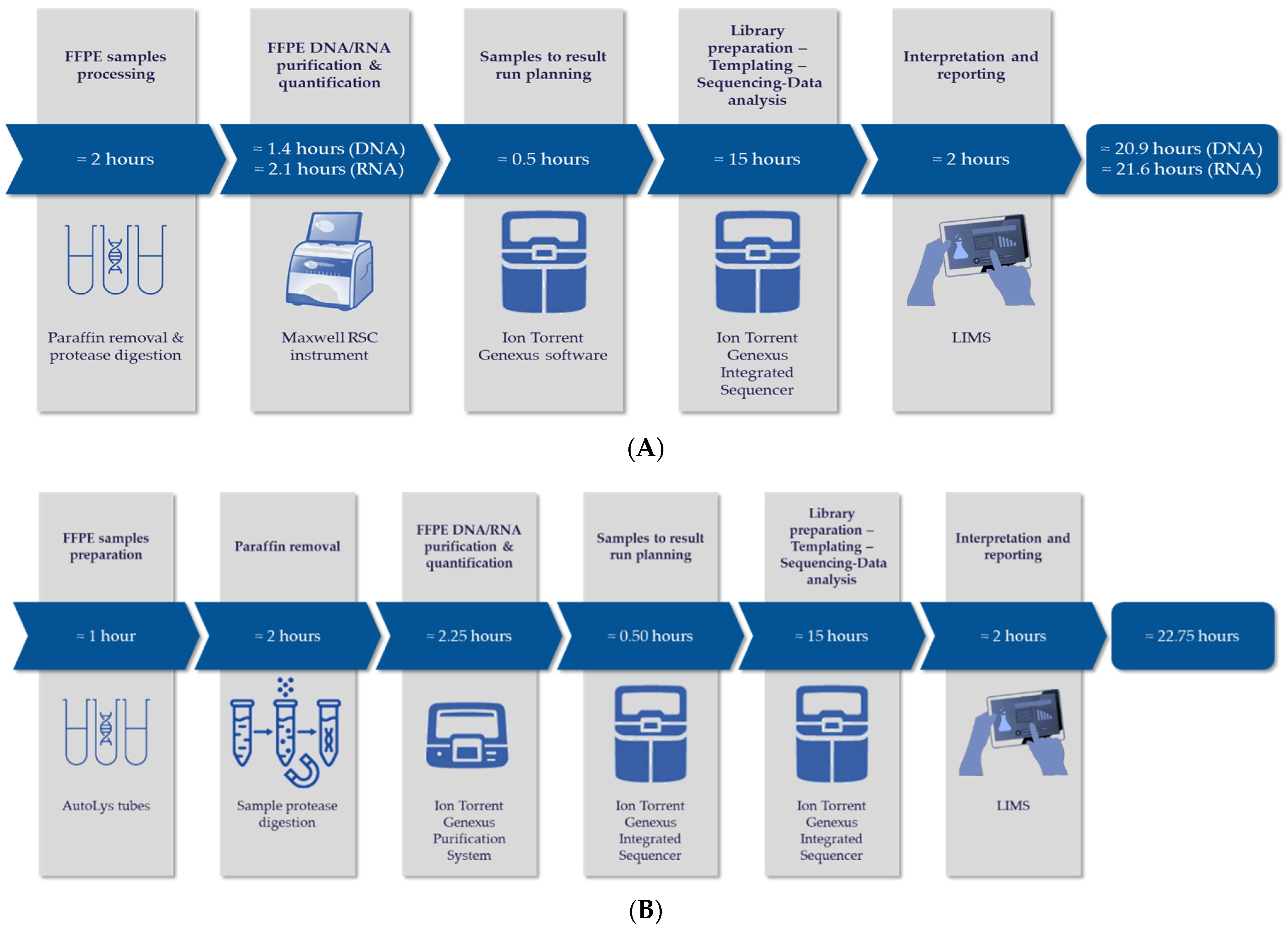
2.2. Ultra-Fast Next-Generation Sequencing
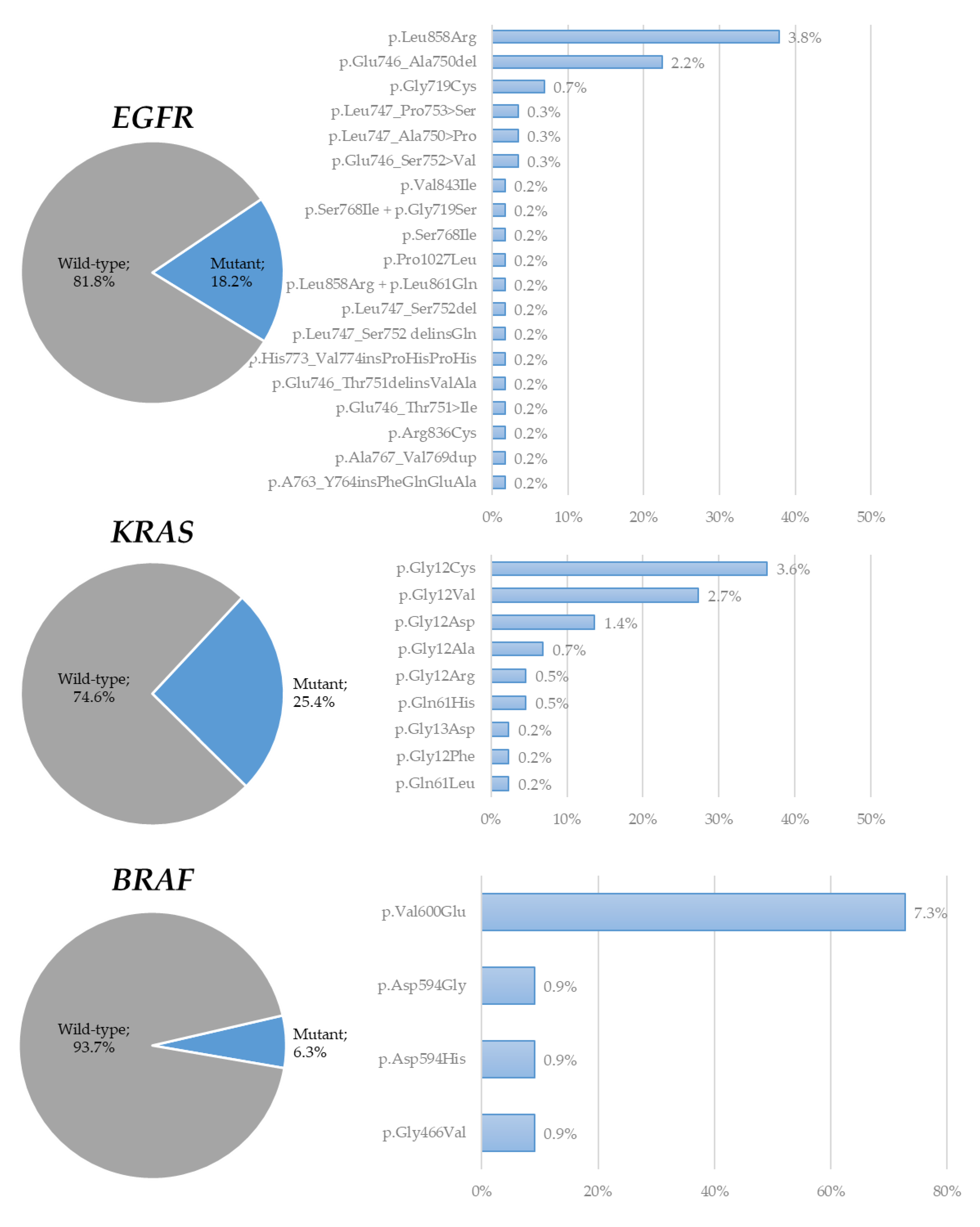
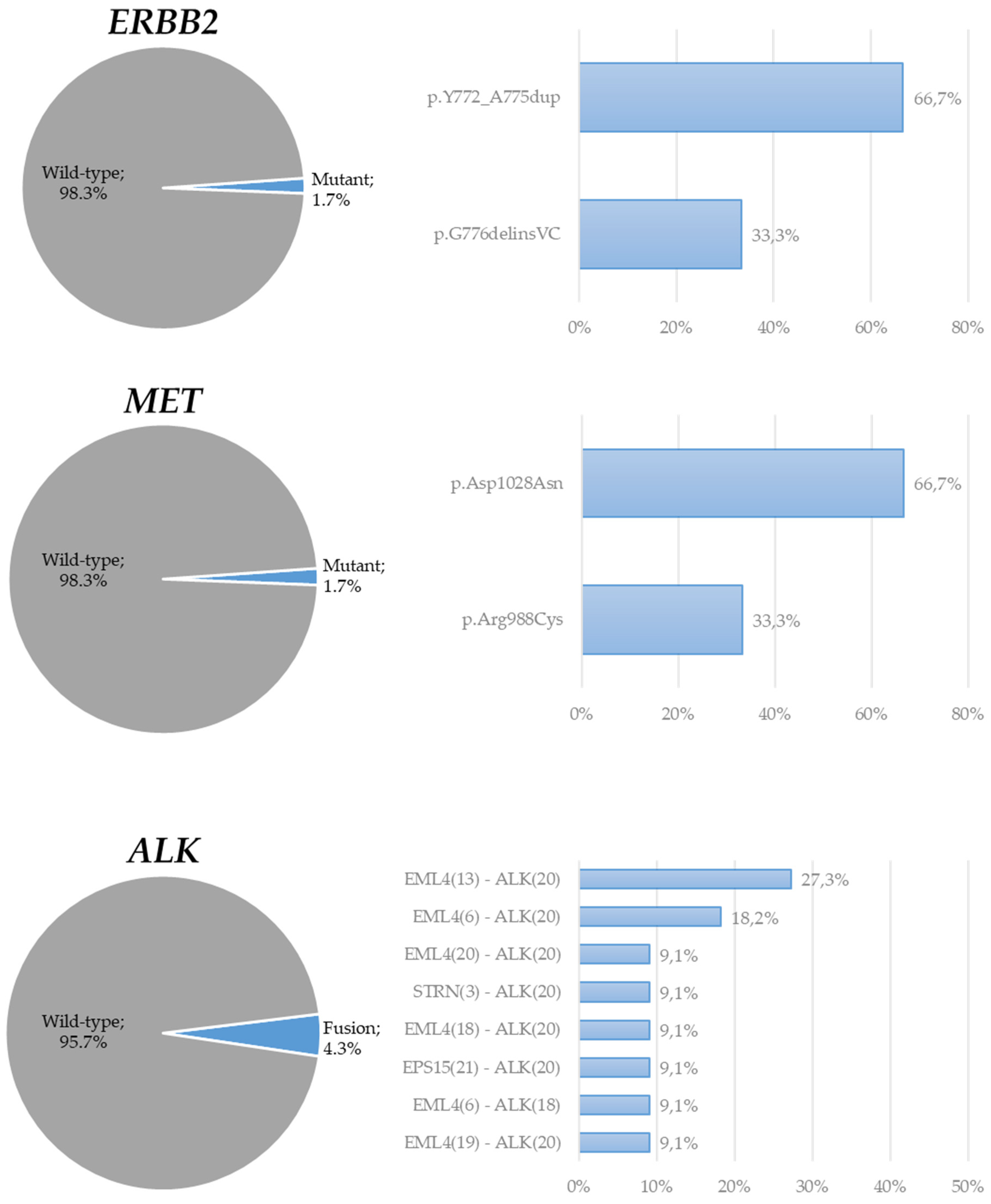
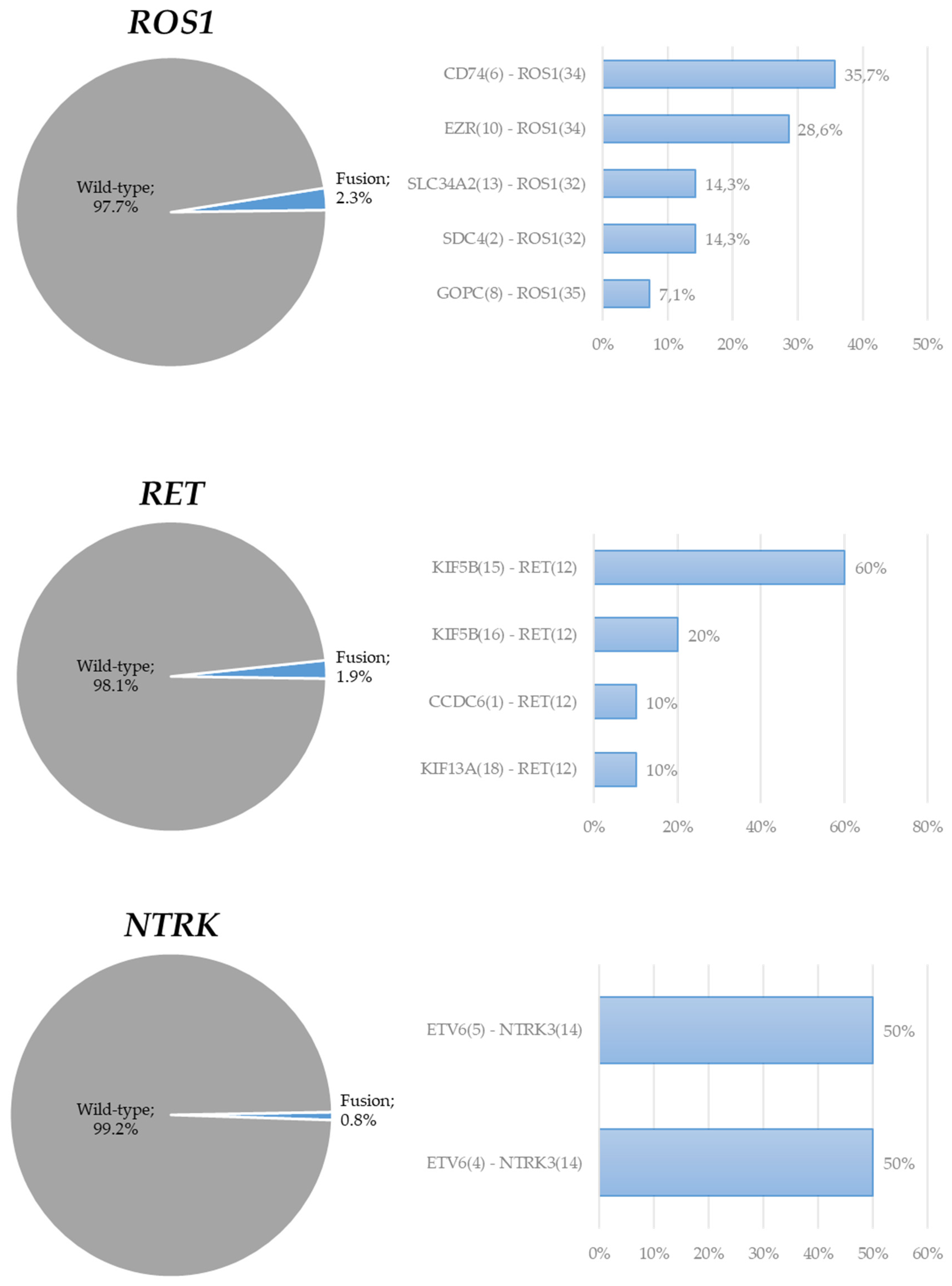
3. Results
4. Discussion
4.1. Advantages and Limitations of a Fast-Next Generation Sequencing
4.1.1. Advantages
4.1.2. Limitations
4.2. Current Challenges Associated with the Rapid Revolution of Predictive Biomarkers in NS-NSCLC
4.3. What Should We Expect in the Future?
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Howlader, N.; Forjaz, G.; Mooradian, M.J.; Meza, R.; Kong, C.Y.; Cronin, K.A.; Mariotto, A.B.; Lowy, D.R.; Feuer, E.J. The Effect of Advances in Lung-Cancer Treatment on Population Mortality. N. Engl. J. Med. 2020, 383, 640–649. [Google Scholar] [CrossRef] [PubMed]
- Melosky, B.; Wheatley-Price, P.; Juergens, R.A.; Sacher, A.; Leighl, N.B.; Tsao, M.S.; Cheema, P.; Snow, S.; Liu, G.; Card, P.B.; et al. The rapidly evolving landscape of novel targeted therapies in advanced non-small cell lung cancer. Lung Cancer 2021, 160, 136–151. [Google Scholar] [CrossRef] [PubMed]
- Garrido, P.; Conde, E.; de Castro, J.; Gomez-Roman, J.J.; Felip, E.; Pijuan, L.; Isla, D.; Sanz, J.; Paz-Ares, L.; Lopez-Rios, F. Updated guidelines for predictive biomarker testing in advanced non-small-cell lung cancer: A National Consensus of the Spanish Society of Pathology and the Spanish Society of Medical Oncology. Clin. Transl. Oncol. 2020, 22, 989–1003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kerr, K.M.; Bibeau, F.; Thunnissen, E.; Botling, J.; Ryska, A.; Wolf, J.; Ohrling, K.; Burdon, P.; Malapelle, U.; Buttner, R. The evolving landscape of biomarker testing for non-small cell lung cancer in Europe. Lung Cancer 2021, 154, 161–175. [Google Scholar] [CrossRef]
- Mosele, F.; Remon, J.; Mateo, J.; Westphalen, C.B.; Barlesi, F.; Lolkema, M.P.; Normanno, N.; Scarpa, A.; Robson, M.; Meric-Bernstam, F.; et al. Recommendations for the use of next-generation sequencing (NGS) for patients with metastatic cancers: A report from the ESMO Precision Medicine Working Group. Ann. Oncol. 2020, 31, 1491–1505. [Google Scholar] [CrossRef]
- Pennell, N.A.; Arcila, M.E.; Gandara, D.R.; West, H. Biomarker Testing for Patients With Advanced Non-Small Cell Lung Cancer: Real-World Issues and Tough Choices. Am. Soc. Clin. Oncol. Educ. Book 2019, 39, 531–542. [Google Scholar] [CrossRef]
- Hanna, N.H.; Robinson, A.G.; Temin, S.; Baker, S., Jr.; Brahmer, J.R.; Ellis, P.M.; Gaspar, L.E.; Haddad, R.Y.; Hesketh, P.J.; Jain, D.; et al. Therapy for Stage IV Non-Small-Cell Lung Cancer With Driver Alterations: ASCO and OH (CCO) Joint Guideline Update. J. Clin. Oncol. 2021, 39, 1040–1091. [Google Scholar] [CrossRef]
- Kazdal, D.; Hofman, V.; Christopoulos, P.; Ilie, M.; Stenzinger, A.; Hofman, P. Fusion-positive non-small cell lung carcinoma: Biological principles, clinical practice, and diagnostic implications. Genes Chromosomes Cancer 2022, 61, 244–260. [Google Scholar] [CrossRef]
- Hellyer, J.A.; White, M.N.; Gardner, R.M.; Cunanan, K.; Padda, S.K.; Das, M.; Ramchandran, K.; Neal, J.W.; Wakelee, H.A. Impact of Tumor Suppressor Gene Co-Mutations on Differential Response to EGFR TKI Therapy in EGFR L858R and Exon 19 Deletion Lung Cancer. Clin. Lung Cancer 2021, S1525-7304(21)00245-X. [Google Scholar] [CrossRef]
- Mograbi, B.; Heeke, S.; Hofman, P. The Importance of STK11/LKB1 Assessment in Non-Small Cell Lung Carcinomas. Diagnostics 2021, 11, 196. [Google Scholar] [CrossRef]
- Nadal, E.; Heeke, S.; Benzaquen, J.; Vilarino, N.; Navarro, A.; Azuara, D.; Varela, M.; Otto, J.; Baixeras, N.; Shahbazian, D.; et al. Two Patients With Advanced-Stage Lung Adenocarcinoma With Radiologic Complete Response to Nivolumab Treatment Harboring an STK11/LKB1 Mutation. JCO Precis Oncol. 2020, 4, 1239–1245. [Google Scholar] [CrossRef] [PubMed]
- Ricciuti, B.; Arbour, K.C.; Lin, J.J.; Vajdi, A.; Vokes, N.; Hong, L.; Zhang, J.; Tolstorukov, M.Y.; Li, Y.Y.; Spurr, L.F.; et al. Diminished Efficacy of Programmed Death-(Ligand)1 Inhibition in STK11- and KEAP1-Mutant Lung Adenocarcinoma Is Affected by KRAS Mutation Status. J. Thorac. Oncol. 2022, 17, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Hanna, N.; Johnson, D.; Temin, S.; Baker, S., Jr.; Brahmer, J.; Ellis, P.M.; Giaccone, G.; Hesketh, P.J.; Jaiyesimi, I.; Leighl, N.B.; et al. Systemic Therapy for Stage IV Non-Small-Cell Lung Cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. J. Clin. Oncol. 2017, 35, 3484–3515. [Google Scholar] [CrossRef] [PubMed]
- Petrelli, F.; Ferrara, R.; Signorelli, D.; Ghidini, A.; Proto, C.; Roudi, R.; Sabet, M.N.; Facelli, S.; Garassino, M.C.; Luciani, A.; et al. Immune checkpoint inhibitors and chemotherapy in first-line NSCLC: A meta-analysis. Immunotherapy 2021, 13, 621–631. [Google Scholar] [CrossRef]
- Ahern, E.; Solomon, B.J.; Hui, R.; Pavlakis, N.; O’Byrne, K.; Hughes, B.G.M. Neoadjuvant immunotherapy for non-small cell lung cancer: Right drugs, right patient, right time? J. Immunother. Cancer 2021, 9, e002248. [Google Scholar] [CrossRef]
- Jones, D.R.; Wu, Y.L.; Tsuboi, M.; Herbst, R.S. Targeted therapies for resectable lung adenocarcinoma: ADAURA opens for thoracic oncologic surgeons. J. Thorac. Cardiovasc. Surg. 2021, 162, 288–292. [Google Scholar] [CrossRef]
- Koch, A.L.; Vellanki, P.J.; Drezner, N.; Li, X.; Mishra-Kalyani, P.S.; Shen, Y.L.; Xia, H.; Li, Y.; Liu, J.; Zirkelbach, J.F.; et al. FDA Approval Summary: Osimertinib for Adjuvant Treatment of Surgically Resected Non-Small Cell Lung Cancer, a Collaborative Project Orbis Review. Clin. Cancer Res. 2021, 27, 6638–6643. [Google Scholar] [CrossRef]
- Hanna, N.H.; Schneider, B.J.; Temin, S.; Baker, S., Jr.; Brahmer, J.; Ellis, P.M.; Gaspar, L.E.; Haddad, R.Y.; Hesketh, P.J.; Jain, D.; et al. Therapy for Stage IV Non-Small-Cell Lung Cancer Without Driver Alterations: ASCO and OH (CCO) Joint Guideline Update. J. Clin. Oncol. 2020, 38, 1608–1632. [Google Scholar] [CrossRef]
- Mazieres, J.; Drilon, A.; Lusque, A.; Mhanna, L.; Cortot, A.B.; Mezquita, L.; Thai, A.A.; Mascaux, C.; Couraud, S.; Veillon, R.; et al. Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: Results from the IMMUNOTARGET registry. Ann. Oncol. 2019, 30, 1321–1328. [Google Scholar] [CrossRef]
- Sheffield, B.S.; Beharry, A.; Diep, J.; Perdrizet, K.; Iafolla, M.A.J.; Raskin, W.; Dudani, S.; Brett, M.A.; Starova, B.; Olsen, B.; et al. Point of Care Molecular Testing: Community-Based Rapid Next-Generation Sequencing to Support Cancer Care. Curr. Oncol. 2022, 29, 1326–1334. [Google Scholar] [CrossRef]
- DiStasio, M.; Chen, Y.; Rangachari, D.; Costa, D.B.; Heher, Y.K.; VanderLaan, P.A. Molecular Testing Turnaround Time for Non-Small Cell Lung Cancer in Routine Clinical Practice Confirms Feasibility of CAP/IASLC/AMP Guideline Recommendations: A Single-center Analysis. Clin. Lung Cancer 2017, 18, e349–e356. [Google Scholar] [CrossRef] [PubMed]
- Heeke, S.; Hofman, V.; Long-Mira, E.; Lespinet, V.; Lalvee, S.; Bordone, O.; Ribeyre, C.; Tanga, V.; Benzaquen, J.; Leroy, S.; et al. Use of the Ion PGM and the GeneReader NGS Systems in Daily Routine Practice for Advanced Lung Adenocarcinoma Patients: A Practical Point of View Reporting a Comparative Study and Assessment of 90 Patients. Cancers 2018, 10, 88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Ahmadi, A.; Ardeshir-Larijani, F.; Fu, P.; Cao, S.; Lipka, M.B.; Dowlati, A.; Bruno, D.S. Next Generation Sequencing of Advanced Non-Small Cell Lung Cancer: Utilization Based on Race and Impact on Survival. Clin. Lung Cancer 2021, 22, 16–22.e11. [Google Scholar] [CrossRef] [PubMed]
- Anand, K.; Phung, T.L.; Bernicker, E.H.; Cagle, P.T.; Olsen, R.J.; Thomas, J.S. Clinical Utility of Reflex Ordered Testing for Molecular Biomarkers in Lung Adenocarcinoma. Clin. Lung Cancer 2020, 21, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Yoon, S.; Lee, D.H.; Jang, S.J.; Chun, S.M.; Kim, S.W. Real-world utility of next-generation sequencing for targeted gene analysis and its application to treatment in lung adenocarcinoma. Cancer Med. 2021, 10, 3197–3204. [Google Scholar] [CrossRef]
- Miller, T.E.; Yang, M.; Bajor, D.; Friedman, J.D.; Chang, R.Y.C.; Dowlati, A.; Willis, J.E.; Sadri, N. Clinical utility of reflex testing using focused next-generation sequencing for management of patients with advanced lung adenocarcinoma. J. Clin. Pathol. 2018, 71, 1108–1115. [Google Scholar] [CrossRef]
- Zacharias, M.; Absenger, G.; Kashofer, K.; Wurm, R.; Lindenmann, J.; Terbuch, A.; Konjic, S.; Sauer, S.; Gollowitsch, F.; Gorkiewicz, G.; et al. Reflex testing in non-small cell lung carcinoma using DNA- and RNA-based next-generation sequencing-a single-center experience. Transl. Lung Cancer Res. 2021, 10, 4221–4234. [Google Scholar] [CrossRef]
- Dagogo-Jack, I.; Azzolli, C.G.; Fintelmann, F.; Mino-Kenudson, M.; Farago, A.F.; Gainor, J.F.; Jiang, G.; Piotrowska, Z.; Heist, R.S.; Lennes, I.T.; et al. Clinical Utility of Rapid EGFR Genotyping in Advanced Lung Cancer. JCO Precis Oncol. 2018, 2018, PO.17.00299. [Google Scholar] [CrossRef]
- Dagogo-Jack, I.; Robinson, H.; Mino-Kenudson, M.; Farago, A.F.; Kamesan, V.; Iafrate, A.J.; Shaw, A.T.; Lennerz, J.K. Expediting Comprehensive Molecular Analysis to Optimize Initial Treatment of Lung Cancer Patients With Minimal Smoking History. J. Thorac. Oncol. 2019, 14, 835–843. [Google Scholar] [CrossRef]
- Camidge, D.R.; Doebele, R.C.; Kerr, K.M. Comparing and contrasting predictive biomarkers for immunotherapy and targeted therapy of NSCLC. Nat. Rev. Clin. Oncol. 2019, 16, 341–355. [Google Scholar] [CrossRef]
- Rosas, D.; Raez, L.E.; Russo, A.; Rolfo, C. Neuregulin 1 Gene (NRG1). A Potentially New Targetable Alteration for the Treatment of Lung Cancer. Cancers 2021, 13, 5038. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, G.I.; LoRusso, P.; Dowlati, A.; Do, K.T.; Jacobson, C.A.; Vaishampayan, U.; Weise, A.; Caimi, P.F.; Eder, J.P.; French, C.A.; et al. A Phase 1 study of RO6870810, a novel bromodomain and extra-terminal protein inhibitor, in patients with NUT carcinoma, other solid tumours, or diffuse large B-cell lymphoma. Br. J. Cancer 2021, 124, 744–753. [Google Scholar] [CrossRef]
- Schoenfeld, A.J.; Bandlamudi, C.; Lavery, J.A.; Montecalvo, J.; Namakydoust, A.; Rizvi, H.; Egger, J.; Concepcion, C.P.; Paul, S.; Arcila, M.E.; et al. The Genomic Landscape of SMARCA4 Alterations and Associations with Outcomes in Patients with Lung Cancer. Clin. Cancer Res. 2020, 26, 5701–5708. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Hong, X.; Song, Z.; Xu, Y.; Li, C.; Wang, G.; Zhang, Y.; Zhao, X.; Zhao, Z.; Zhao, J.; et al. Identification of Deleterious NOTCH Mutation as Novel Predictor to Efficacious Immunotherapy in NSCLC. Clin. Cancer Res. 2020, 26, 3649–3661. [Google Scholar] [CrossRef] [Green Version]
- Hofman, P. What Is New in Biomarker Testing at Diagnosis of Advanced Non-Squamous Non-Small Cell Lung Carcinoma? Implications for Cytology and Liquid Biopsy. J. Mol. Pathol. 2021, 2, 147–172. [Google Scholar] [CrossRef]
- Roy-Chowdhuri, S.; Goswami, R.S.; Chen, H.; Patel, K.P.; Routbort, M.J.; Singh, R.R.; Broaddus, R.R.; Barkoh, B.A.; Manekia, J.; Yao, H.; et al. Factors affecting the success of next-generation sequencing in cytology specimens. Cancer Cytopathol. 2015, 123, 659–668. [Google Scholar] [CrossRef]
- Pisapia, P.; Pepe, F.; Iaccarino, A.; Sgariglia, R.; Nacchio, M.; Conticelli, F.; Salatiello, M.; Tufano, R.; Russo, G.; Gragnano, G.; et al. Next Generation Sequencing in Cytopathology: Focus on Non-Small Cell Lung Cancer. Front. Med. 2021, 8, 633923. [Google Scholar] [CrossRef]
- Cohen, D.; Hondelink, L.M.; Solleveld-Westerink, N.; Uljee, S.M.; Ruano, D.; Cleton-Jansen, A.M.; von der Thusen, J.H.; Ramai, S.R.S.; Postmus, P.E.; Graadt van Roggen, J.F.; et al. Optimizing Mutation and Fusion Detection in NSCLC by Sequential DNA and RNA Sequencing. J. Thorac. Oncol. 2020, 15, 1000–1014. [Google Scholar] [CrossRef] [Green Version]
- Hofman, V.; Benzaquen, J.; Heeke, S.; Lassalle, S.; Poudenx, M.; Long, E.; Lanteri, E.; Bordone, O.; Lespinet, V.; Tanga, V.; et al. Real-world assessment of the BRAF status in non-squamous cell lung carcinoma using VE1 immunohistochemistry: A single laboratory experience (LPCE, Nice, France). Lung Cancer 2020, 145, 58–62. [Google Scholar] [CrossRef]
- Ilie, M.; Long, E.; Hofman, V.; Dadone, B.; Marquette, C.H.; Mouroux, J.; Vignaud, J.M.; Begueret, H.; Merlio, J.P.; Capper, D.; et al. Diagnostic value of immunohistochemistry for the detection of the BRAFV600E mutation in primary lung adenocarcinoma Caucasian patients. Ann. Oncol. 2013, 24, 742–748. [Google Scholar] [CrossRef]
- Lassalle, S.; Hofman, V.; Heeke, S.; Benzaquen, J.; Long, E.; Poudenx, M.; Lanteri, E.; Boutros, J.; Tanga, V.; Zahaf, K.; et al. Targeted Assessment of the EGFR Status as Reflex Testing in Treatment-Naive Non-Squamous Cell Lung Carcinoma Patients: A Single Laboratory Experience (LPCE, Nice, France). Cancers 2020, 12, 955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, T.M.; Morrison, C.; Gold, E.J.; Tradonsky, A.; Layton, A.J. Multiple Biomarker Testing Tissue Consumption and Completion Rates With Single-gene Tests and Investigational Use of Oncomine Dx Target Test for Advanced Non-Small-cell Lung Cancer: A Single-center Analysis. Clin. Lung Cancer 2019, 20, 20–29.e28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christofyllakis, K.; Bittenbring, J.T.; Thurner, L.; Ahlgrimm, M.; Stilgenbauer, S.; Bewarder, M.; Kaddu-Mulindwa, D. Cost-effectiveness of precision cancer medicine-current challenges in the use of next generation sequencing for comprehensive tumour genomic profiling and the role of clinical utility frameworks (Review). Mol. Clin. Oncol. 2022, 16, 21. [Google Scholar] [CrossRef] [PubMed]
- Pennell, N.A.; Mutebi, A.; Zhou, Z.Y.; Ricculli, M.L.; Tang, W.; Wang, H.; Guerin, A.; Arnhart, T.; Dalal, A.; Sasane, M.; et al. Economic Impact of Next-Generation Sequencing Versus Single-Gene Testing to Detect Genomic Alterations in Metastatic Non-Small-Cell Lung Cancer Using a Decision Analytic Model. JCO Precis Oncol. 2019, 3, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Pisapia, P.; Pepe, F.; Baggi, A.; Barberis, M.; Galvano, A.; Gristina, V.; Mastrilli, F.; Novello, S.; Pagni, F.; Pasini, S.; et al. Next generation diagnostic algorithm in non-small cell lung cancer predictive molecular pathology: The KWAY Italian multicenter cost evaluation study. Crit. Rev. Oncol. Hematol. 2022, 169, 103525. [Google Scholar] [CrossRef]
- Pruneri, G.; De Braud, F.; Sapino, A.; Aglietta, M.; Vecchione, A.; Giusti, R.; Marchio, C.; Scarpino, S.; Baggi, A.; Bonetti, G.; et al. Next-Generation Sequencing in Clinical Practice: Is It a Cost-Saving Alternative to a Single-Gene Testing Approach? Pharm. Open 2021, 5, 285–298. [Google Scholar] [CrossRef]
- Tan, A.C.; Lai, G.G.Y.; Tan, G.S.; Poon, S.Y.; Doble, B.; Lim, T.H.; Aung, Z.W.; Takano, A.; Tan, W.L.; Ang, M.K.; et al. Utility of incorporating next-generation sequencing (NGS) in an Asian non-small cell lung cancer (NSCLC) population: Incremental yield of actionable alterations and cost-effectiveness analysis. Lung Cancer 2020, 139, 207–215. [Google Scholar] [CrossRef] [Green Version]
- Volckmar, A.L.; Leichsenring, J.; Kirchner, M.; Christopoulos, P.; Neumann, O.; Budczies, J.; Morais de Oliveira, C.M.; Rempel, E.; Buchhalter, I.; Brandt, R.; et al. Combined targeted DNA and RNA sequencing of advanced NSCLC in routine molecular diagnostics: Analysis of the first 3000 Heidelberg cases. Int. J. Cancer 2019, 145, 649–661. [Google Scholar] [CrossRef]
- Yu, T.M.; Morrison, C.; Gold, E.J.; Tradonsky, A.; Arnold, R.J.G. Budget Impact of Next-Generation Sequencing for Molecular Assessment of Advanced Non-Small Cell Lung Cancer. Value Health 2018, 21, 1278–1285. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Vioix, H.; Liu, F.X.; Singh, B.; Sharma, S.; Sharda, D. Diagnostic and economic value of biomarker testing for targetable mutations in non-small-cell lung cancer: A literature review. Future Oncol. 2022, 18, 505–518. [Google Scholar] [CrossRef]
- De Maglio, G.; Pasello, G.; Dono, M.; Fiorentino, M.; Follador, A.; Sciortino, M.; Malapelle, U.; Tiseo, M. The storm of NGS in NSCLC diagnostic-therapeutic pathway: How to sun the real clinical practice. Crit. Rev. Oncol. Hematol. 2022, 169, 103561. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Xu, F.; Wu, J.; Schubert, J.; Li, M.M. Application of Next Generation Sequencing in Laboratory Medicine. Ann. Lab. Med. 2021, 41, 25–43. [Google Scholar] [CrossRef] [PubMed]
- Skoulidis, F.; Heymach, J.V. Co-occurring genomic alterations in non-small-cell lung cancer biology and therapy. Nat. Rev. Cancer 2019, 19, 495–509. [Google Scholar] [CrossRef] [PubMed]
- Bruno, R.; Fontanini, G. Next Generation Sequencing for Gene Fusion Analysis in Lung Cancer: A Literature Review. Diagnostics 2020, 10, 521. [Google Scholar] [CrossRef]
- Horgan, D.; Curigliano, G.; Riess, O.; Hofman, P.; Buttner, R.; Conte, P.; Cufer, T.; Gallagher, W.M.; Georges, N.; Kerr, K.; et al. Identifying the Steps Required to Effectively Implement Next-Generation Sequencing in Oncology at a National Level in Europe. J. Pers. Med. 2022, 12, 72. [Google Scholar] [CrossRef]
- Schluckebier, L.; Caetano, R.; Garay, O.U.; Montenegro, G.T.; Custodio, M.; Aran, V.; Gil Ferreira, C. Cost-effectiveness analysis comparing companion diagnostic tests for EGFR, ALK, and ROS1 versus next-generation sequencing (NGS) in advanced adenocarcinoma lung cancer patients. BMC Cancer 2020, 20, 875. [Google Scholar] [CrossRef]
- Steuten, L.; Goulart, B.; Meropol, N.J.; Pritchard, D.; Ramsey, S.D. Cost Effectiveness of Multigene Panel Sequencing for Patients With Advanced Non-Small-Cell Lung Cancer. JCO Clin. Cancer Inf. 2019, 3, 1–10. [Google Scholar] [CrossRef]
- Goswami, R.S.; Luthra, R.; Singh, R.R.; Patel, K.P.; Routbort, M.J.; Aldape, K.D.; Yao, H.; Dang, H.D.; Barkoh, B.A.; Manekia, J.; et al. Identification of Factors Affecting the Success of Next-Generation Sequencing Testing in Solid Tumors. Am. J. Clin. Pathol. 2016, 145, 222–237. [Google Scholar] [CrossRef]
- Hofman, P. The challenges of evaluating predictive biomarkers using small biopsy tissue samples and liquid biopsies from non-small cell lung cancer patients. J. Thorac. Dis. 2019, 11, S57–S64. [Google Scholar] [CrossRef]
- Ilie, M.; Hofman, P. Pitfalls in lung cancer molecular pathology: How to limit them in routine practice? Curr. Med. Chem. 2012, 19, 2638–2651. [Google Scholar] [CrossRef]
- Rolfo, C.; Mack, P.; Scagliotti, G.V.; Aggarwal, C.; Arcila, M.E.; Barlesi, F.; Bivona, T.; Diehn, M.; Dive, C.; Dziadziuszko, R.; et al. Liquid Biopsy for Advanced NSCLC: A Consensus Statement From the International Association for the Study of Lung Cancer. J. Thorac. Oncol. 2021, 16, 1647–1662. [Google Scholar] [CrossRef] [PubMed]
- Li, B.T.; Smit, E.F.; Goto, Y.; Nakagawa, K.; Udagawa, H.; Mazieres, J.; Nagasaka, M.; Bazhenova, L.; Saltos, A.N.; Felip, E.; et al. Trastuzumab Deruxtecan in HER2-Mutant Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2022, 386, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Ou, S.I.; Janne, P.A.; Leal, T.A.; Rybkin, I.I.; Sabari, J.K.; Barve, M.A.; Bazhenova, L.; Johnson, M.L.; Velastegui, K.L.; Cilliers, C.; et al. First-in-Human Phase I/IB Dose-Finding Study of Adagrasib (MRTX849) in Patients With Advanced KRAS(G12C) Solid Tumors (KRYSTAL-1). J. Clin. Oncol. 2022, JCO2102752. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Haura, E.B.; Leighl, N.B.; Mitchell, P.; Shu, C.A.; Girard, N.; Viteri, S.; Han, J.Y.; Kim, S.W.; Lee, C.K.; et al. Amivantamab in EGFR Exon 20 Insertion-Mutated Non-Small-Cell Lung Cancer Progressing on Platinum Chemotherapy: Initial Results From the CHRYSALIS Phase I Study. J. Clin. Oncol. 2021, 39, 3391–3402. [Google Scholar] [CrossRef]
- Colli, L.M.; Machiela, M.J.; Zhang, H.; Myers, T.A.; Jessop, L.; Delattre, O.; Yu, K.; Chanock, S.J. Landscape of Combination Immunotherapy and Targeted Therapy to Improve Cancer Management. Cancer Res. 2017, 77, 3666–3671. [Google Scholar] [CrossRef] [Green Version]
- Gotwals, P.; Cameron, S.; Cipolletta, D.; Cremasco, V.; Crystal, A.; Hewes, B.; Mueller, B.; Quaratino, S.; Sabatos-Peyton, C.; Petruzzelli, L.; et al. Prospects for combining targeted and conventional cancer therapy with immunotherapy. Nat. Rev. Cancer 2017, 17, 286–301. [Google Scholar] [CrossRef]
- Socinski, M.A.; Obasaju, C.; Gandara, D.; Hirsch, F.R.; Bonomi, P.; Bunn, P.A., Jr.; Kim, E.S.; Langer, C.J.; Natale, R.B.; Novello, S.; et al. Current and Emergent Therapy Options for Advanced Squamous Cell Lung Cancer. J. Thorac. Oncol. 2018, 13, 165–183. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; Man, S.; Sun, R.; Li, Z.; Wu, Y.; Zuo, D. Recent advances and challenges of immune checkpoint inhibitors in immunotherapy of non-small cell lung cancer. Int. Immunopharmacol. 2020, 85, 106613. [Google Scholar] [CrossRef]
- Greenhalgh, J.; Boland, A.; Bates, V.; Vecchio, F.; Dundar, Y.; Chaplin, M.; Green, J.A. First-line treatment of advanced epidermal growth factor receptor (EGFR) mutation positive non-squamous non-small cell lung cancer. Cochrane Database Syst. Rev. 2021, 3, CD010383. [Google Scholar] [CrossRef]
- Lewis, W.E.; Hong, L.; Mott, F.E.; Simon, G.; Wu, C.C.; Rinsurongkawong, W.; Lee, J.J.; Lam, V.K.; Heymach, J.V.; Zhang, J.; et al. Efficacy of Targeted Inhibitors in Metastatic Lung Squamous Cell Carcinoma With EGFR or ALK Alterations. JTO Clin. Res. Rep. 2021, 2, 100237. [Google Scholar] [CrossRef]
- Stinchcombe, T.E. Targeted therapies for locally advanced or metastatic squamous cell carcinoma of the lung. Curr. Treat. Options Oncol. 2013, 14, 568–579. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, C.; Rolfo, C.D.; Oxnard, G.R.; Gray, J.E.; Sholl, L.M.; Gandara, D.R. Strategies for the successful implementation of plasma-based NSCLC genotyping in clinical practice. Nat. Rev. Clin. Oncol. 2021, 18, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Heitzer, E.; van den Broek, D.; Denis, M.G.; Hofman, P.; Hubank, M.; Mouliere, F.; Paz-Ares, L.; Schuuring, E.; Sultmann, H.; Vainer, G.; et al. Recommendations for a practical implementation of circulating tumor DNA mutation testing in metastatic non-small-cell lung cancer. ESMO Open 2022, 7, 100399. [Google Scholar] [CrossRef] [PubMed]
- Merritt, R.E.; Abdel-Rasoul, M.; Fitzgerald, M.; D’Souza, D.M.; Kneuertz, P.J. The Academic Facility Type Is Associated With Improved Overall Survival for Early-Stage Lung Cancer. Ann. Thorac. Surg. 2021, 111, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Woodard, G.A.; Jones, K.D.; Jablons, D.M. Lung Cancer Staging and Prognosis. Cancer Treat. Res. 2016, 170, 47–75. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, M.H.; Gerlach, D.; Misale, S.; Petronczki, M.; Kraut, N. Expanding the Reach of Precision Oncology by Drugging All KRAS Mutants. Cancer Discov. 2022, 12, 924–937. [Google Scholar] [CrossRef] [PubMed]
- Kartolo, A.; Robinson, A.; Fung, A.S. Optimal first-line immune checkpoint inhibitor treatment in lung cancer: How to choose? Future Oncol. 2022, 18, 635–638. [Google Scholar] [CrossRef]
- Middleton, G.; Robbins, H.; Andre, F.; Swanton, C. A state-of-the-art review of stratified medicine in cancer: Towards a future precision medicine strategy in cancer. Ann. Oncol. 2022, 33, 143–157. [Google Scholar] [CrossRef]
- Tan, A.C.; Tan, D.S.W. Targeted Therapies for Lung Cancer Patients With Oncogenic Driver Molecular Alterations. J. Clin. Oncol. 2022, 40, 611–625. [Google Scholar] [CrossRef]
- Mielgo-Rubio, X.; Calvo, V.; Luna, J.; Remon, J.; Martin, M.; Berraondo, P.; Jarabo, J.R.; Higuera, O.; Conde, E.; De Castro, J.; et al. Immunotherapy Moves to the Early-Stage Setting in Non-Small Cell Lung Cancer: Emerging Evidence and the Role of Biomarkers. Cancers 2020, 12, 3459. [Google Scholar] [CrossRef]
- Friedlaender, A.; Naidoo, J.; Banna, G.L.; Metro, G.; Forde, P.; Addeo, A. Role and impact of immune checkpoint inhibitors in neoadjuvant treatment for NSCLC. Cancer Treat. Rev. 2022, 104, 102350. [Google Scholar] [CrossRef] [PubMed]
- Roller, J.F.; Veeramachaneni, N.K.; Zhang, J. Exploring the Evolving Scope of Neoadjuvant Immunotherapy in NSCLC. Cancers 2022, 14, 741. [Google Scholar] [CrossRef] [PubMed]
- Friedlaender, A.; Addeo, A.; Russo, A.; Gregorc, V.; Cortinovis, D.; Rolfo, C.D. Targeted Therapies in Early Stage NSCLC: Hype or Hope? Int. J. Mol. Sci. 2020, 21, 6329. [Google Scholar] [CrossRef] [PubMed]
- Hofman, P. Next-Generation Sequencing with Liquid Biopsies from Treatment-Naive Non-Small Cell Lung Carcinoma Patients. Cancers 2021, 13, 2049. [Google Scholar] [CrossRef]
- Dagogo-Jack, I.; Shaw, A.T. Tumour heterogeneity and resistance to cancer therapies. Nat. Rev. Clin. Oncol. 2018, 15, 81–94. [Google Scholar] [CrossRef]
- Lazzari, C.; Bulotta, A.; Cangi, M.G.; Bucci, G.; Pecciarini, L.; Bonfiglio, S.; Lorusso, V.; Ippati, S.; Arrigoni, G.; Grassini, G.; et al. Next Generation Sequencing in Non-Small Cell Lung Cancer: Pitfalls and Opportunities. Diagnostics 2020, 10, 1092. [Google Scholar] [CrossRef]
- Huang, B.; Chen, Q.; Allison, D.; El Khouli, R.; Peh, K.H.; Mobley, J.; Anderson, A.; Durbin, E.B.; Goodin, D.; Villano, J.L.; et al. Molecular Tumor Board Review and Improved Overall Survival in Non-Small-Cell Lung Cancer. JCO Precis Oncol. 2021, 5, 1530–1539. [Google Scholar] [CrossRef]
- Luchini, C.; Lawlor, R.T.; Milella, M.; Scarpa, A. Molecular Tumor Boards in Clinical Practice. Trends Cancer 2020, 6, 738–744. [Google Scholar] [CrossRef]
- Ilie, M.; Beaulande, M.; Hamila, M.; Erb, G.; Hofman, V.; Hofman, P. Automated chromogenic multiplexed immunohistochemistry assay for diagnosis and predictive biomarker testing in non-small cell lung cancer. Lung Cancer 2018, 124, 90–94. [Google Scholar] [CrossRef]
- Ilie, M.; Beaulande, M.; Long-Mira, E.; Bontoux, C.; Zahaf, K.; Lalvee, S.; Hamila, M.; Benzaquen, J.; Cohen, C.; Berthet, J.P.; et al. Analytical validation of automated multiplex chromogenic immunohistochemistry for diagnostic and predictive purpose in non-small cell lung cancer. Lung Cancer 2022, 166, 1–8. [Google Scholar] [CrossRef]
- Lengel, H.B.; Connolly, J.G.; Jones, G.D.; Caso, R.; Zhou, J.; Sanchez-Vega, F.; Mastrogiacomo, B.; Isbell, J.M.; Li, B.T.; Liu, Y.; et al. The Emerging Importance of Tumor Genomics in Operable Non-Small Cell Lung Cancer. Cancers 2021, 13, 3656. [Google Scholar] [CrossRef] [PubMed]
- Soh, J.; Hamada, A.; Fujino, T.; Mitsudomi, T. Perioperative Therapy for Non-Small Cell Lung Cancer with Immune Checkpoint Inhibitors. Cancers 2021, 13, 4035. [Google Scholar] [CrossRef] [PubMed]
- Ignatiadis, M.; Sledge, G.W.; Jeffrey, S.S. Liquid biopsy enters the clinic—Implementation issues and future challenges. Nat. Rev. Clin. Oncol. 2021, 18, 297–312. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Zhao, Y.; Huang, W.; Gao, Y.; Xu, W.; Tao, J.; Yang, M.; Li, L.; Ping, W.; Shen, H.; et al. Non-invasive diagnosis of early-stage lung cancer using high-throughput targeted DNA methylation sequencing of circulating tumor DNA (ctDNA). Theranostics 2019, 9, 2056–2070. [Google Scholar] [CrossRef]
- Sands, J.M.; Nguyen, T.; Shivdasani, P.; Sacher, A.G.; Cheng, M.L.; Alden, R.S.; Janne, P.A.; Kuo, F.C.; Oxnard, G.R.; Sholl, L.M. Next-generation sequencing informs diagnosis and identifies unexpected therapeutic targets in lung squamous cell carcinomas. Lung Cancer 2020, 140, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.A.; Hur, J.Y.; Kim, H.J.; Park, J.H.; Hwang, J.J.; Lee, S.A.; Lee, S.E.; Kim, W.S.; Lee, K.Y. Targeted Next-Generation Sequencing Analysis for Recurrence in Early-Stage Lung Adenocarcinoma. Ann. Surg. Oncol. 2021, 28, 3983–3993. [Google Scholar] [CrossRef]
| Characteristics | n (%) | |
|---|---|---|
| 259 (100) | ||
| Age at diagnosis | Median | 69 |
| Range | 34–84 | |
| Gender | Male | 197 (76) |
| Female | 62 (24) | |
| Smoking status | Smokers | 186(72) |
| Non-smokers | 44 (17) | |
| Unknown | 29 (11) | |
| Type of specimens | Bronchial biopsy | 153 (59) |
| Transthoracic biopsy | 47 (18) | |
| Surgical specimen | 39 (15) | |
| Pleural effusion (cellblock) | 13 (5) | |
| EBUS | 7 (3) | |
| Histology | Adenocarcinoma | 250 (97) |
| Large cell carcinoma | 9 (3) | |
| pTNM stage | Stage I | 78 (30) |
| Stage II | 73 (28) | |
| Stage III | 28 (11) | |
| Stage IV | 80 (31) | |
| OPA analyses Overall = 506 | DNA | 252 |
| RNA | 254 | |
| Failed DNA | 10/252 (4%) | |
| Failed RNA | 5/254 (2%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ilié, M.; Hofman, V.; Bontoux, C.; Heeke, S.; Lespinet-Fabre, V.; Bordone, O.; Lassalle, S.; Lalvée, S.; Tanga, V.; Allegra, M.; et al. Setting Up an Ultra-Fast Next-Generation Sequencing Approach as Reflex Testing at Diagnosis of Non-Squamous Non-Small Cell Lung Cancer; Experience of a Single Center (LPCE, Nice, France). Cancers 2022, 14, 2258. https://doi.org/10.3390/cancers14092258
Ilié M, Hofman V, Bontoux C, Heeke S, Lespinet-Fabre V, Bordone O, Lassalle S, Lalvée S, Tanga V, Allegra M, et al. Setting Up an Ultra-Fast Next-Generation Sequencing Approach as Reflex Testing at Diagnosis of Non-Squamous Non-Small Cell Lung Cancer; Experience of a Single Center (LPCE, Nice, France). Cancers. 2022; 14(9):2258. https://doi.org/10.3390/cancers14092258
Chicago/Turabian StyleIlié, Marius, Véronique Hofman, Christophe Bontoux, Simon Heeke, Virginie Lespinet-Fabre, Olivier Bordone, Sandra Lassalle, Salomé Lalvée, Virginie Tanga, Maryline Allegra, and et al. 2022. "Setting Up an Ultra-Fast Next-Generation Sequencing Approach as Reflex Testing at Diagnosis of Non-Squamous Non-Small Cell Lung Cancer; Experience of a Single Center (LPCE, Nice, France)" Cancers 14, no. 9: 2258. https://doi.org/10.3390/cancers14092258
APA StyleIlié, M., Hofman, V., Bontoux, C., Heeke, S., Lespinet-Fabre, V., Bordone, O., Lassalle, S., Lalvée, S., Tanga, V., Allegra, M., Salah, M., Bohly, D., Benzaquen, J., Marquette, C.-H., Long-Mira, E., & Hofman, P. (2022). Setting Up an Ultra-Fast Next-Generation Sequencing Approach as Reflex Testing at Diagnosis of Non-Squamous Non-Small Cell Lung Cancer; Experience of a Single Center (LPCE, Nice, France). Cancers, 14(9), 2258. https://doi.org/10.3390/cancers14092258









