PD-1+ T-Cells Correlate with Nerve Fiber Density as a Prognostic Biomarker in Patients with Resected Perihilar Cholangiocarcinoma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Cohort
2.2. Surgical Techniques, Adjuvant Treatment and Follow-Up
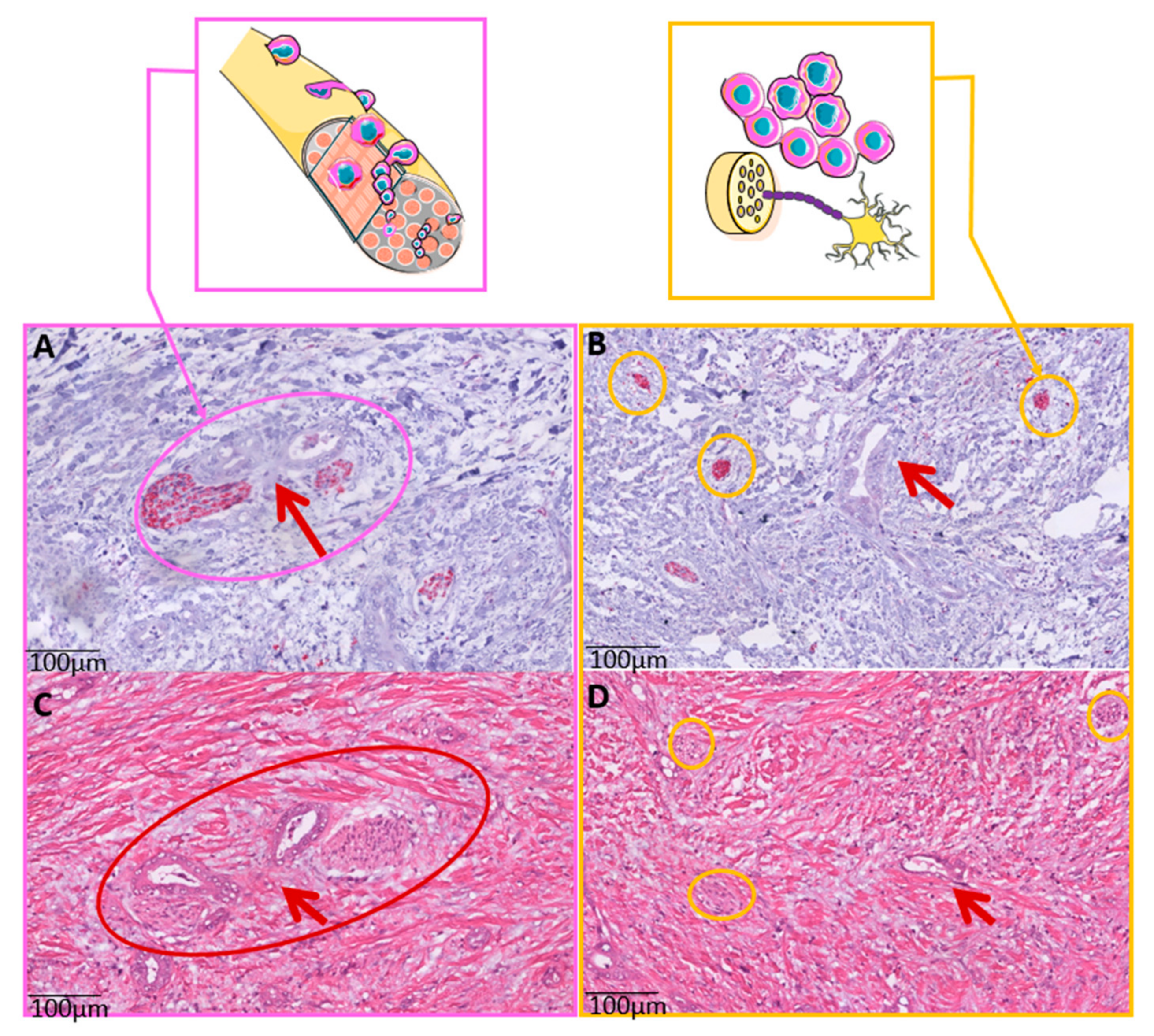
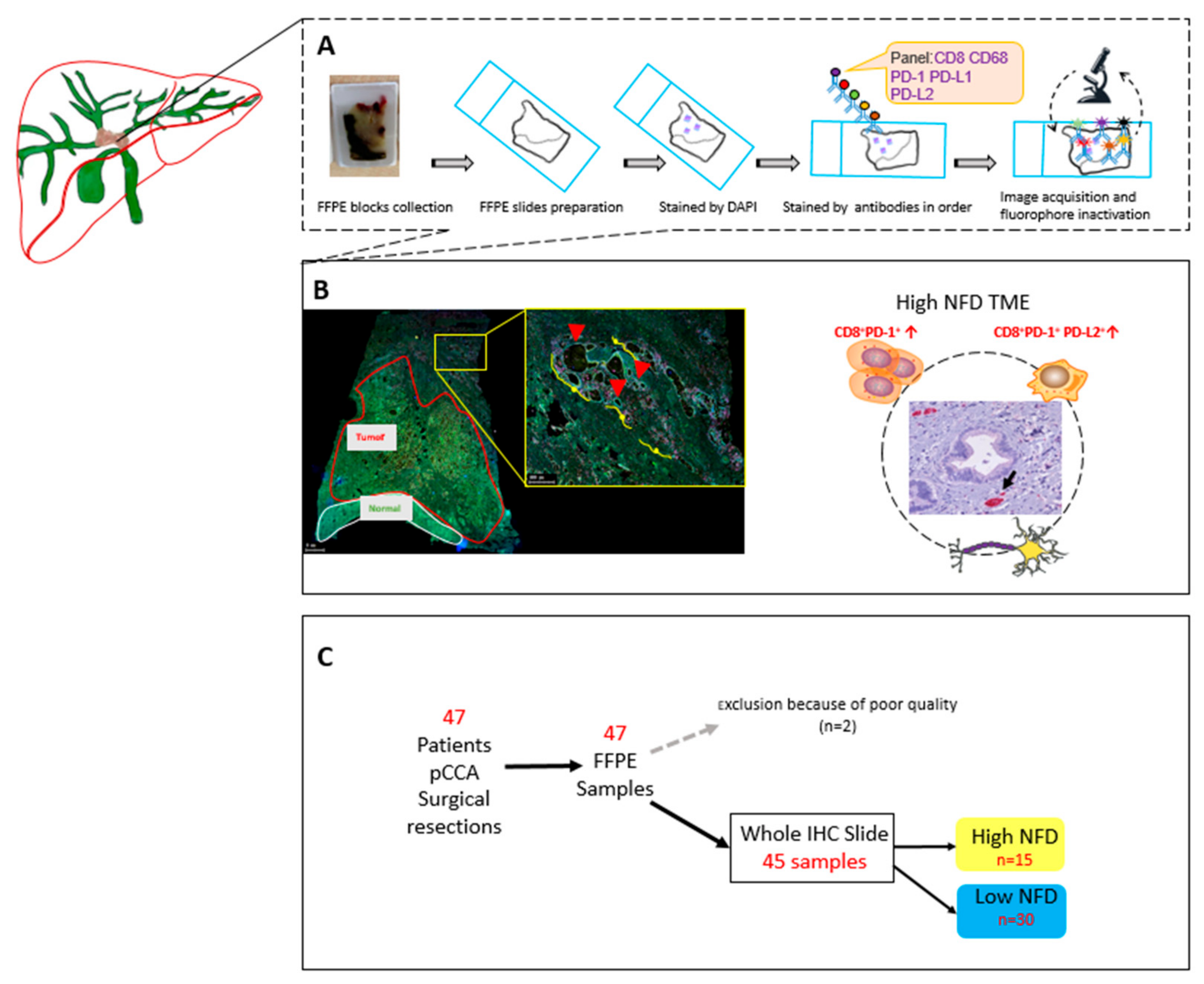
2.3. Whole Slide Immunohistochemistry (IHC) and Nerve Fiber Counts
2.4. Whole Slide Multiplex Immunofluorescence (mIF)
2.5. Statistical Analysis
3. Results
3.1. Patients’ Characteristics
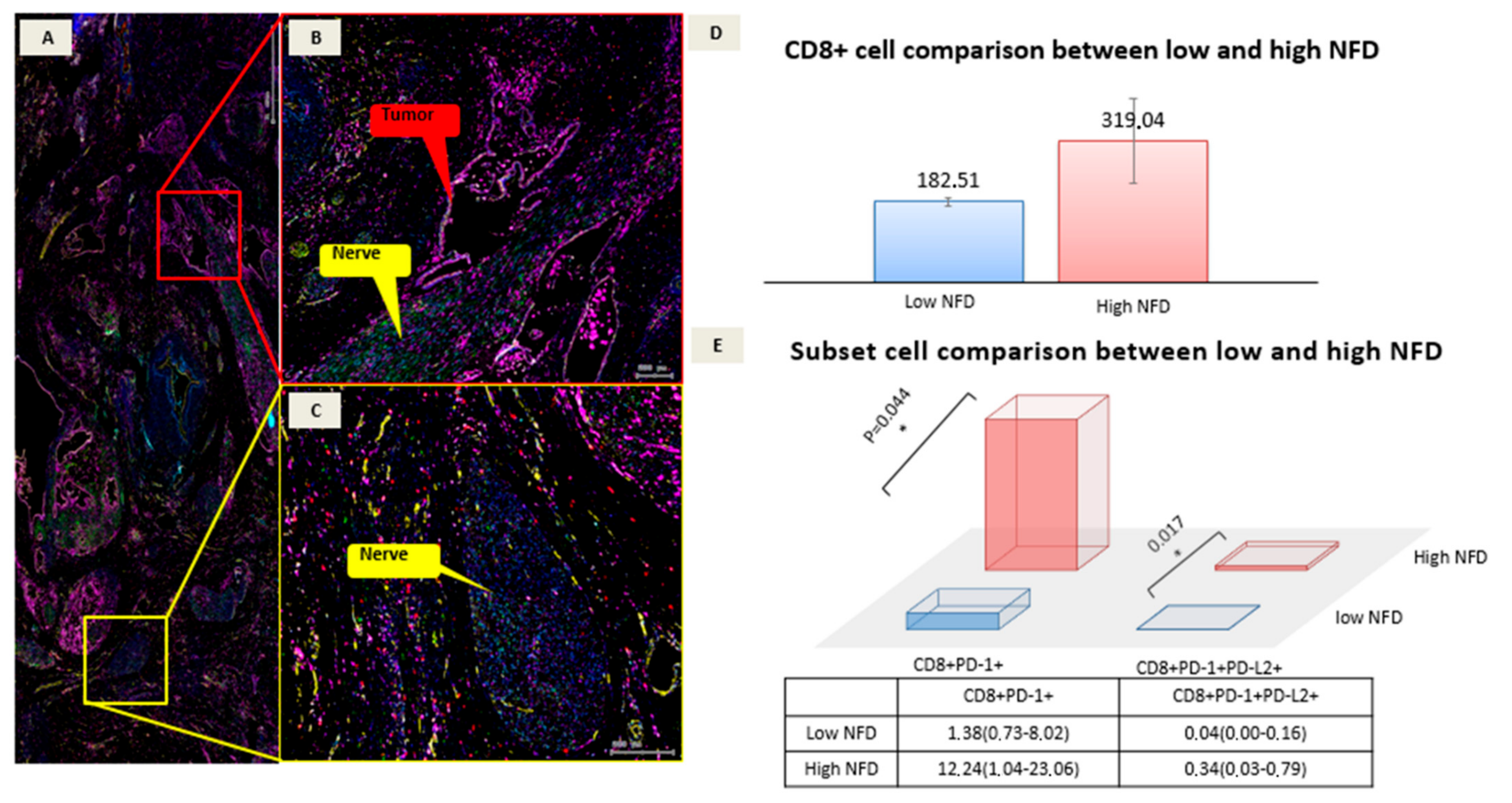
3.2. Multiplex Data
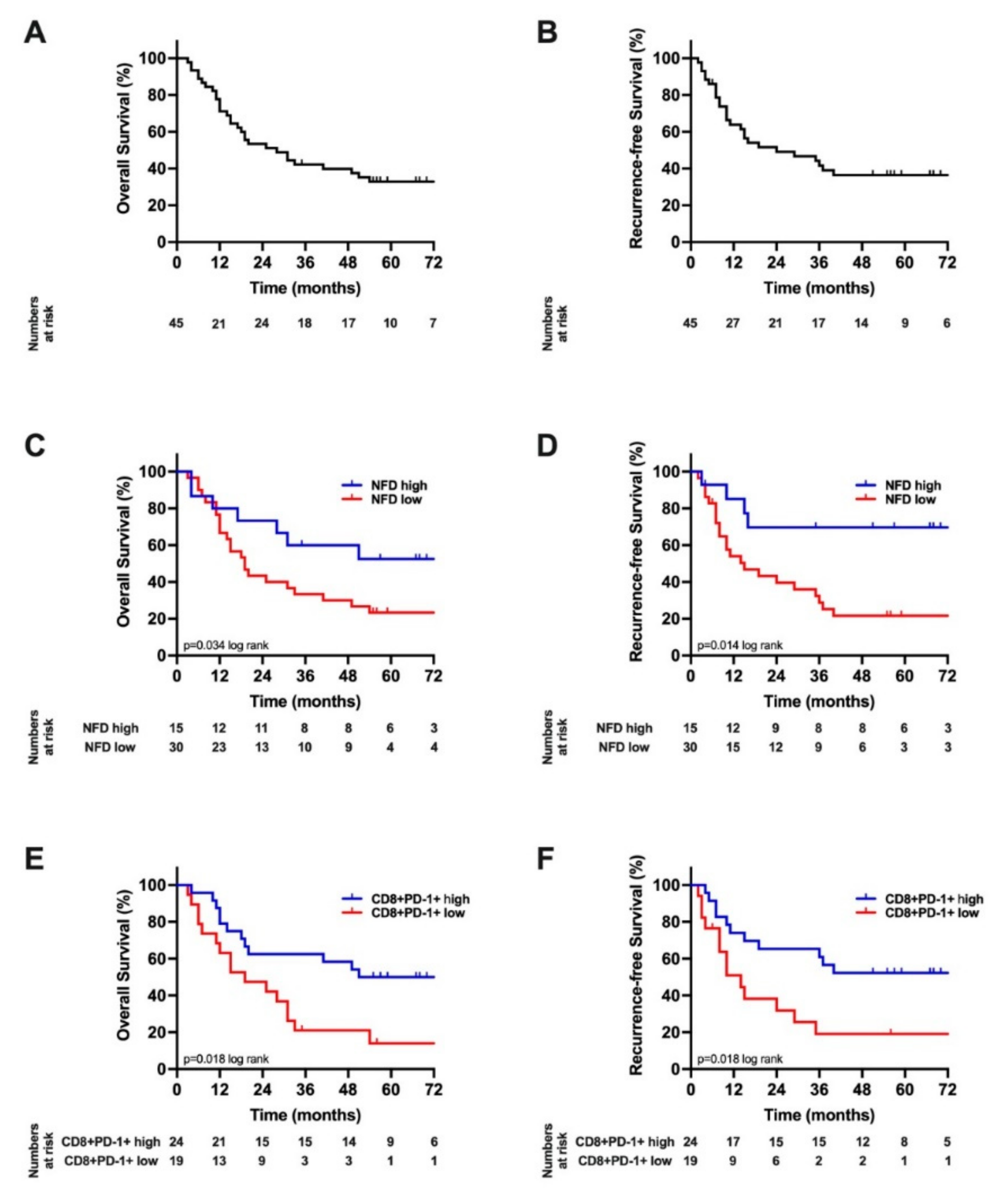
3.3. Survival Analysis
3.4. Cox Regression Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CCA | Cholangiocarcinoma |
| CHCC-CCA | Combined hepatocellular-cholangiocarcinoma |
| DCCA | Distal cholangiocarcinoma |
| FFPE | Formalin fixed paraffin embedded |
| HCC | Hepatocellular carcinoma |
| ICCA | Intrahepatic cholangiocarcinoma |
| MIF | Multiplex Immune Fluorescence |
| NFD | Nerve fib er density |
| OS | Overall survival |
| PCCA | Perihilar cholangiocarcinoma |
| PD-1 | Programmed Death receptor |
| PD-L1 | Programmed Death Ligand 1 |
| PD-L2 | Programmed Death Ligand 2 |
| PGP 9.5 | Protein gene-product 9.5 |
| PNI | Perineural invasion |
| RFS | Recurrence-free survival |
| ROI | Region of interest |
| TME | Tumor microenvironment |
References
- Bertuccio, P.; Malvezzi, M.; Carioli, G.; Hashim, D.; Boffetta, P.; El-Serag, H.B.; La Vecchia, C.; Negri, E. Global trends in mortality from intrahepatic and extrahepatic cholangiocarcinoma. J. Hepatol. 2019, 71, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Valle, J.; Wasan, H.; Palmer, D.H.; Cunningham, D.; Anthoney, A.; Maraveyas, A.; Madhusudan, S.; Iveson, T.; Hughes, S.; Pereira, S.P.; et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N. Engl. J. Med. 2010, 362, 1273–1281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marin, J.J.G.; Prete, M.G.; Lamarca, A.; Tavolari, S.; Landa-Magdalena, A.; Brandi, G.; Segatto, O.; Vogel, A.; Macias, R.I.R.; Rodrigues, P.M.; et al. Current and novel therapeutic opportunities for systemic therapy in biliary cancer. Br. J. Cancer 2020, 123, 1047–1059. [Google Scholar] [CrossRef] [PubMed]
- Piha-Paul, S.A.; Oh, D.Y.; Ueno, M.; Malka, D.; Chung, H.C.; Nagrial, A.; Kelley, R.K.; Ros, W.; Italiano, A.; Nakagawa, K.; et al. Efficacy and safety of pembrolizumab for the treatment of advanced biliary cancer: Results from the KEYNOTE-158 and KEYNOTE-028 studies. Int. J. Cancer 2020, 147, 2190–2198. [Google Scholar] [CrossRef]
- Di Federico, A.; Rizzo, A.; Ricci, A.D.; Frega, G.; Palloni, A.; Tavolari, S.; Brandi, G. Nivolumab: An investigational agent for the treatment of biliary tract cancer. Expert Opin. Investig. Drugs 2021, 30, 325–332. [Google Scholar] [CrossRef]
- Hack, S.P.; Zhu, A.X. Atezolizumab: An investigational agent for the treatment of biliary tract cancer. Expert Opin. Investig. Drugs 2021, 30, 1007–1015. [Google Scholar] [CrossRef]
- Rizzo, A.; Ricci, A.D.; Brandi, G. Recent advances of immunotherapy for biliary tract cancer. Expert Rev. Gastroenterol. Hepatol. 2021, 15, 527–536. [Google Scholar] [CrossRef]
- Ueno, M.; Ikeda, M.; Morizane, C.; Kobayashi, S.; Ohno, I.; Kondo, S.; Okano, N.; Kimura, K.; Asada, S.; Namba, Y.; et al. Nivolumab alone or in combination with cisplatin plus gemcitabine in Japanese patients with unresectable or recurrent biliary tract cancer: A non-randomised, multicentre, open-label, phase 1 study. Lancet. Gastroenterol. Hepatol. 2019, 4, 611–621. [Google Scholar] [CrossRef]
- Banales, J.M.; Marin, J.J.G.; Lamarca, A.; Rodrigues, P.M.; Khan, S.A.; Roberts, L.R.; Cardinale, V.; Carpino, G.; Andersen, J.B.; Braconi, C.; et al. Cholangiocarcinoma 2020: The next horizon in mechanisms and management. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 557–588. [Google Scholar] [CrossRef]
- Zou, S.; Li, J.; Zhou, H.; Frech, C.; Jiang, X.; Chu, J.S.; Zhao, X.; Li, Y.; Li, Q.; Wang, H.; et al. Mutational landscape of intrahepatic cholangiocarcinoma. Nat. Commun. 2014, 5, 5696. [Google Scholar] [CrossRef]
- Andersen, J.B.; Spee, B.; Blechacz, B.R.; Avital, I.; Komuta, M.; Barbour, A.; Conner, E.A.; Gillen, M.C.; Roskams, T.; Roberts, L.R.; et al. Genomic and genetic characterization of cholangiocarcinoma identifies therapeutic targets for tyrosine kinase inhibitors. Gastroenterology 2012, 142, 1021–1031.e15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sia, D.; Hoshida, Y.; Villanueva, A.; Roayaie, S.; Ferrer, J.; Tabak, B.; Peix, J.; Sole, M.; Tovar, V.; Alsinet, C.; et al. Integrative molecular analysis of intrahepatic cholangiocarcinoma reveals 2 classes that have different outcomes. Gastroenterology 2013, 144, 829–840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaisaingmongkol, J.; Budhu, A.; Dang, H.; Rabibhadana, S.; Pupacdi, B.; Kwon, S.M.; Forgues, M.; Pomyen, Y.; Bhudhisawasdi, V.; Lertprasertsuke, N.; et al. Common Molecular Subtypes Among Asian Hepatocellular Carcinoma and Cholangiocarcinoma. Cancer Cell 2017, 32, 57–70.e3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jusakul, A.; Cutcutache, I.; Yong, C.H.; Lim, J.Q.; Huang, M.N.; Padmanabhan, N.; Nellore, V.; Kongpetch, S.; Ng, A.W.T.; Ng, L.M.; et al. Whole-Genome and Epigenomic Landscapes of Etiologically Distinct Subtypes of Cholangiocarcinoma. Cancer Discov. 2017, 7, 1116–1135. [Google Scholar] [CrossRef] [Green Version]
- Sirica, A.E.; Gores, G.J. Desmoplastic stroma and cholangiocarcinoma: Clinical implications and therapeutic targeting. Hepatology 2014, 59, 2397–2402. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, C.T.; Caruso, S.; Maillé, P.; Beaufrère, A.; Augustin, J.; Favre, L.; Pujals, A.; Boulagnon-Rombi, C.; Rhaiem, R.; Amaddeo, G.; et al. Immune profiling of combined hepatocellular-cholangiocarcinoma reveals distinct subtypes and activation of gene signatures predictive of response to immunotherapy. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2021, 28, 540–551. [Google Scholar] [CrossRef]
- Job, S.; Rapoud, D.; Dos Santos, A.; Gonzalez, P.; Desterke, C.; Pascal, G.; Elarouci, N.; Ayadi, M.; Adam, R.; Azoulay, D.; et al. Identification of Four Immune Subtypes Characterized by Distinct Composition and Functions of Tumor Microenvironment in Intrahepatic Cholangiocarcinoma. Hepatology 2020, 72, 965–981. [Google Scholar] [CrossRef] [Green Version]
- Yuan, J.; Hegde, P.S.; Clynes, R.; Foukas, P.G.; Harari, A.; Kleen, T.O.; Kvistborg, P.; Maccalli, C.; Maecker, H.T.; Page, D.B.; et al. Novel technologies and emerging biomarkers for personalized cancer immunotherapy. J. Immunother. Cancer 2016, 4, 3. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez-Larrañaga, M.; González-López, E.; Roa-Bautista, A.; Rodrigues, P.M.; Díaz-González, Á.; Banales, J.M.; López-Hoyos, M.; Santos-Laso, A.; Crespo, J. Immune Checkpoint Inhibitors: The Emerging Cornerstone in Cholangiocarcinoma Therapy? Liver Cancer 2021, 10, 545–560. [Google Scholar] [CrossRef]
- Rizzo, A.; Ricci, A.D.; Brandi, G. Durvalumab: An investigational anti-PD-L1 antibody for the treatment of biliary tract cancer. Expert Opin. Investig. Drugs 2021, 30, 343–350. [Google Scholar] [CrossRef]
- Kang, S.; El-Rayes, B.F.; Akce, M. Evolving Role of Immunotherapy in Advanced Biliary Tract Cancers. Cancers 2022, 14, 1748. [Google Scholar] [CrossRef] [PubMed]
- Vogel, A.; Bathon, M.; Saborowski, A. Immunotherapies in clinical development for biliary tract cancer. Expert Opin. Investig. Drugs 2021, 30, 351–363. [Google Scholar] [CrossRef] [PubMed]
- Bednarsch, J.; Tan, X.; Czigany, Z.; Liu, D.; Lang, S.A.; Sivakumar, S.; Kather, J.N.; Appinger, S.; Rosin, M.; Boroojerdi, S.; et al. The Presence of Small Nerve Fibers in the Tumor Microenvironment as Predictive Biomarker of Oncological Outcome Following Partial Hepatectomy for Intrahepatic Cholangiocarcinoma. Cancers 2021, 13, 3661. [Google Scholar] [CrossRef] [PubMed]
- Bednarsch, J.; Kather, J.; Tan, X.; Sivakumar, S.; Cacchi, C.; Wiltberger, G.; Czigany, Z.; Ulmer, F.; Neumann, U.P.; Heij, L.R. Nerve Fibers in the Tumor Microenvironment as a Novel Biomarker for Oncological Outcome in Patients Undergoing Surgery for Perihilar Cholangiocarcinoma. Liver Cancer 2021, 10, 260–274. [Google Scholar] [CrossRef]
- Bednarsch, J.; Czigany, Z.; Heij, L.R.; Luedde, T.; Loosen, S.H.; Dulk, M.D.; Bruners, P.; Lang, S.A.; Ulmer, T.F.; Neumann, U.P. The prognostic role of in-hospital transfusion of fresh frozen plasma in patients with cholangiocarcinoma undergoing curative-intent liver surgery. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2021, 48, 604–614. [Google Scholar] [CrossRef]
- Primrose, J.N.; Fox, R.P.; Palmer, D.H.; Malik, H.Z.; Prasad, R.; Mirza, D.; Anthony, A.; Corrie, P.; Falk, S.; Finch-Jones, M.; et al. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): A randomised, controlled, multicentre, phase 3 study. Lancet. Oncol. 2019, 20, 663–673. [Google Scholar] [CrossRef] [Green Version]
- Heij, L.R.; Tan, X.; Kather, J.N.; Niehues, J.M.; Sivakumar, S.; Heussen, N.; van der Kroft, G.; Damink, S.W.M.O.; Lang, S.; Aberle, M.R.; et al. Nerve Fibers in the Tumor Microenvironment Are Co-Localized with Lymphoid Aggregates in Pancreatic Cancer. J. Clin. Med. 2021, 10, 490. [Google Scholar] [CrossRef]
- Dievernich, A.; Achenbach, P.; Davies, L.; Klinge, U. Tissue remodeling macrophages morphologically dominate at the interface of polypropylene surgical meshes in the human abdomen. Hernia J. Hernias Abdom. Wall Surg. 2020, 24, 1175–1189. [Google Scholar] [CrossRef]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef]
- Macias, R.I.R.; Kornek, M.; Rodrigues, P.M.; Paiva, N.A.; Castro, R.E.; Urban, S.; Pereira, S.P.; Cadamuro, M.; Rupp, C.; Loosen, S.H.; et al. Diagnostic and prognostic biomarkers in cholangiocarcinoma. Liver Int. Off. J. Int. Assoc. Study Liver 2019, 39 (Suppl. S1), 108–122. [Google Scholar] [CrossRef] [Green Version]
- Tian, L.; Ma, J.; Ma, L.; Zheng, B.; Liu, L.; Song, D.; Wang, Y.; Zhang, Z.; Gao, Q.; Song, K.; et al. PD-1/PD-L1 expression profiles within intrahepatic cholangiocarcinoma predict clinical outcome. World J. Surg. Oncol. 2020, 18, 303. [Google Scholar] [CrossRef] [PubMed]
- Sangro, B.; Melero, I.; Wadhawan, S.; Finn, R.S.; Abou-Alfa, G.K.; Cheng, A.L.; Yau, T.; Furuse, J.; Park, J.W.; Boyd, Z.; et al. Association of inflammatory biomarkers with clinical outcomes in nivolumab-treated patients with advanced hepatocellular carcinoma. J. Hepatol. 2020, 73, 1460–1469. [Google Scholar] [CrossRef] [PubMed]
- Sideras, K.; Biermann, K.; Verheij, J.; Takkenberg, B.R.; Mancham, S.; Hansen, B.E.; Schutz, H.M.; de Man, R.A.; Sprengers, D.; Buschow, S.I.; et al. PD-L1, Galectin-9 and CD8(+) tumor-infiltrating lymphocytes are associated with survival in hepatocellular carcinoma. Oncoimmunology 2017, 6, e1273309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carapeto, F.; Bozorgui, B.; Shroff, R.T.; Chagani, S.; Solis Soto, L.; Foo, W.C.; Wistuba, I.; Meric-Bernstam, F.; Shalaby, A.; Javle, M.; et al. The Immunogenomic Landscape of Resected Intrahepatic Cholangiocarcinoma. Hepatology 2021, 75, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.C.; Zeng, H.Y.; Sun, Q.M.; Meng, Q.N.; Huang, X.Y.; Zhang, P.F.; Yang, X.; Peng, R.; Gao, C.; Wei, C.Y.; et al. Distinct PD-L1/PD1 Profiles and Clinical Implications in Intrahepatic Cholangiocarcinoma Patients with Different Risk Factors. Theranostics 2019, 9, 4678–4687. [Google Scholar] [CrossRef] [PubMed]
- Kitano, Y.; Yamashita, Y.I.; Nakao, Y.; Itoyama, R.; Yusa, T.; Umezaki, N.; Tsukamoto, M.; Yamao, T.; Miyata, T.; Nakagawa, S.; et al. Clinical Significance of PD-L1 Expression in Both Cancer and Stroma Cells of Cholangiocarcinoma Patients. Ann. Surg. Oncol. 2020, 27, 599–607. [Google Scholar] [CrossRef]
- Gani, F.; Nagarajan, N.; Kim, Y.; Zhu, Q.; Luan, L.; Bhaijjee, F.; Anders, R.A.; Pawlik, T.M. Program Death 1 Immune Checkpoint and Tumor Microenvironment: Implications for Patients With Intrahepatic Cholangiocarcinoma. Ann. Surg. Oncol. 2016, 23, 2610–2617. [Google Scholar] [CrossRef]
- Walter, D.; Herrmann, E.; Schnitzbauer, A.A.; Zeuzem, S.; Hansmann, M.L.; Peveling-Oberhag, J.; Hartmann, S. PD-L1 expression in extrahepatic cholangiocarcinoma. Histopathology 2017, 71, 383–392. [Google Scholar] [CrossRef]
- Xu, G.; Sun, L.; Li, Y.; Xie, F.; Zhou, X.; Yang, H.; Du, S.; Xu, H.; Mao, Y. The Clinicopathological and Prognostic Value of PD-L1 Expression in Cholangiocarcinoma: A Meta-Analysis. Front. Oncol. 2019, 9, 897. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, X.Y.; Zhang, Y.; Xu, D.; Dong, J.; Zhang, Z.; Yi, C.H.; Jia, H.L.; Yang, X. Programmed death ligand 1 expression in human intrahepatic cholangiocarcinoma and its association with prognosis and CD8(+) T-cell immune responses. Cancer Manag. Res. 2018, 10, 4113–4123. [Google Scholar] [CrossRef] [Green Version]
- Tumeh, P.C.; Harview, C.L.; Yearley, J.H.; Shintaku, I.P.; Taylor, E.J.; Robert, L.; Chmielowski, B.; Spasic, M.; Henry, G.; Ciobanu, V.; et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014, 515, 568–571. [Google Scholar] [CrossRef]
- Fontugne, J.; Augustin, J.; Pujals, A.; Compagnon, P.; Rousseau, B.; Luciani, A.; Tournigand, C.; Cherqui, D.; Azoulay, D.; Pawlotsky, J.M.; et al. PD-L1 expression in perihilar and intrahepatic cholangiocarcinoma. Oncotarget 2017, 8, 24644–24651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goeppert, B.; Frauenschuh, L.; Zucknick, M.; Stenzinger, A.; Andrulis, M.; Klauschen, F.; Joehrens, K.; Warth, A.; Renner, M.; Mehrabi, A.; et al. Prognostic impact of tumour-infiltrating immune cells on biliary tract cancer. Br. J. Cancer 2013, 109, 2665–2674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Zhou, Y.; Hu, K.; Wang, D.; Wang, Z.; Huang, Y. Perineural invasion as a prognostic factor for intrahepatic cholangiocarcinoma after curative resection and a potential indication for postoperative chemotherapy: A retrospective cohort study. BMC Cancer 2020, 20, 270. [Google Scholar] [CrossRef] [PubMed]
- Kitano, Y.; Okabe, H.; Yamashita, Y.-i.; Nakagawa, S.; Saito, Y.; Umezaki, N.; Tsukamoto, M.; Yamao, T.; Yamamura, K.; Arima, K.; et al. Tumour-infiltrating inflammatory and immune cells in patients with extrahepatic cholangiocarcinoma. Br. J. Cancer 2018, 118, 171–180. [Google Scholar] [CrossRef] [Green Version]
- Affo, S.; Nair, A.; Brundu, F.; Ravichandra, A.; Bhattacharjee, S.; Matsuda, M.; Chin, L.; Filliol, A.; Wen, W.; Song, X.; et al. Promotion of cholangiocarcinoma growth by diverse cancer-associated fibroblast subpopulations. Cancer Cell 2021, 39, 866–882.e811. [Google Scholar] [CrossRef] [PubMed]
| Antibody | Marker | Dilution | Incubation | Theme | Manufacturer |
|---|---|---|---|---|---|
| CD8 | Cytotoxic T | 1:500 | 30 min | CySO | Dako |
| CD68 | Macrophage | 1:6000 | 30 min | Cy7E | Dako |
| PD-1/CD279 | Checkpoint | 1:250 | Over night | Cy47 | Abcam |
| PD-L1/CD274 | Checkpoint | 1:200 | Over night | 46HE | Dako |
| PD-L2/CD273 | Checkpoint | 1:400 | Over night | 43HE | Abcam |
| Variables | NFD Group | ||
|---|---|---|---|
| High (n = 15) | Low (n = 30) | p Value | |
| Demographics | |||
| Gender, m/f (%) | 10 (66.7)/5 (33.3) | 18 (60)/12 (40) | 0.664 |
| Age (years) | 70 (58–72) | 64 (55–73) | 0.563 |
| ASA, n (%) | 0.850 | ||
| I | 1 (6.7) | 1 (3.3) | |
| II | 8 (53.3) | 15 (50.0) | |
| III | 6 (40.0) | 13 (43.3) | |
| IV | 0 | 1 (3.3) | |
| Pathological examination | |||
| T category, n (%) | 0.324 | ||
| T1 | 0 | 0 | |
| T2 | 8 (53.4) | 20 (66.6) | |
| T3 | 6 (40.0) | 6 (20.0) | |
| T4 | 1 (6.7) | 4 (13.3) | |
| N category | 0.832 | ||
| N0 | 7 (46.7) | 13 (43.6) | |
| N1 | 8 (53.3) | 17 (56.7) | |
| Vascular invasion, n (%) | 2 (13.3) | 10 (33.3) | 0.225 |
| Lymphatic invasion, n (%) | 5 (33.3) | 7 (23.3) | 0.611 |
| Perineural invasion, n (%) | 10 (66.7) | 21 (87.5) | 0.733 |
| Tumor grading, n (%) | 0.085 | ||
| G1 | 0 | 0 | |
| G2 | 14 (93.3) | 20 (71.4) | |
| G3 | 0 | 7 (25.0) | |
| G4 | 0 | 1 (3.6) | |
| Multiplex Imaging Data | |||
| CD8-Panel (×10−6) | |||
| CD8+ | 319.04 (131.37–448.66) | 182.51 (118.95–347.54) | 0.195 |
| CD8+PD-1+ | 12.24 (1.04–23.06) | 1.38 (0.73–8.02) | 0.017 |
| CD8+PD-1+PD-L1+ | 0.90 (0.03–1.97) | 0.27 (0.04–0.66) | 0.228 |
| CD8+PD-1+PD-L1+PD-L2+ | 0.14 (0.00–0.52) | 0.02 (0.00–0.08) | 0.150 |
| CD8+PD-1+PD-L2+ | 0.34 (0.03–0.79) | 0.04 (0.00–0.16) | 0.044 |
| CD8+PD-L1+ | 6.53 (1.92–15.76) | 2.83 (1.16–10.42) | 0.306 |
| CD8+PD-L1+PD-L2+ | 0.58 (0.16–5.41) | 0.13 (0.05–0.80) | 0.091 |
| CD8+PD-L2+ | 3.30 (0.81–17.37) | 2.36 (0.37–4.93) | 0.097 |
| CD68-Panel (×10−6) | |||
| CD68+ | 709.33 (348.70–1475.20) | 505.81 (282.94–692.45) | 0.087 |
| CD68+PD-1+ | 5.82 (1.22–15.16) | 2.52 (0.85–5.53) | 0.140 |
| CD68+PD-1+PD-L1+ | 1.01 (0.06–1.90) | 0.37 (0.05–1.09) | 0.363 |
| CD68+PD-1+PD-L1+PLD2+ | 0.01 (0.00–0.47) | 0.02 (0.00–0.14) | 0.946 |
| CD68+PD-1+PD-L2+ | 0.11 (0.02–1.39) | 0.04 (0.00–0.53) | 0.513 |
| CD68+PD-L1+ | 15.31 (1.58–42.57) | 6.64 (3.41–13.88) | 0.195 |
| CD68+PD-L1+PD-L2+ | 1.18 (0.08–4.36) | 0.06 (0.34–0.97) | 0.120 |
| CD68+PD-L2+ | 10.24 (4.03–24.87) | 4.93 (2.56–8.84) | 0.070 |
| Follow-up Data | |||
| Recurrence-free survival (months) | 70 (48–93) | 15 (3–27) | 0.014 |
| Overall survival (months) | 90 (0–196) | 19 (12–27) | 0.037 |
| Variables | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | |
| NFD (low = 1) | 0.47 (0.21–1.06) | 0.070 | excluded | 0.595 |
| Gender (male = 1) | 1.22 (0.60–2.49) | 0.576 | ||
| Age (<65 years = 1) | 1.22 (0.61–2.46) | 0.572 | ||
| ASA (I/II = 1) | 1.14 (0.57–2.30) | 0.707 | ||
| T category (T1/T2 = 1) | 1.53 (0.76–3.09) | 0.233 | ||
| N category (N0 = 1) | 1.70 (0.83–3.49) | 0.149 | ||
| Vascular invasion (No = 1) | 1.75 (0.81–3.78) | 0.154 | ||
| Lymphatic invasion (No = 1) | 1.43 (0.65–3.15) | 0.377 | ||
| Perineural invasion (No = 1) | 1.50 (0.44–5.08) | 0.515 | ||
| Tumor grading (G1/G2 = 1) | 3.22 (1.33–7.82) | 0.010 | 3.67 (1.37–9.82) | 0.010 |
| CD8+PD-1+ (low = 1) | 0.44 (0.21–0.92) | 0.029 | 0.42 (0.19–0.92) | 0.031 |
| CD8+PD-1+PD-L2+ (low = 1) | 0.57 (0.26–1.22) | 0.145 | ||
| Variables | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | |
| NFD (low = 1) | 0.31 (0.12–0.84) | 0.021 | excluded | 0.307 |
| Gender (male = 1) | 1.16 (0.54–2.49) | 0.714 | ||
| Age (<65 years = 1) | 1.03 (0.48–2.20) | 0.941 | ||
| ASA (I/II = 1) | 1.08 (0.51–2.31) | 0.841 | ||
| T category (T1/T2 = 1) | 1.24 (.57–2.73) | 0.589 | ||
| N category (N0 = 1) | 1.53 (0.71–3.31) | 0.277 | ||
| Vascular invasion (No = 1) | 2.24 (0.97–5.16) | 0.059 | excluded | 0.085 |
| Lymphatic invasion (No = 1) | 1.46 (0.63–3.40) | 0.377 | ||
| Perineural invasion (No = 1) | 1.68 (0.39–7.28) | 0.486 | ||
| Tumor grading (G1/G2 = 1) | 4.79 (1.90–12.04) | 0.001 | 5.51 (1.98–15.33) | 0.001 |
| CD8+PD-1+ (low = 1) | 0.40 (0.18–0.89) | 0.024 | 0.40 (0.17–0.96) | 0.039 |
| CD8+PD-1+PD-L2+ (low = 1) | 0.54 (0.23–1.26) | 0.156 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, X.; Bednarsch, J.; Rosin, M.; Appinger, S.; Liu, D.; Wiltberger, G.; Garcia Vallejo, J.; Lang, S.A.; Czigany, Z.; Boroojerdi, S.; et al. PD-1+ T-Cells Correlate with Nerve Fiber Density as a Prognostic Biomarker in Patients with Resected Perihilar Cholangiocarcinoma. Cancers 2022, 14, 2190. https://doi.org/10.3390/cancers14092190
Tan X, Bednarsch J, Rosin M, Appinger S, Liu D, Wiltberger G, Garcia Vallejo J, Lang SA, Czigany Z, Boroojerdi S, et al. PD-1+ T-Cells Correlate with Nerve Fiber Density as a Prognostic Biomarker in Patients with Resected Perihilar Cholangiocarcinoma. Cancers. 2022; 14(9):2190. https://doi.org/10.3390/cancers14092190
Chicago/Turabian StyleTan, Xiuxiang, Jan Bednarsch, Mika Rosin, Simone Appinger, Dong Liu, Georg Wiltberger, Juan Garcia Vallejo, Sven Arke Lang, Zoltan Czigany, Shiva Boroojerdi, and et al. 2022. "PD-1+ T-Cells Correlate with Nerve Fiber Density as a Prognostic Biomarker in Patients with Resected Perihilar Cholangiocarcinoma" Cancers 14, no. 9: 2190. https://doi.org/10.3390/cancers14092190
APA StyleTan, X., Bednarsch, J., Rosin, M., Appinger, S., Liu, D., Wiltberger, G., Garcia Vallejo, J., Lang, S. A., Czigany, Z., Boroojerdi, S., Gaisa, N. T., Boor, P., Bülow, R. D., De Vos-Geelen, J., Valkenburg-van Iersel, L., Clahsen-van Groningen, M. C., de Jong, E. J. M., Groot Koerkamp, B., Doukas, M., ... Heij, L. R. (2022). PD-1+ T-Cells Correlate with Nerve Fiber Density as a Prognostic Biomarker in Patients with Resected Perihilar Cholangiocarcinoma. Cancers, 14(9), 2190. https://doi.org/10.3390/cancers14092190







