Preoperative Management of Perihilar Cholangiocarcinoma
Abstract
:Simple Summary
Abstract
1. Introduction
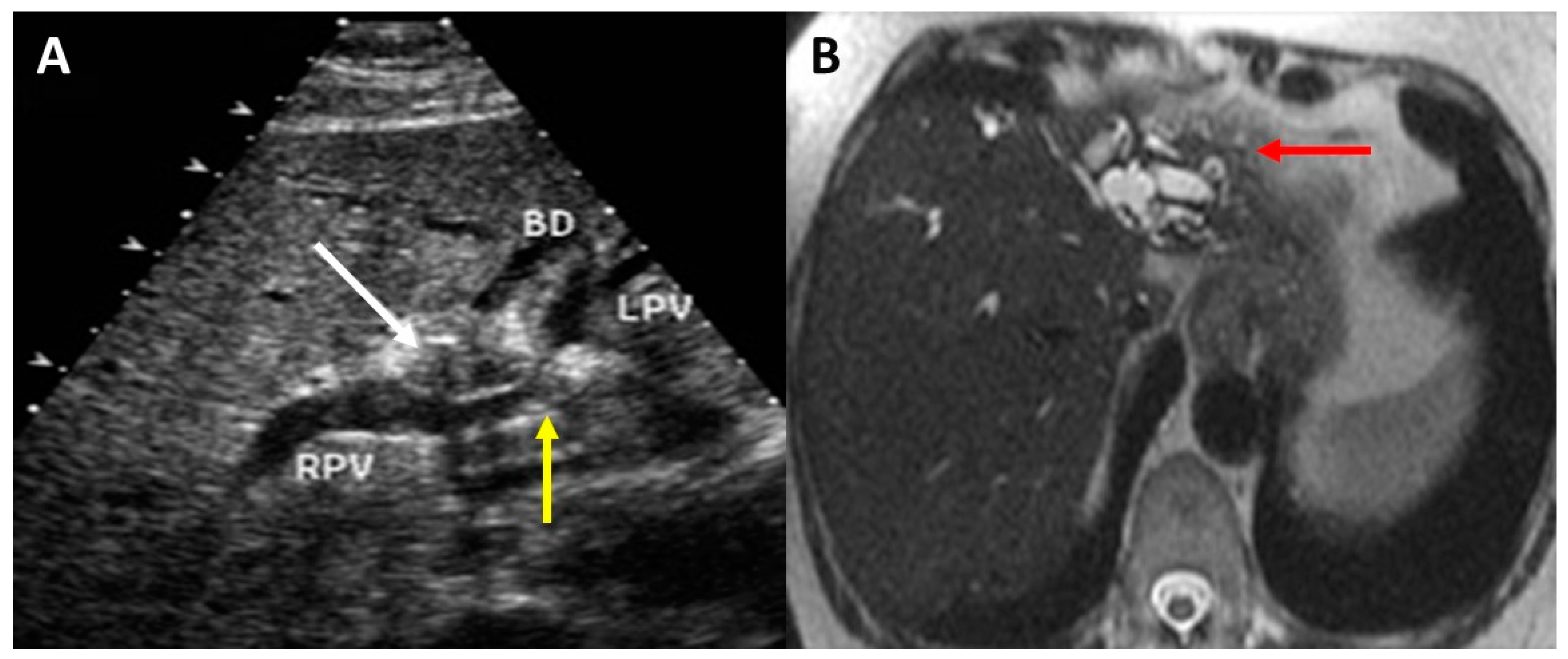
2. Preoperative Imaging
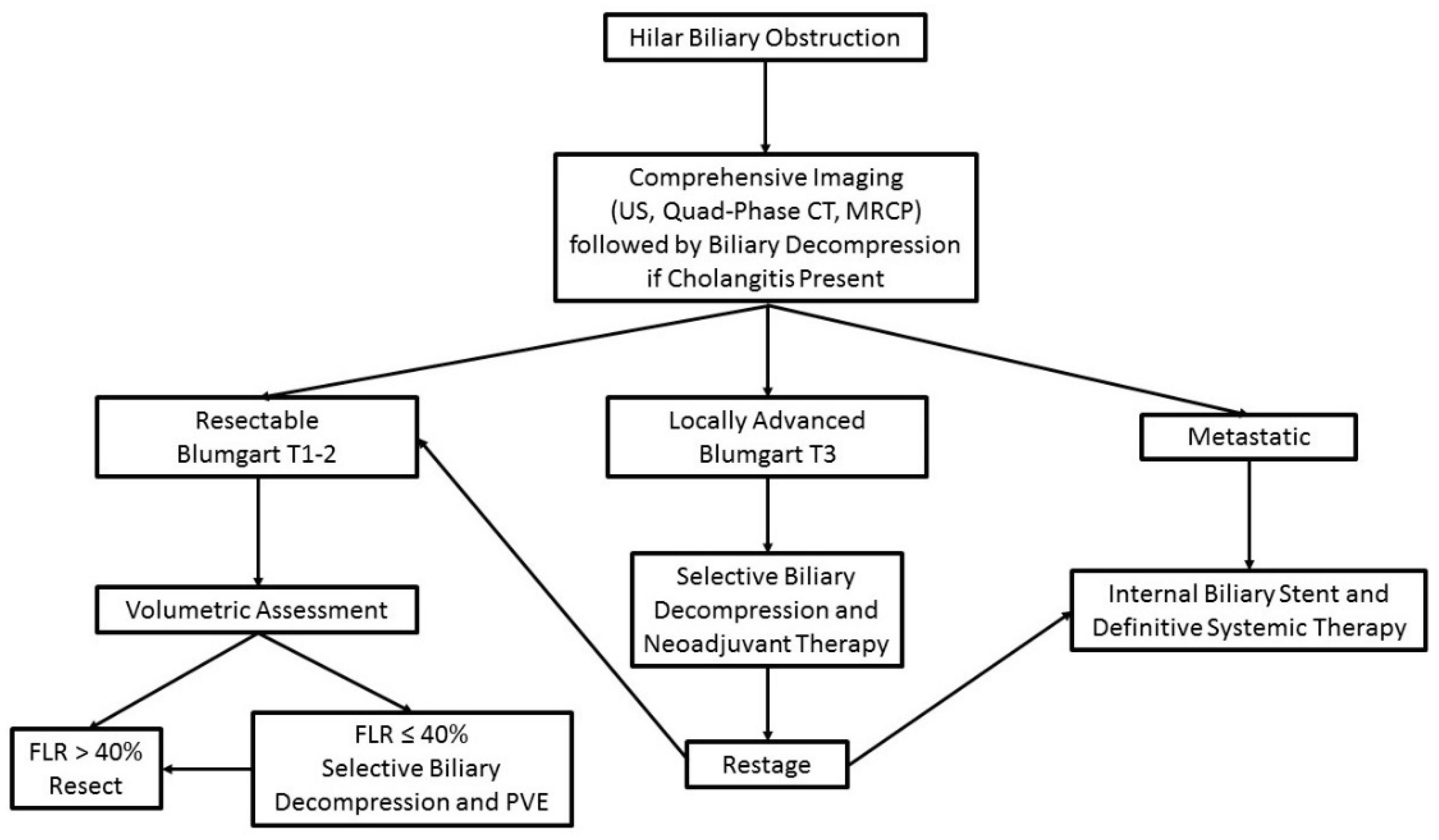
3. Assessment of Resectability and Staging
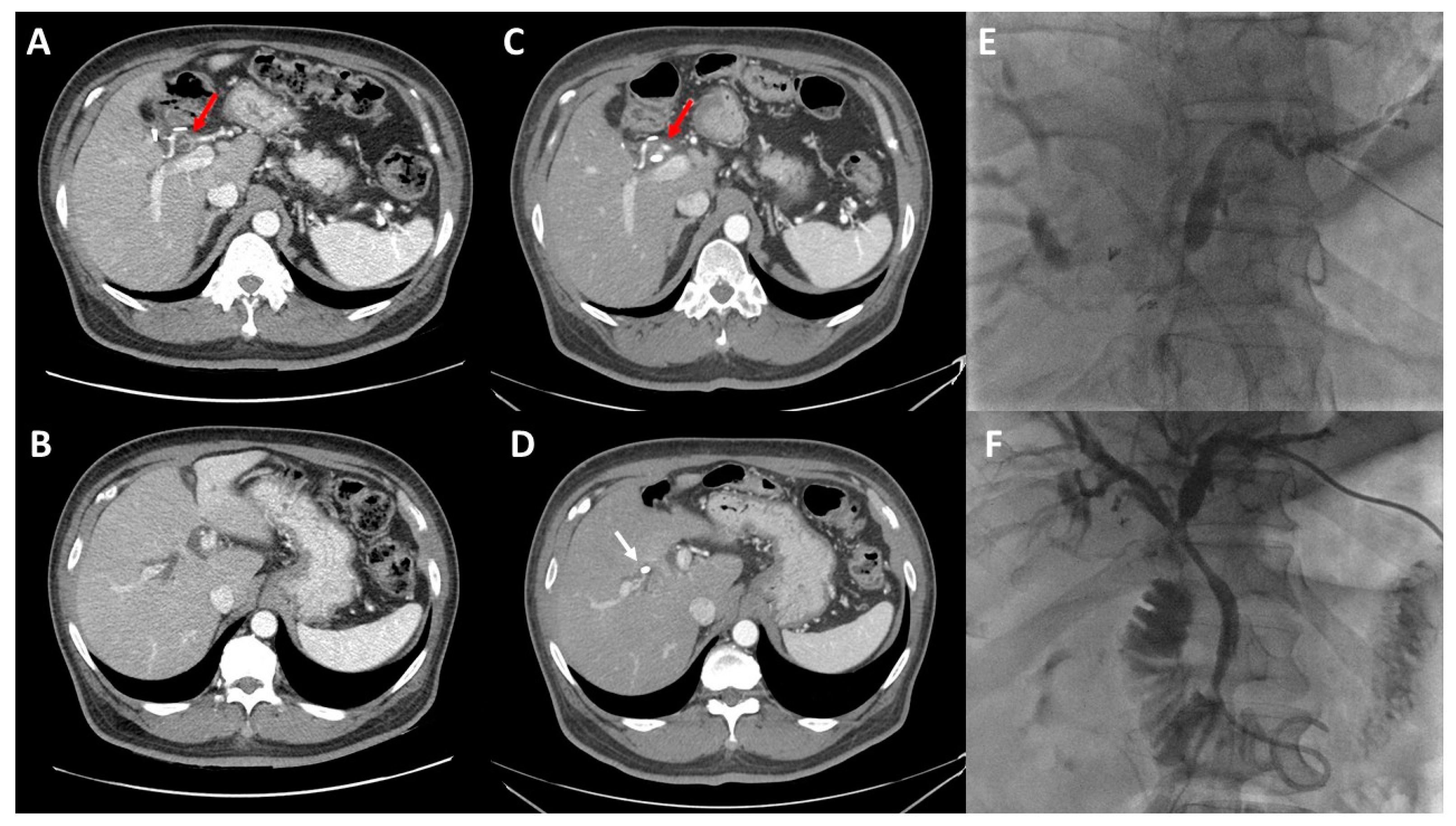
4. Principles of Biliary Drainage
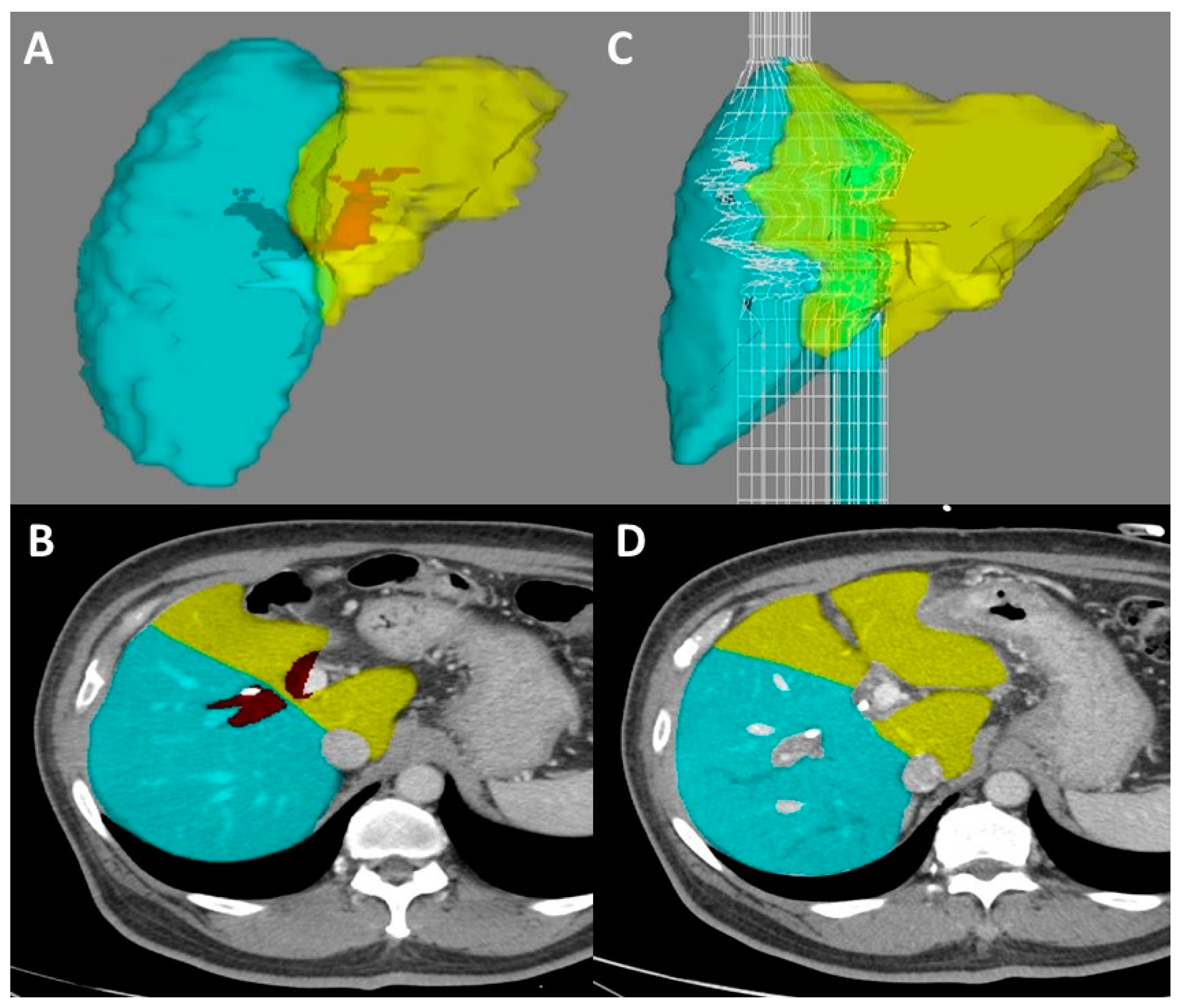
5. Volumetric Analysis and Optimization
6. Diagnostic Laparoscopy
7. Consideration of Liver Transplant
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mansour, J.C.; Aloia, T.A.; Crane, C.H.; Heimbach, J.K.; Nagino, M.; Vauthey, J.N. Hilar cholangiocarcinoma: Expert consensus statement. HPB 2015, 17, 691–699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flemming, J.A.; Zhang-Salomons, J.; Nanji, S.; Booth, C.M. Increased incidence but improved median overall survival for biliary tract cancers diagnosed in Ontario from 1994 through 2012: A population-based study. Cancer 2016, 122, 2534–2543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Groot Koerkamp, B.; Wiggers, J.K.; Allen, P.J.; Besselink, M.G.; Blumgart, L.H.; Busch, O.R.; Coelen, R.J.; D’Angelica, M.I.; DeMatteo, R.P.; Gouma, D.J.; et al. Recurrence Rate and Pattern of Perihilar Cholangiocarcinoma after Curative Intent Resection. J. Am. Coll. Surg. 2015, 221, 1041–1049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klatskin, G. Adenocarcinoma of the Hepatic Duct at Its Bifurcation within the Porta Hepatis. An Unusual Tumor with Distinctive Clinical and Pathological Features. Am. J. Med. 1965, 38, 241–256. [Google Scholar] [CrossRef]
- Belghiti, J.; Hiramatsu, K.; Benoist, S.; Massault, P.; Sauvanet, A.; Farges, O. Seven hundred forty-seven hepatectomies in the 1990s: An update to evaluate the actual risk of liver resection. J. Am. Coll. Surg. 2000, 191, 38–46. [Google Scholar] [CrossRef]
- Jarnagin, W.R.; Fong, Y.; DeMatteo, R.P.; Gonen, M.; Burke, E.C.; Bodniewicz, B.J.; Youssef, B.M.; Klimstra, D.; Blumgart, L.H. Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann. Surg. 2001, 234, 507–517. [Google Scholar] [CrossRef]
- Kingham, T.P.; Correa-Gallego, C.; D’Angelica, M.I.; Gonen, M.; DeMatteo, R.P.; Fong, Y.; Allen, P.J.; Blumgart, L.H.; Jarnagin, W.R. Hepatic parenchymal preservation surgery: Decreasing morbidity and mortality rates in 4152 resections for malignancy. J. Am. Coll. Surg. 2015, 220, 471–479. [Google Scholar] [CrossRef] [Green Version]
- Sano, T.; Shimada, K.; Sakamoto, Y.; Yamamoto, J.; Yamasaki, S.; Kosuge, T. One hundred two consecutive hepatobiliary resections for perihilar cholangiocarcinoma with zero mortality. Ann. Surg. 2006, 244, 240–247. [Google Scholar] [CrossRef]
- Groot Koerkamp, B.; Wiggers, J.K.; Gonen, M.; Doussot, A.; Allen, P.J.; Besselink, M.G.H.; Blumgart, L.H.; Busch, O.R.C.; D’Angelica, M.I.; DeMatteo, R.P.; et al. Survival after resection of perihilar cholangiocarcinoma-development and external validation of a prognostic nomogram. Ann. Oncol. 2015, 26, 1930–1935. [Google Scholar] [CrossRef]
- Ribero, D.; Zimmitti, G.; Aloia, T.A.; Shindoh, J.; Fabio, F.; Amisano, M.; Passot, G.; Ferrero, A.; Vauthey, J.N. Preoperative Cholangitis and Future Liver Remnant Volume Determine the Risk of Liver Failure in Patients Undergoing Resection for Hilar Cholangiocarcinoma. J. Am. Coll. Surg. 2016, 223, 87–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiggers, J.K.; Groot Koerkamp, B.; Cieslak, K.P.; Doussot, A.; van Klaveren, D.; Allen, P.J.; Besselink, M.G.; Busch, O.R.; D’Angelica, M.I.; DeMatteo, R.P.; et al. Postoperative Mortality after Liver Resection for Perihilar Cholangiocarcinoma: Development of a Risk Score and Importance of Biliary Drainage of the Future Liver Remnant. J. Am. Coll. Surg. 2016, 223, 321–331.e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bloom, C.M.; Langer, B.; Wilson, S.R. Role of US in the detection, characterization, and staging of cholangiocarcinoma. Radiographics 1999, 19, 1199–1218. [Google Scholar] [CrossRef] [PubMed]
- Aloia, T.A.; Charnsangavej, C.; Faria, S.; Ribero, D.; Abdalla, E.K.; Vauthey, J.N.; Curley, S.A. High-resolution computed tomography accurately predicts resectability in hilar cholangiocarcinoma. Am. J. Surg. 2007, 193, 702–706. [Google Scholar] [CrossRef]
- Ruys, A.T.; van Beem, B.E.; Engelbrecht, M.R.; Bipat, S.; Stoker, J.; Van Gulik, T.M. Radiological staging in patients with hilar cholangiocarcinoma: A systematic review and meta-analysis. Br. J. Radiol. 2012, 85, 1255–1262. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.Y.; Kim, S.H.; Lee, J.M.; Kim, S.W.; Jang, J.Y.; Han, J.K.; Choi, B.I. Preoperative assessment of resectability of hepatic hilar cholangiocarcinoma: Combined CT and cholangiography with revised criteria. Radiology 2006, 239, 113–121. [Google Scholar] [CrossRef]
- Joo, I.; Lee, J.M.; Yoon, J.H. Imaging Diagnosis of Intrahepatic and Perihilar Cholangiocarcinoma: Recent Advances and Challenges. Radiology 2018, 288, 7–13. [Google Scholar] [CrossRef] [Green Version]
- Bach, A.M.; Hann, L.E.; Brown, K.T.; Getrajdman, G.I.; Herman, S.K.; Fong, Y.; Blumgart, L.H. Portal vein evaluation with US: Comparison to angiography combined with CT arterial portography. Radiology 1996, 201, 149–154. [Google Scholar] [CrossRef]
- Lamarca, A.; Barriuso, J.; Chander, A.; McNamara, M.G.; Hubner, R.A.; ÓReilly, D.; Manoharan, P.; Valle, J.W. 18F-fluorodeoxyglucose positron emission tomography (18FDG-PET) for patients with biliary tract cancer: Systematic review and meta-analysis. J. Hepatol. 2019, 71, 115–129. [Google Scholar] [CrossRef] [Green Version]
- Wiggers, J.K.; Koerkamp, B.G.; van Klaveren, D.; Coelen, R.J.; Nio, C.Y.; Allen, P.J.; Besselink, M.G.; Busch, O.R.; D’Angelica, M.I.; DeMatteo, R.P.; et al. Preoperative Risk Score to Predict Occult Metastatic or Locally Advanced Disease in Patients with Resectable Perihilar Cholangiocarcinoma on Imaging. J. Am. Coll. Surg. 2018, 227, 238–246.e2. [Google Scholar] [CrossRef]
- Roos, E.; Hubers, L.M.; Coelen, R.J.S.; Doorenspleet, M.E.; de Vries, N.; Verheij, J.; Beuers, U.; van Gulik, T.M. IgG4-Associated Cholangitis in Patients Resected for Presumed Perihilar Cholangiocarcinoma: A 30-Year Tertiary Care Experience. Am. J. Gastroenterol. 2018, 113, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, S.; Ebata, T.; Yokoyama, Y.; Igami, T.; Mizuno, T.; Yamaguchi, J.; Onoe, S.; Watanabe, N.; Shimoyama, Y.; Nagino, M. Benign hilar bile duct strictures resected as perihilar cholangiocarcinoma. Br. J. Surg. 2019, 106, 1504–1511. [Google Scholar] [CrossRef] [PubMed]
- Rassam, F.; Roos, E.; van Lienden, K.P.; van Hooft, J.E.; Klumpen, H.J.; van Tienhoven, G.; Bennink, R.J.; Engelbrecht, M.R.; Schoorlemmer, A.; Beuers, U.H.W.; et al. Modern work-up and extended resection in perihilar cholangiocarcinoma: The AMC experience. Langenbecks Arch. Surg. 2018, 403, 289–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glazer, E.S.; Liu, P.; Abdalla, E.K.; Vauthey, J.N.; Curley, S.A. Neither neoadjuvant nor adjuvant therapy increases survival after biliary tract cancer resection with wide negative margins. J. Gastrointest. Surg. 2012, 16, 1666–1671. [Google Scholar] [CrossRef] [Green Version]
- Grendar, J.; Grendarova, P.; Sinha, R.; Dixon, E. Neoadjuvant therapy for downstaging of locally advanced hilar cholangiocarcinoma: A systematic review. HPB 2014, 16, 297–303. [Google Scholar] [CrossRef] [Green Version]
- Bismuth, H.; Nakache, R.; Diamond, T. Management strategies in resection for hilar cholangiocarcinoma. Ann. Surg. 1992, 215, 31–38. [Google Scholar] [CrossRef]
- Burke, E.C.; Jarnagin, W.R.; Hochwald, S.N.; Pisters, P.W.; Fong, Y.; Blumgart, L.H. Hilar Cholangiocarcinoma: Patterns of spread, the importance of hepatic resection for curative operation, and a presurgical clinical staging system. Ann. Surg. 1998, 228, 385–394. [Google Scholar] [CrossRef]
- Zaydfudim, V.M.; Clark, C.J.; Kendrick, M.L.; Que, F.G.; Reid-Lombardo, K.M.; Donohue, J.H.; Farnell, M.B.; Nagorney, D.M. Correlation of staging systems to survival in patients with resected hilar cholangiocarcinoma. Am. J. Surg. 2013, 206, 159–165. [Google Scholar] [CrossRef]
- Matsuo, K.; Rocha, F.G.; Ito, K.; D’Angelica, M.I.; Allen, P.J.; Fong, Y.; Dematteo, R.P.; Gonen, M.; Endo, I.; Jarnagin, W.R. The Blumgart preoperative staging system for hilar cholangiocarcinoma: Analysis of resectability and outcomes in 380 patients. J. Am. Coll. Surg. 2012, 215, 343–355. [Google Scholar] [CrossRef]
- Kawasaki, S.; Imamura, H.; Kobayashi, A.; Noike, T.; Miwa, S.; Miyagawa, S. Results of surgical resection for patients with hilar bile duct cancer: Application of extended hepatectomy after biliary drainage and hemihepatic portal vein embolization. Ann. Surg. 2003, 238, 84–92. [Google Scholar] [CrossRef]
- Kennedy, T.J.; Yopp, A.; Qin, Y.; Zhao, B.; Guo, P.; Liu, F.; Schwartz, L.H.; Allen, P.; D’Angelica, M.; Fong, Y.; et al. Role of preoperative biliary drainage of liver remnant prior to extended liver resection for hilar cholangiocarcinoma. HPB 2009, 11, 445–451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iacono, C.; Ruzzenente, A.; Campagnaro, T.; Bortolasi, L.; Valdegamberi, A.; Guglielmi, A. Role of preoperative biliary drainage in jaundiced patients who are candidates for pancreatoduodenectomy or hepatic resection: Highlights and drawbacks. Ann. Surg. 2013, 257, 191–204. [Google Scholar] [CrossRef] [PubMed]
- Cherqui, D.; Benoist, S.; Malassagne, B.; Humeres, R.; Rodriguez, V.; Fagniez, P.L. Major liver resection for carcinoma in jaundiced patients without preoperative biliary drainage. Arch. Surg. 2000, 135, 302–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lidsky ME and Jarnagin, W.R. Surgical management of hilar cholangiocarcinoma at Memorial Sloan Kettering Cancer Center. Ann. Gastroenterol. Surg. 2018, 2, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Hochwald, S.N.; Burke, E.C.; Jarnagin, W.R.; Fong, Y.; Blumgart, L.H. Association of preoperative biliary stenting with increased postoperative infectious complications in proximal cholangiocarcinoma. Arch. Surg. 1999, 134, 261–266. [Google Scholar] [CrossRef] [Green Version]
- Yokoyama, Y.; Ebata, T.; Igami, T.; Sugawara, G.; Mizuno, T.; Nagino, M. The adverse effects of preoperative cholangitis on the outcome of portal vein embolization and subsequent major hepatectomies. Surgery 2014, 156, 1190–1196. [Google Scholar] [CrossRef]
- Giuliante, F.; Ardito, F.; Aldrighetti, L.; Ferrero, A.; Pinna, A.D.; De Carlis, L.; Cillo, U.; Jovine, E.; Portolani, N.; Gruttadauria, S.; et al. Liver resection for perihilar cholangiocarcinoma: Impact of biliary drainage failure on postoperative outcome. Results of an Italian multicenter study. Surgery 2021, 170, 383–389. [Google Scholar] [CrossRef]
- Coelen, R.J.S.; Roos, E.; Wiggers, J.K.; Besselink, M.G.; Buis, C.I.; Busch, O.R.C.; Dejong, C.H.C.; van Delden, O.M.; van Eijck, C.H.J.; Fockens, P.; et al. Endoscopic versus percutaneous biliary drainage in patients with resectable perihilar cholangiocarcinoma: A multicentre, randomised controlled trial. Lancet Gastroenterol. Hepatol. 2018, 3, 681–690. [Google Scholar] [CrossRef]
- Bednarsch, J.; Czigany, Z.; Heij, L.R.; Luedde, T.; van Dam, R.; Lang, S.A.; Ulmer, T.F.; Hornef, M.W.; Neumann, U.P. Bacterial bile duct colonization in perihilar cholangiocarcinoma and its clinical significance. Sci. Rep. 2021, 11, 2926. [Google Scholar] [CrossRef]
- Dondorf, F.; Graf, M.; Deeb, A.A.; Rohland, O.; Felgendreff, P.; Ardelt, M.; Settmacher, U.; Rauchfuss, F. Pathogen detection in patients with perihilar cholangiocarcinoma: Implications for targeted perioperative antibiotic therapy. Hepatobiliary Pancreat. Dis. Int. 2022. [Google Scholar] [CrossRef]
- Inamdar, S.; Slattery, E.; Bhalla, R.; Sejpal, D.V.; Trindade, A.J. Comparison of Adverse Events for Endoscopic vs Percutaneous Biliary Drainage in the Treatment of Malignant Biliary Tract Obstruction in an Inpatient National Cohort. JAMA Oncol. 2016, 2, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Hameed, A.; Pang, T.; Chiou, J.; Pleass, H.; Lam, V.; Hollands, M.; Johnston, E.; Richardson, A.; Yuen, L. Percutaneous vs. endoscopic pre-operative biliary drainage in hilar cholangiocarcinoma—A systematic review and meta-analysis. HPB 2016, 18, 400–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, X.Q.; Dong, J.H.; Jiang, K.; Huang, X.Q.; Zhang, W.Z. Comparison of percutaneous transhepatic biliary drainage and endoscopic biliary drainage in the management of malignant biliary tract obstruction: A meta-analysis. Dig. Endosc. 2015, 27, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Wiggers, J.K.; Groot Koerkamp, B.; Coelen, R.J.; Rauws, E.A.; Schattner, M.A.; Nio, C.Y.; Brown, K.T.; Gonen, M.; van Dieren, S.; van Lienden, K.P.; et al. Preoperative biliary drainage in perihilar cholangiocarcinoma: Identifying patients who require percutaneous drainage after failed endoscopic drainage. Endoscopy 2015, 47, 1124–1131. [Google Scholar] [CrossRef] [Green Version]
- Kubota, K.; Hasegawa, S.; Iwasaki, A.; Sato, T.; Fujita, Y.; Hosono, K.; Nakajima, A.; Mori, R.; Matsuyama, R.; Endo, I. Stent placement above the sphincter of Oddi permits implementation of neoadjuvant chemotherapy in patients with initially unresectable Klatskin tumor. Endosc. Int. Open 2016, 4, E427–E433. [Google Scholar] [CrossRef] [Green Version]
- Komaya, K.; Ebata, T.; Yokoyama, Y.; Igami, T.; Sugawara, G.; Mizuno, T.; Yamaguchi, J.; Nagino, M. Verification of the oncologic inferiority of percutaneous biliary drainage to endoscopic drainage: A propensity score matching analysis of resectable perihilar cholangiocarcinoma. Surgery 2017, 161, 394–404. [Google Scholar] [CrossRef]
- Wiggers, J.K.; Groot Koerkamp, B.; Coelen, R.J.; Doussot, A.; van Dieren, S.; Rauws, E.A.; Schattner, M.A.; van Lienden, K.P.; Brown, K.T.; Besselink, M.G.; et al. Percutaneous Preoperative Biliary Drainage for Resectable Perihilar Cholangiocarcinoma: No Association with Survival and No Increase in Seeding Metastases. Ann. Surg. Oncol. 2015, 22 (Suppl. 3), S1156–S1163. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.F.; Beal, E.W.; Merath, K.; Ethun, C.G.; Salem, A.; Weber, S.M.; Tran, T.; Poultsides, G.; Son, A.Y.; Hatzaras, I.; et al. Oncologic effects of preoperative biliary drainage in resectable hilar cholangiocarcinoma: Percutaneous biliary drainage has no adverse effects on survival. J. Surg. Oncol. 2018, 117, 1267–1277. [Google Scholar] [CrossRef]
- Elmunzer, B.J.; Smith, Z.L.; Tarnasky, P.; Wang, A.Y.; Yachimski, P.; Banovac, F.; Buscaglia, J.M.; Buxbaum, J.; Chak, A.; Chong, B.; et al. An Unsuccessful Randomized Trial of Percutaneous vs Endoscopic Drainage of Suspected Malignant Hilar Obstruction. Clin. Gastroenterol. Hepatol. 2021, 19, 1282–1284. [Google Scholar] [CrossRef]
- Kawakubo, K.; Kawakami, H.; Kuwatani, M.; Haba, S.; Kudo, T.; Taya, Y.A.; Kawahata, S.; Kubota, Y.; Kubo, K.; Eto, K.; et al. Lower incidence of complications in endoscopic nasobiliary drainage for hilar cholangiocarcinoma. World J. Gastrointest. Endosc. 2016, 8, 385–390. [Google Scholar] [CrossRef]
- Nakai, Y.; Yamamoto, R.; Matsuyama, M.; Sakai, Y.; Takayama, Y.; Ushio, J.; Ito, Y.; Kitamura, K.; Ryozawa, S.; Imamura, T.; et al. Multicenter study of endoscopic preoperative biliary drainage for malignant hilar biliary obstruction: E-POD hilar study. J. Gastroenterol. Hepatol. 2018, 33, 1146–1153. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.X.; Wang, S.P.; Wu, J.; Gao, D.J.; Ye, X.; Wang, T.T.; Zhao, Y.; Hu, B. The risk of acute cholangitis after endoscopic stenting for malignant hilar strictures: A large comprehensive study. J. Gastroenterol. Hepatol. 2020, 35, 1150–1157. [Google Scholar] [CrossRef]
- Vauthey, J.N.; Chaoui, A.; Do, K.A.; Bilimoria, M.M.; Fenstermacher, M.J.; Charnsangavej, C.; Hicks, M.; Alsfasser, G.; Lauwers, G.; Hawkins, I.F.; et al. Standardized measurement of the future liver remnant prior to extended liver resection: Methodology and clinical associations. Surgery 2000, 127, 512–519. [Google Scholar] [CrossRef]
- Shoup, M.; Gonen, M.; D’Angelica, M.; Jarnagin, W.R.; DeMatteo, R.P.; Schwartz, L.H.; Tuorto, S.; Blumgart, L.H.; Fong, Y. Volumetric analysis predicts hepatic dysfunction in patients undergoing major liver resection. J. Gastrointest. Surg. 2003, 7, 325–330. [Google Scholar] [CrossRef]
- Abdalla, E.K.; Denys, A.; Chevalier, P.; Nemr, R.A.; Vauthey, J.N. Total and segmental liver volume variations: Implications for liver surgery. Surgery 2004, 135, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Hemming, A.W.; Reed, A.I.; Howard, R.J.; Fujita, S.; Hochwald, S.N.; Caridi, J.G.; Hawkins, I.F.; Vauthey, J.N. Preoperative portal vein embolization for extended hepatectomy. Ann. Surg. 2003, 237, 686–691. [Google Scholar] [CrossRef]
- Kubota, K.; Makuuchi, M.; Kusaka, K.; Kobayashi, T.; Miki, K.; Hasegawa, K.; Harihara, Y.; Takayama, T. Measurement of liver volume and hepatic functional reserve as a guide to decision-making in resectional surgery for hepatic tumors. Hepatology 1997, 26, 1176–1181. [Google Scholar]
- Makuuchi, M.; Thai, B.L.; Takayasu, K.; Takayama, T.; Kosuge, T.; Gunven, P.; Yamazaki, S.; Hasegawa, H.; Ozaki, H. Preoperative portal embolization to increase safety of major hepatectomy for hilar bile duct carcinoma: A preliminary report. Surgery 1990, 107, 521–527. [Google Scholar]
- Glantzounis, G.K.; Tokidis, E.; Basourakos, S.P.; Ntzani, E.E.; Lianos, G.D.; Pentheroudakis, G. The role of portal vein embolization in the surgical management of primary hepatobiliary cancers. A systematic review. Eur J. Surg. Oncol. 2017, 43, 32–41. [Google Scholar] [CrossRef]
- Olthof, P.B.; Aldrighetti, L.; Alikhanov, R.; Cescon, M.; Groot Koerkamp, B.; Jarnagin, W.R.; Nadalin, S.; Pratschke, J.; Schmelze, M.; Sparrelid, E.; et al. Portal Vein Embolization is Associated with Reduced Liver Failure and Mortality in High-Risk Resections for Perihilar Cholangiocarcinoma. Ann. Surg. Oncol. 2020, 27, 2311–2318. [Google Scholar] [CrossRef] [Green Version]
- Shindoh, J.; Truty, M.J.; Aloia, T.A.; Curley, S.A.; Zimmitti, G.; Huang, S.Y.; Mahvash, A.; Gupta, S.; Wallace, M.J.; Vauthey, J.N. Kinetic growth rate after portal vein embolization predicts posthepatectomy outcomes: Toward zero liver-related mortality in patients with colorectal liver metastases and small future liver remnant. J. Am. Coll. Surg. 2013, 216, 201–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leung, U.; Simpson, A.L.; Araujo, R.L.; Gonen, M.; McAuliffe, C.; Miga, M.I.; Parada, E.P.; Allen, P.J.; D’Angelica, M.I.; Kingham, T.P.; et al. Remnant growth rate after portal vein embolization is a good early predictor of post-hepatectomy liver failure. J. Am. Coll. Surg. 2014, 219, 620–630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Santibanes, E.; Alvarez, F.A.; Ardiles, V. How to avoid postoperative liver failure: A novel method. World J. Surg. 2012, 36, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.; Lee, S.G.; Ko, G.Y.; Kim, B.S.; Sung, K.B.; Kim, M.H.; Lee, S.K.; Hong, H.N. Sequential preoperative ipsilateral hepatic vein embolization after portal vein embolization to induce further liver regeneration in patients with hepatobiliary malignancy. Ann. Surg. 2009, 249, 608–616. [Google Scholar] [CrossRef] [PubMed]
- Schnitzbauer, A.A.; Lang, S.A.; Goessmann, H.; Nadalin, S.; Baumgart, J.; Farkas, S.A.; Fichtner-Feigl, S.; Lorf, T.; Goralcyk, A.; Horbelt, R.; et al. Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings. Ann. Surg. 2012, 255, 405–414. [Google Scholar] [CrossRef]
- Olthof, P.B.; Coelen, R.J.S.; Wiggers, J.K.; Groot Koerkamp, B.; Malago, M.; Hernandez-Alejandro, R.; Topp, S.A.; Vivarelli, M.; Aldrighetti, L.A.; Robles Campos, R.; et al. High mortality after ALPPS for perihilar cholangiocarcinoma: Case-control analysis including the first series from the international ALPPS registry. HPB 2017, 19, 381–387. [Google Scholar] [CrossRef] [Green Version]
- Nagino, M. Value of ALPPS in surgery for Klatskin tumours. Br. J. Surg. 2019, 106, 1574–1575. [Google Scholar] [CrossRef]
- Guiu, B.; Chevallier, P.; Denys, A.; Delhom, E.; Pierredon-Foulongne, M.A.; Rouanet, P.; Fabre, J.M.; Quenet, F.; Herrero, A.; Panaro, F.; et al. Simultaneous trans-hepatic portal and hepatic vein embolization before major hepatectomy: The liver venous deprivation technique. Eur. Radiol. 2016, 26, 4259–4267. [Google Scholar] [CrossRef]
- Heil, J.; Korenblik, R.; Heid, F.; Bechstein, W.O.; Bemelmans, M.; Binkert, C.; Bjornsson, B.; Breitenstein, S.; Detry, O.; Dili, A.; et al. Preoperative portal vein or portal and hepatic vein embolization: DRAGON collaborative group analysis. Br. J. Surg. 2021, 108, 834–842. [Google Scholar] [CrossRef]
- Jarnagin, W.R.; Bowne, W.; Klimstra, D.S.; Ben-Porat, L.; Roggin, K.; Cymes, K.; Fong, Y.; DeMatteo, R.P.; D’Angelica, M.; Koea, J.; et al. Papillary phenotype confers improved survival after resection of hilar cholangiocarcinoma. Ann. Surg. 2005, 241, 703–712. [Google Scholar] [CrossRef]
- Bird, N.; Elmasry, M.; Jones, R.; Elniel, M.; Kelly, M.; Palmer, D.; Fenwick, S.; Poston, G.; Malik, H. Role of staging laparoscopy in the stratification of patients with perihilar cholangiocarcinoma. Br. J. Surg. 2017, 104, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Weber, S.M.; DeMatteo, R.P.; Fong, Y.; Blumgart, L.H.; Jarnagin, W.R. Staging laparoscopy in patients with extrahepatic biliary carcinoma. Analysis of 100 patients. Ann. Surg. 2002, 235, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Coelen, R.J.; Ruys, A.T.; Besselink, M.G.; Busch, O.R.; van Gulik, T.M. Diagnostic accuracy of staging laparoscopy for detecting metastasized or locally advanced perihilar cholangiocarcinoma: A systematic review and meta-analysis. Surg. Endosc. 2016, 30, 4163–4173. [Google Scholar] [CrossRef] [PubMed]
- Meyer, C.G.; Penn, I.; James, L. Liver transplantation for cholangiocarcinoma: Results in 207 patients. Transplantation 2000, 69, 1633–1637. [Google Scholar] [CrossRef]
- De Vreede, I.; Steers, J.L.; Burch, P.A.; Rosen, C.B.; Gunderson, L.L.; Haddock, M.G.; Burgart, L.; Gores, G.J. Prolonged disease-free survival after orthotopic liver transplantation plus adjuvant chemoirradiation for cholangiocarcinoma. Liver Transpl. 2000, 6, 309–316. [Google Scholar] [CrossRef]
- Anderson, B.; Doyle, M.B.M. Surgical Considerations of Hilar Cholangiocarcinoma. Surg. Oncol. Clin. N. Am. 2019, 28, 601–617. [Google Scholar] [CrossRef]
- Rosen, C.B.; Heimbach, J.K.; Gores, G.J. Surgery for cholangiocarcinoma: The role of liver transplantation. HPB 2008, 10, 186–189. [Google Scholar] [CrossRef] [Green Version]
- Croome, K.P.; Rosen, C.B.; Heimbach, J.K.; Nagorney, D.M. Is Liver Transplantation Appropriate for Patients with Potentially Resectable De Novo Hilar Cholangiocarcinoma? J. Am. Coll. Surg. 2015, 221, 130–139. [Google Scholar] [CrossRef]
- Ethun, C.G.; Lopez-Aguiar, A.G.; Anderson, D.J.; Adams, A.B.; Fields, R.C.; Doyle, M.B.; Chapman, W.C.; Krasnick, B.A.; Weber, S.M.; Mezrich, J.D.; et al. Transplantation Versus Resection for Hilar Cholangiocarcinoma: An Argument for Shifting Treatment Paradigms for Resectable Disease. Ann. Surg. 2018, 267, 797–805. [Google Scholar] [CrossRef]
| Stage | Definition |
|---|---|
| T1 | Tumor involving confluence with or without unilateral extension to second-order biliary radicles AND No evidence of vascular involvement or hepatic atrophy |
| T2 | Tumor involving confluence with or without unilateral extension to second-order biliary radicles AND Ipsilateral portal vein involvement and/or ipsilateral hepatic lobar atrophy |
| T3 | Tumor involving confluence with bilateral extension to second-order biliary radicles OR Unilateral extension into second-order biliary radicles with contralateral portal vein involvement and/or hepatic lobar atrophy OR Main/bilateral portal vein involvement |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ellis, R.J.; Soares, K.C.; Jarnagin, W.R. Preoperative Management of Perihilar Cholangiocarcinoma. Cancers 2022, 14, 2119. https://doi.org/10.3390/cancers14092119
Ellis RJ, Soares KC, Jarnagin WR. Preoperative Management of Perihilar Cholangiocarcinoma. Cancers. 2022; 14(9):2119. https://doi.org/10.3390/cancers14092119
Chicago/Turabian StyleEllis, Ryan J., Kevin C. Soares, and William R. Jarnagin. 2022. "Preoperative Management of Perihilar Cholangiocarcinoma" Cancers 14, no. 9: 2119. https://doi.org/10.3390/cancers14092119
APA StyleEllis, R. J., Soares, K. C., & Jarnagin, W. R. (2022). Preoperative Management of Perihilar Cholangiocarcinoma. Cancers, 14(9), 2119. https://doi.org/10.3390/cancers14092119






