Intake Patterns of Specific Alcoholic Beverages by Prostate Cancer Status
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Measurements
2.3. Statistical Analyses
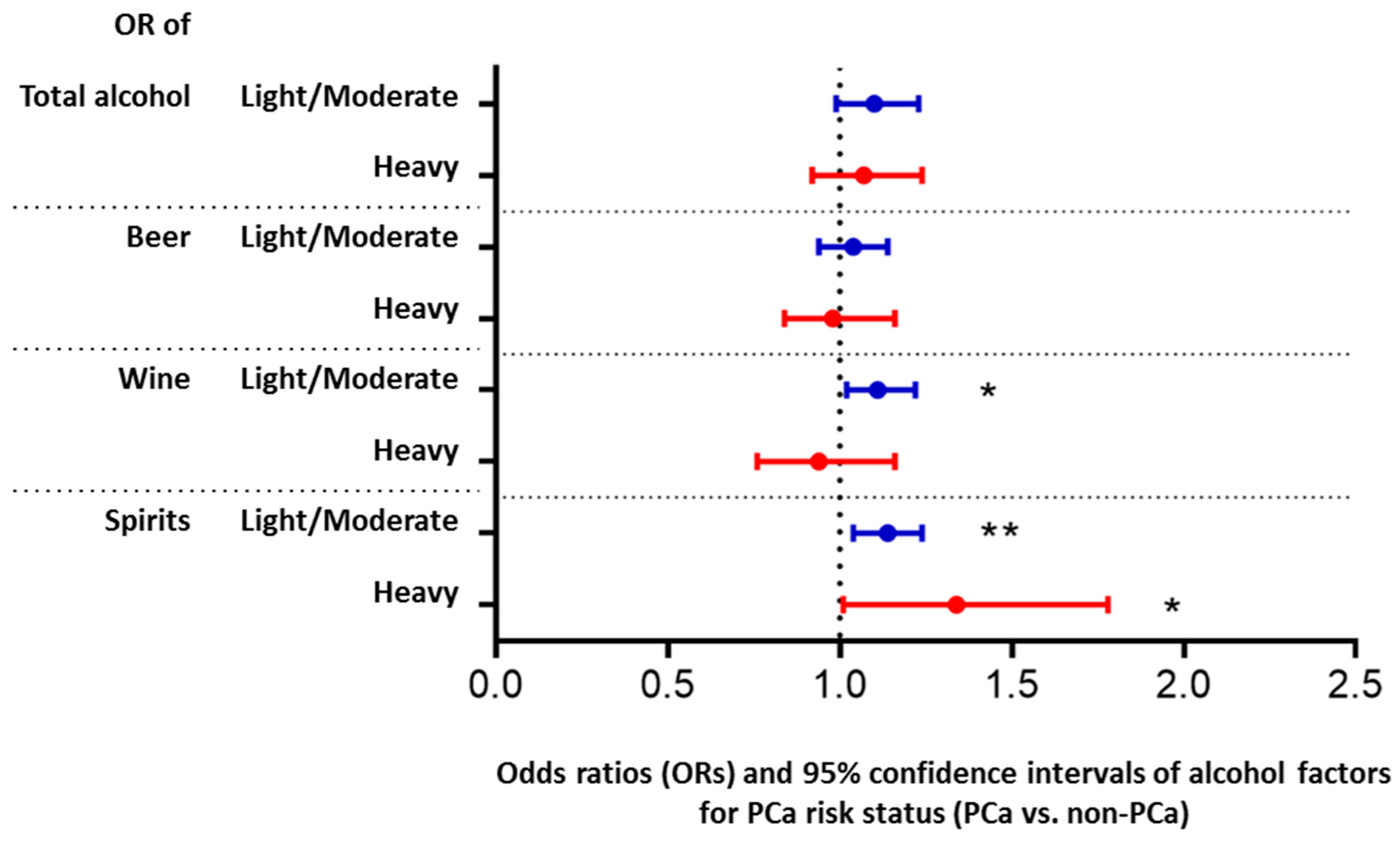
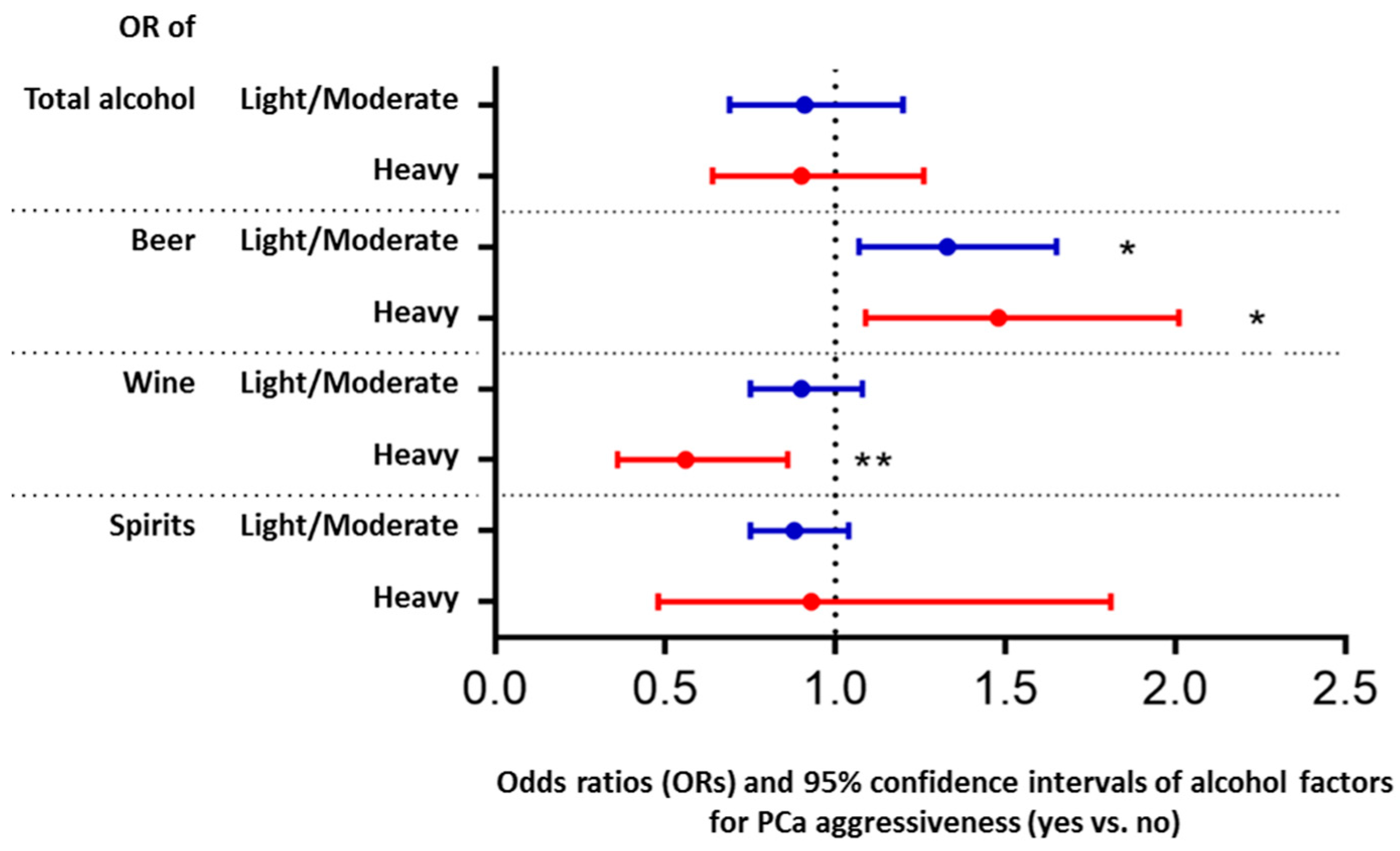
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Stockwell, T.; Roemer, A.; Chikritzhs, T. Is alcohol consumption a risk factor for prostate cancer? A systematic review and meta-analysis. BMC Cancer 2016, 16, 845. [Google Scholar] [CrossRef] [PubMed]
- Demoury, C.; Karakiewicz, P.; Parent, M.E. Association between lifetime alcohol consumption and prostate cancer risk: A case-control study in Montreal, Canada. Cancer Epidemiol. 2016, 45, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Khil, H.; Lee, D.H.; Keum, N.; Giovannucci, E.L. Alcohol Consumption and the Risk of Prostate Cancer: A Dose-Response Meta-Analysis. Nutrients 2020, 12, 2188. [Google Scholar] [CrossRef] [PubMed]
- Ziouziou, I.; Touzani, A.M.; Lahlou, L.; Shariat, S.F.; Sanguedolce, F.; Neuzillet, Y.; Ajdi, F.; Khabbal, Y. Association of Prostate Cancer with Nuts, Seeds, Alcohol and Processed Meats: A Worldwide Population-Based Study. Nutr. Cancer 2021, 73, 2538–2545. [Google Scholar] [CrossRef]
- Bagnardi, V.; Rota, M.; Botteri, E.; Tramacere, I.; Islami, F.; Fedirko, V.; Scotti, L.; Jenab, M.; Turati, F.; Pasquali, E.; et al. Alcohol consumption and site-specific cancer risk: A comprehensive dose-response meta-analysis. Br. J. Cancer 2015, 112, 580–593. [Google Scholar] [CrossRef]
- Watters, J.L.; Park, Y.; Hollenbeck, A.; Schatzkin, A.; Albanes, D. Alcoholic beverages and prostate cancer in a prospective US cohort study. Am. J. Epidemiol. 2010, 172, 773–780. [Google Scholar] [CrossRef]
- Rohrmann, S.; Linseisen, J.; Key, T.J.; Jensen, M.K.; Overvad, K.; Johnsen, N.F.; Tjonneland, A.; Kaaks, R.; Bergmann, M.M.; Weikert, C.; et al. Alcohol consumption and the risk for prostate cancer in the European Prospective Investigation into Cancer and Nutrition. Cancer Epidemiol. Biomark. Prev. 2008, 17, 1282–1287. [Google Scholar] [CrossRef]
- Lumey, L.H.; Pittman, B.; Wynder, E.L. Alcohol use and prostate cancer in U.S. whites: No association in a confirmatory study. Prostate 1998, 36, 250–255. [Google Scholar] [CrossRef]
- Chang, E.T.; Hedelin, M.; Adami, H.O.; Gronberg, H.; Balter, K.A. Alcohol drinking and risk of localized versus advanced and sporadic versus familial prostate cancer in Sweden. Cancer Causes Control 2005, 16, 275–284. [Google Scholar] [CrossRef][Green Version]
- U.S. Department of Agriculture; U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2020–2025, 9th ed.; USDA: Alexandria, VA, USA, 2020. Available online: https://snaped.fns.usda.gov/library/materials/dietary-guidelines-americans-2020-2025 (accessed on 24 November 2021).
- Lin, H.Y.; Fisher, P.; Harris, D.; Tseng, T.S. Alcohol intake patterns for cancer and non-cancer individuals: A population study. Transl. Cancer Res. 2019, 8, S334–S345. [Google Scholar] [CrossRef] [PubMed]
- Simonds, N.I.; Ghazarian, A.A.; Pimentel, C.B.; Schully, S.D.; Ellison, G.L.; Gillanders, E.M.; Mechanic, L.E. Review of the Gene-Environment Interaction Literature in Cancer: What Do We Know? Genet. Epidemiol. 2016, 40, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, F.R.; Al Olama, A.A.; Berndt, S.I.; Benlloch, S.; Ahmed, M.; Saunders, E.J.; Dadaev, T.; Leongamornlert, D.; Anokian, E.; Cieza-Borrella, C.; et al. Association analyses of more than 140,000 men identify 63 new prostate cancer susceptibility loci. Nat. Genet. 2018, 50, 928–936. [Google Scholar] [CrossRef] [PubMed]
- Glossary-Alcohol, National Health Interview Survey. Available online: https://www.cdc.gov/nchs/nhis/alcohol/alcohol_glossary.htm (accessed on 17 December 2021).
- WHO. Body Mass Index-BMI. Available online: https://www.euro.who.int/en/health-topics/disease-prevention/nutrition/a-healthy-lifestyle/body-mass-index-bmi (accessed on 24 November 2021).
- Papa, N.P.; MacInnis, R.J.; Jayasekara, H.; English, D.R.; Bolton, D.; Davis, I.D.; Lawrentschuk, N.; Millar, J.L.; Pedersen, J.; Severi, G.; et al. Total and beverage-specific alcohol intake and the risk of aggressive prostate cancer: A case-control study. Prostate Cancer Prostatic Dis. 2017, 20, 305–310. [Google Scholar] [CrossRef]
- Downer, M.K.; Kenfield, S.A.; Stampfer, M.J.; Wilson, K.M.; Dickerman, B.A.; Giovannucci, E.L.; Rimm, E.B.; Wang, M.; Mucci, L.A.; Willett, W.C.; et al. Alcohol Intake and Risk of Lethal Prostate Cancer in the Health Professionals Follow-Up Study. J. Clin. Oncol. 2019, 37, 1499–1511. [Google Scholar] [CrossRef]
- Tayie, F.A.; Beck, G.L. Alcoholic beverage consumption contributes to caloric and moisture intakes and body weight status. Nutrition 2016, 32, 799–805. [Google Scholar] [CrossRef]
- Sanford, N.N.; Sher, D.J.; Xu, X.; Ahn, C.; D’Amico, A.V.; Aizer, A.A.; Mahal, B.A. Alcohol Use Among Patients with Cancer and Survivors in the United States, 2000–2017. J. Natl. Compr. Cancer Netw. 2020, 18, 69–79. [Google Scholar] [CrossRef]
- Frazelle, M.L.; Friend, P.J. Optimizing the Teachable Moment for Health Promotion for Cancer Survivors and Their Families. J. Adv. Pract. Oncol. 2016, 7, 422–433. [Google Scholar]
- Shield, K.D.; Parry, C.; Rehm, J. Chronic diseases and conditions related to alcohol use. Alcohol Res. 2013, 35, 155–173. [Google Scholar]
- American Cancer Society. Survival Rates for Prostate Cancer. Available online: https://www.cancer.org/cancer/prostate-cancer/detection-diagnosis-staging/survival-rates.html (accessed on 9 December 2021).
- Xiao, H.; Tan, F.; Goovaerts, P.; Ali, A.; Adunlin, G.; Huang, Y.; Gwede, C. Construction of a comorbidity index for prostate cancer patients linking state cancer registry with inpatient and outpatient data. J. Regist. Manag. 2013, 40, 159–164. [Google Scholar]
- Boakye, D.; Gunther, K.; Niedermaier, T.; Haug, U.; Ahrens, W.; Nagrani, R. Associations between comorbidities and advanced stage diagnosis of lung, breast, colorectal, and prostate cancer: A systematic review and meta-analysis. Cancer Epidemiol. 2021, 75, 102054. [Google Scholar] [CrossRef] [PubMed]
- Nolen-Hoeksema, S. Gender differences in risk factors and consequences for alcohol use and problems. Clin. Psychol. Rev. 2004, 24, 981–1010. [Google Scholar] [CrossRef] [PubMed]
- Thibodeau, M.; Pickering, G.J. The role of taste in alcohol preference, consumption and risk behavior. Crit. Rev. Food Sci. Nutr. 2019, 59, 676–692. [Google Scholar] [CrossRef] [PubMed]
| Total Alcohol Intake 1 | Beer Intake 1 | Wine Intake 1 | Spirits Intake 1 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Infrequent | Light/ Moderate | Heavy | Infrequent | Light/ Moderate | Heavy | Infrequent | Light/ Moderate | Heavy | Infrequent | Light/ Moderate | Heavy | |
| N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | |
| Total | 2016 (21.4) | 6056 (64.3) | 1343 (14.3) | 3513 (35.5) | 5520 (55.7) | 872 (8.8) | 3889 (40.4) | 5278 (54.9) | 456 (4.7) | 5415 (55.8) | 4067 (42.0) | 215 (2.2) |
| Prostate cancer (PCa) | ||||||||||||
| No Yes | 1124 (25.4) 892 (17.9) | 2728 (61.7) 3328 (66.6) | 569 (12.9) 774 (15.5) *** | 1829 (39.6) 1684 (31.8) | 2419 (52.4) 3101 (58.6) | 367 (8.0) 505 (9.6) *** | 2009 (44.4) 1880 (36.9) | 2329 (51.5) 2949 (57.9) | 188 (4.1) 268 (5.2) *** | 2664 (58.9) 2751 (53.2) | 1760 (38.9) 2307 (44.6) | 102 (2.2) 113 (2.2) *** |
| Age (mean ± SD) 2 | 64.4 ± 6.2 | 63.2 ± 7.0 | 63.8 ± 7.2 *** | 64.7 ± 6. 5 | 63.0 ± 7.0 | 62.7 ± 7.2 *** | 63.9 ± 6.7 | 63.2 ± 7.0 | 66.0 ± 7.0 *** | 63.8 ± 6.8 | 63.3 ± 7.1 | 65.1 ± 6.6 *** |
| BMI status 3 | ||||||||||||
| Normal/Underweight | 460 (17.3) | 1798 (67.7) | 398 (15.0) | 816 (28.9) | 1692 (59.9) | 316 (11.2) | 972 (35.6) | 1650 (60.4) | 110 (4.0) | 1551 (56.6) | 1151 (42.0) | 39 (1.4) |
| Overweight | 929 (20.0) | 3026 (65.1) | 692 (14.9) | 1717 (35.3) | 2732 (56.2) | 410 (8.4) | 1833 (38.6) | 2659 (56.1) | 252 (5.3) | 2597 (54.3) | 2062 (43.1) | 122 (2.6) |
| Obese | 608 (31.0) | 1125 (57.3) | 229 (11.7) *** | 943 (46.2) | 974 (47.8) | 123 (6.0) *** | 1010 (50.8) | 889 (44.7) | 91 (4.6) *** | 1173 (58.3) | 787 (39.1) | 53 (2.6) *** |
| Smoking | ||||||||||||
| Never | 896 (24.4) | 2391 (65.2) | 382 (10.4) | 1467 (38.1) | 2152 (55.9) | 228 (5.9) | 1565 (41.7) | 2042 (54.5) | 142 (3.8) | 2353 (62.7) | 1345 (35.9) | 53 (1.4) |
| Former | 970 (20.9) | 2933 (63.2) | 740 (15.9) | 1745 (36.0) | 2628 (54.2) | 474 (9.8) | 1880 (39.7) | 2616 (55.2) | 240 (5.1) | 2540 (53.1) | 2110 (44.1) | 134 (2.8) |
| Current | 145 (13.7) | 697 (65.8) | 217 (20.5)*** | 290 (25.0) | 704 (60.8) | 164 (14.2) *** | 428 (39.3) | 590 (54.1) | 72 (6.6) *** | 492 (44.1) | 596 (53.4) | 28 (2.5) *** |
| Region | ||||||||||||
| Europe | 175 (4.4) | 2930 (74.4) | 833 (21.2) | 613 (14.0) | 3125 (71.5) | 635 (14.5) | 1089 (26.6) | 2702 (65.9) | 310 (7.5) | 1940 (46.6) | 2187 (52.6) | 35 (0.8) |
| USA | 1841 (33.6) | 3126 (57.1) | 510 (9.3) *** | 2900 (52.4) | 2395 (43.3) | 237 (4.3) *** | 2800 (50.7) | 2576 (46.7) | 146 (2.6) *** | 3475 (62.8) | 1880 (34.0) | 180 (3.2) *** |
| Total Alcohol Intake 1 | Beer Intake 1 | Wine Intake 1 | Spirits Intake 1 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Infrequent | Light/ Moderate | Heavy | Infrequent | Light/ Moderate | Heavy | Infrequent | Light/ Moderate | Heavy | Infrequent | Light/ Moderate | Heavy | |
| N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | |
| Total | 892 (17.9) | 3321 (66.6) | 772 (15.5) | 1682 (31.9) | 3095 (58.6) | 504 (9.5) | 1879 (36.9) | 2942 (57.8) | 267 (5.3) | 2746 (53.2) | 2303 (44.6) | 113 (2.2) |
| PCa aggressiveness | ||||||||||||
| No Yes | 810 (19.1) 82 (11.1) | 2802 (66.0) 519 (70.2) | 634 (14.9) 138 (18.7) *** | 1518 (34.0) 164 (20.0) | 2547 (57.1) 548 (67.0) | 398 (8.9) 106 (13.0) *** | 1621 (37.5) 258 (33.6) | 2477 (57.3) 465 (60.6) | 223 (5.2) 44 (5.7) | 2334 (53.3) 412 (52.4) | 1940 (44.3) 363 (46.2) | 102 (2.3) 11 (1.4) |
| Age (mean ± SD) 2 | 64.2± 6.0 | 63.1± 7.1 | 63.6± 7.3 *** | 64.8± 6.4 | 62.9± 7.2 | 62.7± 7.3 *** | 63.7± 6.7 | 63.0± 7.1 | 66.0± 7.2 *** | 63.8± 7.0 | 63.0± 7.1 | 64.4± 6.4 *** |
| BMI status 3 | ||||||||||||
| Normal/Underweight | 206 (13.4) | 1094 (71.0) | 241 (15.6) | 409 (24.7) | 1050 (63.4) | 197 (11.9) | 530 (33.4) | 999 (63.0) | 57 (3.6) | 860 (53.7) | 713 (44.5) | 28 (1.8) |
| Overweight | 432 (17.7) | 1619 (66.4) | 386 (15.8) | 850 (33.2) | 1480 (57.9) | 228 (8.9) | 883 (35.6) | 1447 (58.4) | 148 (6.0) | 1321 (52.4) | 1139 (45.2) | 59 (2.3) |
| Obese | 250 (27.4) | 533 (58.5) | 128 (14.1) *** | 409 (43.1) | 479 (50.4) | 62 (6.5) *** | 424 (45.9) | 439 (47.6) | 60 (6.5) *** | 511 (54.4) | 402 (42.8) | 26 (2.8) |
| Smoking | ||||||||||||
| Never | 410 (20.4) | 1367 (68.0) | 233 (11.6) | 720 (33.8) | 1275 (59.8) | 137 (6.4) | 777 (37.8) | 1191 (57.9) | 89 (4.3) | 1250 (60.4) | 792 (38.2) | 29 (1.4) |
| Former | 416 (17.7) | 1520 (64.7) | 412 (17.6) | 816 (33.2) | 1379 (56.1) | 262 (10.7) | 882 (36.9) | 1368 (57.3) | 138 (5.8) | 1220 (50.2) | 1141 (47.0) | 68 (2.8) |
| Current | 65 (10.8) | 410 (68.3) | 125 (20.8) *** | 139 (21.1) | 418 (63.5) | 101 (15.4) *** | 213 (34.6) | 363 (59.0) | 39 (6.3) | 259 (40.9) | 358 (56.6) | 16 (2.5) *** |
| Region | ||||||||||||
| Europe | 105 (4.1) | 1909 (74.7) | 541 (21.2) | 399 (14.1) | 2014 (71.4) | 409 (14.5) | 692 (26.2) | 1752 (66.4) | 196 (7.4) | 1261 (46.7) | 1418 (52.5) | 22 (0.8) |
| USA | 787 (32.4) | 1412 (58.1) | 231 (9.5) *** | 1283 (52.2) | 1081 (44.0) | 95 (3.9) *** | 1187 (48.5) | 1190 (48.6) | 71 (2.9) *** | 1485 (60.3) | 885 (36.0) | 91 (3.7) *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, H.-Y.; Tseng, T.-S.; Wang, X.; Fang, Z.; Zea, A.H.; Wang, L.; Pow-Sang, J.; Tangen, C.M.; Goodman, P.J.; Wolk, A.; et al. Intake Patterns of Specific Alcoholic Beverages by Prostate Cancer Status. Cancers 2022, 14, 1981. https://doi.org/10.3390/cancers14081981
Lin H-Y, Tseng T-S, Wang X, Fang Z, Zea AH, Wang L, Pow-Sang J, Tangen CM, Goodman PJ, Wolk A, et al. Intake Patterns of Specific Alcoholic Beverages by Prostate Cancer Status. Cancers. 2022; 14(8):1981. https://doi.org/10.3390/cancers14081981
Chicago/Turabian StyleLin, Hui-Yi, Tung-Sung Tseng, Xinnan Wang, Zhide Fang, Arnold H. Zea, Liang Wang, Julio Pow-Sang, Catherine M. Tangen, Phyllis J. Goodman, Alicja Wolk, and et al. 2022. "Intake Patterns of Specific Alcoholic Beverages by Prostate Cancer Status" Cancers 14, no. 8: 1981. https://doi.org/10.3390/cancers14081981
APA StyleLin, H.-Y., Tseng, T.-S., Wang, X., Fang, Z., Zea, A. H., Wang, L., Pow-Sang, J., Tangen, C. M., Goodman, P. J., Wolk, A., Håkansson, N., Kogevinas, M., Llorca, J., Brenner, H., Schöttker, B., Castelao, J. E., Gago-Dominguez, M., Gamulin, M., Lessel, D., ... Park, J. Y. (2022). Intake Patterns of Specific Alcoholic Beverages by Prostate Cancer Status. Cancers, 14(8), 1981. https://doi.org/10.3390/cancers14081981








