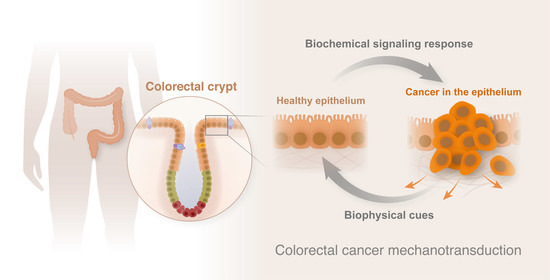
Mechanobiology of Colorectal Cancer
Abstract
Simple Summary
Abstract
1. A Brief Introduction to Colorectal Cancer (CRC)
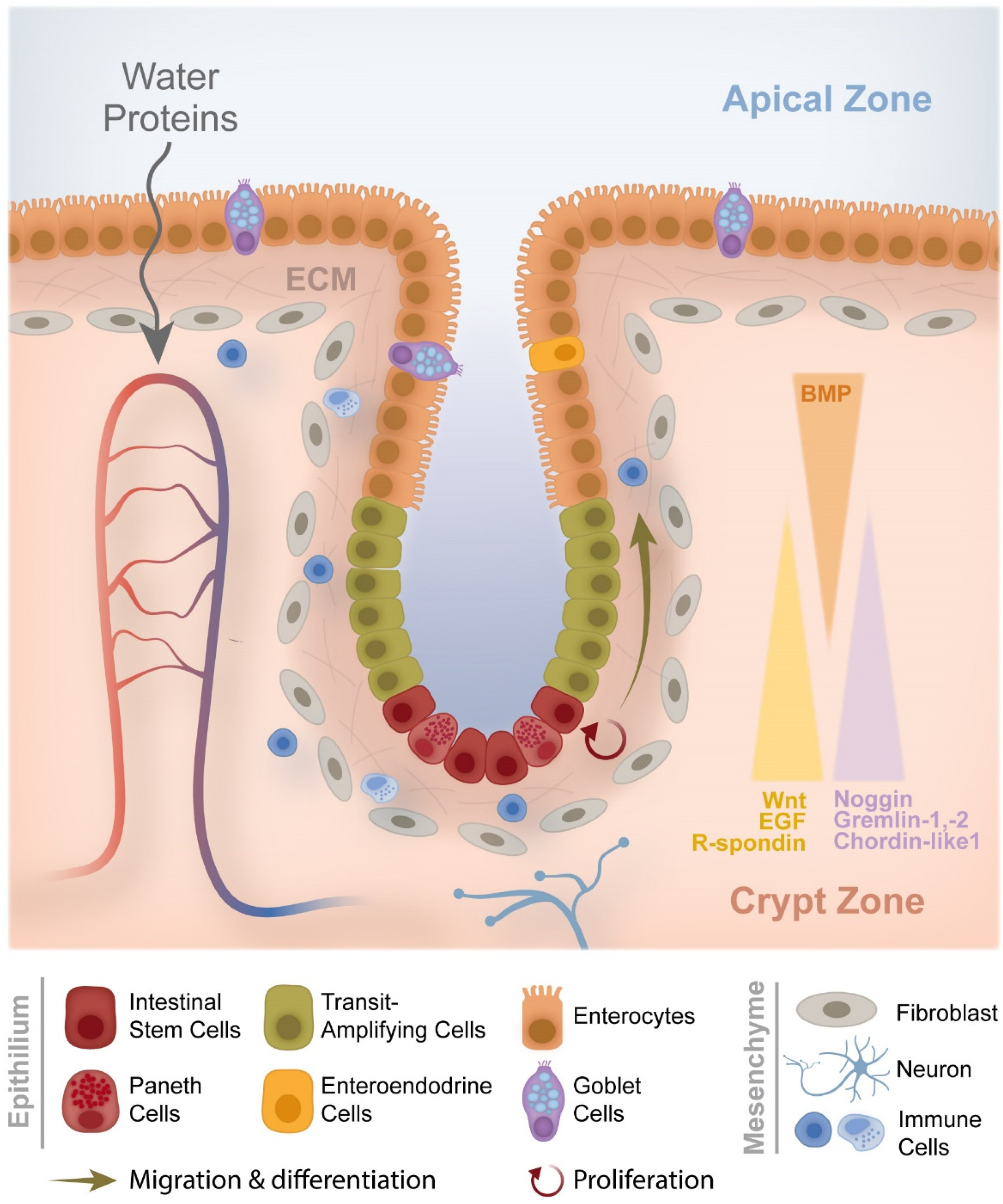
1.1. From Crypt Dysfunction to Polyp Formation
1.2. CRC Formation and Development
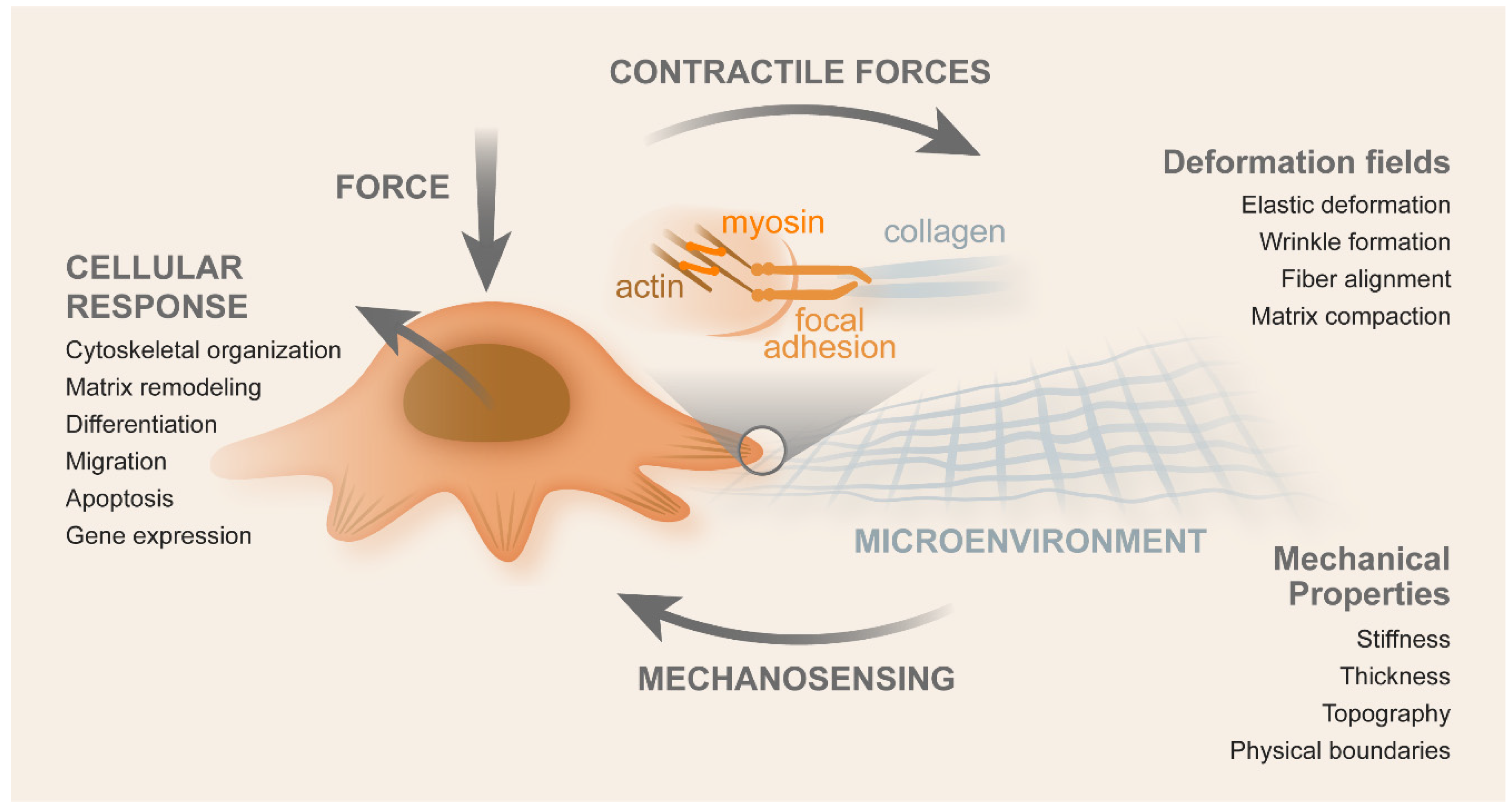
2. CRC Mechanotransduction
2.1. Contributors for Mechanotransduction
2.1.1. Nuclear Envelope
2.1.2. Cytoskeleton
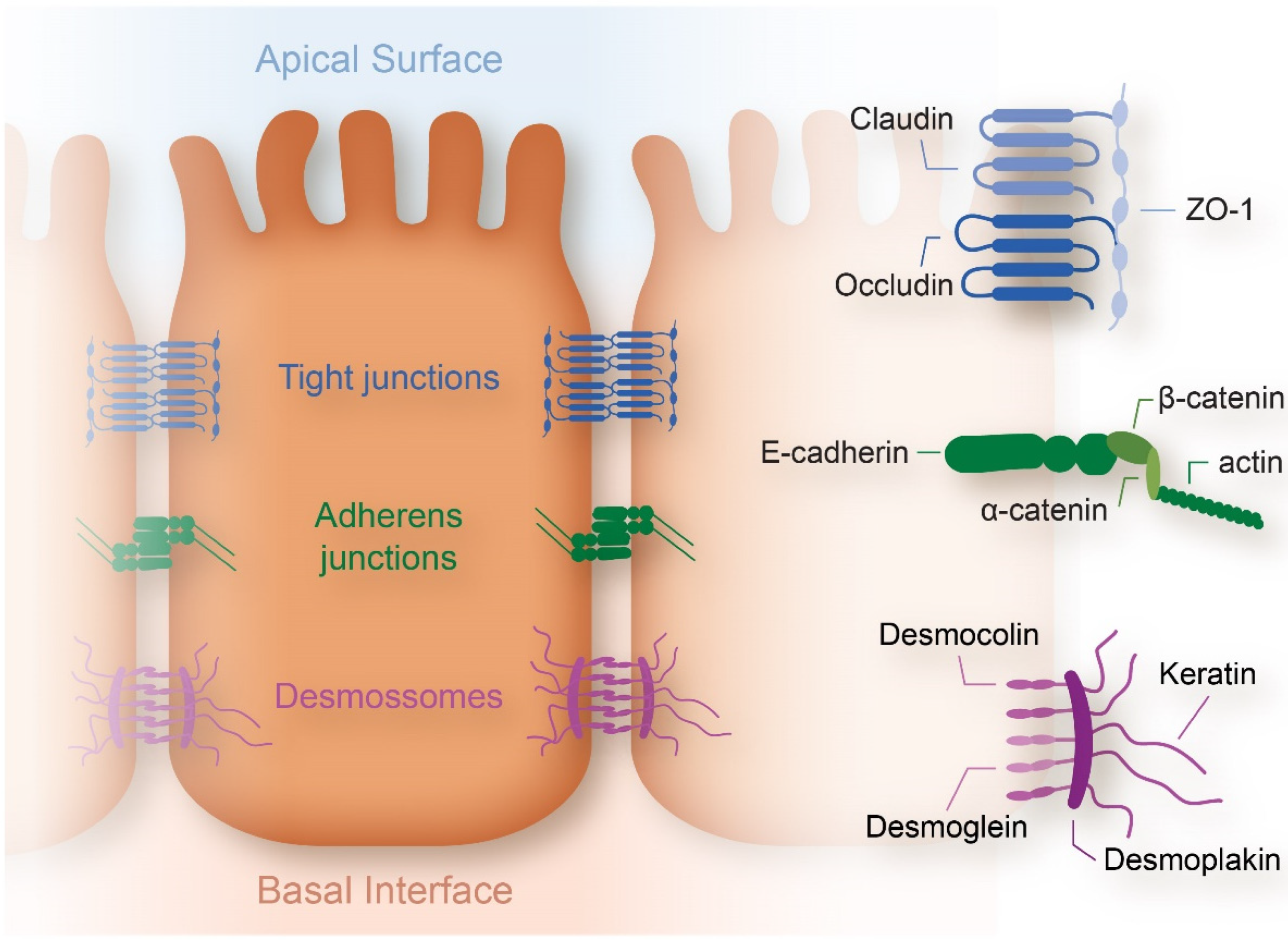
2.1.3. Cell Membrane
2.1.4. Stroma and ECM
2.2. Mechanics of CRC Cells
2.3. Techniques to Characterize Mechanotransduction
2.4. Biophysical Cues vs. Biochemical Signaling
2.5. Role of Cancer-Associated Fibroblasts (CAFs)
2.6. Impact of Shear Stress on Metastization
3. Mechanobiology in CRC Therapeutics
4. Conclusions and Assessment
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.P.; Rai, S.; Pandey, A.; Singh, N.K.; Srivastava, S. Molecular subtypes of colorectal cancer: An emerging therapeutic opportunity for personalized medicine. Genes Dis. 2021, 8, 133–145. [Google Scholar] [CrossRef]
- Pandurangan, A.K.; Divya, T.; Kumar, K.; Dineshbabu, V.; Velavan, B.; Sudhandiran, G. Colorectal carcinogenesis: Insights into the cell death and signal transduction pathways: A review. World J. Gastrointest. Oncol. 2018, 10, 244–259. [Google Scholar] [CrossRef]
- Shussman, N.; Wexner, S.D. Colorectal polyps and polyposis syndromes. Gastroenterol. Rep. 2014, 2, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Gargalionis, A.N.; Papavassiliou, K.A.; Basdra, E.K.; Papavassiliou, A.G. mTOR Signaling Components in Tumor Mechanobiology. Int. J. Mol. Sci. 2022, 23, 1825. [Google Scholar] [CrossRef]
- Audrey Ferrand LO-R. Intestinal Stem Cells. 2017. Available online: https://encyclopedia.pub/4539 (accessed on 21 December 2020).
- Peifer, M. Developmental biology: Colon construction. Nature 2002, 420, 274–275. [Google Scholar] [CrossRef] [PubMed]
- Jezkova, J.; Williams, J.S.; Pinto, F.; Sammut, S.J.; Williams, G.T.; Gollins SMcFarlane, R.J.; Reis, R.M.R.; Wakeman, J.A. Brachyury identifies a class of enteroendocrine cells in normal human intestinal crypts and colorectal cancer. Oncotarget 2016, 7, 11478–11486. [Google Scholar] [CrossRef]
- Onfroy-Roy, L.; Hamel, D.; Foncy, J.; Malaquin, L.; Ferrand, A. Extracellular Matrix Mechanical Properties and Regulation of the Intestinal Stem Cells: When Mechanics Control Fate. Cells 2020, 9, 2629. [Google Scholar] [CrossRef]
- Sideris, M.; Papagrigoriadis, S. Molecular biomarkers and classification models in the evaluation of the prognosis of colorectal cancer. Anticancer Res. 2014, 34, 2061–2068. [Google Scholar]
- Colussi, D.; Brandi, G.; Bazzoli, F.; Ricciardiello, L. Molecular Pathways Involved in Colorectal Cancer: Implications for Disease Behavior and Prevention. Int. J. Mol. Sci. 2013, 14, 16365–16385. [Google Scholar] [CrossRef]
- Witold, K.; Anna, K.; Maciej, T.; Jakub, J. Adenomas—Genetic factors in colorectal cancer prevention. Rep. Pract. Oncol. Radiother. 2018, 23, 75–83. [Google Scholar] [CrossRef]
- Lee, S.-J.; Yun, C.C. Colorectal cancer cells—Proliferation, survival and invasion by lysophosphatidic acid. Int. J. Biochem. Cell Biol. 2010, 42, 1907–1910. [Google Scholar] [CrossRef] [PubMed]
- Balda, M.S.; Matter, K. Tight junctions at a glance. J. Cell Sci. 2008, 121, 3677–3682. [Google Scholar] [CrossRef] [PubMed]
- Trepat, X.; Chen, Z.; Jacobson, K. Cell migration. Compr. Physiol. 2012, 2, 2369–2392. [Google Scholar] [PubMed]
- Lechuga, S.; Ivanov, A.I. Actin cytoskeleton dynamics during mucosal inflammation: A view from broken epithelial barriers. Curr. Opin. Physiol. 2021, 19, 10–16. [Google Scholar] [CrossRef]
- Wong, W.-M.; Mandir, N.; Goodlad, R.A.; Wong, B.C.Y.; Garcia, S.B.; Lam, S.-K.; Wright, N.A. Histogenesis of human colorectal adenomas and hyperplastic polyps: The role of cell proliferation and crypt fission. Gut 2002, 50, 212–217. [Google Scholar] [CrossRef]
- Araki, K.; Ogata, T.; Kobayashi, M.; Yatani, R. A morphological study on the histogenesis of human colorectal hyperplastic polyps. Gastroenterology 1995, 109, 1468–1474. [Google Scholar] [CrossRef]
- Van Leeuwen, I.M.M.; Byrne, H.M.; Jensen, O.E.; King, J.R. Crypt dynamics and colorectal cancer: Advances in mathematical modelling. Cell Prolif. 2006, 39, 157–181. [Google Scholar] [CrossRef]
- Nelson, M.R.; Howard, D.; Jensen, O.E.; King, J.R.; Rose, F.R.A.J.; Waters, S.L. Growth-induced buckling of an epithelial layer. Biomech. Model. Mechanobiol. 2011, 10, 883–900. [Google Scholar] [CrossRef]
- Brodland, G.W.; Conte, V.; Cranston, P.G.; Veldhuis, J.; Narasimhan, S.; Hutson, M.S.; Jacinto, A.; Ulrich, F.; Baum, B.; Miodownik, M. Video force microscopy reveals the mechanics of ventral furrow invagination in Drosophila. Proc. Natl. Acad. Sci. USA 2010, 107, 22111–22116. [Google Scholar] [CrossRef]
- Wyatt, T.P.; Fouchard, J.; Lisica, A.; Khalilgharibi, N.; Baum, B.; Recho, P.; Kabla, A.J.; Charras, G.T. Actomyosin controls planarity and folding of epithelia in response to compression. Nat. Mater. 2020, 19, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Trushko, A.; Di Meglio, I.; Merzouki, A.; Blanch-Mercader, C.; Abuhattum, S.; Guck, J.; Alessandri, K.; Nassoy, P.; Kruse, K.; Chopard, B.; et al. Buckling of an Epithelium Growing under Spherical Confinement. Dev. Cell 2020, 54, 655.e6–668.e6. [Google Scholar] [CrossRef] [PubMed]
- Latorre, E.; Kale, S.; Casares, L.; Gómez-González, M.; Uroz, M.; Valon, L.; Nair, R.V.; Garreta, E.; Montserrat, N.; del Campo, A.; et al. Active superelasticity in three-dimensional epithelia of controlled shape. Nature 2018, 563, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Gómez-González, M.; Latorre, E.; Arroyo, M.; Trepat, X. Measuring mechanical stress in living tissues. Nat. Rev. Phys. 2020, 2, 300–317. [Google Scholar] [CrossRef]
- Preston, S.L.; Wong, W.-M.; Chan, A.O.-O.; Poulsom, R.; Jeffery, R.; Goodlad, R.A.; Mandir, N.; Elia, G.; Novelli, M.; Bodmer, W.F.; et al. Bottom-up histogenesis of colorectal adenomas: Origin in the monocryptal adenoma and initial expansion by crypt fission. Cancer Res. 2003, 63, 3819–3825. [Google Scholar] [PubMed]
- Crosnier, C.; Stamataki, D.; Lewis, J. Organizing cell renewal in the intestine: Stem cells, signals and combinatorial control. Nat. Rev. Genet. 2006, 7, 349–359. [Google Scholar] [CrossRef]
- Pothuraju, R.; Krishn, S.R.; Gautam, S.K.; Pai, P.; Ganguly, K.; Chaudhary, S.; Rachagani, S.; Kaur, S.; Batra, S.K. Mechanistic and Functional Shades of Mucins and Associated Glycans in Colon Cancer. Cancers 2020, 12, 649. [Google Scholar] [CrossRef] [PubMed]
- Sellers, R.S.; Morton, D. The Colon: From Banal to Brilliant. Toxicol. Pathol. 2014, 42, 67–81. [Google Scholar] [CrossRef]
- Kumar, V.; Abbas, A.K.; Aster, J.C. Robbins & Cotran Pathologic Basis of Disease, 9th ed.; Elsevier: Amsterdam, The Netherlands, 2014; 1408p. [Google Scholar]
- Society TAC. Colorectal Cancer Stages The American Cancer Society. 2021. Available online: https://www.cancer.org/cancer/colon-rectal-cancer/detection-diagnosis-staging/staged.html (accessed on 29 June 2020).
- Muto, T.; Bussey, H.J.; Morson, B.C. The evolution of cancer of the colon and rectum. Cancer 1975, 36, 2251–2270. [Google Scholar] [CrossRef]
- Thiery, J.P.; Acloque, H.; Huang, R.Y.J.; Nieto, M.A. Epithelial-Mesenchymal Transitions in Development and Disease. Cell 2009, 139, 871–890. [Google Scholar] [CrossRef]
- Swaminathan, V.; Gloerich, M. Decoding mechanical cues by molecular mechanotransduction. Curr. Opin. Cell Biol. 2021, 72, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Panciera, T.; Azzolin, L.; Cordenonsi, M.; Piccolo, S. Mechanobiology of YAP and TAZ in physiology and disease. Nat. Rev. Mol. Cell Biol. 2017, 18, 758–770. [Google Scholar] [CrossRef] [PubMed]
- Zanconato, F.; Cordenonsi, M.; Piccolo, S. YAP/TAZ at the Roots of Cancer. Cancer Cell 2016, 29, 783–803. [Google Scholar] [CrossRef] [PubMed]
- Sarah Libring, L.S. Cancer mechanobiology: Interaction of biomaterials with cancer cells. In Biomaterials for Cancer Therapeutics, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2020; p. 782. [Google Scholar]
- Yu, W.; Sharma, S.; Rao, E.; Rowat, A.C.; Gimzewski, J.K.; Han, D.; Rao, J. Cancer Cell Mechanobiology: A New Frontier for Cancer Research. Front. Cell Dev. Biol. 2021, 9, 775012. [Google Scholar] [CrossRef]
- Lever, E.; Sheer, D. The role of nuclear organization in cancer. J. Pathol. 2010, 220, 114–125. [Google Scholar] [CrossRef]
- Dahl, K.; Engler, A.; Pajerowski, J.D.; Discher, D.E. Power-Law Rheology of Isolated Nuclei with Deformation Mapping of Nuclear Substructures. Biophys. J. 2005, 89, 2855–2864. [Google Scholar] [CrossRef]
- Dahl, K.N.; Kahn, S.M.; Wilson, K.L.; Discher, D. The nuclear envelope lamina network has elasticity and a compressibility limit suggestive of a molecular shock absorber. J. Cell Sci. 2004, 117, 4779–4786. [Google Scholar] [CrossRef]
- Kim, D.-H.; Hah, J.; Wirtz, D. Mechanics of the Cell Nucleus. Adv. Exp. Med. Biol. 2018, 1092, 41–55. [Google Scholar] [CrossRef]
- Lammerding, J.; Fong, L.G.; Ji, J.Y.; Reue, K.; Stewart, C.L.; Young, S.; Lee, R.T. Lamins A and C but Not Lamin B1 Regulate Nuclear Mechanics. J. Biol. Chem. 2006, 281, 25768–25780. [Google Scholar] [CrossRef]
- Swift, J.; Ivanovska, I.L.; Buxboim, A.; Harada, T.; Dingal, P.C.D.P.; Pinter, J.; Pajerowski, J.D.; Spinler, K.R.; Shin, J.-W.; Tewari, M.; et al. Nuclear Lamin-A Scales with Tissue Stiffness and Enhances Matrix-Directed Differentiation. Science 2013, 341, 1240104. [Google Scholar] [CrossRef]
- Pennacchio, F.A.; Nastały, P.; Poli, A.; Maiuri, P. Tailoring Cellular Function: The Contribution of the Nucleus in Mechanotransduction. Front. Bioeng. Biotechnol. 2021, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Mierke, C.T. Viscoelasticity Acts as a Marker for Tumor Extracellular Matrix Characteristics. Front. Cell Dev. Biol. 2021, 9, 785138. [Google Scholar] [CrossRef] [PubMed]
- Fackler, O.T.; Grosse, R. Cell motility through plasma membrane blebbing. J. Cell Biol. 2008, 181, 879–884. [Google Scholar] [CrossRef] [PubMed]
- De Nicola, M.; Cerella, C.; D’Alessio, M.; Coppola, S.; Magrini, A.; Bergamaschi, A.; Ghibelli, L. The cleavage mode of apoptotic nuclear vesiculation is related to plasma membrane blebbing and depends on actin reorganization. In Signal Transduction Pathways, Part A: Apoptotic and Extracellular Signaling; Diederich, M., Ed.; Annals of the New York Academy of Sciences 1090; Wiley-Blackwell: Malden, MA, USA, 2006; pp. 69–78. [Google Scholar]
- Charras, G.; Paluch, E. Blebs lead the way: How to migrate without lamellipodia. Nat. Rev. Mol. Cell Biol. 2008, 9, 730–736. [Google Scholar] [CrossRef] [PubMed]
- Ebata, T.; Hirata, H.; Kawauchi, K. Functions of the Tumor Suppressors p53 and Rb in Actin Cytoskeleton Remodeling. BioMed Res. Int. 2016, 2016, 9231057. [Google Scholar] [CrossRef] [PubMed]
- Pachenari, M.; Seyedpour, S.; Janmaleki, M.; Shayan, S.B.; Taranejoo, S.; Hosseinkhani, H. Mechanical properties of cancer cytoskeleton depend on actin filaments to microtubules content: Investigating different grades of colon cancer cell lines. J. Biomech. 2014, 47, 373–379. [Google Scholar] [CrossRef]
- Broders-Bondon, F.; Ho-Bouldoires, T.H.N.; Sanchez, M.E.F.; Farge, E. Mechanotransduction in tumor progression: The dark side of the force. J. Cell Biol. 2018, 217, 1571–1587. [Google Scholar] [CrossRef]
- Biber, G.; Ben-Shmuel, A.; Sabag, B.; Barda-Saad, M. Actin regulators in cancer progression and metastases: From structure and function to cytoskeletal dynamics. Int. Rev. Cell Mol. Biol. 2020, 356, 131–196. [Google Scholar] [CrossRef]
- Lin, Y.N.; Izbicki, J.R.; König, A.; Habermann, J.K.; Blechner, C.; Lange, T.; Lange, T.; Schumacher, U.; Windhorst, S. Expression of DIAPH1 is up-regulated in colorectal cancer and its down-regulation strongly reduces the metastatic capacity of colon carcinoma cells. Int. J. Cancer 2014, 134, 1571–1582. [Google Scholar] [CrossRef]
- Kumar, S.; Weaver, V.M. Mechanics, malignancy, and metastasis: The force journey of a tumor cell. Cancer Metastasis Rev. 2009, 28, 113–127. [Google Scholar] [CrossRef]
- Suresh, S. Biomechanics and biophysics of cancer cells. Acta Biomater. 2007, 3, 413–438. [Google Scholar] [CrossRef] [PubMed]
- Bellovin, D.I.; Bates, R.C.; Muzikansky, A.; Rimm, D.L.; Mercurio, A.M. Altered localization of p120 catenin during epithelial to mesenchymal transition of colon carcinoma is prognostic for aggressive disease. Cancer Res. 2005, 65, 10938–10945. [Google Scholar] [CrossRef] [PubMed]
- Ketene, A.N.; Schmelz, E.M.; Roberts, P.C.; Agah, M. The effects of cancer progression on the viscoelasticity of ovarian cell cytoskeleton structures. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Janmey, P.A.; Euteneuer, U.; Traub, P.; Schliwa, M. Viscoelastic properties of vimentin compared with other filamentous biopolymer networks. J. Cell Biol. 1991, 113, 155–160. [Google Scholar] [CrossRef]
- Lindberg, U.; Karlsson, R.; Lassing, I.; Schutt, C.E.; Hoglund, A.S. The microfilament system and malignancy. Semin. Cancer Biol. 2008, 18, 2–11. [Google Scholar] [CrossRef]
- Creekmore, A.; Silkworth, W.T.; Cimini, D.; Jensen, R.V.; Roberts, P.C.; Schmelz, E.M. Changes in Gene Expression and Cellular Architecture in an Ovarian Cancer Progression Model. PLoS ONE 2011, 6, e17676. [Google Scholar] [CrossRef]
- Ren, K.; Gao, J.; Han, D. AFM Force Relaxation Curve Reveals That the Decrease of Membrane Tension Is the Essential Reason for the Softening of Cancer Cells. Front. Cell Dev. Biol. 2021, 9, 663021. [Google Scholar] [CrossRef]
- Zhou, B.; Zong, S.; Zhong, W.; Tian, Y.; Wang, L.; Zhang, Q.; Zhang, R.; Li, L.; Wang, W.; Zhao, J.; et al. Interaction between laminin-5γ2 and integrin β1 promotes the tumor budding of colorectal cancer via the activation of Yes-associated proteins. Oncogene 2020, 39, 1527–1542. [Google Scholar] [CrossRef]
- Lecuit, T.; Yap, A. E-cadherin junctions as active mechanical integrators in tissue dynamics. Nat. Cell Biol. 2015, 17, 533–539. [Google Scholar] [CrossRef]
- Lu, W.; Kang, Y. Epithelial-Mesenchymal Plasticity in Cancer Progression and Metastasis. Dev. Cell 2019, 49, 361–374. [Google Scholar] [CrossRef]
- Maffeis, V.; Nicolè, L.; Cappellesso, R. RAS, Cellular Plasticity, and Tumor Budding in Colorectal Cancer. Front. Oncol. 2019, 9, 1255. [Google Scholar] [CrossRef]
- Tsai, J.H.; Yang, J. Epithelial–mesenchymal plasticity in carcinoma metastasis. Genes Dev. 2013, 27, 2192–2206. [Google Scholar] [CrossRef] [PubMed]
- Ieda, T.; Tazawa, H.; Okabayashi, H.; Yano, S.; Shigeyasu, K.; Kuroda, S.; Ohara, T.; Noma, K.; Kishimoto, H.; Nishizaki, M.; et al. Visualization of epithelial-mesenchymal transition in an inflammatory microenvironment–colorectal cancer network. Sci. Rep. 2019, 9, 16378. [Google Scholar] [CrossRef] [PubMed]
- Boesch, M.; Spizzo, G.; Seeber, A. Concise Review: Aggressive Colorectal Cancer: Role of Epithelial Cell Adhesion Molecule in Cancer Stem Cells and Epithelial-to-Mesenchymal Transition. Stem Cells Transl. Med. 2018, 7, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Maetzel, D.; Denzel, S.; Mack, B.; Canis, M.; Went, P.; Benk, M.; Kieu, C.; Papior, P.; Baeuerle, P.A.; Munz, M.; et al. Nuclear signalling by tumour-associated antigen EpCAM. Nat. Cell Biol. 2009, 11, 162–171. [Google Scholar] [CrossRef]
- Balzar, M.; Prins, F.A.; Bakker, H.A.; Fleuren, G.J.; Warnaar, S.O.; Litvinov, S.V. The Structural Analysis of Adhesions Mediated by Ep-CAM. Exp. Cell Res. 1999, 246, 108–121. [Google Scholar] [CrossRef] [PubMed]
- Winter, M.J.; Nagelkerken, B.; Mertens, A.E.; Rees-Bakker, H.A.; Bruijn, I.H.B.-D.; Litvinov, S.V. Expression of Ep-CAM shifts the state of cadherin-mediated adhesions from strong to weak. Exp. Cell Res. 2003, 285, 50–58. [Google Scholar] [CrossRef]
- Trzpis, M.; McLaughlin, P.M.; de Leij, L.M.; Harmsen, M.C. Epithelial Cell Adhesion Molecule: More than a Carcinoma Marker and Adhesion Molecule. Am. J. Pathol. 2007, 171, 386–395. [Google Scholar] [CrossRef]
- Zeuner, A.; Todaro, M.; Stassi, G.; De Maria, R. Colorectal Cancer Stem Cells: From the Crypt to the Clinic. Cell Stem Cell 2014, 15, 692–705. [Google Scholar] [CrossRef]
- Kelley, L.C.; Lohmer, L.L.; Hagedorn, E.J.; Sherwood, D.R. Traversing the basement membrane in vivo: A diversity of strategies. J. Cell Biol. 2014, 204, 291–302. [Google Scholar] [CrossRef]
- LeBleu, V.S.; MacDonald, B.; Kalluri, R. Structure and Function of Basement Membranes. Exp. Biol. Med. 2007, 232, 1121–1129. [Google Scholar] [CrossRef] [PubMed]
- Belli, C.; Trapani, D.; Viale, G.; D’Amico, P.; Duso, B.A.; Della Vigna, P.; Orsi, F.; Curigliano, G. Targeting the microenvironment in solid tumors. Cancer Treat. Rev. 2018, 65, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Linder, S.; Wiesner, C.; Himmel, M. Degrading Devices: Invadosomes in Proteolytic Cell Invasion. Annu. Rev. Cell Dev. Biol. 2011, 27, 185–211. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; Zeisberg, M. Fibroblasts in cancer. Nat. Rev. Cancer 2006, 6, 392–401. [Google Scholar] [CrossRef]
- Hotary, K.B.; Yana, I.; Sabeh, F.; Li, X.-Y.; Holmbeck, K.; Birkedal-Hansen, H.; Allen, E.D.; Hiraoka, N.; Weiss, S.J. Matrix Metalloproteinases (MMPs) Regulate Fibrin-invasive Activity via MT1-MMP–dependent and –independent Processes. J. Exp. Med. 2002, 195, 295–308. [Google Scholar] [CrossRef]
- Peddareddigari, V.G.; Wang, D.; DuBois, R.N. The Tumor Microenvironment in Colorectal Carcinogenesis. Cancer Microenviron. 2010, 3, 149–166. [Google Scholar] [CrossRef]
- Stylianou, A.; Lekka, M.; Stylianopoulos, T. AFM assessing of nanomechanical fingerprints for cancer early diagnosis and classification: From single cell to tissue level. Nanoscale 2018, 10, 20930–20945. [Google Scholar] [CrossRef]
- McKee, C.T.; Last, J.A.; Russell, P.; Murphy, C.J. Indentation Versus Tensile Measurements of Young’s Modulus for Soft Biological Tissues. Tissue Eng. Part B-Rev. 2011, 17, 155–164. [Google Scholar] [CrossRef]
- Mohammadi, H.; McCulloch, C.A. Impact of elastic and inelastic substrate behaviors on mechanosensation. Soft Matter 2014, 10, 408–420. [Google Scholar] [CrossRef]
- Romero-Lopez, M.; Trinh, A.L.; Sobrino, A.; Hatch, M.M.S.; Keating, M.T.; Fimbres, C.; Lewis, D.E.; Gershon, P.D.; Botvinick, E.L.; Digman, M.; et al. Recapitulating the human tumor microenvironment: Colon tumor-derived zxtracellular matrix promotes angiogenesis and tumor cell growth. Biomaterials 2017, 116, 118–129. [Google Scholar] [CrossRef]
- Mohammadi, H.; Sahai, E. Mechanisms and impact of altered tumour mechanics. Nat. Cell Biol. 2018, 20, 766–774. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Kuhlenschmidt, T.B.; Zhou, J.; Bell, P.; Wang, F.; Kuhlenschmidt, M.S.; Saif, T.A. Mechanical Force Affects Expression of an In Vitro Metastasis-Like Phenotype in HCT-8 Cells. Biophys. J. 2010, 99, 2460–2469. [Google Scholar] [CrossRef] [PubMed]
- Gao, H. Probing mechanical principles of cell–nanomaterial interactions. J. Mech. Phys. Solids 2014, 62, 312–339. [Google Scholar] [CrossRef]
- Pinto, M.L.; Rios, E.; Silva, A.C.; Neves, S.C.; Caires, H.R.; Pinto, A.T.; Durães, C.; Carvalho, F.A.; Cardoso, A.P.; Santos, N.C.; et al. Decellularized human colorectal cancer matrices polarize macrophages towards an anti-inflammatory phenotype promoting cancer cell invasion via CCL18. Biomaterials 2017, 124, 211–224. [Google Scholar] [CrossRef]
- Condeelis, J.; Pollard, J.W. Macrophages: Obligate Partners for Tumor Cell Migration, Invasion, and Metastasis. Cell 2006, 124, 263–266. [Google Scholar] [CrossRef]
- Vermeulen, S.J.; Nollet, F.; Teugels, E.; Vennekens, K.M.; Malfait, F.; Philippé, J.; Speleman, F.; Bracke, M.E.; van Roy, F.M.; Mareel, M.M.; et al. The alphaE-catenin gene (CTNNA1) acts as an invasion-suppressor gene in human colon cancer cells. Oncogene 1999, 18, 905–915. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, S.J.; Bruyneel, E.A.; Bracke, M.E.; De Bruyne, G.K.; Vennekens, K.M.; Vleminckx, K.L.; Berx, G.; Van Roy, F.M.; Mareel, M.M. Transition from the noninvasive to the invasive phenotype and loss of alpha-catenin in human colon cancer cells. Cancer Res. 1995, 55, 4722–4728. [Google Scholar]
- Tang, X.; Kuhlenschmidt, T.B.; Li, Q.; Ali, S.; Lezmi, S.; Chen, H.; Pires-Alves, M.; Laegreid, W.W.; Saif, T.A.; Kuhlenschmidt, M.S. A mechanically-induced colon cancer cell population shows increased metastatic potential. Mol. Cancer 2014, 13, 131. [Google Scholar] [CrossRef]
- Ali, M.Y.; Saif, M.T.A. Substrate Stiffness Mediated Metastasis Like Phenotype of Colon Cancer Cells is Independent of Cell to Gel Adhesion. Cell. Mol. Bioeng. 2014, 7, 532–543. [Google Scholar] [CrossRef]
- Ciasca, G.; Papi, M.; Minelli, E.; Palmieri, V.; De Spirito, M. Changes in cellular mechanical properties during onset or progression of colorectal cancer. World J. Gastroenterol. 2016, 22, 7203–7214. [Google Scholar] [CrossRef]
- Levental, K.R.; Yu, H.; Kass, L.; Lakins, J.N.; Egeblad, M.; Erler, J.T.; Fong, S.F.T.; Csiszar, K.; Giaccia, A.; Weninger, W.; et al. Matrix Crosslinking Forces Tumor Progression by Enhancing Integrin Signaling. Cell 2009, 139, 891–906. [Google Scholar] [CrossRef]
- Provenzano, P.P.; Eliceiri, K.W.; Campbell, J.M.; Inman, D.R.; White, J.G.; Keely, P.J. Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC Med. 2006, 4, 38. [Google Scholar] [CrossRef]
- Willis, A.; Sabeh, F.; Li, X.-Y.; Weiss, S. Extracellular matrix determinants and the regulation of cancer cell invasion stratagems. J. Microsc. 2013, 251, 250–260. [Google Scholar] [CrossRef]
- Nebuloni, M.; Albarello, L.; Andolfo, A.; Magagnotti, C.; Genovese, L.; Locatelli, I.; Tonon, G.; Longhi, E.; Zerbi, P.; Allevi, R.; et al. Insight On Colorectal Carcinoma Infiltration by Studying Perilesional Extracellular Matrix. Sci. Rep. 2016, 6, 22522. [Google Scholar] [CrossRef]
- Reidy, E.; Leonard, N.A.; Treacy, O.; Ryan, A.E. A 3D View of Colorectal Cancer Models in Predicting Therapeutic Responses and Resistance. Cancers 2021, 13, 227. [Google Scholar] [CrossRef]
- Pape, J.; Emberton, M.; Cheema, U. 3D Cancer Models: The Need for a Complex Stroma, Compartmentalization and Stiffness. Front. Bioeng. Biotechnol. 2021, 9, 276. [Google Scholar] [CrossRef]
- Lange, J.R.; Fabry, B. Cell and tissue mechanics in cell migration. Exp. Cell Res. 2013, 319, 2418–2423. [Google Scholar] [CrossRef]
- Discher, D.E.; Janmey, P.; Wang, Y.-L. Tissue Cells Feel and Respond to the Stiffness of Their Substrate. Science 2005, 310, 1139–1143. [Google Scholar] [CrossRef]
- Palmieri, V.; Lucchetti, D.; Maiorana, A.; Papi, M.; Maulucci, G.; Calapà, F.; Ciasca, G.; Giordano, R.; Sgambato, A.; De Spirito, M. Mechanical and structural comparison between primary tumor and lymph node metastasis cells in colorectal cancer. Soft Matter 2015, 11, 5719–5726. [Google Scholar] [CrossRef]
- Boccaccio, A.; Uva, A.; Papi, M.; Fiorentino, M.; De Spirito, M.; Monno, G. Nanoindentation characterisation of human colorectal cancer cells considering cell geometry, surface roughness and hyperelastic constitutive behaviour. Nanotechnology 2017, 28, 45703. [Google Scholar] [CrossRef]
- Ball, M.; Grant, D.M.; Lo, W.; Scotchford, C.A. The effect of different surface morphology and roughness on osteoblast-like cells. J. Biomed. Mater. Res. Part A 2008, 86, 637–647. [Google Scholar] [CrossRef]
- Liu, J.; Tan, Y.; Zhang, H.; Zhang, Y.; Xu, P.; Chen, J.; Poh, Y.-C.; Tang, K.; Wang, N.; Huang, B. Soft fibrin gels promote selection and growth of tumorigenic cells. Nat. Mater. 2012, 11, 734–741. [Google Scholar] [CrossRef]
- Tang, X.; Wen, Q.; Kuhlenschmidt, T.B.; Kuhlenschmidt, M.S.; Janmey, P.A.; Saif, T.A. Attenuation of Cell Mechanosensitivity in Colon Cancer Cells during In Vitro Metastasis. PLoS ONE 2012, 7, e50443. [Google Scholar] [CrossRef]
- Lammermann, T.; Sixt, M. Mechanical modes of ‘amoeboid’ cell migration. Curr. Opin. Cell Biol. 2009, 21, 636–644. [Google Scholar] [CrossRef]
- Sahai, E.; Marshall, C.J. Differing modes of tumour cell invasion have distinct requirements for Rho/ROCK signalling and extracellular proteolysis. Nat. Cell Biol. 2003, 5, 711–719. [Google Scholar] [CrossRef]
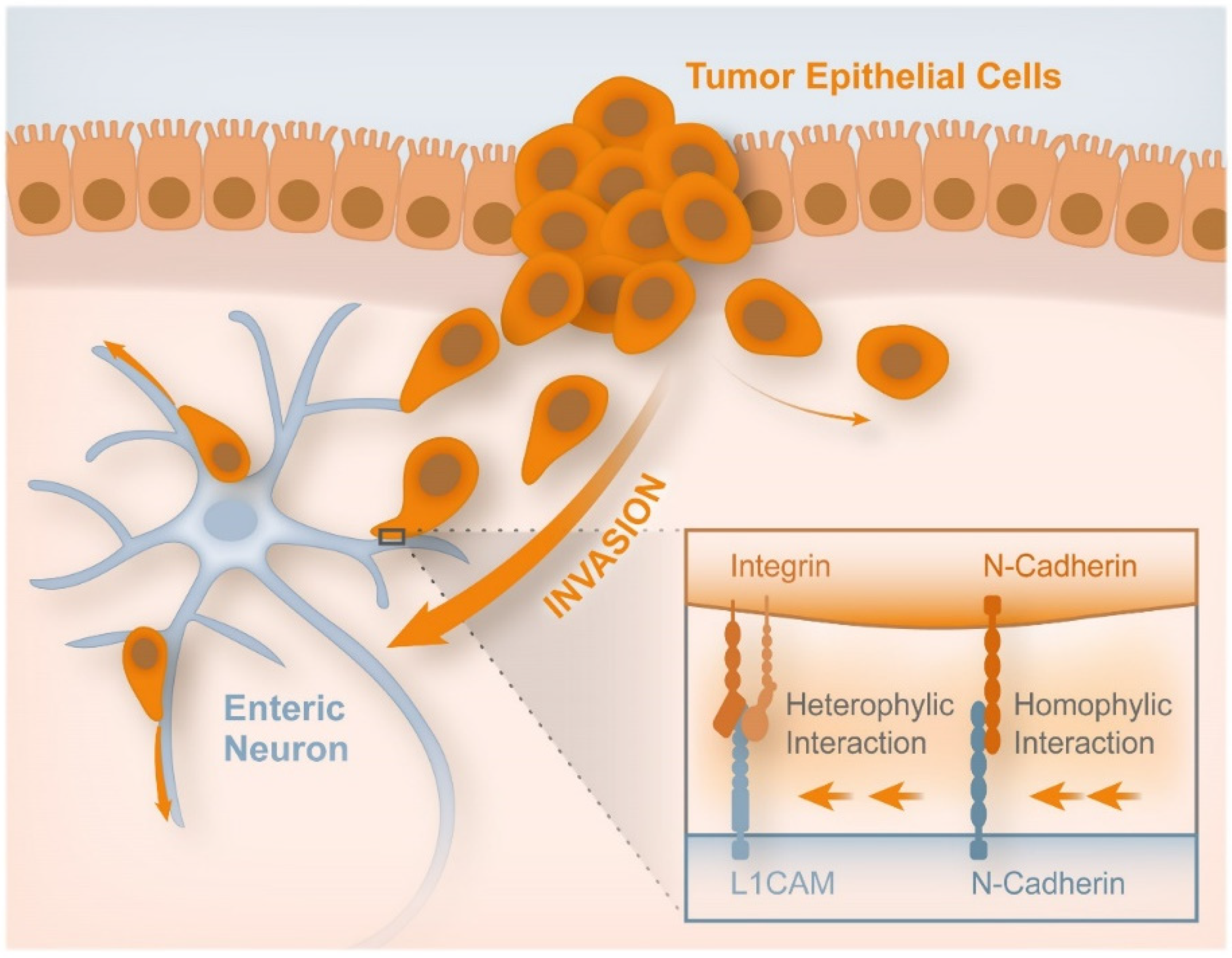
- Duchalais, E.; Guilluy, C.; Nedellec, S.; Touvron, M.; Bessard, A.; Touchefeu, Y.; Bossard, C.; Boudin, H.; Louarn, G.; Neunlist, M.; et al. Colorectal Cancer Cells Adhere to and Migrate Along the Neurons of the Enteric Nervous System. Cell. Mol. Gastroenterol. Hepatol. 2018, 5, 31–49. [Google Scholar] [CrossRef]
- Deptuła, P.; Łysik, D.; Pogoda, K.; Cieśluk, M.; Namiot, A.; Mystkowska, J.; Król, G.; Głuszek, S.; Janmey, P.A.; Bucki, R. Tissue Rheology as a Possible Complementary Procedure to Advance Histological Diagnosis of Colon Cancer. ACS Biomater. Sci. Eng. 2020, 6, 5620–5631. [Google Scholar] [CrossRef]
- De Sousa, J.S.; Freire, R.S.; Sousa, F.D.; Radmacher, M.; Silva, A.F.B.; Ramos, M.V.; Monteiro-Moreira, A.C.O.; Mesquita, F.P.; Moraes, M.E.A.; Montenegro, R.C.; et al. Double power-law viscoelastic relaxation of living cells encodes motility trends. Sci. Rep. 2020, 10, 4749. [Google Scholar] [CrossRef]
- Zemła, J.; Danilkiewicz, J.; Orzechowska, B.; Pabijan, J.; Seweryn, S.; Lekka, M. Atomic force microscopy as a tool for assessing the cellular elasticity and adhesiveness to identify cancer cells and tissues. Semin. Cell Dev. Biol. 2018, 73, 115–124. [Google Scholar] [CrossRef]
- Ebenstein, D.M.; Pruitt, L.A. Nanoindentation of biological materials. Nano Today 2006, 1, 26–33. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Tsukune, M.; Miyashita, T.; Fujie, M.G. Simple empirical model for identifying rheological properties of soft biological tissues. Phys. Rev. E 2017, 95, 022418. [Google Scholar] [CrossRef]
- Ozturk, A.; Grajo, J.R.; Dhyani, M.; Anthony, B.W.; Samir, A.E. Principles of ultrasound elastography. Abdom. Radiol. 2018, 43, 773–785. [Google Scholar] [CrossRef]
- Johnson, C.L.; Telzer, E.H. Magnetic resonance elastography for examining developmental changes in the mechanical properties of the brain. Dev. Cogn. Neurosci. 2018, 33, 176–181. [Google Scholar] [CrossRef]
- Fovargue, D.; Nordsletten, D.; Sinkus, R. Stiffness reconstruction methods for MR elastography. NMR Biomed. 2018, 31, e3935. [Google Scholar] [CrossRef]
- Daza, R.; González-Bermúdez, B.; Cruces, J.; De la Fuente, M.; Plaza, G.R.; Arroyo-Hernández, M.; Elices, M.; Pérez-Rigueiro, J.; Guinea, G.V. Comparison of cell mechanical measurements provided by Atomic Force Microscopy (AFM) and Micropipette Aspiration (MPA). J. Mech. Behav. Biomed. Mater. 2019, 95, 103–115. [Google Scholar] [CrossRef]
- Lai, C.-W.; Hsiung, S.-K.; Yeh, C.-L.; Chiou, A.; Lee, G.-B. A cell delivery and pre-positioning system utilizing microfluidic devices for dual-beam optical trap-and-stretch. Sens. Actuators B Chem. 2008, 135, 388–397. [Google Scholar] [CrossRef]
- Laurent, V.M.; He’non, S.; Planus, E.; Fodil, R.; Balland, M.; Isabey, D.; Gallet, F. Assessment of Mechanical Properties of Adherent Living Cells by Bead Micromanipulation: Comparison of Magnetic Twisting Cytometry vs Optical Tweezers. J. Biomech. Eng. 2002, 124, 408–421. [Google Scholar] [CrossRef]
- Peng, Y.; Shi, C.; Wu, X.; Zhu, Y.; Zhuang, S. Terahertz Imaging and Spectroscopy in Cancer Diagnostics: A Technical Review. BME Front. 2020, 2020, 2547609. [Google Scholar] [CrossRef]
- Wahaia, F.; Kašalynas, I.; Venckevicius, R.; Seliuta, D.; Valusis, G.; Urbanowicz, A.; Molis, G.; Carneiro, F.; Silva, C.D.C.; Granja, P. Terahertz absorption and reflection imaging of carcinoma-affected colon tissues embedded in paraffin. J. Mol. Struct. 2016, 1107, 214–219. [Google Scholar] [CrossRef]
- Cross, S.E.; Jin, Y.-S.; Rao, J.; Gimzewski, J.K. Nanomechanical analysis of cells from cancer patients. Nat. Nanotechnol. 2007, 2, 780–783. [Google Scholar] [CrossRef]
- Thanki, K.; Nicholls, M.E.; Gajjar, A.; Senagore, A.J.; Qiu, S.; Szabo, C.; Hellmich, M.R.; Chao, C. Consensus Molecular Subtypes of Colorectal Cancer and their Clinical Implications. Int. Biol. Biomed. J. 2017, 3, 105–111. [Google Scholar]
- Smit, W.L.; Spaan, C.N.; de Boer, R.J.; Ramesh, P.; Garcia, T.M.; Meijer, B.J.; Vermeulen, J.L.M.; Lezzerini, M.; MacInnes, A.W.; Koster, J.; et al. Driver mutations of the adenoma-carcinoma sequence govern the intestinal epithelial global translational capacity. Proc. Natl. Acad. Sci. USA 2020, 117, 25560–25570. [Google Scholar] [CrossRef]
- Lekka, M.; Laidler, P. Applicability of AFM in cancer detection. Nat. Nanotechnol. 2009, 4, 72. [Google Scholar] [CrossRef]
- Lekka, M.; Gil, D.; Pogoda, K.; Dulińska-Litewka, J.; Jach, R.; Gostek, J.; Klymenko, O.; Prauzner-Bechcicki, S.; Stachura, Z.; Wiltowska-Zuber, J.; et al. Cancer cell detection in tissue sections using AFM. Arch. Biochem. Biophys. 2012, 518, 151–156. [Google Scholar] [CrossRef]
- Tian, M.; Li, Y.; Liu, W.; Jin, L.; Jiang, X.; Wang, X.; Ding, Z.; Peng, Y.; Zhou, J.; Fan, J.; et al. The nanomechanical signature of liver cancer tissues and its molecular origin. Nanoscale 2015, 7, 12998–13010. [Google Scholar] [CrossRef]
- Sanchez, M.E.F.; Barbier, S.; Whitehead, J.; Béalle, G.; Michel, A.; Latorre-Ossa, H.; Rey, C.; Fouassier, L.; Claperon, A.; Brullé, L.; et al. Mechanical induction of the tumorigenic β-catenin pathway by tumour growth pressure. Nature 2015, 523, 92–95. [Google Scholar] [CrossRef]
- Whitehead, J.; Vignjevic, D.; Fütterer, C.; Beaurepaire, E.; Robine, S.; Farge, E. Mechanical factors activate beta-catenin-dependent oncogene expression in APC mouse colon. HFSP J. 2008, 2, 286–294. [Google Scholar] [CrossRef]
- Shih, I.-M.; Wang, T.-L.; Traverso, G.; Romans, K.; Hamilton, S.R.; Ben-Sasson, S.; Kinzler, K.W.; Vogelstein, B. Top-down morphogenesis of colorectal tumors. Proc. Natl. Acad. Sci. USA 2001, 98, 2640–2645. [Google Scholar] [CrossRef]
- Bienz, M.; Hamada, F. Adenomatous polyposis coli proteins and cell adhesion. Curr. Opin. Cell Biol. 2004, 16, 528–535. [Google Scholar] [CrossRef]
- Kroboth, K.; Newton, I.P.; Kita, K.; Dikovskaya, D.; Zumbrunn, J.; Waterman, C.; Näthke, I.S. Lack of Adenomatous Polyposis Coli Protein Correlates with a Decrease in Cell Migration and Overall Changes in Microtubule Stability. Mol. Biol. Cell 2007, 18, 910–918. [Google Scholar] [CrossRef]
- Nusse, R.; Brown, A.; Papkoff, J.; Scambler, P.; Shackleford, G.; McMahon, A.; Moon, R.; Varmus, H. A new nomenclature for int-1 and related genes: The Wnt gene family. Cell 1991, 64, 231. [Google Scholar] [CrossRef]
- Boland, C.R.; Goel, A. Microsatellite instability in colorectal cancer. Gastroenterology 2010, 138, 2073.e3–2087.e3. [Google Scholar] [CrossRef]
- Zessner-Spitzenberg, J.; Thomas, A.L.; Krett, N.L.; Jung, B. TGFβ and activin A in the tumor microenvironment in colorectal cancer. Gene Rep. 2019, 17, 100501. [Google Scholar] [CrossRef]
- Yin, L.; Lin, Y.; Wang, X.; Su, Y.; Hu, H.; Li, C.; Wang, L.; Jiang, Y. The family of apoptosis-stimulating proteins of p53 is dysregulated in colorectal cancer patients. Oncol. Lett. 2018, 15, 6409–6417. [Google Scholar] [CrossRef]
- Van Haastert, P.J.M.; Keizer-Gunnink, I.; Kortholt, A. Coupled excitable Ras and F-actin activation mediates spontaneous pseudopod formation and directed cell movement. Mol. Biol. Cell. 2017, 28, 922–934. [Google Scholar] [CrossRef]
- Nobes, C.D.; Hall, A. Rho, Rac and CDC42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stresses fibers, lamellipodia, and filopodia. Cell 1995, 81, 53–62. [Google Scholar] [CrossRef]
- Mouillet-Richard, S.; Laurent-Puig, P. YAP/TAZ Signalling in Colorectal Cancer: Lessons from Consensus Molecular Subtypes. Cancers 2020, 12, 3160. [Google Scholar] [CrossRef]
- Ou, C.; Sun, Z.; Li, S.; Li, G.; Li, X.; Ma, J. Dual roles of yes-associated protein (YAP) in colorectal cancer. Oncotarget 2017, 8, 75727–75741. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, H.; Xue, X.; Shah, Y.M. Hypoxia-inducible factor 2α (HIF-2α) promotes colon cancer growth by potentiating Yes-associated protein 1 (YAP1) activity. J. Biol. Chem. 2017, 292, 17046–17056. [Google Scholar] [CrossRef]
- Pinheiro, D.; Bellaïche, Y. Mechanical Force-Driven Adherens Junction Remodeling and Epithelial Dynamics. Dev. Cell 2018, 47, 3–19. [Google Scholar] [CrossRef]
- Piccolo, S.; Dupont, S.; Cordenonsi, M. The Biology of YAP/TAZ: Hippo Signaling and Beyond. Physiol. Rev. 2014, 94, 1287–1312. [Google Scholar] [CrossRef]
- Elosegui-Artola, A.; Andreu, I.; Beedle, A.E.M.; Lezamiz, A.; Uroz, M.; Kosmalska, A.J.; Oria, R.; Kechagia, J.Z.; Rico-Lastres, P.; Le Roux, A.-L.; et al. Force Triggers YAP Nuclear Entry by Regulating Transport across Nuclear Pores. Cell 2017, 171, 1397.e14–1410.e14. [Google Scholar] [CrossRef]
- Liu, H.; Wang, N.; Zhang, Z.; Wang, H.; Du, J.; Tang, J. Effects of Tumor Necrosis Factor-α on Morphology and Mechanical Properties of HCT116 Human Colon Cancer Cells Investigated by Atomic Force Microscopy. Scanning 2017, 2017, 2027079. [Google Scholar] [CrossRef]
- Sancho, E.; Batlle, E.; Clevers, H. Signaling pathways in intestinal development and cancer. Annu. Rev. Cell Dev. Biol. 2004, 20, 695–723. [Google Scholar] [CrossRef]
- Tsuji, T.; Sasaki, Y.; Tanaka, M.; Hanabata, N.; Hada, R.; Munakata, A. Microvessel morphology and vascular endothelial growth factor expression in human colonic carcinoma with or without metastasis. Lab. Investig. 2002, 82, 555–562. [Google Scholar] [CrossRef][Green Version]
- Gagné, D.; Benoit, Y.D.; Groulx, J.F.; Vachon, P.H.; Beaulieu, J.F. ILK supports RhoA/ROCK-mediated contractility of human intestinal epithelial crypt cells by inducing the fibrillogenesis of endogenous soluble fibronectin during the spreading process. BMC Mol. Cell Biol. 2020, 21, 14. [Google Scholar] [CrossRef]
- Bremnes, R.M.; Dønnem, T.; Al-Saad, S.; Al-Shibli, K.; Andersen, S.; Sirera, R.; Camps, C.; Marinez, I.; Busund, L.-T. The Role of Tumor Stroma in Cancer Progression and Prognosis: Emphasis on Carcinoma-Associated Fibroblasts and Non-small Cell Lung Cancer. J. Thorac. Oncol. 2011, 6, 209–217. [Google Scholar] [CrossRef]
- Krndija, D.; Schmid, H.; Eismann, J.-L.; Lother, U.; Adler, G.; Oswald, F.; Seufferlein, T.; von Wichert, G. Substrate stiffness and the receptor-type tyrosine-protein phosphatase alpha regulate spreading of colon cancer cells through cytoskeletal contractility. Oncogene 2010, 29, 2724–2738. [Google Scholar] [CrossRef]
- Martino, F.; Perestrelo, A.R.; Vinarsky, V.; Pagliari, S.; Forte, G. Cellular Mechanotransduction: From Tension to Function. Front. Physiol. 2018, 9, 824. [Google Scholar] [CrossRef]
- Fearon, E.R. Molecular Genetics of Colorectal Cancer. Annu. Rev. Pathol. Mech. Dis. 2011, 6, 479–507. [Google Scholar] [CrossRef]
- Guinney, J.; Dienstmann, R.; Wang, X.; De Reyniès, A.; Schlicker, A.; Soneson, C.; Marisa, L.; Roepman, P.; Nyamundanda, G.; Angelino, P.; et al. The consensus molecular subtypes of colorectal cancer. Nat. Med. 2015, 21, 1350–1356. [Google Scholar] [CrossRef] [PubMed]
- Slattery, M.L.; Mullany, L.E.; Wolff, R.K.; Sakoda, L.C.; Samowitz, W.S.; Herrick, J.S. The p53-signaling pathway and colorectal cancer: Interactions between downstream p53 target genes and miRNAs. Genomics 2019, 111, 762–771. [Google Scholar] [CrossRef] [PubMed]
- Aoki, K.; Taketo, M.M. Adenomatous polyposis coli (APC): A multi-functional tumor suppressor gene. J. Cell Sci. 2007, 120, 3327–3335. [Google Scholar] [CrossRef] [PubMed]
- Zeng, R.; Dong, J. The Hippo Signaling Pathway in Drug Resistance in Cancer. Cancers 2021, 13, 318. [Google Scholar] [CrossRef] [PubMed]
- Mohri, Z.; Hernandez, A.D.R.; Krams, R. The emerging role of YAP/TAZ in mechanotransduction. J. Thorac. Dis. 2017, 9, E507–E509. [Google Scholar] [CrossRef]
- Nukuda, A.; Sasaki, C.T.; Ishihara, S.; Mizutani, T.; Nakamura, K.; Ayabe, T.; Kawabata, K.; Haga, H. Stiff substrates increase YAP-signaling-mediated matrix metalloproteinase-7 expression. Oncogenesis 2015, 4, e165. [Google Scholar] [CrossRef]
- Lee, K.-W.; Lee, S.S.; Kim, S.-B.; Sohn, B.H.; Lee, H.-S.; Jang, H.-J.; Park, Y.-Y.; Kopetz, S.; Kim, S.S.; Oh, S.C.; et al. Significant Association of Oncogene YAP1 with Poor Prognosis and Cetuximab Resistance in Colorectal Cancer Patients. Clin. Cancer Res. 2015, 21, 357–364. [Google Scholar] [CrossRef]
- Lee, H.J.; Diaz, M.F.; Price, K.M.; Ozuna, J.A.; Zhang, S.; Sevick-Muraca, E.M.; Hagan, J.; Wenzel, P.L. Fluid shear stress activates YAP1 to promote cancer cell motility. Nat. Commun. 2017, 8, 14122. [Google Scholar] [CrossRef]
- Szulzewsky, F.; Holland, E.C.; Vasioukhin, V. YAP1 and its fusion proteins in cancer initiation, progression and therapeutic resistance. Dev. Biol. 2021, 475, 205–221. [Google Scholar] [CrossRef]
- Shin, N.; Son, G.M.; Shin, D.-H.; Kwon, M.-S.; Park, B.-S.; Kim, H.-S.; Ryu, D.; Kang, C.-D. Cancer-Associated Fibroblasts and Desmoplastic Reactions Related to Cancer Invasiveness in Patients With Colorectal Cancer. Ann. Coloproctology 2019, 35, 36–46. [Google Scholar] [CrossRef]
- Kuzet, S.-E.; Gaggioli, C. Fibroblast activation in cancer: When seed fertilizes soil. Cell Tissue Res. 2016, 365, 607–619. [Google Scholar] [CrossRef] [PubMed]
- Mukaida, N.; Sasaki, S. Fibroblasts, an inconspicuous but essential player in colon cancer development and progression. World J. Gastroenterol. 2016, 22, 5301–5316. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Gaggioli, C.; Hooper, S.; Hidalgo-Carcedo, C.; Grosse, R.; Marshall, J.F.; Harrington, K.; Sahai, E. Fibroblast-led collective invasion of carcinoma cells with differing roles for RhoGTPases in leading and following cells. Nat. Cell Biol. 2007, 9, 1392–1400. [Google Scholar] [CrossRef]
- Labernadie, A.; Kato, T.; Brugués, A.; Serra-Picamal, X.; Derzsi, S.; Arwert, E.; Weston, A.; González-Tarragó, V.; Elosegui-Artola, A.; Albertazzi, L.; et al. A mechanically active heterotypic E-cadherin/N-cadherin adhesion enables fibroblasts to drive cancer cell invasion. Nat. Cell Biol. 2017, 19, 224–237. [Google Scholar] [CrossRef]
- Glentis, A.; Oertle, P.; Mariani, P.; Chikina, A.; El Marjou, F.; Attieh, Y.; Zaccarini, F.; Lae, M.; Loew, D.; Dingli, F.; et al. Cancer-associated fibroblasts induce metalloprotease-independent cancer cell invasion of the basement membrane. Nat. Commun. 2017, 8, 924. [Google Scholar] [CrossRef]
- Mierke, C.T. Mechanical Cues Affect Migration and Invasion of Cells From Three Different Directions. Front. Cell Dev. Biol. 2020, 8, 30. [Google Scholar] [CrossRef]
- Peng, C.; Zou, X.; Xia, W.; Gao, H.; Li, Z.; Liu, N.; Xu, Z.; Gao, C.; He, Z.; Niu, W.; et al. Integrin αvβ6 plays a bi-directional regulation role between colon cancer cells and cancer-associated fibroblasts. Biosci. Rep. 2018, 38, BSR20180243. [Google Scholar] [CrossRef]
- Erdogan, B.; Webb, D.J. Cancer-associated fibroblasts modulate growth factor signaling and extracellular matrix remodeling to regulate tumor metastasis. Biochem. Soc. Trans. 2017, 45, 229–236. [Google Scholar] [CrossRef]
- Jang, I.; Beningo, K.A. Integrins, CAFs and Mechanical Forces in the Progression of Cancer. Cancers 2019, 11, 721. [Google Scholar] [CrossRef]
- Attieh, Y.; Clark, A.G.; Grass, C.; Richon, S.; Pocard, M.; Mariani, P.; Elkhatib, N.; Betz, T.; Gurchenkov, B.; Vignjevic, D.M. Cancer-associated fibroblasts lead tumor invasion through integrin-β3–dependent fibronectin assembly. J. Cell Biol. 2017, 216, 3509–3520. [Google Scholar] [CrossRef] [PubMed]
- Bao, S.; Ouyang, G.; Bai, X.; Huang, Z.; Ma, C.; Liu, M.; Shao, R.; Anderson, R.M.; Rich, J.N.; Wang, X.-F. Periostin potently promotes metastatic growth of colon cancer by augmenting cell survival via the Akt/PKB pathway. Cancer Cell 2004, 5, 329–339. [Google Scholar] [CrossRef]
- Shiga, K.; Hara, M.; Nagasaki, T.; Sato, T.; Takahashi, H.; Takeyama, H. Cancer-Associated Fibroblasts: Their Characteristics and Their Roles in Tumor Growth. Cancers 2015, 7, 2443–2458. [Google Scholar] [CrossRef] [PubMed]
- Staudacher, J.; Bauer, J.; Jana, A.; Tian, J.; Carroll, T.; Mancinelli, G.; Özden, Ö.; Krett, N.; Guzman, G.; Kerr, D.; et al. Activin signaling is an essential component of the TGF-β induced pro-metastatic phenotype in colorectal cancer. Sci. Rep. 2017, 7, 5569. [Google Scholar] [CrossRef]
- Yoshinaga, K.; Mimori, K.; Inoue, H.; Kamohara, Y.; Yamashita, K.; Tanaka, F.; Mori, M. Activin A enhances MMP-7 activity via the transcription factor AP-1 in an esophageal squamous cell carcinoma cell line. Int. J. Oncol. 2008, 33, 453–459. [Google Scholar] [CrossRef]
- Storm, C.; Pastore, J.J.; MacKintosh, F.; Lubensky, T.C.; Janmey, P.A. Nonlinear elasticity in biological gels. Nature 2005, 435, 191–194. [Google Scholar] [CrossRef]
- Calvo, F.; Ege, N.; Grande-Garcia, A.; Hooper, S.; Jenkins, R.P.; Chaudhry, S.I.; Harrington, K.; Williamson, P.; Moeendarbary, E.; Charras, G.; et al. Mechanotransduction and YAP-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Nat. Cell Biol. 2013, 15, 637–646. [Google Scholar] [CrossRef]
- Madsen, C.D.; Pedersen, J.T.; Venning, F.A.; Singh, L.B.; Moeendarbary, E.; Charras, G.; Cox, T.R.; Sahai, E.; Erler, J.T. Hypoxia and loss of PHD2 inactivate stromal fibroblasts to decrease tumour stiffness andmetastasis. EMBO Rep. 2015, 16, 1394–1408. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhou, Q.; Wu, X.; Xu, S.; Hu, X.; Tao, X.; Li, B.; Peng, J.; Li, D.; Shen, L.; et al. VCAM-1 secreted from cancer-associated fibroblasts enhances the growth and invasion of lung cancer cells through AKT and MAPK signaling. Cancer Lett. 2020, 473, 62–73. [Google Scholar] [CrossRef]
- Avvisato, C.L.; Yang, X.; Shah, S.; Hoxter, B.; Li, W.; Gaynor, R.; Pestell, R.; Tozeren, A.; Byers, S.W. Mechanical force modulates global gene expression and beta-catenin signaling in colon cancer cells. J. Cell Sci. 2007, 120, 2672–2682. [Google Scholar] [CrossRef]
- Basson, M.D. Paradigms for mechanical signal transduction in the intestinal epithelium—Category: Molecular, cell, and developmental biology. Digestion 2003, 68, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Delon, L.; Guo, Z.; Oszmiana, A.; Chien, C.-C.; Gibson, R.; Prestidge, C.; Thierry, B. A systematic investigation of the effect of the fluid shear stress on Caco-2 cells towards the optimization of epithelial organ-on-chip models. Biomaterials 2019, 225, 119521. [Google Scholar] [CrossRef]
- Lam, W.A.; Rosenbluth, M.J.; Fletcher, D.A. Chemotherapy exposure increases leukemia cell stiffness. Blood 2007, 109, 3505–3508. [Google Scholar] [CrossRef] [PubMed]
- Grzanka, A.; Grzanka, D.; Zuryń, A.; Grzanka, A.A.; Safiejko-Mroczka, B. Reorganization of actin in K-562 and HL-60 cells treated with taxol. Neoplasma 2006, 53, 56–61. [Google Scholar]
- Levee, M.G.; Dabrowska, M.I.; Lelli, J.L.; Hinshaw, D.B. Actin polymerization and depolymerization during apoptosis in HL-60 cells. Am. J. Physiol. Cell Physiol. 1996, 271, C1981–C1992. [Google Scholar] [CrossRef]
- Kristi, N.; Gafur, A.; Kong, L.; Ma, X.; Ye, Z.; Wang, G. Atomic Force Microscopy in Mechanoimmunology Analysis: A New Perspective for Cancer Immunotherapy. Biotechnol. J. 2020, 15, e1900559. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yang, C.; Cheng, H.; Huang, S.; Zheng, Y. CAR-T cells for Colorectal Cancer: Target-selection and strategies for improved activity and safety. J. Cancer 2021, 12, 1804–1814. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Umezu-Goto, M.; Murph, M.; Lu, Y.; Liu, W.; Zhang, F.; Yu, S.; Stephens, L.C.; Cui, X.; Murrow, G.; et al. Expression of Autotaxin and Lysophosphatidic Acid Receptors Increases Mammary Tumorigenesis, Invasion, and Metastases. Cancer Cell 2009, 15, 539–550. [Google Scholar] [CrossRef]
- Shida, D.; Watanabe, T.; Aoki, J.; Hama, K.; Kitayama, J.; Sonoda, H.; Kishi, Y.; Yamaguchi, H.; Sasaki, S.; Sako, A.; et al. Aberrant expression of lysophosphatidic acid (LPA) receptors in human colorectal cancer. Lab. Investig. 2004, 84, 1352–1362. [Google Scholar] [CrossRef]
- Cortes, E.; Sarper, M.; Robinson, B.; Lachowski, D.; Chronopoulos, A.; Thorpe, S.D.; Lee, D.A.; Hernández, A.E.D.R. GPER is a mechanoregulator of pancreatic stellate cells and the tumor microenvironment. EMBO Rep. 2019, 20, e46556. [Google Scholar] [CrossRef]
- Kong, X.; Cheng, R.; Wang, J.; Fang, Y.; Hwang, K.C. Nanomedicines inhibiting tumor metastasis and recurrence and their clinical applications. Nano Today 2021, 36, 101004. [Google Scholar] [CrossRef]
- Yu, M.; Stott, S.; Toner, M.; Maheswaran, S.; Haber, D.A. Circulating tumor cells: Approaches to isolation and characterization. J. Cell Biol. 2011, 192, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Bankó, P.; Lee, S.Y.; Nagygyörgy, V.; Zrínyi, M.; Chae, C.H.; Cho, D.H.; Telekes, A. Technologies for circulating tumor cell separation from whole blood. J. Hematol. Oncol. 2019, 12, 20. [Google Scholar] [CrossRef]
- Tauro, B.J.; Greening, D.W.; Mathias, R.A.; Ji, H.; Mathivanan, S.; Scott, A.M.; Simpson, R.J. Comparison of ultracentrifugation, density gradient separation, and immunoaffinity capture methods for isolating human colon cancer cell line LIM1863-derived exosomes. Methods 2012, 56, 293–304. [Google Scholar] [CrossRef]
- Glia, A.; Deliorman, M.; Qasaimeh, M.A. (Eds.) Atomic force microscopy for single cell analysis and mechanophenotyping of circulating tumor cells. In Proceedings of the 2020 International Conference on Manipulation Automation and Robotics at Small Scales (MARSS), Toronto, ON, Canada, 13–17 July 2020. [Google Scholar]
- Liu, J.; Qu, Y.; Wang, G.; Wang, X.; Zhang, W.; Li, J.; Wang, Z.; Li, D.; Jiang, J. Study of morphological and mechanical features of multinuclear and mononuclear SW480 cells by atomic force microscopy. Microsc. Res. Tech. 2018, 81, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Serratì, S.; Porcelli, L.; Fragassi, F.; Garofoli, M.; Di Fonte, R.; Fucci, L.; Iacobazzi, R.; Palazzo, A.; Margheri, F.; Cristiani, G.; et al. The Interaction between Reactive Peritoneal Mesothelial Cells and Tumor Cells via Extracellular Vesicles Facilitates Colorectal Cancer Dissemination. Cancers 2021, 13, 2505. [Google Scholar] [CrossRef]
- Barroso, E.M.L. Development of Raman Spectroscopy Tools for Surgery Guidance in Head & Neck Oncology. Ph.D. Thesis, Erasmus University Rotterdam, London, UK, 2018. [Google Scholar]


| Technique | Principle | Refs. |
|---|---|---|
| Atomic force microscopy (AFM) | Mechanical characterization of samples in physiological conditions; allows quantification of mechanical properties of cells and tissue samples and also quantification of molecules and cells interactions by force spectroscopy, using dynamic conditions. | [82,112,113,114] |
| Microindentation | Quantification of micro-mechanical properties of biomaterials, hydrogels and biological samples down to cell-length scale, in physiological and dynamic conditions | [115] |
| Rheometry | Quantification of mechanical properties of soft tissues, thus being a promising technique for cancer diagnosis, as well as for assessment of effectiveness of anticancer treatments, in dynamic conditions | [116] |
| Shear wave elastography and magnetic resonance elastography | Characterization of healthy and diseased tissues in a non-invasive manner, with the generation of cross-sectional images, showing the stiffness of the tissue. | [117,118] |
| Elastography | Quantification of stiffness of the tissue is evaluated by the tissue response to an externally applied mechanical stimulus, leading to a measurable tissue deformation. | [119] |
| Micropippete aspiration | Allows study of a whole cell which, under the suction pressure, undergoes a deformation process, thereby allowing measurement of geometry of the cell inside the capillar. | [120] |
| Optical stretching | Formation of an optical trap, resulting from the two opposing laser beams, presenting a Gaussian intensity distribution, able to capture the cell that is in suspension and stretching it in situ; it acts on the entire cell membrane, making it possible to measure its viscoelastic properties. | [121] |
| Magnetic twisting cytometry | It is based in the application of a twisting field, generating rotational shear stresses in several directions; it allows the attachment of specific beads to the cytoskeleton through the membrane mechanoreceptors, applying local mechanical stress to live cells. | [122] |
| Terahertz waves | It is used in cancer diagnosis, not through mechanical properties but using imaging and spectroscopy (in a range of 0.1 and 10 THz). | [123,124] |
| Biophysical Cues | Biochemical Pathway Activation | Cell Process Activation | Prognosis | Therapeutic Target | Refs. |
|---|---|---|---|---|---|
| Cell stress (UV, hypoxia) ECM stiffness | p53 | Loss of cell adhesion promoting EMT Stroma enriched in CAFs DNA damage Filopodia formation | Good | Promote the wild-type p53 Avoid CAFs differentiation Avoid the ECM stiffness Regulating the expression of E- and N-cadherins, p53 prevents EMT | [39,66,156,157] |
| ECM stiffness | APC (loss) APC (overexpression) | Decrease of microtubule stability Shape changes in cells Cell deformation Loss of cell adhesion Induce cell protrusions Alter cell shape Cellular asymmetry by inducing longer cellular protrusion | Good | Validating the Wnt pathway as a therapeutic target | [13,132,135,158] |
| Cell friction forces ECM rigidity Fluid shear stress Forces applied on the nucleus | Hippo ON (YAP/TAZ off) Hippo ON (YAP/TAZ off) Hippo OFF (YAP/TAZ on) | Cell size increment Cell size increment MMP-7 increasing (break the BM) Cell stretching and density increasing Β-catenin inside the nucleus More research must be performed | Poor | The therapy must be applied according to the CMS | [39,142,143,147,159,160,161,162,163,164] |
| ECM stiffness | KRAS | EMT Cellular plasticity PI3K activation Cytoskeletal deformability | Poor | Targeting EMT | [66] |
| ECM via interaction with fibronectin through tenascin-C ECM stiffness | Rho-family | Filopodia formation Lamellopodia formation Podosomes formation Contractability Activation of actin regulation Stress fibers increase Loss of cell polarity | Poor | Target the integrins and ECM connections; target the tenascin-C Targeting VEGF and EGFR | [39,50,141,153] |
| ECM stiffness Shear stress Compression Cell adhesion | Wnt (on) Wnt (off) | APC mutation activation Nucleus with β-catenin inside More studies are need | Good | [95] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brás, M.M.; Sousa, S.R.; Carneiro, F.; Radmacher, M.; Granja, P.L. Mechanobiology of Colorectal Cancer. Cancers 2022, 14, 1945. https://doi.org/10.3390/cancers14081945
Brás MM, Sousa SR, Carneiro F, Radmacher M, Granja PL. Mechanobiology of Colorectal Cancer. Cancers. 2022; 14(8):1945. https://doi.org/10.3390/cancers14081945
Chicago/Turabian StyleBrás, Maria Manuela, Susana R. Sousa, Fátima Carneiro, Manfred Radmacher, and Pedro L. Granja. 2022. "Mechanobiology of Colorectal Cancer" Cancers 14, no. 8: 1945. https://doi.org/10.3390/cancers14081945
APA StyleBrás, M. M., Sousa, S. R., Carneiro, F., Radmacher, M., & Granja, P. L. (2022). Mechanobiology of Colorectal Cancer. Cancers, 14(8), 1945. https://doi.org/10.3390/cancers14081945