Emerging Biomarkers for Immunotherapy in Glioblastoma
Abstract
Simple Summary
Abstract
1. Introduction
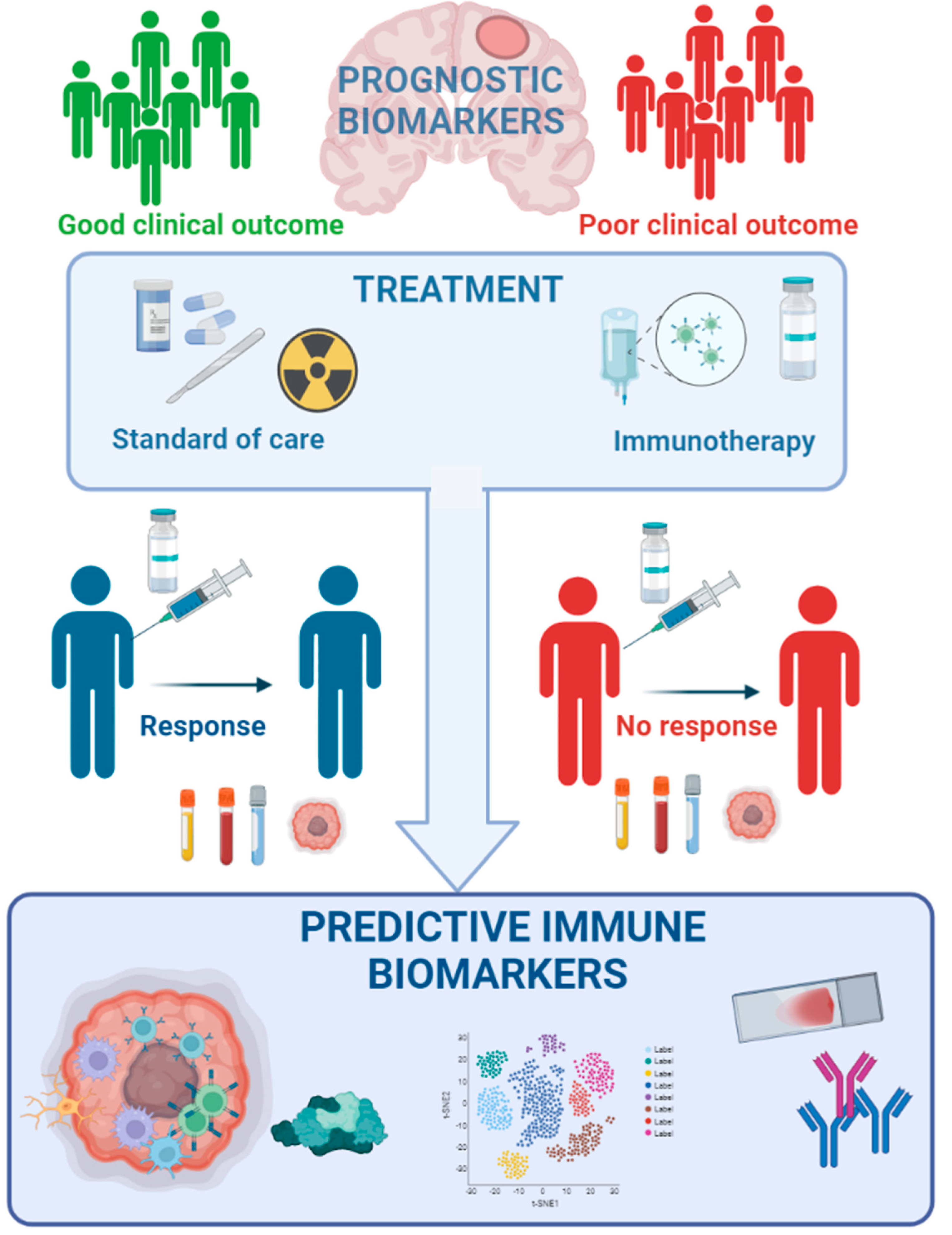
2. Current Prognostic Biomarkers in Clinical Use
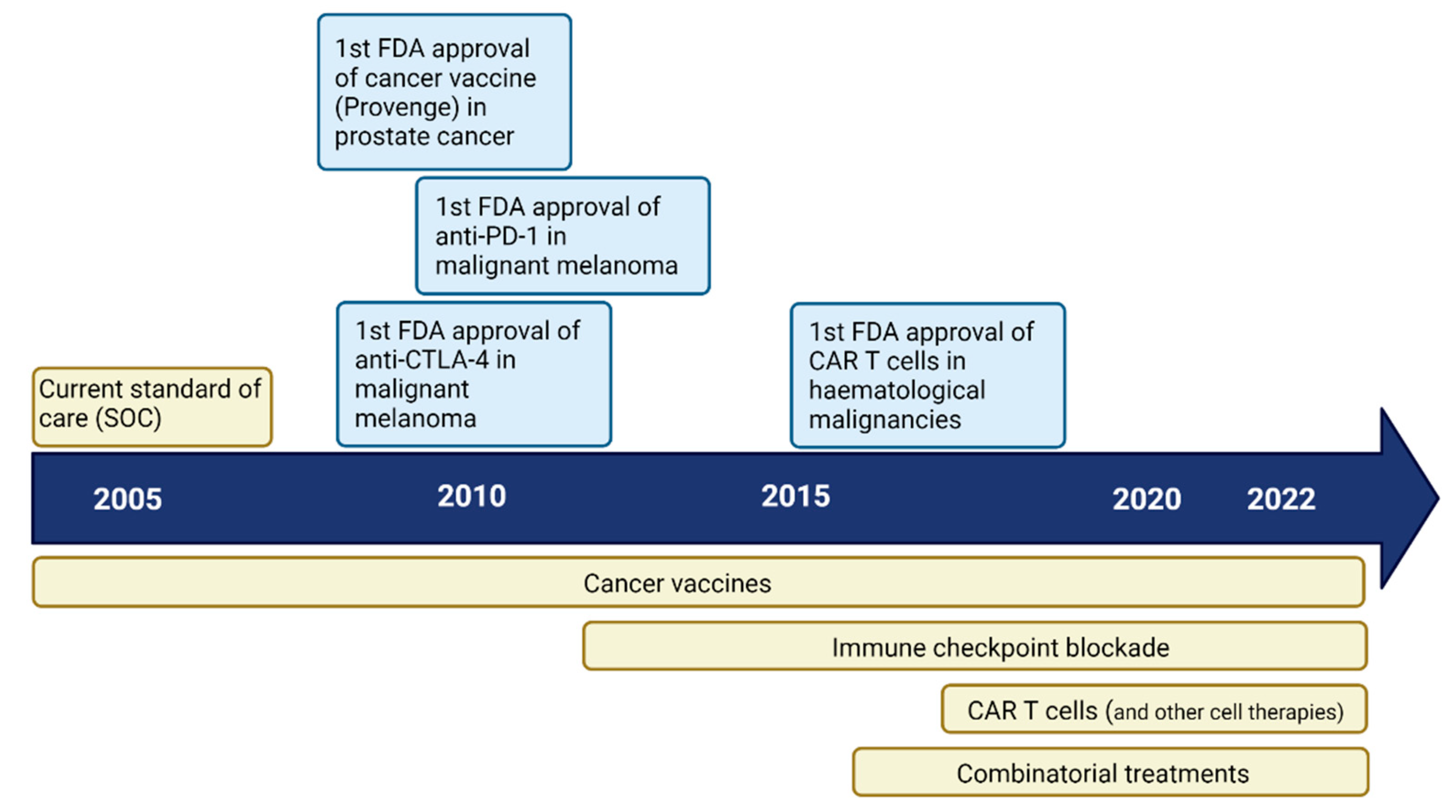
3. Immunotherapy for Glioblastoma
4. Tumour Microenvironment (TME) in Glioblastoma
4.1. Tumour-Associated Macrophages (TAM) and Myeloid-Derived Suppressor Cells (MDSCs)
4.2. GBM Tumour Biology: Molecular Plasticity and Heterogeneity
5. Current State and Future of Immunotherapy in GBM
5.1. Immune Checkpoint Blockade (ICB)
5.2. Therapeutic Vaccines
5.3. CAR T Cell Therapies
6. Combination with Multimodal Therapies
7. Discussion
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ostrom, Q.T.; Cioffi, G.; Gittleman, H.; Patil, N.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2012–2016. Neuro-Oncology 2019, 21, v1–v100. [Google Scholar] [CrossRef] [PubMed]
- Yaghi, N.K.; Gilbert, M.R. Immunotherapeutic Approaches for Glioblastoma Treatment. Biomedicines 2022, 10, 427. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.M.; Choi, J.; Lim, M. Mechanisms of immunotherapy resistance: Lessons from glioblastoma. Nat. Immunol. 2019, 20, 1100–1109. [Google Scholar] [CrossRef]
- Weller, M.; Butowski, N.; Tran, D.D.; Recht, L.D.; Lim, M.; Hirte, H.; Ashby, L.; Mechtler, L.; Goldlust, S.A.; Iwamoto, F.; et al. Rindopepimut with temozolomide for patients with newly diagnosed, EGFRvIII-expressing glioblastoma (ACT IV): A randomised, double-blind, international phase 3 trial. Lancet Oncol. 2017, 18, 1373–1385. [Google Scholar] [CrossRef]
- O’Rourke, D.M.; Nasrallah, M.P.; Desai, A.; Melenhorst, J.J.; Mansfield, K.; Morrissette, J.J.D.; Martinez-Lage, M.; Brem, S.; Maloney, E.; Shen, A.; et al. A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci. Transl. Med. 2017, 9, eaaa0984. [Google Scholar] [CrossRef] [PubMed]
- Omuro, A.; Vlahovic, G.; Lim, M.; Sahebjam, S.; Baehring, J.; Cloughesy, T.; Voloschin, A.; Ramkissoon, S.H.; Ligon, K.L.; Latek, R.; et al. Nivolumab with or without ipilimumab in patients with recurrent glioblastoma: Results from exploratory phase I cohorts of CheckMate 143. Neuro-Oncology 2018, 20, 674–686. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Hegi, M.E.; Mason, W.P.; van den Bent, M.J.; Taphoorn, M.J.B.; Janzer, R.C.; Ludwin, S.K.; Allgeier, A.; Fisher, B.; Belanger, K.; et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009, 10, 459–466. [Google Scholar] [CrossRef]
- Scott, J.N.; Rewcastle, N.B.; Brasher, P.M.; Fulton, D.; MacKinnon, J.A.; Hamilton, M.; Cairncross, J.G.; Forsyth, P. Which Glioblastoma Multiforme Patient Will Become a Long-Term Survivor? A Population-Based Study. Ann. Neurol. 1999, 46, 183–188. [Google Scholar] [CrossRef]
- Bi, W.L.; Beroukhim, R. Beating the odds: Extreme long-term survival with glioblastoma. Neuro-Oncology 2014, 16, 1159–1160. [Google Scholar] [CrossRef]
- Castro, L.N.G.; Wesseling, P. The cIMPACT-NOW updates and their significance to current neuro-oncology practice. Neuro-Oncol. Pract. 2021, 8, 4–10. [Google Scholar] [CrossRef]
- Combs, S.E.; Rieken, S.; Wick, W.; Abdollahi, A.; Von Deimling, A.; Debus, J.; Hartmann, C. Prognostic significance of IDH-1 and MGMT in patients with glioblastoma: One step forward, and one step back? Radiat. Oncol. 2011, 6, 115. [Google Scholar] [CrossRef] [PubMed]
- Amelot, A.; De Cremoux, P.; Quillien, V.; Polivka, M.; Adle-Biassette, H.; Lehmann-Che, J.; Françoise, L.; Carpentier, A.F.; George, B.; Mandonnet, E.; et al. IDH-Mutation Is a Weak Predictor of Long-Term Survival in Glioblastoma Patients. PLoS ONE 2015, 10, e0130596. [Google Scholar] [CrossRef] [PubMed]
- Wick, W.; Meisner, C.; Hentschel, B.; Platten, M.; Schilling, A.; Wiestler, B.; Sabel, M.; Koeppen, S.; Ketter, R.; Weiler, M.; et al. Prognostic or predictive value of MGMT promoter methylation in gliomas depends on IDH1 mutation. Neurology 2013, 81, 1515–1522. [Google Scholar] [CrossRef] [PubMed]
- Hegi, M.E.; Diserens, A.-C.; Gorlia, T.; Hamou, M.-F.; De Tribolet, N.; Weller, M.; Kros, J.M.; Hainfellner, J.A.; Mason, W.; Mariani, L.; et al. MGMT Gene Silencing and Benefit from Temozolomide in Glioblastoma. N. Engl. J. Med. 2005, 352, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Gan, H.K.; Kaye, A.H.; Luwor, R.B. The EGFRvIII variant in glioblastoma multiforme. J. Clin. Neurosci. 2009, 16, 748–754. [Google Scholar] [CrossRef] [PubMed]
- Montano, N.; D’Alessandris, Q.G.; Izzo, A.; Fernandez, E.; Pallini, R. Biomarkers for glioblastoma multiforme: Status quo. J. Clin. Transl. Res. 2016, 2, 3–10. [Google Scholar]
- Felsberg, J.; Hentschel, B.; Kaulich, K.; Gramatzki, D.; Zacher, A.; Malzkorn, B.; Kamp, M.; Sabel, M.C.; Simon, M.; Westphal, M.; et al. Epidermal Growth Factor Receptor Variant III (EGFRvIII) Positivity in EGFR-Amplified Glioblastomas: Prognostic Role and Comparison between Primary and Recurrent Tumors. Clin. Cancer Res. 2017, 23, 6846–6855. [Google Scholar] [CrossRef]
- Nguyen, H.N.; Lie, A.; Li, T.; Chowdhury, R.; Liu, F.; Ozer, B.; Wei, B.; Green, R.M.; Ellingson, B.M.; Wang, H.-J.; et al. Human TERT Promoter Mutation Enables Survival Advantage from MGMT Promoter Methylation in IDH1 Wild-Type Primary Glioblastoma Treated by Standard Chemoradiotherapy. Neuro-Oncology 2017, 19, 394–404. [Google Scholar] [CrossRef]
- Chen, P.-L.; Roh, W.; Reuben, A.; Cooper, Z.A.; Spencer, C.N.; Prieto, P.A.; Miller, J.P.; Bassett, R.L.; Gopalakrishnan, V.; Wani, K.; et al. Analysis of Immune Signatures in Longitudinal Tumor Samples Yields Insight into Biomarkers of Response and Mechanisms of Resistance to Immune Checkpoint Blockade. Cancer Discov. 2016, 6, 827–837. [Google Scholar] [CrossRef]
- Fecci, P.; Ochiai, H.; Mitchell, D.A.; Grossi, P.M.; Sweeney, A.E.; Archer, G.E.; Cummings, T.; Allison, J.; Bigner, D.D.; Sampson, J. Systemic CTLA-4 Blockade Ameliorates Glioma-Induced Changes to the CD4+ T Cell Compartment without Affecting Regulatory T-Cell Function. Clin. Cancer Res. 2007, 13, 2158–2167. [Google Scholar] [CrossRef]
- Zeng, J.; See, A.P.; Phallen, J.; Jackson, C.M.; Belcaid, Z.; Ruzevick, J.; Durham, N.; Meyer, C.; Harris, T.J.; Albesiano, E.; et al. Anti-PD-1 Blockade and Stereotactic Radiation Produce Long-Term Survival in Mice with Intracranial Gliomas. Int. J. Radiat. Oncol. Biol. Phys. 2013, 86, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kwon, M.; Kim, K.H.; Kim, T.-S.; Hong, S.-H.; Kim, C.G.; Kang, S.-G.; Moon, J.H.; Kim, E.H.; Park, S.-H.; et al. Immune Checkpoint Inhibitor-Induced Reinvigoration of Tumor-Infiltrating CD8+ T Cells is Determined by Their Differentiation Status in Glioblastoma. Clin. Cancer Res. 2019, 25, 2549–2559. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Chen, A.; Gartrell, R.D.; Silverman, A.M.; Aparicio, L.; Chu, T.; Bordbar, D.; Shan, D.; Samanamud, J.; Mahajan, A.; et al. Immune and genomic correlates of response to anti-PD-1 immunotherapy in glioblastoma. Nat. Med. 2019, 25, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Sager, O.; Dincoglan, F.; Demiral, S.; Uysal, B.; Gamsiz, H.; Dirican, B.; Beyzadeoğlu, M. A concise review of immunotherapy for glioblastoma. Neuroimmunol. Neuroinflamm. 2018, 5, 25. [Google Scholar] [CrossRef]
- Medikonda, R.; Dunn, G.; Rahman, M.; Fecci, P.; Lim, M. A review of glioblastoma immunotherapy. J. Neuro-Oncol. 2021, 151, 41–53. [Google Scholar] [CrossRef]
- Lim, M.; Xia, Y.; Bettegowda, C.; Weller, M. Current state of immunotherapy for glioblastoma. Nat. Rev. Clin. Oncol. 2018, 15, 422–442. [Google Scholar] [CrossRef]
- Charles, N.A.; Holland, E.C.; Gilbertson, R.; Glass, R.; Kettenmann, H. The brain tumor microenvironment. Glia 2012, 60, 502–514. [Google Scholar] [CrossRef]
- Hambardzumyan, D.; Gutmann, D.H.; Kettenmann, H. The role of microglia and macrophages in glioma maintenance and progression. Nat. Neurosci. 2016, 19, 20–27. [Google Scholar] [CrossRef]
- Chen, Z.; Hambardzumyan, D. Immune Microenvironment in Glioblastoma Subtypes. Front. Immunol. 2018, 9, 1004. [Google Scholar] [CrossRef]
- Preusser, M.; Berghoff, A.S.; Thallinger, C.; Zielinski, C. CECOG educational illustrations: The blood–brain barrier and its relevance for targeted cancer therapies and immuno-oncology. ESMO Open 2017, 2, e000194. [Google Scholar] [CrossRef][Green Version]
- Tomaszewski, W.; Sanchez-Perez, L.; Gajewski, T.F.; Sampson, J.H. Brain Tumor Microenvironment and Host State: Implications for Immunotherapy. Clin. Cancer Res. 2019, 25, 4202–4210. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Meng, Q.; Bartek, J.; Poiret, T.; Persson, O.; Rane, L.; Rangelova, E.; Illies, C.; Peredo, I.H.; Luo, X.; et al. Tumor-infiltrating lymphocytes (TILs) from patients with glioma. OncoImmunology 2017, 6, e1252894. [Google Scholar] [CrossRef] [PubMed]
- Woroniecka, K.; Chongsathidkiet, P.; Rhodin, K.; Kemeny, H.; DeChant, C.; Farber, S.H.; Elsamadicy, A.A.; Cui, X.; Koyama, S.; Jackson, C.; et al. T-Cell Exhaustion Signatures Vary with Tumor Type and Are Severe in Glioblastoma. Clin. Cancer Res. 2018, 24, 4175–4186. [Google Scholar] [CrossRef] [PubMed]
- Woroniecka, K.I.; Rhodin, K.E.; Chongsathidkiet, P.; Keith, K.A.; Fecci, P.E. T-cell Dysfunction in Glioblastoma: Applying a New Framework. Clin. Cancer Res. 2018, 24, 3792–3802. [Google Scholar] [CrossRef]
- Galon, J.; Costes, A.; Sanchez-Cabo, F.; Kirilovsky, A.; Mlecnik, B.; Lagorce-Pagès, C.; Tosolini, M.; Camus, M.; Berger, A.; Wind, P.; et al. Type, Density, and Location of Immune Cells within Human Colorectal Tumors Predict Clinical Outcome. Science 2006, 313, 1960–1964. [Google Scholar] [CrossRef]
- Angell, H.K.; Bruni, D.; Barrett, J.C.; Herbst, R.; Galon, J. The Immunoscore: Colon Cancer and Beyond. Clin. Cancer Res. 2020, 26, 332–339. [Google Scholar] [CrossRef]
- Han, S.; Ma, E.; Wang, X.; Yu, C.; Dong, T.; Zhan, W.; Wei, X.; Liang, G.; Feng, S. Rescuing defective tumor-infiltrating T-cell proliferation in glioblastoma patients. Oncol. Lett. 2016, 12, 2924–2929. [Google Scholar] [CrossRef]
- Ooi, Y.C.; Tran, P.; Ung, N.; Thill, K.; Trang, A.; Fong, B.M.; Nagasawa, D.T.; Lim, M.; Yang, I. The role of regulatory T-cells in glioma immunology. Clin. Neurol. Neurosurg. 2014, 119, 125–132. [Google Scholar] [CrossRef]
- Han, S.; Zhang, C.; Li, Q.; Dong, J.; Liu, Y.; Huang, Y.; Jiang, T.; Wu, A. Tumour-infiltrating CD4+ and CD8+ lymphocytes as predictors of clinical outcome in glioma. Br. J. Cancer 2014, 110, 2560–2568. [Google Scholar] [CrossRef]
- Mohme, M.; Neidert, M.C. Tumor-Specific T Cell Activation in Malignant Brain Tumors. Front. Immunol. 2020, 11, 205. [Google Scholar] [CrossRef]
- Yang, I.; Tihan, T.; Han, S.J.; Wrensch, M.R.; Wiencke, J.; Sughrue, M.E.; Parsa, A.T. CD8+ T-cell infiltrate in newly diagnosed glioblastoma is associated with long-term survival. J. Clin. Neurosci. 2010, 17, 1381–1385. [Google Scholar] [CrossRef] [PubMed]
- Lohr, J.; Ratliff, T.; Huppertz, A.; Ge, Y.; Dictus, C.; Ahmadi, R.; Grau, S.; Hiraoka, N.; Eckstein, V.; Ecker, R.C.; et al. Effector T-Cell Infiltration Positively Impacts Survival of Glioblastoma Patients and Is Impaired by Tumor-Derived TGF-β. Clin. Cancer Res. 2011, 17, 4296–4308. [Google Scholar] [CrossRef] [PubMed]
- Komohara, Y.; Ohnishi, K.; Kuratsu, J.; Takeya, M. Possible involvement of the M2 anti-inflammatory macrophage phenotype in growth of human gliomas. J. Pathol. 2008, 216, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Bieńkowski, M.; Preusser, M. Prognostic role of tumour-infiltrating inflammatory cells in brain tumours: Literature review. Curr. Opin. Neurol. 2015, 28, 647–658. [Google Scholar] [CrossRef]
- Gieryng, A.; Pszczolkowska, D.; Walentynowicz, K.A.; Rajan, W.D.; Kaminska, B. Immune microenvironment of gliomas. Lab. Investig. 2017, 97, 498–518. [Google Scholar] [CrossRef]
- Roesch, S.; Rapp, C.; Dettling, S.; Herold-Mende, C. When Immune Cells Turn Bad—Tumor-Associated Microglia/Macrophages in Glioma. Int. J. Mol. Sci. 2018, 19, 436. [Google Scholar] [CrossRef]
- Pimenta, M.G.; Otero, Á.; Guzman, D.A.A.; Pascual-Argente, D.; Martín, L.R.; Sousa-Casasnovas, P.; García-Martin, A.; de Oca, J.C.R.M.; Villaseñor, J.J.M.; Carretero, L.T.; et al. Tumor cell and immune cell profiles in primary human glioblastoma: Impact on patient outcome. Brain Pathol. 2021, 31, 365–380. [Google Scholar] [CrossRef]
- Del Bianco, P.; Pinton, L.; Magri, S.; Canè, S.; Masetto, E.; Basso, D.; Padovan, M.; Volpin, F.; D’Avella, D.; Lombardi, G.; et al. Myeloid Diagnostic and Prognostic Markers of Immune Suppression in the Blood of Glioma Patients. Front. Immunol. 2021, 12, 809826. [Google Scholar] [CrossRef]
- Lakshmanachetty, S.; Cruz-Cruz, J.; Hoffmeyer, E.; Cole, A.; Mitra, S. New Insights into the Multifaceted Role of Myeloid-Derived Suppressor Cells (MDSCs) in High-Grade Gliomas: From Metabolic Reprograming, Immunosuppression, and Therapeutic Resistance to Current Strategies for Targeting MDSCs. Cells 2021, 10, 893. [Google Scholar] [CrossRef]
- Diaz-Montero, C.M.; Finke, J.; Montero, A.J. Myeloid-Derived Suppressor Cells in Cancer: Therapeutic, Predictive, and Prognostic Implications. Semin. Oncol. 2014, 41, 174–184. [Google Scholar] [CrossRef]
- Bayik, D.; Zhou, Y.; Park, C.; Hong, C.; Vail, D.; Silver, D.J.; Lauko, A.; Roversi, G.; Watson, D.C.; Lo, A.; et al. Myeloid-Derived Suppressor Cell Subsets Drive Glioblastoma Growth in a Sex-Specific Manner. Cancer Discov. 2020, 10, 1210–1225. [Google Scholar] [CrossRef] [PubMed]
- Sottoriva, A.; Spiteri, I.; Piccirillo, S.G.M.; Touloumis, A.; Collins, V.P.; Marioni, J.C.; Curtis, C.; Watts, C.; Tavaré, S. Intratumor heterogeneity in human glioblastoma reflects cancer evolutionary dynamics. Proc. Natl. Acad. Sci. USA 2013, 110, 4009–4014. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, N.; Gielen, G.H.; Rauschenbach, L.; Kebir, S.; Till, A.; Reinartz, R.; Simon, M.; Niehusmann, P.; Kleinschnitz, C.; Herrlinger, U.; et al. Longitudinal heterogeneity in glioblastoma: Moving targets in recurrent versus primary tumors. J. Transl. Med. 2019, 17, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Alexandrov, L.B.; Nik-Zainal, S.; Wedge, D.C.; Aparicio, S.A.J.R.; Behjati, S.; Biankin, A.V.; Bignell, G.R.; Bolli, N.; Borg, A.; Børresen-Dale, A.-L.; et al. Signatures of mutational processes in human cancer. Nature 2013, 500, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Gromeier, M.; Brown, M.C.; Zhang, G.; Lin, X.; Chen, Y.; Wei, Z.; Beaubier, N.; Yan, H.; He, Y.; Desjardins, A.; et al. Very low mutation burden is a feature of inflamed recurrent glioblastomas responsive to cancer immunotherapy. Nat. Commun. 2021, 12, 352. [Google Scholar] [CrossRef] [PubMed]
- Louveau, A.; Smirnov, I.; Keyes, T.J.; Eccles, J.D.; Rouhani, S.J.; Peske, J.D.; Derecki, N.C.; Castle, D.; Mandell, J.W.; Lee, K.S.; et al. Structural and functional features of central nervous system lymphatic vessels. Nature 2015, 523, 337–341. [Google Scholar] [CrossRef]
- Allen, B.D.; Limoli, C.L. Breaking barriers: Neurodegenerative repercussions of radiotherapy induced damage on the blood-brain and blood-tumor barrier. Free Radic. Biol. Med. 2021, 178, 189–201. [Google Scholar] [CrossRef]
- Reardon, D.A.; Brandes, A.A.; Omuro, A.; Mulholland, P.; Lim, M.; Wick, A.; Baehring, J.; Ahluwalia, M.S.; Roth, P.; Bähr, O.; et al. Effect of Nivolumab vs Bevacizumab in Patients with Recurrent Glioblastoma: The CheckMate 143 Phase 3 Randomized Clinical Trial. JAMA Oncol. 2020, 6, 1003–1010. [Google Scholar] [CrossRef]
- Sampson, J.H.; Omuro, A.M.P.; Preusser, M.; Lim, M.; Butowski, N.A.; Cloughesy, T.F.; Strauss, L.C.; Latek, R.R.; Paliwal, P.; Weller, M.; et al. A randomized, phase 3, open-label study of nivolumab versus temozolomide (TMZ) in combination with radiotherapy (RT) in adult patients (pts) with newly diagnosed, O-6-methylguanine DNA methyltransferase (MGMT)-unmethylated glioblastoma (GBM): CheckMate-498. J. Clin. Oncol. 2016, 34, TPS2079. [Google Scholar] [CrossRef]
- Weller, M.; Lim, M.; Idbaih, A.; Steinbach, J.; Finocchiaro, G.; Raval, R.; Ashby, L.; Ansstas, G.; Baehring, J.; Taylor, J.; et al. CTIM-25. A Randomized Phase 3 Study of Nivolumab or Placebo Combined with Radiotherapy Plus Temozolomide in Patients with Newly Diagnosed Glioblastoma with Methylated Mgmt Promoter: Checkmate 548. Neuro-Oncology 2021, 23, vi55–vi56. [Google Scholar] [CrossRef]
- Schalper, K.A.; Rodriguez-Ruiz, M.E.; Diez-Valle, R.; López-Janeiro, A.; Porciuncula, A.; Idoate, M.A.; Inogés, S.; De Andrea, C.; De Cerio, A.L.-D.; Tejada, S.; et al. Neoadjuvant nivolumab modifies the tumor immune microenvironment in resectable glioblastoma. Nat. Med. 2019, 25, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Cloughesy, T.F.; Mochizuki, A.Y.; Orpilla, J.R.; Hugo, W.; Lee, A.H.; Davidson, T.B.; Wang, A.C.; Ellingson, B.M.; Rytlewski, J.A.; Sanders, C.M.; et al. Neoadjuvant anti-PD-1 immunotherapy promotes a survival benefit with intratumoral and systemic immune responses in recurrent glioblastoma. Nat. Med. 2019, 25, 477–486. [Google Scholar] [CrossRef] [PubMed]
- De Groot, J.F.; Penas-Prado, M.; Mandel, J.J.; O’Brien, B.J.; Weathers, S.-P.S.; Zhou, S.; Hunter, K.; Alfaro-Munoz, K.; Fuller, G.N.; Huse, J.; et al. Window-of-opportunity clinical trial of a PD-1 inhibitor in patients with recurrent glioblastoma. J. Clin. Oncol. 2018, 36, 2008. [Google Scholar] [CrossRef]
- Reardon, D.A.; Kaley, T.J.; Dietrich, J.; Clarke, J.L.; Dunn, G.; Lim, M.; Cloughesy, T.F.; Gan, H.K.; Park, A.J.; Schwarzenberger, P.; et al. Phase II study to evaluate safety and efficacy of MEDI4736 (durvalumab) + radiotherapy in patients with newly diagnosed unmethylated MGMT glioblastoma (new unmeth GBM). J. Clin. Oncol. 2019, 37, 2032. [Google Scholar] [CrossRef]
- Awada, G.; Ben Salama, L.; De Cremer, J.; Schwarze, J.K.; Fischbuch, L.; Seynaeve, L.; Du Four, S.; Vanbinst, A.-M.; Michotte, A.; Everaert, H.; et al. Axitinib plus avelumab in the treatment of recurrent glioblastoma: A stratified, open-label, single-center phase 2 clinical trial (GliAvAx). J. Immunother. Cancer 2020, 8, e001146. [Google Scholar] [CrossRef]
- Jacques, F.H.; Nicholas, G.; Lorimer, I.A.J.; Foko, V.S.; Prevost, J.; Dumais, N.; Milne, K.; Nelson, B.H.; Woulfe, J.; Jansen, G.; et al. Avelumab in newly diagnosed glioblastoma. Neuro-Oncol. Adv. 2021, 3, vdab118. [Google Scholar] [CrossRef]
- Reardon, D.A.; Desjardins, A.; Vredenburgh, J.J.; O’Rourke, D.M.; Tran, D.D.; Fink, K.L.; Nabors, L.B.; Li, G.; Bota, D.A.; Lukas, R.V.; et al. Rindopepimut with Bevacizumab for Patients with Relapsed EGFRvIII-Expressing Glioblastoma (ReACT): Results of a Double-Blind Randomized Phase II Trial. Clin. Cancer Res. 2020, 26, 1586–1594. [Google Scholar] [CrossRef]
- Platten, M.; Schilling, D.; Bunse, L.; Wick, A.; Bunse, T.; Riehl, D.; Karapanagiotou-Schenkel, I.; Harting, I.; Sahm, F.; Schmitt, A.; et al. A mutation-specific peptide vaccine targeting IDH1R132H in patients with newly diagnosed malignant astrocytomas: A first-in-man multicenter phase I clinical trial of the German Neurooncology Working Group (NOA-16). J. Clin. Oncol. 2018, 36, 2001. [Google Scholar] [CrossRef]
- Ahluwalia, M.S.; Reardon, D.A.; Abad, A.P.; Curry, W.T.; Wong, E.T.; Belal, A.; Qiu, J.; Mogensen, K.; Schilero, C.; Casucci, D.; et al. Phase II trial of SurVaxM combined with standard therapy in patients with newly diagnosed glioblastoma. J. Clin. Oncol. 2018, 36, 2041. [Google Scholar] [CrossRef]
- Rampling, R.; Peoples, S.; Mulholland, P.; James, A.; Al-Salihi, O.; Twelves, C.J.; McBain, C.; Jefferies, S.; Jackson, A.; Stewart, W.; et al. A Cancer Research UK First Time in Human Phase I Trial of IMA950 (Novel Multipeptide Therapeutic Vaccine) in Patients with Newly Diagnosed Glioblastoma. Clin. Cancer Res. 2016, 22, 4776–4785. [Google Scholar] [CrossRef]
- Migliorini, D.; Dutoit, V.; Allard, M.; Hallez, N.G.; Marinari, E.; Widmer, V.; Philippin, G.; Corlazzoli, F.; Gustave, R.; Kreutzfeldt, M.; et al. Phase I/II trial testing safety and immunogenicity of the multipeptide IMA950/poly-ICLC vaccine in newly diagnosed adult malignant astrocytoma patients. Neuro-Oncology 2019, 21, 923–933. [Google Scholar] [CrossRef] [PubMed]
- Dutoit, V.; Marinari, E.; Dietrich, P.-Y.; Migliorini, D. CTIM-08. Combination of the Ima950/Poly-Iclc Multipeptide Vaccine with Pembrolizumab in Relapsing Glioblastoma Patients. Neuro-Oncology 2020, 22, ii34. [Google Scholar] [CrossRef]
- Hilf, N.; Kuttruff-Coqui, S.; Frenzel, K.; Bukur, V.; Stevanović, S.; Gouttefangeas, C.; Platten, M.; Tabatabai, G.; Dutoit, V.; Van Der Burg, S.H.; et al. Actively personalized vaccination trial for newly diagnosed glioblastoma. Nature 2019, 565, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Keskin, D.B.; Anandappa, A.J.; Sun, J.; Tirosh, I.; Mathewson, N.D.; Li, S.; Oliveira, G.; Giobbie-Hurder, A.; Felt, K.; Gjini, E.; et al. Neoantigen vaccine generates intratumoral T cell responses in phase Ib glioblastoma trial. Nature 2019, 565, 234–239. [Google Scholar] [CrossRef]
- Wen, P.Y.; Reardon, D.A.; Armstrong, T.S.; Phuphanich, S.; Aiken, R.D.; Landolfi, J.C.; Curry, W.T.; Zhu, J.-J.; Glantz, M.; Peereboom, D.M.; et al. A Randomized Double-Blind Placebo-Controlled Phase II Trial of Dendritic Cell Vaccine ICT-107 in Newly Diagnosed Patients with Glioblastoma. Clin. Cancer Res. 2019, 25, 5799–5807. [Google Scholar] [CrossRef]
- Liau, L.M.; Ashkan, K.; Tran, D.D.; Campian, J.L.; Trusheim, J.E.; Cobbs, C.S.; Heth, J.A.; Salacz, M.; Taylor, S.; D’Andre, S.D.; et al. First results on survival from a large Phase 3 clinical trial of an autologous dendritic cell vaccine in newly diagnosed glioblastoma. J. Transl. Med. 2018, 16, 142. [Google Scholar] [CrossRef]
- Batich, K.; Mitchell, D.; Healy, P.; Herndon, J.; Broadwater, G.; Michael, G.; Huang, M.-N.; Hotchkiss, K.; Sanchez-Perez, L.; Nair, S.; et al. CTIM-10. Reproducibility of Clinical Trials Using CMV-Targeted Dendritic Cell Vaccines in Patients with Glioblastoma. Neuro-Oncology 2021, 23, vi51. [Google Scholar] [CrossRef]
- Rapp, M.; Grauer, O.M.; Kamp, M.; Sevens, N.; Zotz, N.; Sabel, M.; Sorg, R.V. A randomized controlled phase II trial of vaccination with lysate-loaded, mature dendritic cells integrated into standard radiochemotherapy of newly diagnosed glioblastoma (GlioVax): Study protocol for a randomized controlled trial. Trials 2018, 19, 293. [Google Scholar] [CrossRef]
- Vik-Mo, E.O.; Nyakas, M.; Mikkelsen, B.V.; Moe, M.C.; Due-Tønnesen, P.; Suso, E.M.I.; Sæbøe-Larssen, S.; Sandberg, C.; Brinchmann, J.E.; Helseth, E.; et al. Therapeutic vaccination against autologous cancer stem cells with mRNA-transfected dendritic cells in patients with glioblastoma. Cancer Immunol. Immunother. 2013, 62, 1499–1509. [Google Scholar] [CrossRef]
- Ji, N.; Zhang, Y.; Liu, Y.; Xie, J.; Wang, Y.; Hao, S.; Gao, Z. Heat shock protein peptide complex-96 vaccination for newly diagnosed glioblastoma: A phase I, single-arm trial. JCI Insight 2018, 3, 99145. [Google Scholar] [CrossRef]
- Bloch, O.; Lim, M.; Sughrue, M.E.; Komotar, R.J.; Abrahams, J.M.; O’Rourke, D.; D’Ambrosio, A.; Bruce, J.N.; Parsa, A.T. Autologous Heat Shock Protein Peptide Vaccination for Newly Diagnosed Glioblastoma: Impact of Peripheral PD-L1 Expression on Response to Therapy. Clin. Cancer Res. 2017, 23, 3575–3584. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Du, Y.; Zhang, Y.; Ji, N. Immunotherapy with heat shock protein 96 to treat gliomas. Chin. Neurosurg. J. 2020, 6, 31. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.E.; Badie, B.; Barish, M.E.; Weng, L.; Ostberg, J.R.; Chang, W.-C.; Naranjo, A.; Starr, R.; Wagner, J.; Wright, C.; et al. Bioactivity and Safety of IL13Rα2-Redirected Chimeric Antigen Receptor CD8+ T Cells in Patients with Recurrent Glioblastoma. Clin. Cancer Res. 2015, 21, 4062–4072. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.E.; Alizadeh, D.; Starr, R.; Weng, L.; Wagner, J.R.; Naranjo, A.; Ostberg, J.R.; Blanchard, M.S.; Kilpatrick, J.; Simpson, J.; et al. Regression of Glioblastoma after Chimeric Antigen Receptor T-Cell Therapy. N. Engl. J. Med. 2016, 375, 2561–2569. [Google Scholar] [CrossRef]
- Suryadevara, C.M.; Desai, R.; Abel, M.L.; Riccione, K.A.; Batich, K.A.; Shen, S.H.; Chongsathidkiet, P.; Gedeon, P.; Elsamadicy, A.A.; Snyder, D.J.; et al. Temozolomide lymphodepletion enhances CAR abundance and correlates with antitumor efficacy against established glioblastoma. OncoImmunology 2018, 7, e1434464. [Google Scholar] [CrossRef]
- Ahmed, N.; Brawley, V.; Hegde, M.; Bielamowicz, K.; Kalra, M.; Landi, D.; Robertson, C.; Gray, T.L.; Diouf, O.; Wakefield, A.; et al. HER2-Specific Chimeric Antigen Receptor–Modified Virus-Specific T Cells for Progressive Glioblastoma: A Phase 1 Dose-Escalation Trial. JAMA Oncol. 2017, 3, 1094–1101. [Google Scholar] [CrossRef]
- Goff, S.L.; Morgan, R.A.; Yang, J.C.; Sherry, R.M.; Robbins, P.F.; Restifo, N.P.; Feldman, S.A.; Lu, Y.-C.; Lu, L.; Zheng, Z.; et al. Pilot Trial of Adoptive Transfer of Chimeric Antigen Receptor–Transduced T Cells Targeting EGFRvIII in Patients with Glioblastoma. J. Immunother. 2019, 42, 126–135. [Google Scholar] [CrossRef]
- Badie, B.; Barish, M.E.; Chaudhry, A.; D’Apuzzo, M.; Forman, S.J.; Portnow, J.; Wang, S.; Ressler, J.A.; Simpson, J.; Kilpatrick, J.; et al. A phase 1 study to evaluate chimeric antigen receptor (CAR) T cells incorporating a chlorotoxin tumor-targeting domain for patients with MMP2+ Recurrent or progressive glioblastoma (NCT04214392). J. Clin. Oncol. 2021, 39, TPS2662. [Google Scholar] [CrossRef]
- Bagley, S.; Desai, A.; Binder, Z.; Nasrallah, M.; Hwang, W.-T.; Maloney-Wilensky, E.; Prior, T.; Brem, S.; O’Rourke, D. RBTT-12. A Phase I Study of Egfrviii-Directed Car T Cells Combined with PD-1 Inhibition in Patients with Newly, Diagnosed, Mgmt-Unmethylated Glioblastoma: Trial In Progress. Neuro-Oncology 2019, 21, vi221. [Google Scholar] [CrossRef]
- Reardon, D.A.; Omuro, A.; Brandes, A.A.; Rieger, J.; Wick, A.; Sepulveda, J.; Phuphanich, S.; De Souza, P.; Ahluwalia, M.S.; Lim, M.; et al. OS10.3 Randomized Phase 3 Study Evaluating the Efficacy and Safety of Nivolumab vs Bevacizumab in Patients With Recurrent Glioblastoma: CheckMate 143. Neuro-Oncology 2017, 19, iii21. [Google Scholar] [CrossRef]
- Bouffet, E.; Larouche, V.; Campbell, B.B.; Merico, D.; De Borja, R.; Aronson, M.; Durno, C.; Krueger, J.; Cabric, V.; Ramaswamy, V.; et al. Immune Checkpoint Inhibition for Hypermutant Glioblastoma Multiforme Resulting From Germline Biallelic Mismatch Repair Deficiency. J. Clin. Oncol. 2016, 34, 2206–2211. [Google Scholar] [CrossRef] [PubMed]
- Aslan, K.; Turco, V.; Blobner, J.; Sonner, J.K.; Liuzzi, A.R.; Núñez, N.G.; De Feo, D.; Kickingereder, P.; Fischer, M.; Green, E.; et al. Heterogeneity of response to immune checkpoint blockade in hypermutated experimental gliomas. Nat. Commun. 2020, 11, 931. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, Y. Identification of glioblastoma immune subtypes and immune landscape based on a large cohort. Hereditas 2021, 158, 30. [Google Scholar] [CrossRef] [PubMed]
- Magri, S.; Musca, B.; Bonaudo, C.; Tushe, A.; Russo, M.G.; Masetto, E.; Zagonel, V.; Lombardi, G.; Della Puppa, A.; Mandruzzato, S. Sustained Accumulation of Blood-Derived Macrophages in the Immune Microenvironment of Patients with Recurrent Glioblastoma after Therapy. Cancers 2021, 13, 6178. [Google Scholar] [CrossRef] [PubMed]
- Swartz, A.M.; Shen, S.H.; Salgado, M.A.; Congdon, K.L.; Sanchez-Perez, L. Promising vaccines for treating glioblastoma. Expert Opin. Biol. Ther. 2018, 18, 1159–1170. [Google Scholar] [CrossRef]
- Sampson, J.H.; Heimberger, A.B.; Archer, G.E.; Aldape, K.D.; Friedman, A.H.; Friedman, H.S.; Gilbert, M.R.; Ii, J.E.H.; McLendon, R.E.; Mitchell, D.A.; et al. Immunologic Escape after Prolonged Progression-Free Survival with Epidermal Growth Factor Receptor Variant III Peptide Vaccination in Patients with Newly Diagnosed Glioblastoma. J. Clin. Oncol. 2010, 28, 4722–4729. [Google Scholar] [CrossRef]
- Sampson, J.H.; Aldape, K.D.; Archer, G.E.; Coan, A.; Desjardins, A.; Friedman, A.H.; Friedman, H.S.; Gilbert, M.R.; Herndon, J.E.; McLendon, R.E.; et al. Greater chemotherapy-induced lymphopenia enhances tumor-specific immune responses that eliminate EGFRvIII-expressing tumor cells in patients with glioblastoma. Neuro-Oncology 2011, 13, 324–333. [Google Scholar] [CrossRef]
- Schumacher, T.; Bunse, L.; Pusch, S.; Sahm, F.; Wiestler, B.; Quandt, J.; Menn, O.; Osswald, M.; Oezen, I.; Ott, M.; et al. A vaccine targeting mutant IDH1 induces antitumour immunity. Nature 2014, 512, 324–327. [Google Scholar] [CrossRef]
- Platten, M.; Bunse, L.; Wick, A.; Bunse, T.; Le Cornet, L.; Harting, I.; Sahm, F.; Sanghvi, K.; Tan, C.L.; Poschke, I.; et al. A vaccine targeting mutant IDH1 in newly diagnosed glioma. Nature 2021, 592, 463–468. [Google Scholar] [CrossRef]
- Jaskoll, T.; Chen, H.; Zhou, Y.M.; Wu, D.W.; Melnick, M. Developmental expression of survivin during embryonic submandibular salivary gland development. BMC Dev. Biol. 2001, 1, 5. [Google Scholar] [CrossRef]
- Dutoit, V.; Herold-Mende, C.; Hilf, N.; Schoor, O.; Beckhove, P.; Bucher, J.; Dorsch, K.; Flohr, S.; Fritsche, J.; Lewandrowski, P.; et al. Exploiting the glioblastoma peptidome to discover novel tumour-associated antigens for immunotherapy. Brain 2012, 135, 1042–1054. [Google Scholar] [CrossRef] [PubMed]
- Phuphanich, S.; Wheeler, C.J.; Rudnick, J.D.; Mazer, M.; Wang, H.; Nuño, M.A.; Richardson, J.E.; Fan, X.; Ji, J.; Chu, R.M.; et al. Phase I trial of a multi-epitope-pulsed dendritic cell vaccine for patients with newly diagnosed glioblastoma. Cancer Immunol. Immunother. 2013, 62, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Cobbs, C.S.; Harkins, L.; Samanta, M.; Gillespie, G.Y.; Bharara, S.; King, P.H.; Nabors, L.B.; Cobbs, C.G.; Britt, W.J. Human cytomegalovirus infection and expression in human malignant glioma. Cancer Res. 2002, 62, 3347–3350. [Google Scholar] [PubMed]
- Rahbar, A.; Peredo, I.; Solberg, N.W.; Taher, C.; Dzabic, M.; Xu, X.; Skarman, P.; Fornara, O.; Tammik, C.; Yaiw, K.; et al. Discordant humoral and cellular immune responses to Cytomegalovirus (CMV) in glioblastoma patients whose tumors are positive for CMV. OncoImmunology 2015, 4, e982391. [Google Scholar] [CrossRef]
- Söderberg-Nauclér, C.; Johnsen, J.I. Cytomegalovirus in human brain tumors: Role in pathogenesis and potential treatment options. World J. Exp. Med. 2015, 5, 1–10. [Google Scholar] [CrossRef]
- Garcia-Martinez, A.; Alenda, C.; Irles, E.; Ochoa, E.; Quintanar, T.; Rodriguez-Lescure, A.; Soto, J.L.; Barbera, V.M. Lack of cytomegalovirus detection in human glioma. Virol. J. 2017, 14, 216. [Google Scholar] [CrossRef]
- Rahman, M.; Dastmalchi, F.; Karachi, A.; Mitchell, D. The role of CMV in glioblastoma and implications for immunotherapeutic strategies. OncoImmunology 2019, 8, e1514921. [Google Scholar] [CrossRef]
- Reap, E.A.; Suryadevara, C.M.; Batich, K.A.; Sanchez-Perez, L.; Archer, G.E.; Schmittling, R.J.; Norberg, P.K.; Herndon, J.E.; Healy, P.; Congdon, K.L.; et al. Dendritic Cells Enhance Polyfunctionality of Adoptively Transferred T Cells That Target Cytomegalovirus in Glioblastoma. Cancer Res. 2018, 78, 256–264. [Google Scholar] [CrossRef]
- Crane, C.A.; Han, S.J.; Ahn, B.; Oehlke, J.; Kivett, V.; Fedoroff, A.; Butowski, N.; Chang, S.M.; Clarke, J.; Berger, M.S.; et al. Individual Patient-Specific Immunity against High-Grade Glioma after Vaccination with Autologous Tumor Derived Peptides Bound to the 96 KD Chaperone Protein. Clin. Cancer Res. 2013, 19, 205–214. [Google Scholar] [CrossRef]
- Rafiq, S.; Hackett, C.S.; Brentjens, R.J. Engineering strategies to overcome the current roadblocks in CAR T cell therapy. Nat. Rev. Clin. Oncol. 2020, 17, 147–167. [Google Scholar] [CrossRef]
- Yan, M.; Schwaederle, M.; Arguello, D.; Millis, S.Z.; Gatalica, Z.; Kurzrock, R. HER2 expression status in diverse cancers: Review of results from 37,992 patients. Cancer Metastasis Rev. 2015, 34, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Nehama, D.; Di Ianni, N.; Musio, S.; Du, H.; Patané, M.; Pollo, B.; Finocchiaro, G.; Park, J.J.; Dunn, D.E.; Edwards, D.S.; et al. B7-H3-redirected chimeric antigen receptor T cells target glioblastoma and neurospheres. eBioMedicine 2019, 47, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.; Okeke, E.; Clay, M.; Haydar, D.; Justice, J.; O’Reilly, C.; Pruett-Miller, S.; Papizan, J.; Moore, J.; Zhou, S.; et al. Route of 41BB/41BBL Costimulation Determines Effector Function of B7-H3-CAR.CD28ζ T Cells. Mol. Ther. Oncolytics 2020, 18, 202–214. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Wang, Y.; Huang, J.; Zhang, Z.; Liu, F.; Xu, J.; Guo, G.; Wang, W.; Tong, A.; Zhou, L. Administration of B7-H3 targeted chimeric antigen receptor-T cells induce regression of glioblastoma. Signal Transduct. Target. Ther. 2021, 6, 125. [Google Scholar] [CrossRef]
- Wang, D.; Starr, R.; Chang, W.-C.; Aguilar, B.; Alizadeh, D.; Wright, S.L.; Yang, X.; Brito, A.; Sarkissian, A.; Ostberg, J.R.; et al. Chlorotoxin-directed CAR T cells for specific and effective targeting of glioblastoma. Sci. Transl. Med. 2020, 12, eaaw2672. [Google Scholar] [CrossRef] [PubMed]
- Seyfrid, M.; Maich, W.T.; Shaikh, V.M.; Tatari, N.; Upreti, D.; Piyasena, D.; Subapanditha, M.; Savage, N.; McKenna, D.; Mikolajewicz, N.; et al. CD70 as an actionable immunotherapeutic target in recurrent glioblastoma and its microenvironment. J. Immunother. Cancer 2022, 10, e003289. [Google Scholar] [CrossRef]
- Jin, L.; Ge, H.; Long, Y.; Yang, C.; Chang, Y.E.; Mu, L.; Sayour, E.J.; De Leon, G.; Wang, Q.J.; Yang, J.C.; et al. CD70, a novel target of CAR T-cell therapy for gliomas. Neuro-Oncology 2018, 20, 55–65. [Google Scholar] [CrossRef]
- Golinelli, G.; Grisendi, G.; Prapa, M.; Bestagno, M.; Spano, C.; Rossignoli, F.; Bambi, F.; Sardi, I.; Cellini, M.; Horwitz, E.M.; et al. Targeting GD2-positive glioblastoma by chimeric antigen receptor empowered mesenchymal progenitors. Cancer Gene Ther. 2020, 27, 558–570. [Google Scholar] [CrossRef]
- Prapa, M.; Chiavelli, C.; Golinelli, G.; Grisendi, G.; Bestagno, M.; Di Tinco, R.; Dall’Ora, M.; Neri, G.; Candini, O.; Spano, C.; et al. GD2 CAR T cells against human glioblastoma. Precis. Oncol. 2021, 5, 1–14. [Google Scholar] [CrossRef]
- Burger, M.C.; Zhang, C.; Harter, P.N.; Romanski, A.; Strassheimer, F.; Senft, C.; Tonn, T.; Steinbach, J.P.; Wels, W.S. CAR-Engineered NK Cells for the Treatment of Glioblastoma: Turning Innate Effectors into Precision Tools for Cancer Immunotherapy. Front. Immunol. 2019, 10, 2683. [Google Scholar] [CrossRef]
- Shaim, H.; Shanley, M.; Basar, R.; Daher, M.; Gumin, J.; Zamler, D.B.; Uprety, N.; Wang, F.; Huang, Y.; Gabrusiewicz, K.; et al. Targeting the αv integrin/TGF-β axis improves natural killer cell function against glioblastoma stem cells. J. Clin. Investig. 2021, 131, 142116. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.Y.; Choi, J.; Jackson, C.; Lim, M. Combination immunotherapy strategies for glioblastoma. J. Neuro-Oncol. 2021, 151, 375–391. [Google Scholar] [CrossRef] [PubMed]
- Bausart, M.; Préat, V.; Malfanti, A. Immunotherapy for glioblastoma: The promise of combination strategies. J. Exp. Clin. Cancer Res. 2022, 41, 35. [Google Scholar] [CrossRef] [PubMed]
- Kjeldsen, J.W.; Lorentzen, C.L.; Martinenaite, E.; Ellebaek, E.; Donia, M.; Holmstroem, R.B.; Klausen, T.W.; Madsen, C.O.; Ahmed, S.M.; Weis-Banke, S.E.; et al. A phase 1/2 trial of an immune-modulatory vaccine against IDO/PD-L1 in combination with nivolumab in metastatic melanoma. Nat. Med. 2021, 27, 2212–2223. [Google Scholar] [CrossRef] [PubMed]
- Blitz, S.E.; Kappel, A.D.; Gessler, F.A.; Klinger, N.V.; Arnaout, O.; Lu, Y.; Peruzzi, P.P.; Smith, T.R.; Chiocca, E.A.; Friedman, G.K.; et al. Tumor-Associated Macrophages/Microglia in Glioblastoma Oncolytic Virotherapy: A Double-Edged Sword. Int. J. Mol. Sci. 2022, 23, 1808. [Google Scholar] [CrossRef]
- Wu, A.; Maxwell, R.; Xia, Y.; Cardarelli, P.; Oyasu, M.; Belcaid, Z.; Kim, E.; Hung, A.; Luksik, A.S.; Garzon-Muvdi, T.; et al. Combination anti-CXCR4 and anti-PD-1 immunotherapy provides survival benefit in glioblastoma through immune cell modulation of tumor microenvironment. J. Neuro-Oncol. 2019, 143, 241–249. [Google Scholar] [CrossRef]
- Ghobrial, I.M.; Liu, C.-J.; Redd, R.A.; Perez, R.P.; Baz, R.; Zavidij, O.; Sklavenitis-Pistofidis, R.; Richardson, P.G.; Anderson, K.C.; Laubach, J.P.; et al. A Phase Ib/II Trial of the First-in-Class Anti-CXCR4 Antibody Ulocuplumab in Combination with Lenalidomide or Bortezomib Plus Dexamethasone in Relapsed Multiple Myeloma. Clin. Cancer Res. 2020, 26, 344–353. [Google Scholar] [CrossRef]
- Bockorny, B.; Semenisty, V.; Macarulla, T.; Borazanci, E.; Wolpin, B.M.; Stemmer, S.M.; Golan, T.; Geva, R.; Borad, M.J.; Pedersen, K.S.; et al. BL-8040, a CXCR4 antagonist, in combination with pembrolizumab and chemotherapy for pancreatic cancer: The COMBAT trial. Nat. Med. 2020, 26, 878–885. [Google Scholar] [CrossRef]
- Jacobs, S.M.; Wesseling, P.; de Keizer, B.; Tolboom, N.; Ververs, F.F.T.; Krijger, G.C.; Westerman, B.A.; Snijders, T.J.; Robe, P.A.; van der Kolk, A.G. CXCR4 expression in glioblastoma tissue and the potential for PET imaging and treatment with [68Ga]Ga-Pentixafor/[177Lu]Lu-Pentixather. Eur. J. Nucl. Med. Mol. Imaging 2021, 49, 481–491. [Google Scholar] [CrossRef]
- Isci, D.; D’Uonnolo, G.; Wantz, M.; Rogister, B.; Lombard, A.; Chevigné, A.; Szpakowska, M.; Neirinckx, V. Patient-Oriented Perspective on Chemokine Receptor Expression and Function in Glioma. Cancers 2021, 14, 130. [Google Scholar] [CrossRef]
- Linhares, P.; Carvalho, B.; Vaz, R.; Costa, B.M. Glioblastoma: Is There Any Blood Biomarker with True Clinical Relevance? Int. J. Mol. Sci. 2020, 21, 5809. [Google Scholar] [CrossRef] [PubMed]
- McGranahan, N.; Furness, A.J.S.; Rosenthal, R.; Ramskov, S.; Lyngaa, R.; Saini, S.K.; Jamal-Hanjani, M.; Wilson, G.A.; Birkbak, N.J.; Hiley, C.T.; et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science 2016, 351, 1463–1469. [Google Scholar] [CrossRef]
- Łuksza, M.; Riaz, N.; Makarov, V.; Balachandran, V.P.; Hellmann, M.D.; Solovyov, A.; Rizvi, N.A.; Merghoub, T.; Levine, A.J.; Chan, T.A.; et al. A neoantigen fitness model predicts tumour response to checkpoint blockade immunotherapy. Nature 2017, 551, 517–520. [Google Scholar] [CrossRef] [PubMed]
- Balachandran, V.P.; Łuksza, M.; Zhao, J.N.; Makarov, V.; Moral, J.A.; Remark, R.; Herbst, B.; Askan, G.; Bhanot, U.; Senbabaoglu, Y.; et al. Identification of unique neoantigen qualities in long-term survivors of pancreatic cancer. Nature 2017, 551, 512–516. [Google Scholar] [CrossRef] [PubMed]
- Ratnam, N.M.; Frederico, S.C.; Gonzalez, J.A.; Gilbert, M.R. Clinical correlates for immune checkpoint therapy: Significance for CNS malignancies. Neuro-Oncol. Adv. 2020, 3, vdaa161. [Google Scholar] [CrossRef] [PubMed]
- Khalsa, J.; Shah, K. Immune Profiling of Syngeneic Murine and Patient GBMs for Effective Translation of Immunotherapies. Cells 2021, 10, 491. [Google Scholar] [CrossRef]
- Mohme, M.; Schliffke, S.; Maire, C.L.; Rünger, A.; Glau, L.; Mende, K.C.; Matschke, J.; Gehbauer, C.; Akyüz, N.; Zapf, S.; et al. Immunophenotyping of Newly Diagnosed and Recurrent Glioblastoma Defines Distinct Immune Exhaustion Profiles in Peripheral and Tumor-infiltrating Lymphocytes. Clin. Cancer Res. 2018, 24, 4187–4200. [Google Scholar] [CrossRef]
- Abdelfattah, N.; Kumar, P.; Wang, C.; Leu, J.-S.; Flynn, W.F.; Gao, R.; Baskin, D.S.; Pichumani, K.; Ijare, O.B.; Wood, S.L.; et al. Single-cell analysis of human glioma and immune cells identifies S100A4 as an immunotherapy target. Nat. Commun. 2022, 13, 767. [Google Scholar] [CrossRef]
- Casarrubios, M.; Cruz-Bermúdez, A.; Nadal, E.; Insa, A.; Campelo, M.D.R.G.; Lázaro, M.; Dómine, M.; Majem, M.; Rodríguez-Abreu, D.; Martínez-Martí, A.; et al. Pretreatment Tissue TCR Repertoire Evenness Is Associated with Complete Pathologic Response in Patients with NSCLC Receiving Neoadjuvant Chemoimmunotherapy. Clin. Cancer Res. 2021, 27, 5878–5890. [Google Scholar] [CrossRef]
- Mandeville, R.; Lamoureux, G.; Legault-Poisson, S.; Poisson, R. Biological Markers and Breast Cancer. A Multiparametric Study. II. Depressed Immune Competence. Cancer 1982, 50, 1280–1288. [Google Scholar] [CrossRef]
- Domchek, S.M.; Recio, A.; Mick, R.; Clark, C.E.; Carpenter, E.L.; Fox, K.R.; DeMichele, A.; Schuchter, L.M.; Leibowitz, M.S.; Wexler, M.H.; et al. Telomerase-Specific T-Cell Immunity in Breast Cancer: Effect of Vaccination on Tumor Immunosurveillance. Cancer Res. 2007, 67, 10546–10555. [Google Scholar] [CrossRef] [PubMed]
| Trial Name Clinical Trials.gov Identifier | Phase | Target | Treatment | Indication | Sample Size Recruitment Status | Primary Endpoints | Results | Immunological Response | Comment | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| Immune Checkpoint Blockade (ICB) | ||||||||||
| CheckMate 143 NCT02017717 | III | PD-1 | Nivo −/+ Ipi | rGBM | 626 Randomized Active not recruiting Ongoing | OS | No impact | Did not meet primary endpoint | [58] | |
| CheckMate 498 NCT02617589 | III | PD-1 | Nivo + Rad | MGMT un-methylated nGBM | 550 Randomized Completed Ongoing | OS | No impact | Did not meet primary endpoint | [59] | |
| CheckMate 548 NCT02667587 | III | PD-1 | Nivo + SOC | MGMT methylated nGBM | 693 Randomized Active, not recruiting Ongoing | OS | No impact | Did not meet primary endpoint | [60] | |
| MK-3475 NCT02337491 | II | PD-1 + VEGF | Pem + Bev | rGBM | 80 Randomized Completed Terminated | OS | No impact | Did not meet primary endpoint | ||
| NeoNivo NCT02550249 | II | PD-1 | Nivo(neoad), surgery, Nivo (ad) | nGBM rGBM requiring surgery | 30 Single-arm Completed Terminated | OS | Survival benefit 7.3 months | Increased chemokine transcript expression Immune cell infiltration TCR clonal diversity in tumour. | No obvious clinical benefit | [61] |
| MK-3475 NCT02852655 | I | PD-1 | Pem (neoad), surgery, Pem (ad) | rGBM requiring surgery | 35 Randomized Completed Terminated | OS | 13.7 months vs. 7.5 months | Pre-surgical ICB enables a selective, primary tumour-specific T-cell clonal modulation. | Neoadjuvant ICB enhanced both local and systemic antitumour immune response. | [62] |
| NCT02337686 | II | PD-1 | Pem (neoad), surgery, Pem (ad) | rGBM | 15 Active, not recruiting Ongoing | PFS6 | Unpublished | Rare CD8+ T cells and abundant of CD68+ MΦs in GBM tissue. | Comparison of TIL and PD-L1 scores pre- and post-treatment associated with survival | [63] |
| Durvalumab NCT02336165 | II | PD-L1 | Dur + Rad | nGBM un-methylated MGMT | 40 Completed Terminated | Safety OS12 | First study report of anti-PD-L1 for new GBM | [64] | ||
| GliAVax NCT03291314 | II | PD-L1 + VEGFR | Ave + Axi | rGBM | 52 Completed Terminated | PFS6 | No impact | Well-tolerated Did not meet the threshold for activity | [65] | |
| NCT03047473 | II | PD-L1 | Ave + SOC | nGBM | 30 Active, not recruiting Terminated | PFS, OS | Median PFS: 9.7 months Median OS: 15.3 months. | No pre-treatment biomarkers showed any predictive value. No significant treatment effect. | ORR 23.3% | [66] |
| Trial Name Clinical Trials.gov Identifier | Phase | Target | Treatment | Indication | Sample Size Recruitment Status | Primary Endpoints | Results | Immunological Response | Comment | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| Peptide Vaccine Trials in GBM | ||||||||||
| ACT IV (CDX-110) NCT01480479 | III | EGFRvIII | Rindo + TMZ | nGBM EGFRvIII+ | 745 Randomized Completed Terminated | OS | No impact | Increased antigen-specific antibody titres. T-cell response NA. | Loss of EGFRvIII in recurrent tumour | [4] |
| ReACT NCT01498328 | II | EGFRvIII | Rindo + Bev | rGBM EGFRvIII+ | 70 Randomized Completed Terminated | PFS6 | Positive trend Improved OS | Humoral response YES T-cell response NA. | Further validation needed due to small study size. | [67] |
| NOA-16 NCT02454634 | I | IDH1 IDH1R132H mutation | IDH1 vaccine −/+ TMZ | IDH1R132H-mutated, Grade III-IV gliomas | 39 CompletedTerminated | Safety Tolerability Immunogenicity | Safe vaccine | Detection of mutation-specific humoral and T-cell responses. | Pseudo progressions after vaccine may indicate intra-tumoural immune reactions | [68] |
| SurVaxM NCT02455557 | II | Survivin | SurvaxM vaccine + TMZ | nGBM | 66 Active, not recruiting Ongoing | PFS6 | PFS6: 97%, OS12: 94% | Increased survivin-specific IgG titre post-treatment, baseline and CD8+ T-cell responses. | Positive trend. Immunogenicity and minimal toxicity. | [69] |
| IMA-950 NCT01222221 | I | Multi-peptide (IMA-950) | IMA-950 vaccine + SOC | nGBM | 45 Completed Terminated | Safety Immunogenicity Response vs. single or multiple tumour-associated peptide | Safe vaccine and immunogenic | Ninety percent of patients showed CD8+ T-cell immune response to at least one TAA, with 50% responding to two or more TAAs. | Steroids did not affect immune responses to vaccine. | [70] |
| IMA-950 NCT01920191 | I/II | Multi-peptide (IMA-950) | IMA-950 vaccine adjuvated with poly-ICLC + SOC | nGBM HLA-A2+ | 19 Completed Terminated | Safety Tolerability | Safe vaccine and immunogenic | CD8+ T-cell responses to a single or multiple peptides observed in 63.2% and 36.8% respectively. Sustained Th1 CD4+ T-cell responses. | Beneficial effect of adjuvant + vaccines co-injection. | [71] |
| IMA-950 NCT03665545 | I/II | Multi-peptide (IMA-950) + PD-1 | IMA-950 /poly-ICLC + anti-PD1 (Pem) | rGBM | 24 Randomized Recruiting Ongoing | Incidence of adverse events Safety Tolerability (PFS, OS) | Preliminary results show vaccine-specific CD4 and CD8 T-cell responses in both groups in blood. | [72] | ||
| GAPVAC 101 NCT02149225 | I | Personalized multiple peptide | APVAC1 + APVAC2 /poly-ICLC + TMZ | nGBM | 16 Completed Terminated | Safety Immunological response CD8 specific response | Safe and positive trend for immunological response | Short, non-mutated APVAC1 antigens induced sustained CD8 memory responses. Mutated APVAC2 antigens induced predominantly CD4 Th1 type responses. | Median PFS and OS: 14.2 and 29 months from diagnosis, respectively. | [73] |
| NeoVax NCT02287428 | I | Personalized neoantigen peptide −/+ PD-1 | NeoVax + TMZ −/+ Pem | MGMT un-methylated nGBM | 56 Recruiting Ongoing | Feasibility and safety | Pending | In no dexamethasone patients circulating polyfunctional neoantigen-specific CD4+and CD8+T-cell responses enriched in a memory phenotype. Increased number of TILs. | Neoantigen-specific T cells from blood can migrate into tumour. | [74] |
| Dendritic Cell (DC) Vaccine Trials in GBM | ||||||||||
| ICT-107 NCT01280552 | II | Autologous DCs pulsed with peptides targeting GBM tumour/stem cell-associated antigens | ICT-107 DC vacc + TMZ | nGBM HLA-A1+ and/or HLA-A2+ | 278 Randomized Completed Terminated | OS OS in HLA-A2 | No difference in OS. PFS significantly improved | Robust systemic response HLA-A2 subgroup showed increased ICT-107 activity clinically and immunologically | HLA-A2 primary tumour antigen expression was higher than for HLA-A1 HLA-A2 patients had higher immune response and meaningful therapeutic benefit whereas only HLA-A1 MGMT methylated patients had an OS benefit. | [75] |
| ICT-107 NCT02546102 | III | Autologous DCs pulsed with peptides targeting GBM tumour/stem cell-associated antigens | ICT-107 DC vacc + TMZ | nGBM HLA-A2+ | 14 Randomized Suspended (lack of funding) | OS | ||||
| DCVax-L NCT00045968 | III | Autologous DCs pulsed with tumour lysate | DCVax-L + SOC | nGBM | 348 Randomized Unknown Completed | PFS | 23.1 months median OS vs. 17 months | Increased frequency of CD4+ T cells | Due to the crossover design, nearly 90% of the population received DCVax-L at some point in the trial. | [76] |
| DCVax-L NCT03014804 | II | Autologous DCs pulsed with tumour lysate −/+ PD-1 | DCVax-L + SOC −/+ Nivo | rGBM | 0 Withdrawn | Safety and tolerability | None | Withdrawn (Final contract negotiations) | ||
| ATTAC II NCT02465268 | II | CMV pp65 autologous DCs | pp65 DC vaccine | nGBM | 175 Randomized Recruiting Ongoing | OS | [77] | |||
| ELEVATE NCT02366728 | II | CMV pp65-LAMP mRNA, autologous DCs | Benefit of tetanus-diphtheria (Td) toxoid pre-conditioning on DC migration and evaluation of synergy among vaccination | GBM | 64 Randomized Completed Terminated | OS | Not yet available | Confirmed that pre-conditioning with (Td) toxoid significantly increased DC migration to the lymph nodes. | [77] | |
| DERIVe NCT03688178 | II | CMV pp65-LAMP mRNA, autologous DCs | Benefit of Td toxoid pre-conditioning on DC migration and evaluation of synergy among vaccination | GBM | 112 Randomized Recruiting Ongoing | Safety OS | [77] | |||
| GLIOVAX NCT03395587 | II | Tumour lysate-loaded mature DCs | DC vaccine + SOC | GBM | 136 Randomized Recruiting Ongoing | OS | No impact | Encouraging, but cannot provide robust evidence of clinical efficacy because of non- controlled studies or low patient numbers. | [78] | |
| NCT00846456 | I/II | DCs with mRNA from tumour stem cells + hTert/Survivin mRNA | DC vaccine with mRNA from tumour stem cells + hTert/Survivin mRNA | GBM | 20 Completed Terminated | Safety, Immunological response | PFS longer compared to matched control patients | Peripheral vaccine-induced immune response | Several patients alive at 2 years after diagnosis. | [79] |
| DEN-STEM NCT03548571 | II/III | DCs with mRNA from tumour stem cells + hTert/Survivin mRNA | DC vaccine with mRNA from tumour stem cells + hTERT/Survivin mRNA | GBM | 60 randomized Active | PFS | Not yet available | |||
| Heat Shock Protein Complex Trial in GBM | ||||||||||
| Heat Shock Protein gp96 NCT02122822 | I | HSP gp96-peptide complex from patient’s tumour cells | HSPgp96 vaccination + SOC | nGBM | 20 Completed Terminated | Safety and effectiveness | Safe and effective | Tumour-specific immune response was significantly increased after vaccination | Tumour-specific immune response after vaccination, instead of which before vaccination, correlated with good survival in vaccinated patients. | [80] |
| Heat Shock Protein gp96 NCT03018288 | II | HSP gp96-peptide complex from patient’s tumour cells + PD-1 | HSP gp96 vaccination + SOC −/+ Pem | nGBM | 90 Randomized Active, not recruiting Ongoing | 1 year OS | Pending | [81,82] | ||
| Trial Name Clinical Trials.gov Identifier | Phase | Target | Treatment | Indication | Sample Size Recruitment Status | Primary Endpoints | Results | Immunological Response | Comment | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| CAR T cell Trials in GBM | ||||||||||
| IL13Ra2 NCT00730613 | I | IL13Ra2 | IL13Ra2 CAR intracranial CD3z 1st generation CAR | rGBM | 3 Completed Terminated | Safety and feasibility | Safe and feasible No survival benefit | Evidence for transient anti-glioma responses was observed in 2 of the patients. Reduced IL13Rα2 expression within the tumour following treatment. | First-in-human pilot | [83] |
| IL13Ra2 NCT02208362 | I | IL13Ra2 | IL13Ra2 CAR 4-1BB-CD3z 2nd generation Intracavitary and intraventricular infusions | rGBM | 82 Active, not recruiting Ongoing | Safety and feasibility | Pending | One patient had dramatic clinical response sustained for 7.5 months. Reduction in size of all intracranial and spinal tumours. | [84] | |
| ExCeL NCT02664363 | I | EGFRvIII | EGFRvIII CAR + TMZDI (dose-intensified) | nGBM | 3 Terminated (Study funding ended) Terminated | Max tolerated dose Safety | Safe Feasible | TMZDI pre-treatment prompted dramatic CAR proliferation and enhanced persistence in circulation. | [85] | |
| EGFRvIII NCT02209376 | I | EGFRvIII | EGFRvIII CAR 4-1BB-CD3z 2nd generation CAR | rGBM | 11 Terminated by the sponsor | Safety and feasibility | No clinical response | Detectable transient expansion of CAR T EGFRvIII cells in peripheral blood. CAR T EGFRvIII migrated into the tumour. Increased expression of inhibitory molecules and infiltration by regulatory T cells after CAR T EGFRvIII infusion. | [5] | |
| HER2 NCT01109095 | I | HER2 virus specific | Virus-specific T cells expressing HER2 CAR 2nd generation | rGBM | 16 Completed Terminated prematurely | Safety and feasibility | Median OS of 11.1 months after T-cell infusion and 24.5 months after diagnosis. | Three patients alive with no disease progression at last follow-up. | [86] | |
| EGFRvIII NCT01454596 | I/II | EGFRvIII | EGFRvIII CAR CD28-4-1BB-CD3z 3rd generation | rGBM | 18 Completed Terminated | Safety, Feasibility, PFS6 | no OR | [87] | ||
| EGFRvIII NCT02844062 | I | EGFRvIII | EGFRvIII CAR | rGBM | 20 Unknown Terminated | Safety, Feasibility | ||||
| EGFRvIII NCT03283631 | I | EGFRvIII | EGFRvIII CAR | GBM | 24 Terminated Terminated | Max tolerated dose | ||||
| HER2 NCT02442297 | I | HER2 | HER2 CAR 2nd generation CAR T cells | GBM | 28 Recruiting Ongoing | Safety | ||||
| HER2 NCT03389230 | I | HER2 | HER2 CAR 4-1BB 2nd generation CAR T cells | GBM | 42 Recruiting Ongoing | Safety | ||||
| EphA2 NCT02575261 | I/II | EphA2 | EphA2 autologous CAR T cells | GBM EphA2+ | 0 Withdrawn | Safety, effectiveness | ||||
| Anti-PD-L1 CSR T cells NCT02937844 | I | Anti-PD-L1 chimeric switch receptor | Chimeric switch receptor with PD-1 extracellular domain fused to the costimulatory molecule CD28. | rGBM | 20 Unknown Terminated | Safety, Efficacy | ||||
| B7-H3 CAR T cells NCT04077866 | I/II | B7-H3 | B7-H3 autologous CAR T cells + TMZ | rGBM | 40 Randomized Recruiting Ongoing | Safety, Efficacy, OS | ||||
| B7-H3 NCT04385173 | I | B7-H3 | B7-H3 autologous CAR T cells + TMZ | rGBM | 12 Recruiting Ongoing | Safety, Feasibility, OS, PFS | ||||
| Chlorotoxin NCT04214392 | I | Chlorotoxin tumour-targeting domain | Chlorotoxin-CD28-CD3zeta 2nd generation CAR | rGBM | 36 Recruiting Ongoing | Toxicity, Safety | Strong CLTX binding to tumour cells was observed in of the majority of primary GBM lines. | [88] | ||
| Trial Name Clinical Trials.gov Identifier | Phase | Target | Treatment | Indication | Sample Size Recruitment Status | Primary Endpoints | Results | Immunological Response | Comment | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| Combinatorial Trials in GBM | ||||||||||
| NCT03726515 | I | EGFRvIII + PD-1 | EGFRvIII CAR-T + Pem | EGFRvIII+, MGMT unmethylated nGBM | 7 Completed Terminated | Safety | [89] | |||
| NCT04003649 | I | IL13Ra2 −/+ PD-1 −/+ CTLA-4 | IL13Ra2-CAR T cells +/− Nivo and Ipi | rGBM | 60 Randomized Recruiting | Adverse events, Toxicity, Feasibility, OS | ||||
| NCT02873390 | I | PD-1/EGFR | PD-1 Antibody expressing CAR-T cells for EGFR+ advanced solid tumour | Advanced malignancies incl. GBM | 20 | OR, PFS, OS | ||||
| AVERT NCT02529072 | I | PD-1 | Nivo with DC vaccines for recurrent brain tumours | GBM | 6 Randomized Completed | Safety | ||||
| NeoVax NCT03422094 | I | Personalized neoantigen peptide vaccine + PD-1 −/+ CTLA-4 | NeoVax+ TMZ+ Ipi −/+ Nivo | MGMT unmethylated nGBM | 3 Terminated | Safety, Feasibility, Immunogenicity | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mensali, N.; Inderberg, E.M. Emerging Biomarkers for Immunotherapy in Glioblastoma. Cancers 2022, 14, 1940. https://doi.org/10.3390/cancers14081940
Mensali N, Inderberg EM. Emerging Biomarkers for Immunotherapy in Glioblastoma. Cancers. 2022; 14(8):1940. https://doi.org/10.3390/cancers14081940
Chicago/Turabian StyleMensali, Nadia, and Else Marit Inderberg. 2022. "Emerging Biomarkers for Immunotherapy in Glioblastoma" Cancers 14, no. 8: 1940. https://doi.org/10.3390/cancers14081940
APA StyleMensali, N., & Inderberg, E. M. (2022). Emerging Biomarkers for Immunotherapy in Glioblastoma. Cancers, 14(8), 1940. https://doi.org/10.3390/cancers14081940






