Febrile Neutropenia Duration Is Associated with the Severity of Gut Microbiota Dysbiosis in Pediatric Allogeneic Hematopoietic Stem Cell Transplantation Recipients
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
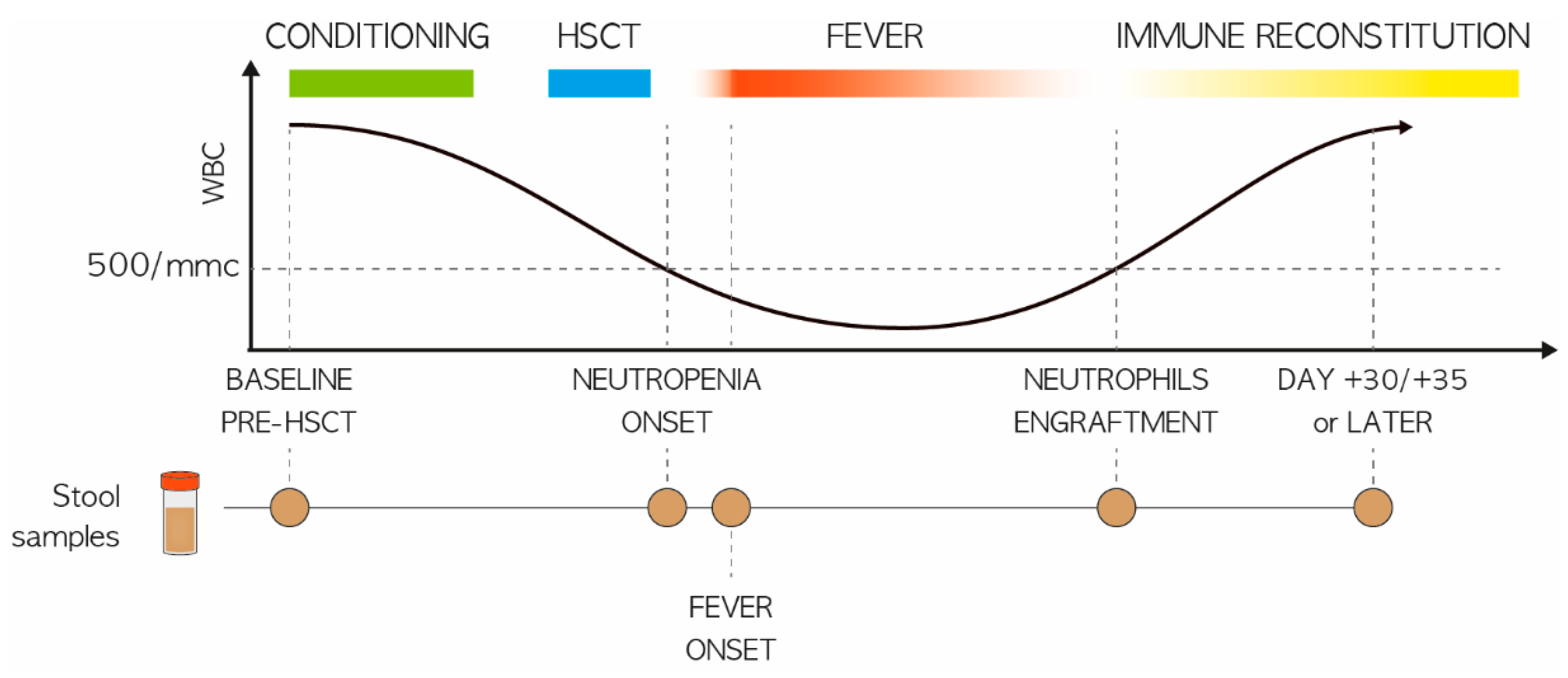
2.1. Study Population and Stool Sampling
2.2. Microbial DNA Extraction and 16S rRNA Gene Library Preparation
2.3. Bioinformatic and Statistical Analysis
3. Results
3.1. Study Cohort Description
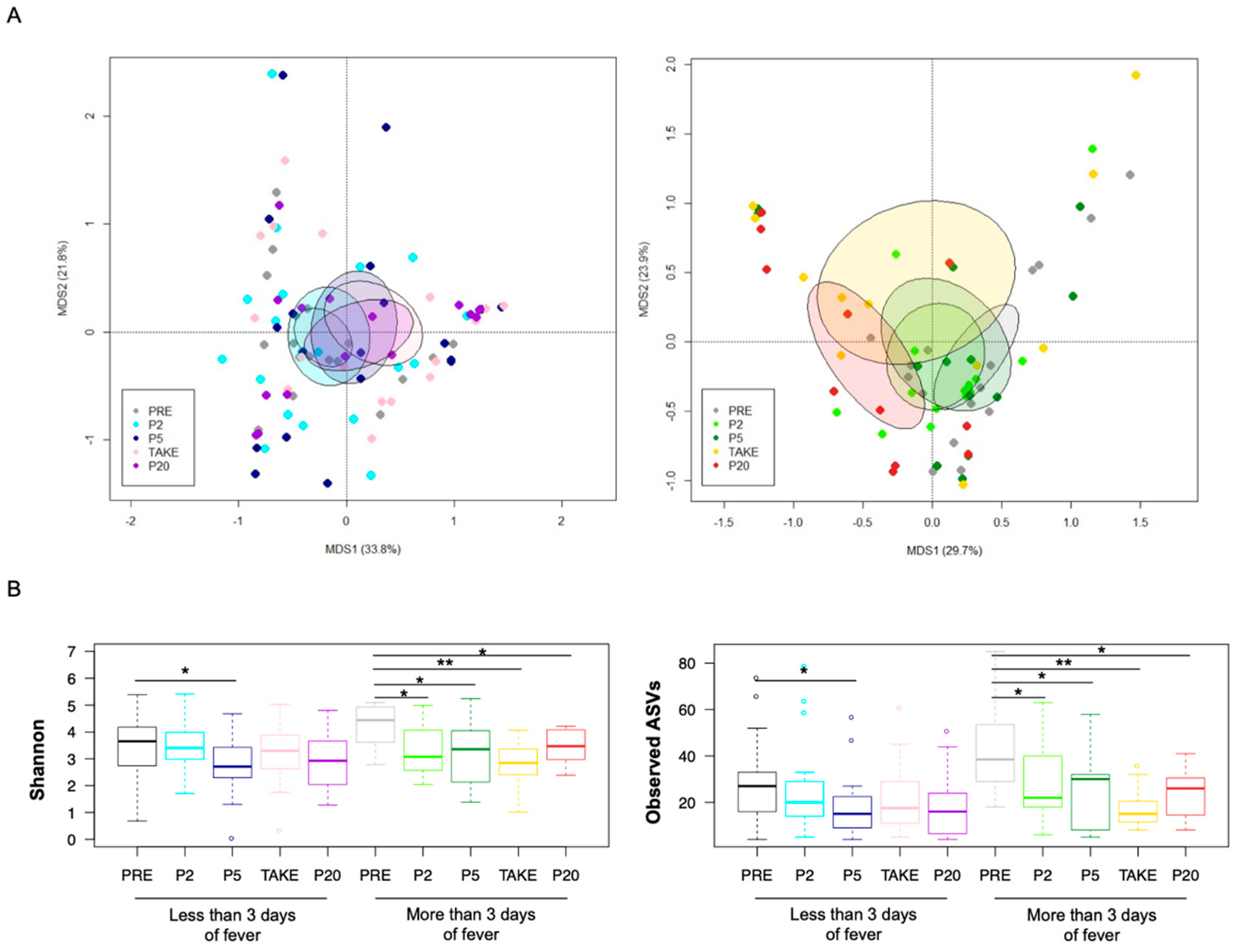
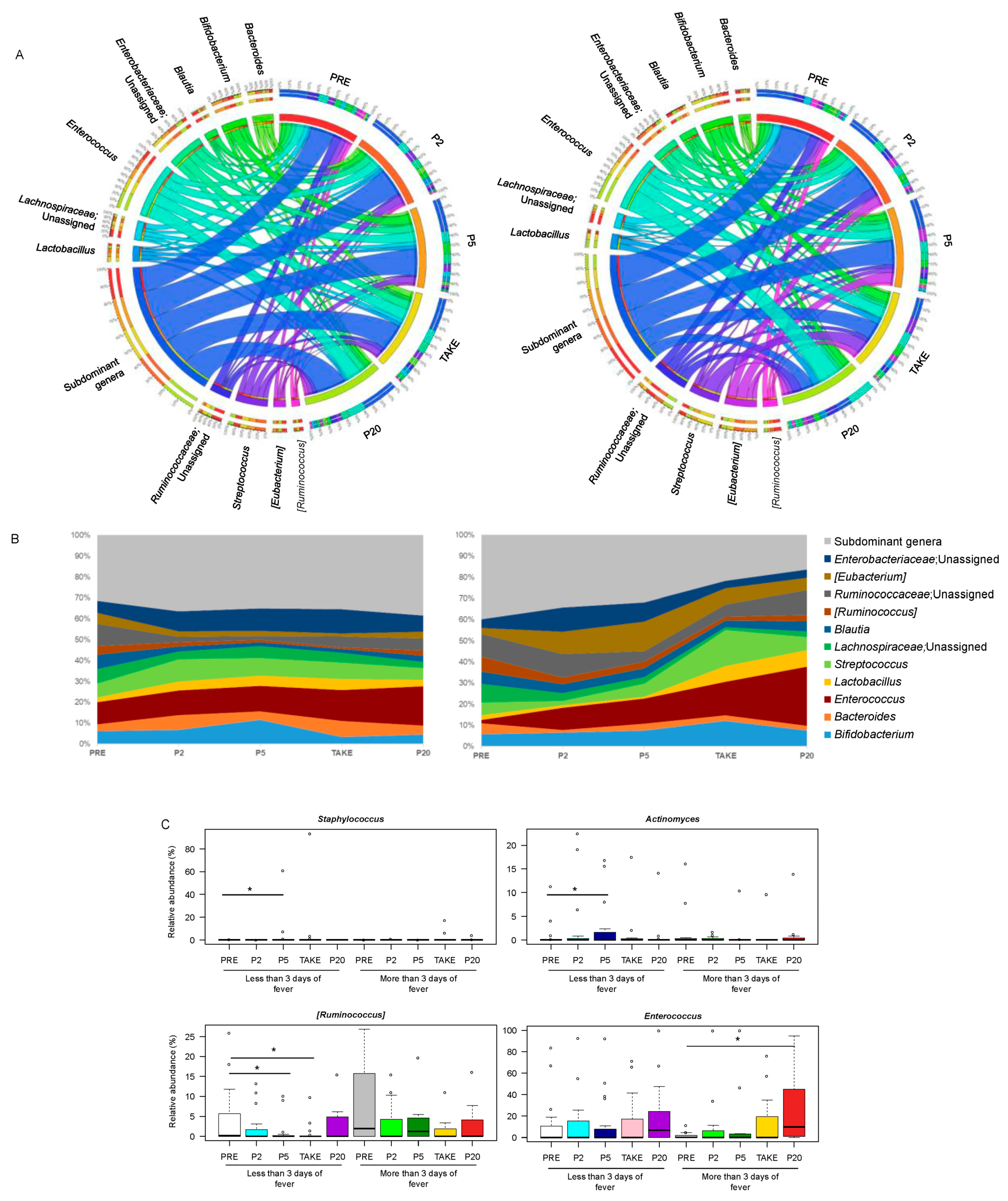
3.2. Gut Microbiota Dynamics in Pediatric Allo-HSCT Patients in Relation to the Duration of Febrile Neutropenia
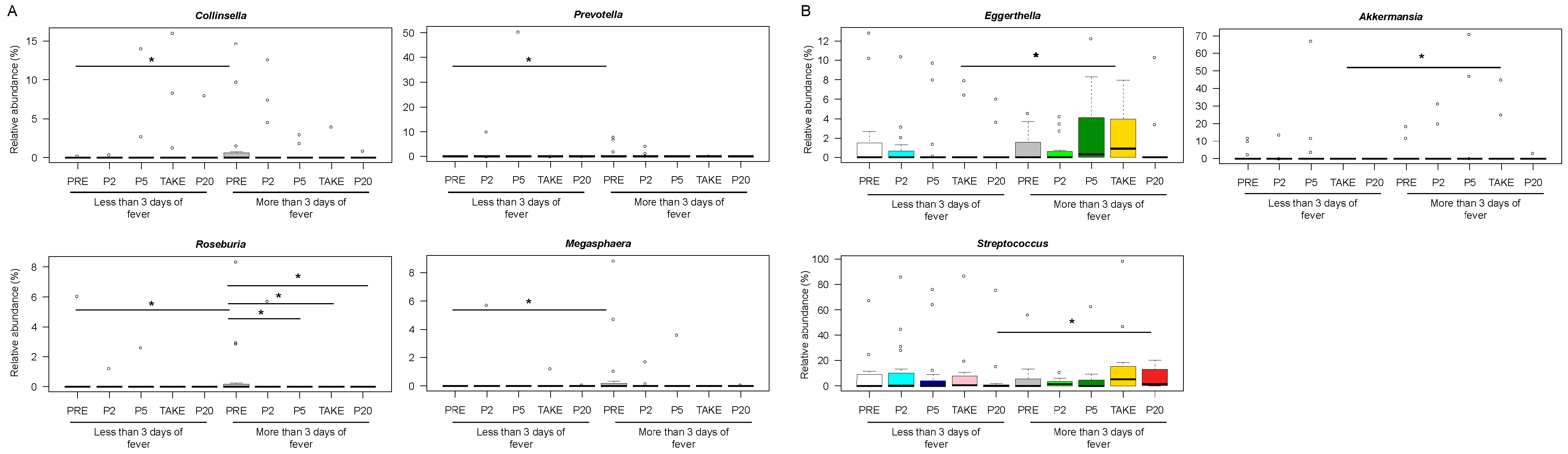
3.3. Early and Late Gut Microbiota Signatures of Febrile Neutropenia Duration in Pediatric Allo-HSCT Patients
3.4. Association between Febrile Neutropenia Duration and Development of Other Transplant Complications
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barton, C.D.; Waugh, L.K.; Nielsen, M.J.; Paulus, S. Febrile neutropenia in children treated for malignancy. J. Infect. 2015, 71, S27–S35. [Google Scholar] [CrossRef] [PubMed]
- Lehrnbecher, T.; Robinson, P.; Fisher, B.; Alexander, S.; Ammann, R.A.; Beauchemin, M.; Carlesse, F.; Groll, A.H.; Haeusler, G.M.; Santolaya, M.; et al. Guideline for the management of fever and neutropenia in children with cancer and hematopoietic stem-cell transplantation recipients: 2017 update. J. Clin. Oncol. 2017, 35, 2082–2094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lehrnbecher, T.; Averbuch, D.; Castagnola, E.; Cesaro, S.; Ammann, R.A.; Garcia-Vidal, C.; Kanerva, J.; Lanternier, F.; Mesini, A.; Mikulska, M.; et al. 8th European Conference on Infections in Leukaemia: 2020 guidelines for the use of antibiotics in paediatric patients with cancer or post-haematopoietic cell transplantation. Lancet Oncol. 2021, 22, e270–e280. [Google Scholar] [CrossRef]
- Carreras, E.; Dufour, C.; Mohty, M.; Kröger, N. The EBMT Handbook: Hematopoietic Stem Cell Transplantation and Cellular Therapies; Springer International Publishing: Cham, Switzerland, 2019; ISBN 3030022781. [Google Scholar]
- Masetti, R.; Muratore, E.; Leardini, D.; Zama, D.; Turroni, S.; Brigidi, P.; Esposito, S.; Pession, A. Gut microbiome in pediatric acute leukemia: From predisposition to cure. Blood Adv. 2021, 5, 4619–4629. [Google Scholar] [CrossRef] [PubMed]
- Masetti, R.; Zama, D.; Leardini, D.; Muratore, E.; Turroni, S.; Prete, A.; Brigidi, P.; Pession, A. The gut microbiome in pediatric patients undergoing allogeneic hematopoietic stem cell transplantation. Pediatr. Blood Cancer 2020, 67, e28711. [Google Scholar] [CrossRef] [PubMed]
- Biagi, E.; Zama, D.; Nastasi, C.; Consolandi, C.; Fiori, J.; Rampelli, S.; Turroni, S.; Centanni, M.; Severgnini, M.; Peano, C.; et al. Gut microbiota trajectory in pediatric patients undergoing hematopoietic SCT. Bone Marrow Transplant. 2015, 50, 992–998. [Google Scholar] [CrossRef] [Green Version]
- Zama, D.; Bossù, G.; Leardini, D.; Muratore, E.; Biagi, E.; Prete, A.; Pession, A.; Masetti, R. Insights into the role of intestinal microbiota in hematopoietic stem-cell transplantation. Ther. Adv. Hematol. 2020, 11, 2040620719896961. [Google Scholar] [CrossRef] [Green Version]
- Stein-Thoeringer, C.K.; Nichols, K.B.; Lazrak, A.; Docampo, M.D.; Slingerland, A.E.; Slingerland, J.B.; Clurman, A.G.; Armijo, G.; Gomes, A.L.C.; Shono, Y.; et al. Lactose drives Enterococcus expansion to promote graft-versus-host disease. Science 2019, 366, 1143–1149. [Google Scholar] [CrossRef]
- D’Amico, F.; Biagi, E.; Rampelli, S.; Fiori, J.; Zama, D.; Soverini, M.; Barone, M.; Leardini, D.; Muratore, E.; Prete, A.; et al. Enteral Nutrition in Pediatric Patients Undergoing Hematopoietic SCT Promotes the Recovery of Gut Microbiome Homeostasis. Nutrients 2019, 11, 2958. [Google Scholar] [CrossRef] [Green Version]
- Simms-Waldrip, T.R.; Sunkersett, G.; Coughlin, L.A.; Savani, M.R.; Arana, C.; Kim, J.; Kim, M.; Zhan, X.; Greenberg, D.E.; Xie, Y.; et al. Antibiotic-Induced Depletion of Anti-inflammatory Clostridia Is Associated with the Development of Graft-versus-Host Disease in Pediatric Stem Cell Transplantation Patients. Biol. Blood Marrow Transplant. 2017, 23, 820–829. [Google Scholar] [CrossRef] [Green Version]
- Zama, D.; Gori, D.; Muratore, E.; Leardini, D.; Rallo, F.; Turroni, S.; Prete, A.; Brigidi, P.; Pession, A.; Masetti, R. Enteral versus Parenteral Nutrition as Nutritional Support after Allogeneic Hematopoietic Stem Cell Transplantation: A Systematic Review and Meta-Analysis. Transplant. Cell. Ther. 2021, 27, 180.e1–180.e8. [Google Scholar] [CrossRef]
- Peled, J.U.; Gomes, A.L.C.; Devlin, S.M.; Littmann, E.R.; Taur, Y.; Sung, A.D.; Weber, D.; Hashimoto, D.; Slingerland, A.E.; Slingerland, J.B.; et al. Microbiota as Predictor of Mortality in Allogeneic Hematopoietic-Cell Transplantation. N. Engl. J. Med. 2020, 382, 822–834. [Google Scholar] [CrossRef] [PubMed]
- Peled, J.U.; Devlin, S.M.; Staffas, A.; Lumish, M.; Khanin, R.; Littmann, E.R.; Ling, L.; Kosuri, S.; Maloy, M.; Slingerland, J.B.; et al. Intestinal microbiota and Relapse after hematopoietic-Cell transplantation. J. Clin. Oncol. 2017, 35, 1650–1659. [Google Scholar] [CrossRef] [Green Version]
- Biagi, E.; Zama, D.; Rampelli, S.; Turroni, S.; Brigidi, P.; Consolandi, C.; Severgnini, M.; Picotti, E.; Gasperini, P.; Merli, P.; et al. Early gut microbiota signature of aGvHD in children given allogeneic hematopoietic cell transplantation for hematological disorders. BMC Med. Genomics 2019, 12, 49. [Google Scholar] [CrossRef] [PubMed]
- Masetti, R.; Biagi, E.; Zama, D.; Muratore, E.; Amico, F.D.; Leardini, D.; Turroni, S.; Prete, A.; Brigidi, P.; Pession, A. Early modifications of the gut microbiome in children with hepatic sinusoidal obstruction syndrome after hematopoietic stem cell transplantation. Sci. Rep. 2021, 11, 14307. [Google Scholar] [CrossRef] [PubMed]
- Kelly, M.S.; Ward, D.V.; Severyn, C.J.; Arshad, M.; Heston, S.M.; Jenkins, K.; Martin, P.L.; McGill, L.; Stokhuyzen, A.; Bhattarai, S.K.; et al. Gut Colonization Preceding Mucosal Barrier Injury Bloodstream Infection in Pediatric Hematopoietic Stem Cell Transplantation Recipients. Biol. Blood Marrow Transplant. 2019, 25, 2274–2280. [Google Scholar] [CrossRef]
- Taur, Y.; Xavier, J.B.; Lipuma, L.; Ubeda, C.; Goldberg, J.; Gobourne, A.; Lee, Y.J.; Dubin, K.A.; Socci, N.D.; Viale, A.; et al. Intestinal domination and the risk of bacteremia in patients undergoing allogeneic hematopoietic stem cell transplantation. Clin. Infect. Dis. 2012, 55, 905–914. [Google Scholar] [CrossRef]
- Murthy, H.S.; Gharaibeh, R.Z.; Al-Mansour, Z.; Kozlov, A.; Trikha, G.; Newsome, R.C.; Gauthier, J.; Farhadfar, N.; Wang, Y.; Kelly, D.L.; et al. Baseline Gut Microbiota Composition is Associated with Major Infections Early after Hematopoietic Cell Transplantation. Biol. Blood Marrow Transplant. 2020, 26, 2001–2010. [Google Scholar] [CrossRef]
- Rashidi, A.; Ebadi, M.; Rehman, T.U.; Elhusseini, H.; Nalluri, H.; Kaiser, T.; Ramamoorthy, S.; Holtan, S.G.; Khoruts, A.; Weisdorf, D.J.; et al. Altered microbiota-host metabolic cross talk preceding neutropenic fever in patients with acute leukemia. Blood Adv. 2021, 5, 3937–3950. [Google Scholar] [CrossRef]
- Yu, Z.; Morrison, M. Improved extraction of PCR-quality community DNA from digesta and fecal samples. Biotechniques 2004, 36, 808–812. [Google Scholar] [CrossRef]
- D’Amico, F.; Perrone, A.M.; Rampelli, S.; Coluccelli, S.; Barone, M.; Ravegnini, G.; Fabbrini, M.; Brigidi, P.; De Iaco, P.; Turroni, S. Gut Microbiota Dynamics during Chemotherapy in Epithelial Ovarian Cancer Patients Are Related to Therapeutic Outcome. Cancers 2021, 13, 3999. [Google Scholar] [CrossRef] [PubMed]
- Masella, A.P.; Bartram, A.K.; Truszkowski, J.M.; Brown, D.G.; Neufeld, J.D. PANDAseq: Paired-end assembler for illumina sequences. BMC Bioinform. 2012, 13, 31. [Google Scholar] [CrossRef] [Green Version]
- Bolyen, E.; Rideout, J.R.; Chase, J.; Pitman, T.A.; Shiffer, A.; Mercurio, W.; Dillon, M.R.; Caporaso, J.G. An Introduction to Applied Bioinformatics: A free, open, and interactive text. J. Open Source Educ. 2018, 1, 27. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. VSEARCH: A versatile open source tool for metagenomics. PeerJ 2016, 4, e2584. [Google Scholar] [CrossRef]
- Culhane, A.C.; Thioulouse, J.; Perrière, G.; Higgins, D.G. MADE4: An R package for multivariate analysis of gene expression data. Bioinformatics 2005, 21, 2789–2790. [Google Scholar] [CrossRef] [Green Version]
- Krzywinski, M.; Schein, J.; Birol, I.; Connors, J.; Gascoyne, R.; Horsman, D.; Jones, S.J.; Marra, M.A. Circos: An information aesthetic for comparative genomics. Genome Res. 2009, 19, 1639–1645. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Wu, W.; Zheng, H.M.; Li, P.; McDonald, D.; Sheng, H.F.; Chen, M.X.; Chen, Z.H.; Ji, G.Y.; Zheng, Z.D.X.; et al. Regional variation limits applications of healthy gut microbiome reference ranges and disease models. Nat. Med. 2018, 24, 1532–1535. [Google Scholar] [CrossRef]
- Ingham, A.C.; Kielsen, K.; Cilieborg, M.S.; Lund, O.; Holmes, S.; Aarestrup, F.M.; Müller, K.G.; Pamp, S.J. Specific gut microbiome members are associated with distinct immune markers in pediatric allogeneic hematopoietic stem cell transplantation. Microbiome 2019, 7, 131. [Google Scholar] [CrossRef]
- Ingham, A.C.; Kielsen, K.; Mordhorst, H.; Ifversen, M.; Aarestrup, F.M.; Müller, K.G.; Pamp, S.J. Microbiota long-term dynamics and prediction of acute graft-versus-host disease in pediatric allogeneic stem cell transplantation. Microbiome 2021, 9, 148. [Google Scholar] [CrossRef]
- Lebreton, F.; Manson, A.L.; Saavedra, J.T.; Straub, T.J.; Earl, A.M.; Gilmore, M.S. Tracing the Enterococci from Paleozoic Origins to the Hospital. Cell 2017, 169, 849–861.e13. [Google Scholar] [CrossRef] [Green Version]
- Cancello, R.; Turroni, S.; Rampelli, S.; Cattaldo, S.; Candela, M.; Cattani, L.; Mai, S.; Vietti, R.; Scacchi, M.; Brigidi, P.; et al. Effect of short-term dietary intervention and probiotic mix supplementation on the gut microbiota of elderly obese women. Nutrients 2019, 11, 3011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seregin, S.S.; Golovchenko, N.; Schaf, B.; Chen, J.; Pudlo, N.A.; Mitchell, J.; Baxter, N.T.; Zhao, L.; Schloss, P.D.; Martens, E.C.; et al. NLRP6 Protects Il10-/- Mice from Colitis by Limiting Colonization of Akkermansia muciniphila. Cell Rep. 2017, 19, 2174. [Google Scholar] [CrossRef] [PubMed]
- Dejea, C.M.; Fathi, P.; Craig, J.M.; Boleij, A.; Taddese, R.; Geis, A.L.; Wu, X.; DeStefano Shields, C.E.; Hechenbleikner, E.M.; Huso, D.L.; et al. Patients with familial adenomatous polyposis harbor colonic biofilms containing tumorigenic bacteria. Science 2018, 359, 592–597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saresella, M.; Marventano, I.; Barone, M.; La Rosa, F.; Piancone, F.; Mendozzi, L.; D’Arma, A.; Rossi, V.; Pugnetti, L.; Roda, G.; et al. Alterations in Circulating Fatty Acid Are Associated With Gut Microbiota Dysbiosis and Inflammation in Multiple Sclerosis. Front. Immunol. 2020, 11, 1390. [Google Scholar] [CrossRef] [PubMed]
- Depommier, C.; Everard, A.; Druart, C.; Plovier, H.; Van Hul, M.; Vieira-Silva, S.; Falony, G.; Raes, J.; Maiter, D.; Delzenne, N.M.; et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: A proof-of-concept exploratory study. Nat. Med. 2019, 25, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- Routy, B.; Le Chatelier, E.; Derosa, L.; Duong, C.P.M.; Alou, M.T.; Daillère, R.; Fluckiger, A.; Messaoudene, M.; Rauber, C.; Roberti, M.P.; et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 2018, 359, 91–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masetti, R.; Zama, D.; Leardini, D.; Muratore, E.; Turroni, S.; Brigidi, P.; Pession, A. Microbiome-derived metabolites in allogeneic hematopoietic stem cell transplantation. Int. J. Mol. Sci. 2021, 22, 1197. [Google Scholar] [CrossRef]
- Van Der Velden, W.J.F.M.; Herbers, A.H.E.; Brüggemann, R.J.M.; Feuth, T.; Peter Donnelly, J.; Blijlevens, N.M.A. Citrulline and albumin as biomarkers for gastrointestinal mucositis in recipients of hematopoietic SCT. Bone Marrow Transplant. 2013, 48, 977–981. [Google Scholar] [CrossRef]
- Krell, D.; Jones, A.L. Impact of effective prevention and management of febrile neutropenia. Br. J. Cancer 2009, 101, S23–S26. [Google Scholar] [CrossRef]
- Lonetti, A.; Pession, A.; Masetti, R. Targeted Therapies for Pediatric AML: Gaps and Perspective. Front Pediatr. 2019, 7, 463. [Google Scholar] [CrossRef] [PubMed]
- Pession, A.; Zama, D.; Muratore, E.; Leardini, D.; Gori, D.; Guaraldi, F.; Prete, A.; Turroni, S.; Brigidi, P.; Masetti, R. Fecal Microbiota Transplantation in Allogeneic Hematopoietic Stem Cell Transplantation Recipients: A Systematic Review. J. Pers. Med. 2021, 11, 100. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Overall (N = 37) | Bologna (N = 12) | Verona (N = 12) | Wroclaw (N = 13) |
|---|---|---|---|---|
| Age at HSCT—year | 8.3 (1.0–18.0) | 8.8 (1.1–18.0) | 9.0 (1.2–17.6) | 7.2 (1.0–13.7) |
| Malignant disease—no. (%) | 22 (59) | 8 (67) | 8 (67) | 6 (46) |
| Donor—no. (%) | ||||
| MUD | 20 (54) | 6 (50) | 7 (58) | 7 (54) |
| MMUD | 7 (19) | 2 (17) | 4 (33) | 1 (8) |
| Haplo | 5 (14) | 3 (25) | 0 (19) | 2 (15) |
| MSD | 5 (14) | 1 (8) | 1 (8) | 3 (23) |
| Graft type—no. (%) | ||||
| BM | 22 (60) | 9 (75) | 8 (67) | 5 (38) |
| PBSC | 15 (40) | 3 (25) | 4 (33) | 8 (62) |
| Intensity of conditioning regimen—no. (%) | ||||
| MAC | 24 (65) | 10 (83) | 7 (58) | 7 (54) |
| RIC | 13 (35) | 2 (17) | 5 (42) | 6 (46) |
| Fever > 3 Days (n = 16) | Fever ≤ 3 Days (n = 21) | p | |
|---|---|---|---|
| Center: | 0.136 | ||
| Bologna | 8 (50.0%) | 4 (19.0%) | |
| Wroclaw | 4 (25.0%) | 9 (42.9%) | |
| Verona | 4 (25.0%) | 8 (38.1%) | |
| Underlying disease: | 0.742 | ||
| Malignant | 10 (62.5%) | 12 (57.1%) | |
| Non-malignant | 6 (37.5%) | 9 (42.9%) | |
| Antibiotic prophylaxis: | 0.141 | ||
| Yes | 6 (37.5%) | 13 (61.9%) | |
| No | 10 (62.5%) | 8 (38.1%) | |
| Type of conditioning: | 0.260 | ||
| MAC | 12 (75.0%) | 12 (57.1%) | |
| RIC | 4 (25.0%) | 9 (42.9%) | |
| Use of granulocyte colony-stimulating factor (G-CSF): | 0.384 | ||
| Yes | 14 (87.5%) | 16 (76.2%) | |
| No | 2 (12.5%) | 5 (23.8%) | |
| Corticosteroid for GvHD: | 0.208 | ||
| Yes | 8 (50.0%) | 7 (33.3%) | |
| No | 2 (12.5%) | 0 (0%) | |
| Bloodstream infections: | 0.384 | ||
| Yes | 2 (12.5%) | 5 (23.8%) | |
| No | 14 (87.5%) | 16 (76.2%) | |
| GvHD (any grade): | 0.104 | ||
| Yes | 10 (62.5%) | 7 (33.3%) | |
| No | 6 (37.5%) | 14 (66.7%) | |
| GvHD (grade II-IV): | 0.705 | ||
| Yes | 4 (25.0%) | 4 (19.0%) | |
| No | 12 (75.0%) | 17 (81.0%) | |
| Nutrition: | 1.00 | ||
| EN | 6 (37.5%) | 8 (38.1%) | |
| PN | 10 (62.5%%) | 13 (61.9%) | |
| Mucositis: | 0.733 | ||
| Grade 3–4 | 7 (43.8%) | 7 (33.3%) | |
| Grade ≤ 2 | 9 (56.2%) | 14 (66.7%) | |
| Graft source: | 0.176 | ||
| BM | 12 (75.0%) | 10 (47.6%) | |
| PBSC | 4 (25.0%) | 11 (52.4%) | |
| Donor: | 0.460 | ||
| MUD | 7 (43.8%) | 13 (61.9%) | |
| MMUD | 4 (25.0%) | 4 (19.0%) | |
| Haplo | 4 (25.0%) | 2 (9.5%) | |
| MSD | 1 (6.2%) | 3 (14.3%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masetti, R.; D’Amico, F.; Zama, D.; Leardini, D.; Muratore, E.; Ussowicz, M.; Fraczkiewicz, J.; Cesaro, S.; Caddeo, G.; Pezzella, V.; et al. Febrile Neutropenia Duration Is Associated with the Severity of Gut Microbiota Dysbiosis in Pediatric Allogeneic Hematopoietic Stem Cell Transplantation Recipients. Cancers 2022, 14, 1932. https://doi.org/10.3390/cancers14081932
Masetti R, D’Amico F, Zama D, Leardini D, Muratore E, Ussowicz M, Fraczkiewicz J, Cesaro S, Caddeo G, Pezzella V, et al. Febrile Neutropenia Duration Is Associated with the Severity of Gut Microbiota Dysbiosis in Pediatric Allogeneic Hematopoietic Stem Cell Transplantation Recipients. Cancers. 2022; 14(8):1932. https://doi.org/10.3390/cancers14081932
Chicago/Turabian StyleMasetti, Riccardo, Federica D’Amico, Daniele Zama, Davide Leardini, Edoardo Muratore, Marek Ussowicz, Jowita Fraczkiewicz, Simone Cesaro, Giulia Caddeo, Vincenza Pezzella, and et al. 2022. "Febrile Neutropenia Duration Is Associated with the Severity of Gut Microbiota Dysbiosis in Pediatric Allogeneic Hematopoietic Stem Cell Transplantation Recipients" Cancers 14, no. 8: 1932. https://doi.org/10.3390/cancers14081932
APA StyleMasetti, R., D’Amico, F., Zama, D., Leardini, D., Muratore, E., Ussowicz, M., Fraczkiewicz, J., Cesaro, S., Caddeo, G., Pezzella, V., Belotti, T., Gottardi, F., Tartari, P., Brigidi, P., Turroni, S., & Prete, A. (2022). Febrile Neutropenia Duration Is Associated with the Severity of Gut Microbiota Dysbiosis in Pediatric Allogeneic Hematopoietic Stem Cell Transplantation Recipients. Cancers, 14(8), 1932. https://doi.org/10.3390/cancers14081932












