Blood Plasma Stabilized Gold Nanoclusters for Personalized Tumor Theranostics
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
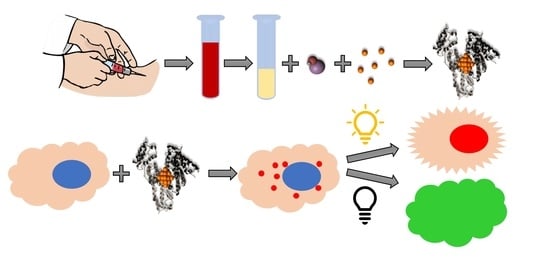
2.2. Au NCs Synthesis
2.3. Characterization of Au NCs
2.4. Generation of Reactive Oxygen Species
2.5. Cell Culturing
2.6. Biocompatibility Assay
2.7. Accumulation of Au NCs in Cell Monolayers
2.8. The Phototoxicity of Au NCs in Cells
3. Results and Discussion
3.1. Optimization of Au NCs Synthesis
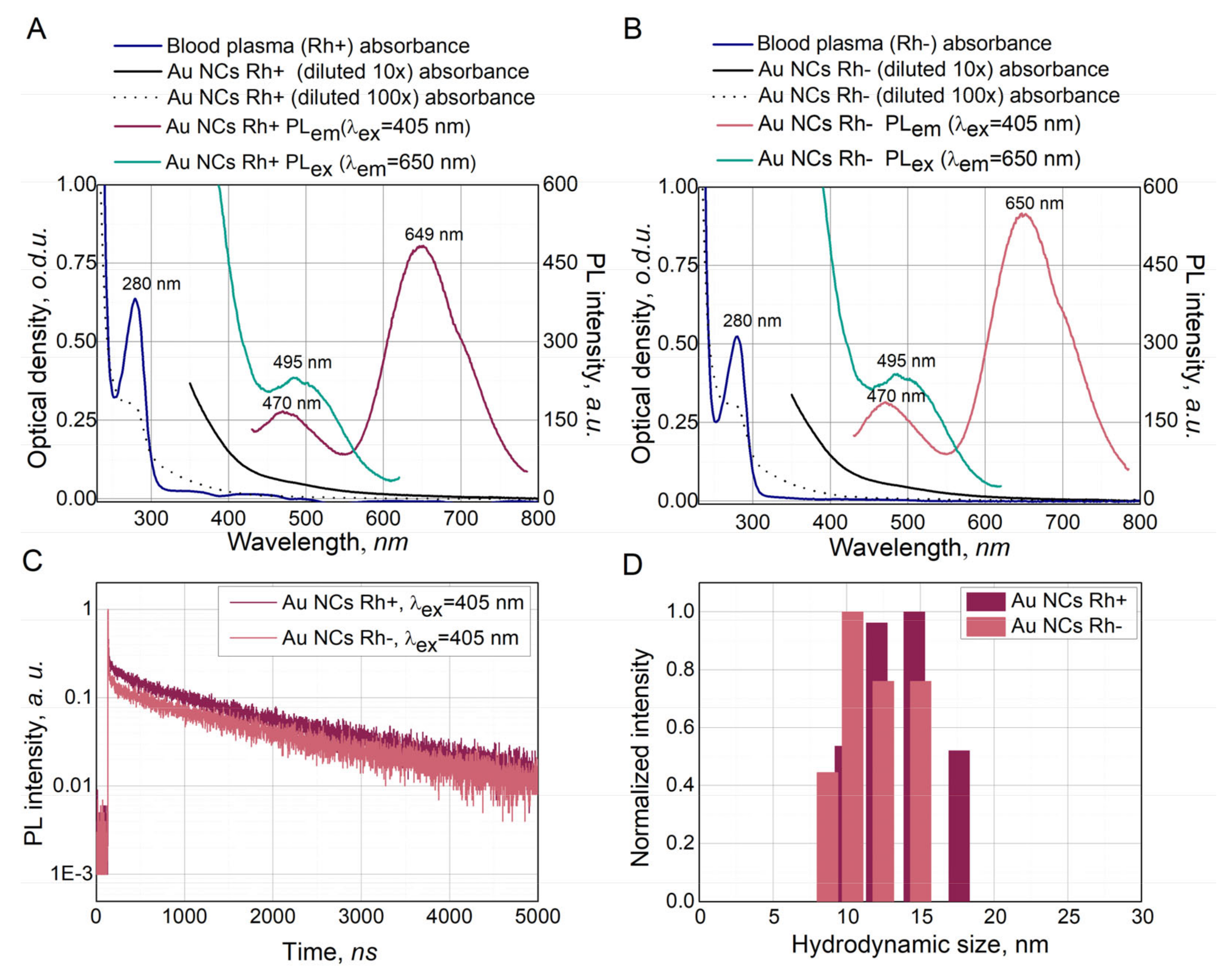
3.2. Characterization of Synthesized Au NCs
3.3. Singlet Oxygen and Other Reactive Oxygen Species Generation
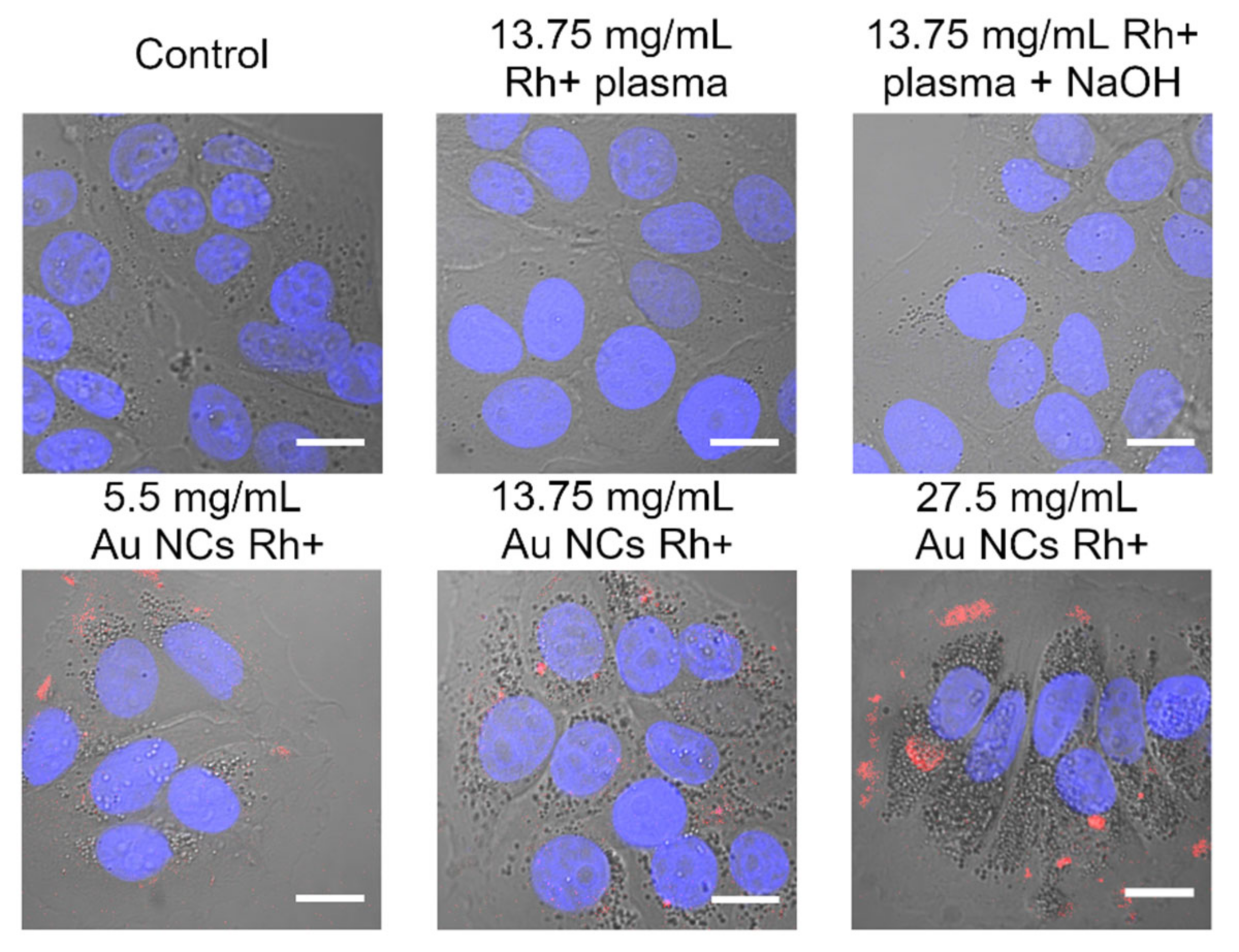
3.4. Au NCs Accumulation in Breast Cancer Cells
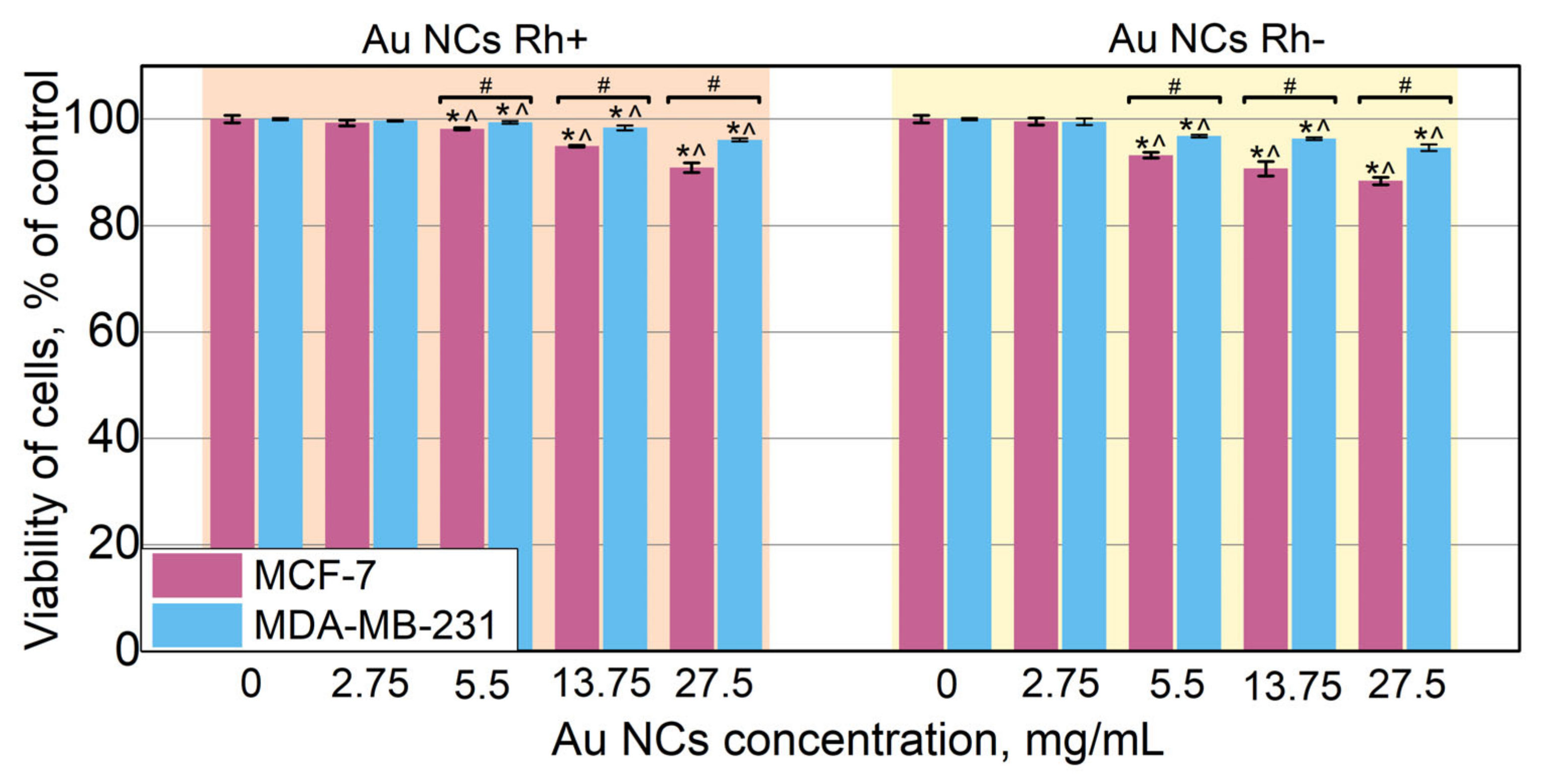
3.5. Biocompatibility of Au NCs
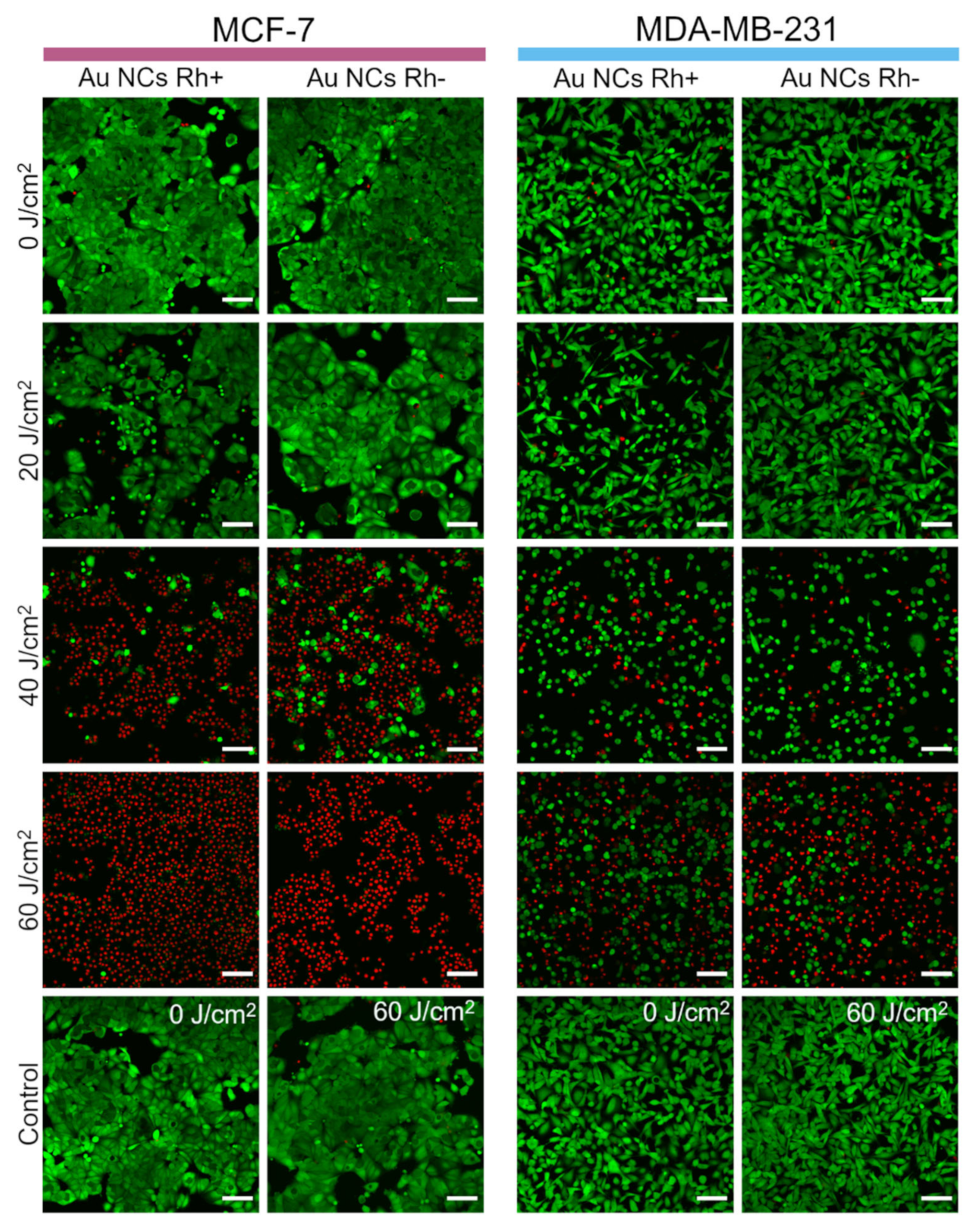
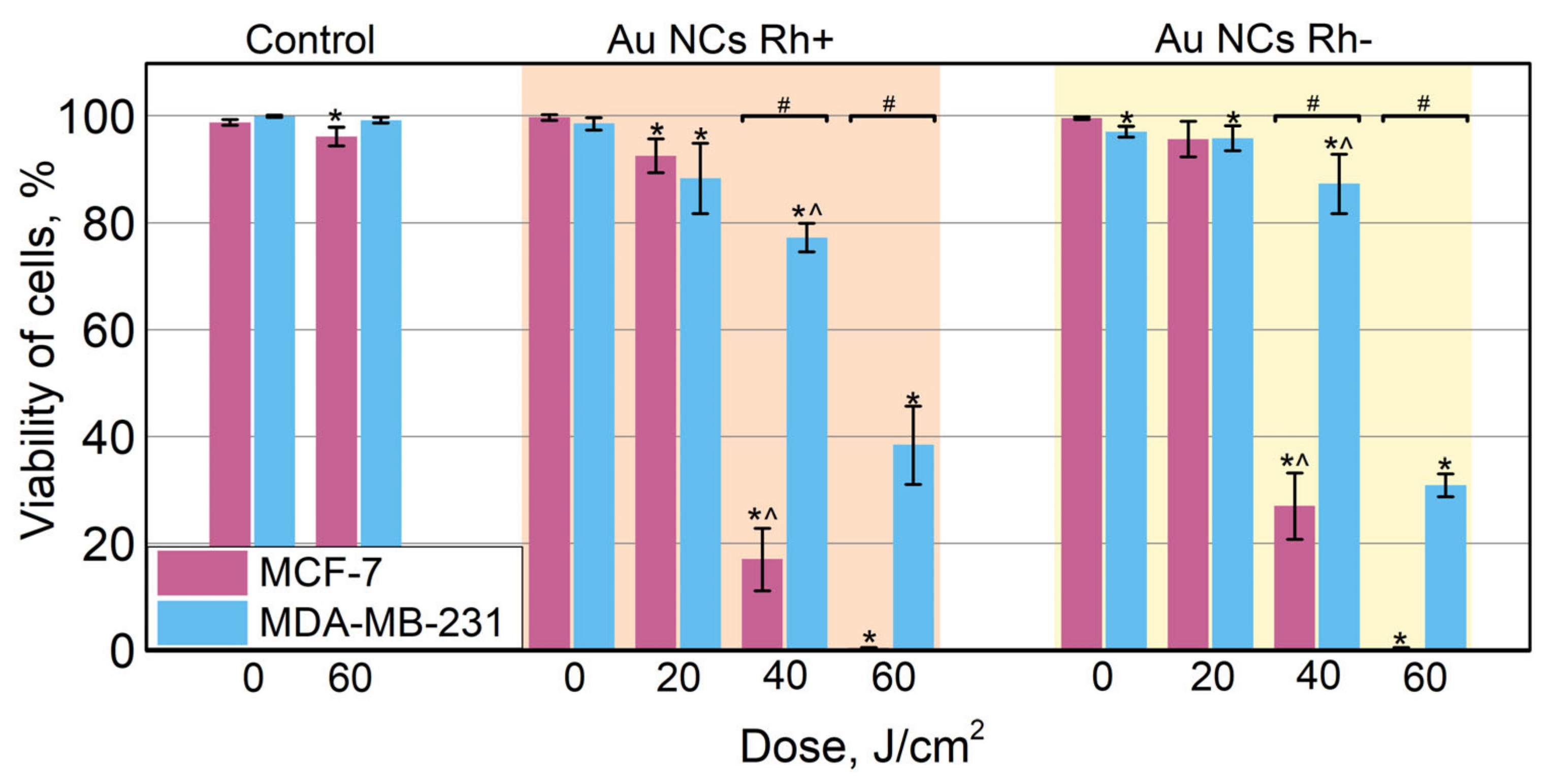
3.6. Photodynamic Effect
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Castano, A.P.; Demidova, T.N.; Hamblin, M.R. Mechanisms in Photodynamic Therapy: Part Three—Photosensitizer Pharmacokinetics, Biodistribution, Tumor Localization and Modes of Tumor Destruction. Photodiagnosis Photodyn. Ther. 2005, 2, 91–106. [Google Scholar] [CrossRef] [Green Version]
- Steponkiene, S.; Valanciunaite, J.; Skripka, A.; Rotomskis, R. Cellular Uptake and Photosensitizing Properties of Quantum Dot-Chlorin E6 Complex: In Vitro Study. J. Biomed. Nanotechnol. 2014, 10, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Skripka, A.; Karabanovas, V.; Jarockyte, G.; Marin, R.; Tam, V.; Cerruti, M.; Rotomskis, R.; Vetrone, F. Decoupling Theranostics with Rare Earth Doped Nanoparticles. Adv. Funct. Mater. 2019, 29, 1807105. [Google Scholar] [CrossRef]
- Zhang, C.; Li, C.; Liu, Y.; Zhang, J.; Bao, C.; Liang, S.; Wang, Q.; Yang, Y.; Fu, H.; Wang, K.; et al. Gold Nanoclusters-Based Nanoprobes for Simultaneous Fluorescence Imaging and Targeted Photodynamic Therapy with Superior Penetration and Retention Behavior in Tumors. Adv. Funct. Mater. 2015, 25, 1314–1325. [Google Scholar] [CrossRef]
- Xia, F.; Hou, W.; Liu, Y.; Wang, W.; Han, Y.; Yang, M.; Zhi, X.; Li, C.; Qi, D.; Li, T.; et al. Cytokine Induced Killer Cells-Assisted Delivery of Chlorin E6 Mediated Self-Assembled Gold Nanoclusters to Tumors for Imaging and Immuno-Photodynamic Therapy. Biomaterials 2018, 170, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ho-Wu, R.; Yau, S.H.; Goodson, T. Efficient Singlet Oxygen Generation in Metal Nanoclusters for Two-Photon Photodynamic Therapy Applications. J. Phys. Chem. B 2017, 121, 10073–10080. [Google Scholar] [CrossRef]
- Poderys, V.; Jarockyte, G.; Bagdonas, S.; Karabanovas, V.; Rotomskis, R. Protein-Stabilized Gold Nanoclusters for PDT: ROS and Singlet Oxygen Generation. J. Photochem. Photobiol. B Biol. 2020, 204, 111802. [Google Scholar] [CrossRef]
- Combes, G.F.; Vučković, A.-M.; Perić Bakulić, M.; Antoine, R.; Bonačić-Koutecky, V.; Trajković, K. Nanotechnology in Tumor Biomarker Detection: The Potential of Liganded Nanoclusters as Nonlinear Optical Contrast Agents for Molecular Diagnostics of Cancer. Cancers 2021, 13, 4206. [Google Scholar] [CrossRef]
- Cifuentes-Rius, A.; Deepagan, V.G.; Xie, J.; Voelcker, N.H. Bright Future of Gold Nanoclusters in Theranostics. ACS Appl. Mater. Interfaces 2021, 13, 49581–49588. [Google Scholar] [CrossRef]
- Higbee-Dempsey, E.; Amirshaghaghi, A.; Case, M.J.; Miller, J.; Busch, T.M.; Tsourkas, A. Indocyanine Green–Coated Gold Nanoclusters for Photoacoustic Imaging and Photothermal Therapy. Adv. Ther. 2019, 2, 1900088. [Google Scholar] [CrossRef]
- Jiang, X.; Du, B.; Huang, Y.; Yu, M.; Zheng, J. Cancer Photothermal Therapy with ICG-Conjugated Gold Nanoclusters. Bioconj. Chem. 2020, 31, 1522–1528. [Google Scholar] [CrossRef] [PubMed]
- Kefayat, A.; Ghahremani, F.; Motaghi, H.; Amouheidari, A. Ultra-Small but Ultra-Effective: Folic Acid-Targeted Gold Nanoclusters for Enhancement of Intracranial Glioma Tumors’ Radiation Therapy Efficacy. Nanomed. Nanotechnol. Biol. Med. 2019, 16, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Samani, R.K.; Tavakoli, M.B.; Maghsoudinia, F.; Motaghi, H.; Hejazi, S.H.; Mehrgardi, M.A. Trastuzumab and Folic Acid Functionalized Gold Nanoclusters as a Dual-Targeted Radiosensitizer for Megavoltage Radiation Therapy of Human Breast Cancer. Eur. J. Pharm. Sci. 2020, 153, 105487. [Google Scholar] [CrossRef] [PubMed]
- Latorre, A.; Latorre, A.; Castellanos, M.; Rodriguez Diaz, C.; Lazaro-Carrillo, A.; Aguado, T.; Lecea, M.; Romero-Pérez, S.; Calero, M.; Sanchez-Puelles, J.M.; et al. Multifunctional Albumin-Stabilized Gold Nanoclusters for the Reduction of Cancer Stem Cells. Cancers 2019, 11, 969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Govindaraju, S.; Roshini, A.; Lee, M.-H.; Yun, K. Kaempferol Conjugated Gold Nanoclusters Enabled Efficient for Anticancer Therapeutics to A549 Lung Cancer Cells. Int. J. Nanomed. 2019, 14, 5147–5157. [Google Scholar] [CrossRef] [Green Version]
- Demann, E.T.K.; Stein, P.S.; Haubenreich, J.E. Gold as an Implant in Medicine and Dentistry. J. Long-Term Eff. Med. Implant. 2005, 15, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Syburra, T.; Schurr, U.; Rahn, M.; Graves, K.; Genoni, M. Gold-Coated Pacemaker Implantation after Allergic Reactions to Pacemaker Compounds. Europace 2010, 12, 749–750. [Google Scholar] [CrossRef]
- Xie, J.; Zheng, Y.; Ying, J.Y. Protein-Directed Synthesis of Highly Fluorescent Gold Nanoclusters. J. Am. Chem Soc. 2009, 131, 888–889. [Google Scholar] [CrossRef]
- Wei, H.; Wang, Z.; Yang, L.; Tian, S.; Hou, C.; Lu, Y. Lysozyme-Stabilized Gold Fluorescent Cluster: Synthesis and Application as Hg2+ Sensor. Analyst 2010, 135, 1406–1410. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.-H.; Tseng, W.-L. (Lysozyme Type VI)-Stabilized Au8 Clusters: Synthesis Mechanism and Application for Sensing of Glutathione in a Single Drop of Blood. Small 2012, 8, 1912–1919. [Google Scholar] [CrossRef]
- Kawasaki, H.; Yoshimura, K.; Hamaguchi, K.; Arakawa, R. Trypsin-Stabilized Fluorescent Gold Nanocluster for Sensitive and Selective Hg2+ Detection. Anal. Sci. 2011, 27, 591–596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dixon, J.M.; Egusa, S. Common Motif at the Red Luminophore in Bovine Serum Albumin–, Ovalbumin–, Trypsin–, and Insulin–Gold Complexes. J. Phys. Chem. Lett. 2021, 12, 2865–2870. [Google Scholar] [CrossRef]
- Liu, C.-L.; Wu, H.-T.; Hsiao, Y.-H.; Lai, C.-W.; Shih, C.-W.; Peng, Y.-K.; Tang, K.-C.; Chang, H.-W.; Chien, Y.-C.; Hsiao, J.-K.; et al. Insulin-Directed Synthesis of Fluorescent Gold Nanoclusters: Preservation of Insulin Bioactivity and Versatility in Cell Imaging. Angew. Chem. Int. Ed. Engl. 2011, 50, 7056–7060. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.R.; Rahn, I.; Johnson, S.; Patel, R.; Guo, J.; Orbulescu, J.; Micic, M.; Whyte, J.D.; Blackwelder, P.; Leblanc, R.M. Human Insulin Fibril-Assisted Synthesis of Fluorescent Gold Nanoclusters in Alkaline Media under Physiological Temperature. Colloids Surf. B Biointerfaces 2013, 105, 167–172. [Google Scholar] [CrossRef]
- Peralta, D.V.; He, J.; Wheeler, D.A.; Zhang, J.Z.; Tarr, M.A. Encapsulating Gold Nanomaterials into Size-Controlled Human Serum Albumin Nanoparticles for Cancer Therapy Platforms. J. Microencapsul. 2014, 31, 824–831. [Google Scholar] [CrossRef]
- Russell, B.A.; Jachimska, B.; Kralka, I.; Mulheran, P.A.; Chen, Y. Human Serum Albumin Encapsulated Gold Nanoclusters: Effects of Cluster Synthesis on Natural Protein Characteristics. J. Mater. Chem. B 2016, 4, 6876–6882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chakraborty, S.; Nandy, A.; Ghosh, S.; Das, N.K.; Parveen, S.; Datta, S.; Mukherjee, S. Protein-Templated Gold Nanoclusters as Specific Bio-Imaging Probes for the Detection of Hg(Ii) Ions in in Vivo and in Vitro Systems: Discriminating between MDA-MB-231 and MCF10A Cells. Analyst 2021, 146, 1455–1463. [Google Scholar] [CrossRef]
- Suo, Z.; Hou, X.; Hu, Z.; Liu, Y.; Xing, F.; Feng, L. Fibrinogen-Templated Gold Nanoclusters for Fluorometric Determination of Cysteine and Mercury(II). Microchim. Acta 2019, 186, 799. [Google Scholar] [CrossRef]
- Xavier, P.L.; Chaudhari, K.; Verma, P.K.; Pal, S.K.; Pradeep, T. Luminescent Quantum Clusters of Gold in Transferrin Family Protein, Lactoferrin Exhibiting FRET. Nanoscale 2010, 2, 2769–2776. [Google Scholar] [CrossRef]
- Le Guével, X.; Daum, N.; Schneider, M. Synthesis and Characterization of Human Transferrin-Stabilized Gold Nanoclusters. Nanotechnology 2011, 22, 275103. [Google Scholar] [CrossRef]
- El-Sayed, N.; Schneider, M. Advances in Biomedical and Pharmaceutical Applications of Protein-Stabilized Gold Nanoclusters. J. Mater. Chem. B 2020, 8, 8952–8971. [Google Scholar] [CrossRef] [PubMed]
- Kratz, F.; Elsadek, B. Clinical Impact of Serum Proteins on Drug Delivery. J. Control. Release 2012, 161, 429–445. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, G.; Li, Q.; Jiang, H.; Liu, C.; Amatore, C.; Wang, X. In Vivo Self-Bio-Imaging of Tumors through in Situ Biosynthesized Fluorescent Gold Nanoclusters. Sci. Rep. 2013, 3, 1157. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liu, X.; Fu, C.; Tan, L.; Liu, H. Biosynthesis of Fluorescent Gold Nanoclusters for in Vitro and in Vivo Tumor Imaging. Opt. Commun. 2015, 355, 567–574. [Google Scholar] [CrossRef]
- Ahmed, S.; Annu; Ikram, S.; Yudha, S.S. Biosynthesis of Gold Nanoparticles: A Green Approach. J. Photochem. Photobiol. B Biol. 2016, 161, 141–153. [Google Scholar] [CrossRef]
- Tepale, N.; Fernández-Escamilla, V.V.A.; Carreon-Alvarez, C.; González-Coronel, V.J.; Luna-Flores, A.; Carreon-Alvarez, A.; Aguilar, J. Nanoengineering of Gold Nanoparticles: Green Synthesis, Characterization, and Applications. Crystals 2019, 9, 612. [Google Scholar] [CrossRef] [Green Version]
- Rotomskis, R. Optical Biopsy of Cancer: Nanotechnological Aspects. Tumori 2008, 94, 200–205. [Google Scholar] [CrossRef]
- Kubiliute, R.; Slektaite, A.; Burkanas, M.; Grigiene, R.; Rotomskis, R. Gold Nanoparticles as a Contrast Agent for X-Ray Imaging. In Proceedings of the Medical Physics in the Baltic States, Kaunas, Lithuania, 4–6 November 2021; Adliene, D., Ed.; Kaunas Univ Technology Press: Kaunas, Lithuania, 2011; p. 27. [Google Scholar]
- Cole, L.E.; Ross, R.D.; Tilley, J.M.; Vargo-Gogola, T.; Roeder, R.K. Gold Nanoparticles as Contrast Agents in X-Ray Imaging and Computed Tomography. Nanomedicine 2015, 10, 321–341. [Google Scholar] [CrossRef] [Green Version]
- Lippitz, M.; van Dijk, M.A.; Orrit, M. Third-Harmonic Generation from Single Gold Nanoparticles. Nano Lett. 2005, 5, 799–802. [Google Scholar] [CrossRef]
- Stoscheck, C.M. Quantitation of Protein. In Methods in Enzymology; Deutscher, M.P., Ed.; Academic Press: Cambridge, MA, USA, 1990; Volume 182, pp. 50–68. [Google Scholar]
- Henderson, L.M.; Chappell, J.B. Dihydrorhodamine 123: A Fluorescent Probe for Superoxide Generation? Eur. J. Biochem. 1993, 217, 973–980. [Google Scholar] [CrossRef]
- Baracca, A.; Sgarbi, G.; Solaini, G.; Lenaz, G. Rhodamine 123 as a Probe of Mitochondrial Membrane Potential: Evaluation of Proton Flux through F(0) during ATP Synthesis. Biochim. Biophys. Acta 2003, 1606, 137–146. [Google Scholar] [CrossRef] [Green Version]
- Gollmer, A.; Arnbjerg, J.; Blaikie, F.H.; Pedersen, B.W.; Breitenbach, T.; Daasbjerg, K.; Glasius, M.; Ogilby, P.R. Singlet Oxygen Sensor Green®: Photochemical Behavior in Solution and in a Mammalian Cell. Photochem. Photobiol. 2011, 87, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Ragàs, X.; Jiménez-Banzo, A.; Sánchez-García, D.; Batllori, X.; Nonell, S. Singlet Oxygen Photosensitisation by the Fluorescent Probe Singlet Oxygen Sensor Green. Chem. Commun. 2009, 2920–2922. [Google Scholar] [CrossRef]
- Lin, H.; Shen, Y.; Chen, D.; Lin, L.; Wilson, B.C.; Li, B.; Xie, S. Feasibility Study on Quantitative Measurements of Singlet Oxygen Generation Using Singlet Oxygen Sensor Green. J. Fluoresc. 2013, 23, 41–47. [Google Scholar] [CrossRef]
- Poderys, V.; Matulionytė-Safinė, M.; Rupšys, D.; Rotomskis, R. Protein Stabilized Au Nanoclusters: Spectral Properties and Photostability. Lith. J. Phys. 2016, 56, 55–65. [Google Scholar] [CrossRef] [Green Version]
- Sarkar, S.; Sengupta, A.; Hazra, P.; Mandal, P. Blue Emission in Proteins. arXiv 2014, arXiv:1404.6859. [Google Scholar]
- Wen, X.; Yu, P.; Toh, Y.-R.; Hsu, A.-C.; Lee, Y.-C.; Tang, J. Fluorescence Dynamics in BSA-Protected Au25 Nanoclusters. J. Phys. Chem. C 2012, 116, 19032–19038. [Google Scholar] [CrossRef]
- Yu, P.; Wen, X.; Toh, Y.-R.; Tang, J. Temperature-Dependent Fluorescence in Au10 Nanoclusters. J. Phys. Chem. C 2012, 116, 6567–6571. [Google Scholar] [CrossRef]
- Wong, X.Y.; Quesada-González, D.; Manickam, S.; New, S.Y.; Muthoosamy, K.; Merkoçi, A. Integrating Gold Nanoclusters, Folic Acid and Reduced Graphene Oxide for Nanosensing of Glutathione Based on “Turn-off” Fluorescence. Sci. Rep. 2021, 11, 2375. [Google Scholar] [CrossRef]
- Zhu, M.; Aikens, C.M.; Hollander, F.J.; Schatz, G.C.; Jin, R. Correlating the Crystal Structure of A Thiol-Protected Au25 Cluster and Optical Properties. J. Am. Chem. Soc. 2008, 130, 5883–5885. [Google Scholar] [CrossRef]
- Yang, T.; Dai, S.; Tan, H.; Zong, Y.; Liu, Y.; Chen, J.; Zhang, K.; Wu, P.; Zhang, S.; Xu, J.; et al. Mechanism of Photoluminescence in Ag Nanoclusters: Metal-Centered Emission versus Synergistic Effect in Ligand-Centered Emission. J. Phys. Chem. C 2019, 123, 18638–18645. [Google Scholar] [CrossRef]
- With, T.K. Porphyrins in Erythrocytes and Blood Plasma: Preliminary Studies with Qualitative Thin-Layer Chromatography. Clin. Sci. Mol. Med. 1977, 52, 463–467. [Google Scholar] [CrossRef] [PubMed]
- Matulionyte, M.; Dapkute, D.; Budenaite, L.; Jarockyte, G.; Rotomskis, R. Photoluminescent Gold Nanoclusters in Cancer Cells: Cellular Uptake, Toxicity, and Generation of Reactive Oxygen Species. Int. J. Mol. Sci. 2017, 18, 378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, L.; Shang, L.; Nienhaus, G.U. Mechanistic Aspects of Fluorescent Gold Nanocluster Internalization by Live HeLa Cells. Nanoscale 2013, 5, 1537–1543. [Google Scholar] [CrossRef]
- Ungor, D.; Barbasz, A.; Czyżowska, A.; Csapó, E.; Oćwieja, M. Cytotoxicity Studies of Protein-Stabilized Fluorescent Gold Nanoclusters on Human Lymphocytes. Colloids Surf. B Biointerfaces 2021, 200, 111593. [Google Scholar] [CrossRef]
- Zhang, Z.; Yao, Y.; Yuan, Q.; Lu, C.; Zhang, X.; Yuan, J.; Hou, K.; Zhang, C.; Du, Z.; Gao, X.; et al. Gold Clusters Prevent Breast Cancer Bone Metastasis by Suppressing Tumor-Induced Osteoclastogenesis. Theranostics 2020, 10, 4042–4055. [Google Scholar] [CrossRef]
- Escudero-Francos, M.A.; Cepas, V.; González-Menéndez, P.; Badía-Laíño, R.; Díaz-García, M.E.; Sainz, R.M.; Mayo, J.C.; Hevia, D. Cellular Uptake and Tissue Biodistribution of Functionalized Gold Nanoparticles and Nanoclusters. J. Biomed. Nanotechnol. 2017, 13, 167–179. [Google Scholar] [CrossRef]
- Dong, L.; Li, M.; Zhang, S.; Li, J.; Shen, G.; Tu, Y.; Zhu, J.; Tao, J. Cytotoxicity of BSA-Stabilized Gold Nanoclusters: In Vitro and In Vivo Study. Small 2015, 11, 2571–2581. [Google Scholar] [CrossRef]
- Retnakumari, A.; Setua, S.; Menon, D.; Ravindran, P.; Muhammed, H.; Pradeep, T.; Nair, S.; Koyakutty, M. Molecular-Receptor-Specific, Non-Toxic, near-Infrared-Emitting Au Cluster-Protein Nanoconjugates for Targeted Cancer Imaging. Nanotechnology 2010, 21, 55103. [Google Scholar] [CrossRef]
- Czyżowska, A.; Barbasz, A.; Szyk-Warszyńska, L.; Oćwieja, M.; Csapó, E.; Ungor, D. The Surface-Dependent Biological Effect of Protein-Gold Nanoclusters on Human Immune System Mimetic Cells. Colloids Surf. A Physicochem. Eng. Asp. 2021, 620, 126569. [Google Scholar] [CrossRef]
- Damalakiene, L.; Karabanovas, V.; Bagdonas, S.; Valius, M.; Rotomskis, R. Intracellular Distribution of Nontargeted Quantum Dots after Natural Uptake and Microinjection. Int. J. Nanomed. 2013, 8, 555–568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, X.; Wu, Y.; Jin, S.; Tian, Y.; Zhang, X.; Zhao, Y.; Yu, L.; Liang, X.-J. Gold Nanoparticles Induce Autophagosome Accumulation through Size-Dependent Nanoparticle Uptake and Lysosome Impairment. ACS Nano 2011, 5, 8629–8639. [Google Scholar] [CrossRef] [PubMed]
- Van Cruchten, S.; Van den Broeck, W. Morphological and Biochemical Aspects of Apoptosis, Oncosis and Necrosis. Anat. Histol. Embryol. 2002, 31, 214–223. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jarockyte, G.; Poderys, V.; Barzda, V.; Karabanovas, V.; Rotomskis, R. Blood Plasma Stabilized Gold Nanoclusters for Personalized Tumor Theranostics. Cancers 2022, 14, 1887. https://doi.org/10.3390/cancers14081887
Jarockyte G, Poderys V, Barzda V, Karabanovas V, Rotomskis R. Blood Plasma Stabilized Gold Nanoclusters for Personalized Tumor Theranostics. Cancers. 2022; 14(8):1887. https://doi.org/10.3390/cancers14081887
Chicago/Turabian StyleJarockyte, Greta, Vilius Poderys, Virginijus Barzda, Vitalijus Karabanovas, and Ricardas Rotomskis. 2022. "Blood Plasma Stabilized Gold Nanoclusters for Personalized Tumor Theranostics" Cancers 14, no. 8: 1887. https://doi.org/10.3390/cancers14081887
APA StyleJarockyte, G., Poderys, V., Barzda, V., Karabanovas, V., & Rotomskis, R. (2022). Blood Plasma Stabilized Gold Nanoclusters for Personalized Tumor Theranostics. Cancers, 14(8), 1887. https://doi.org/10.3390/cancers14081887