Prognostic Analysis of HPV Status in Sinonasal Squamous Cell Carcinoma
Abstract
:Simple Summary
Abstract
1. Introduction
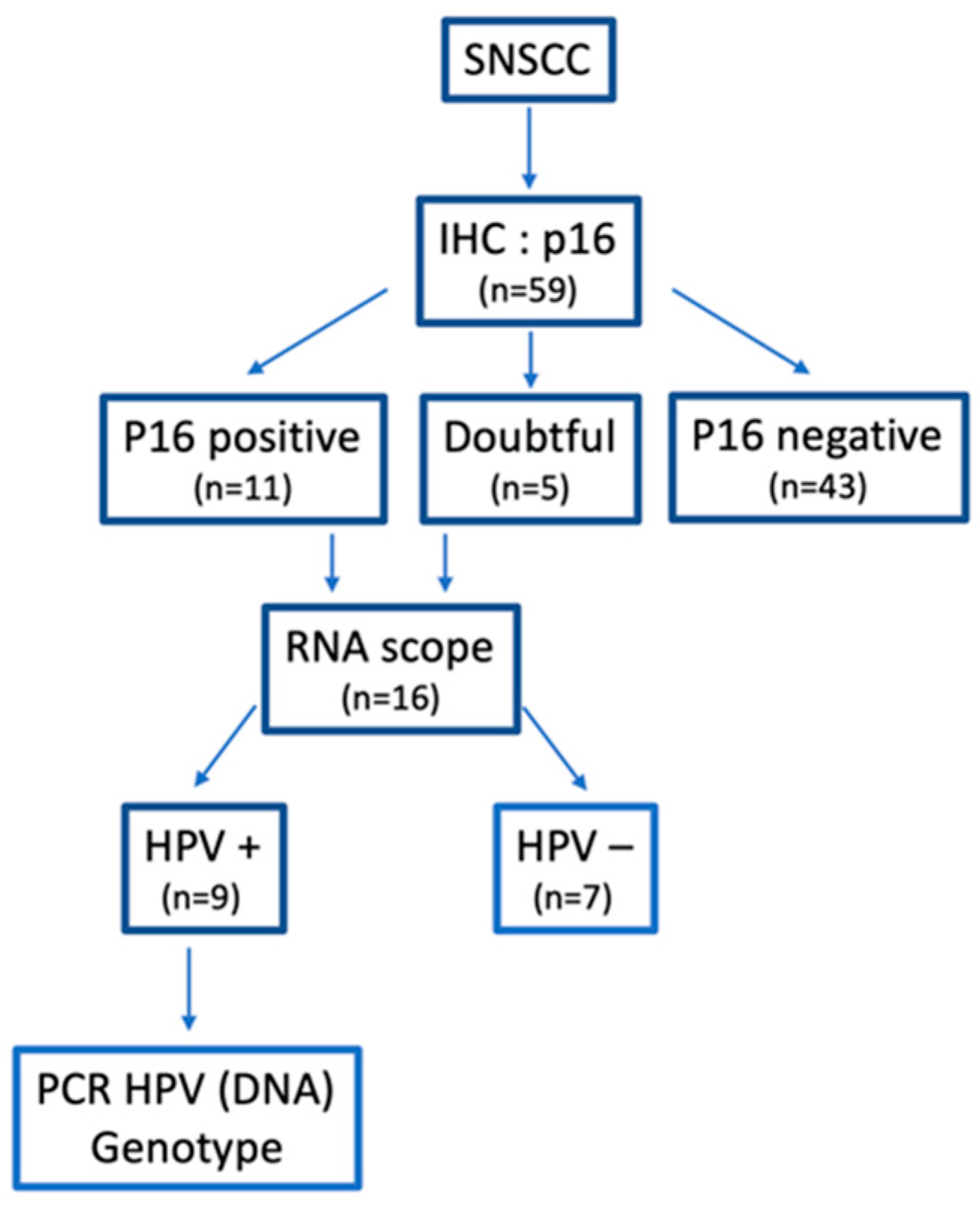
2. Materials and Methods
2.1. Patient Selection
2.2. Data
2.3. Immunohistochemistry
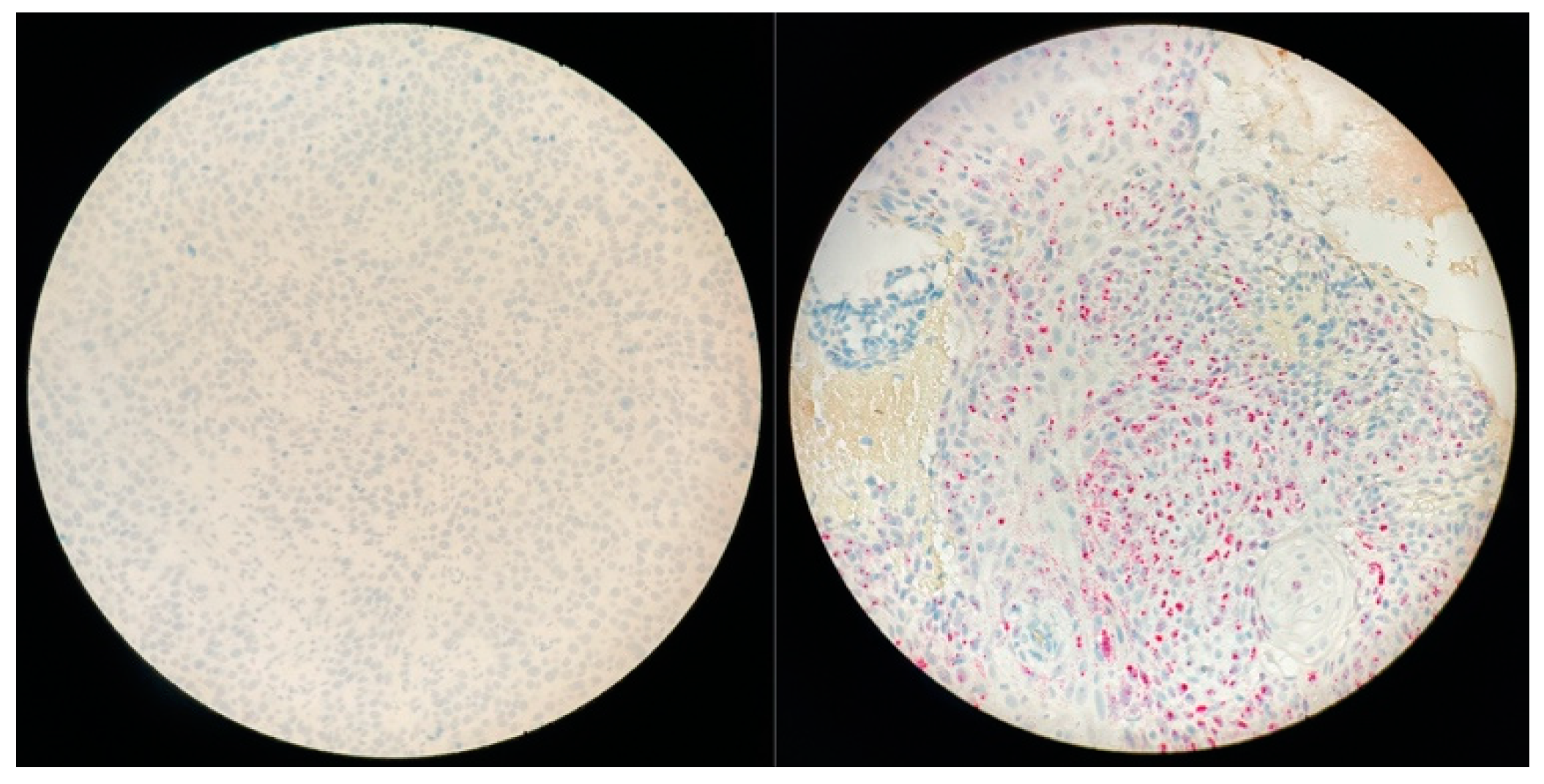
2.4. In Situ Hybridization (ISH): RNAscope
2.5. PCR DNA
2.6. Statistical Analysis
3. Results
3.1. Patients and Tumor Characteristics
3.2. Therapeutic Algorithm
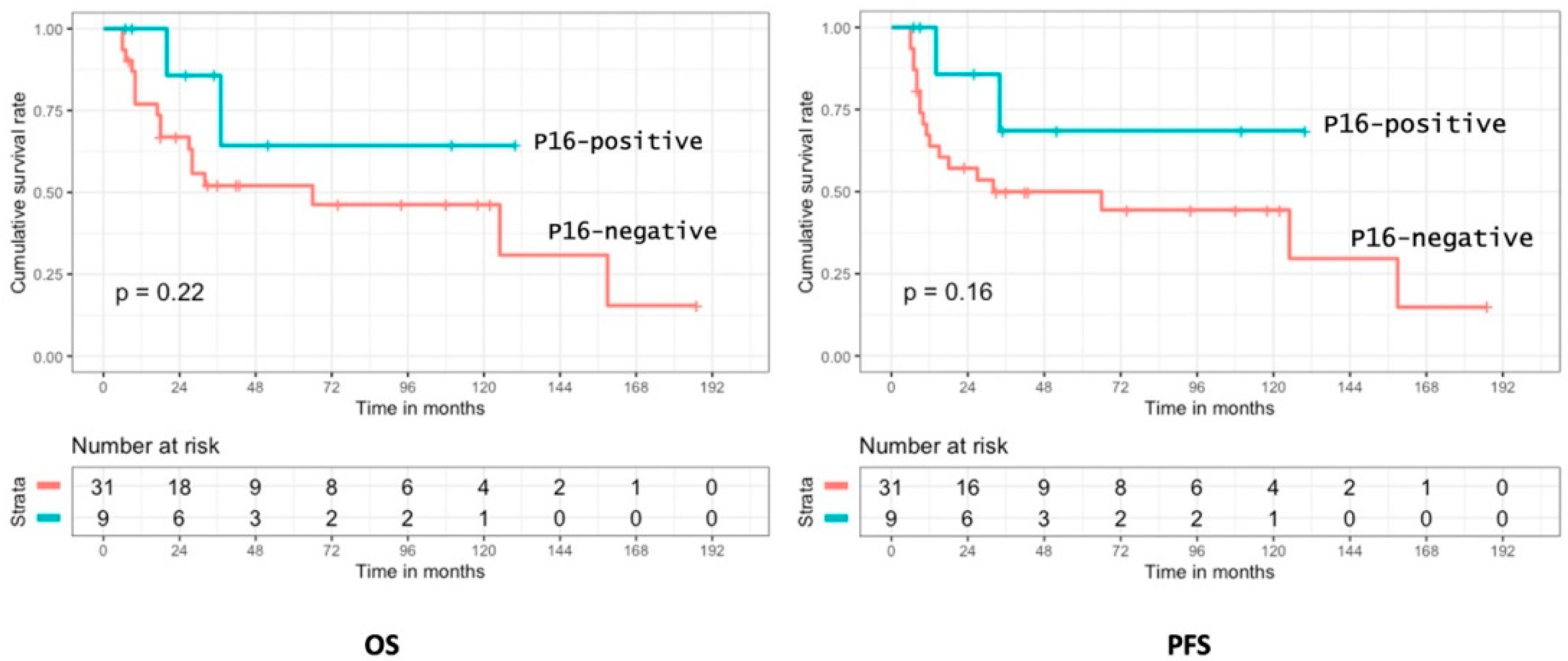
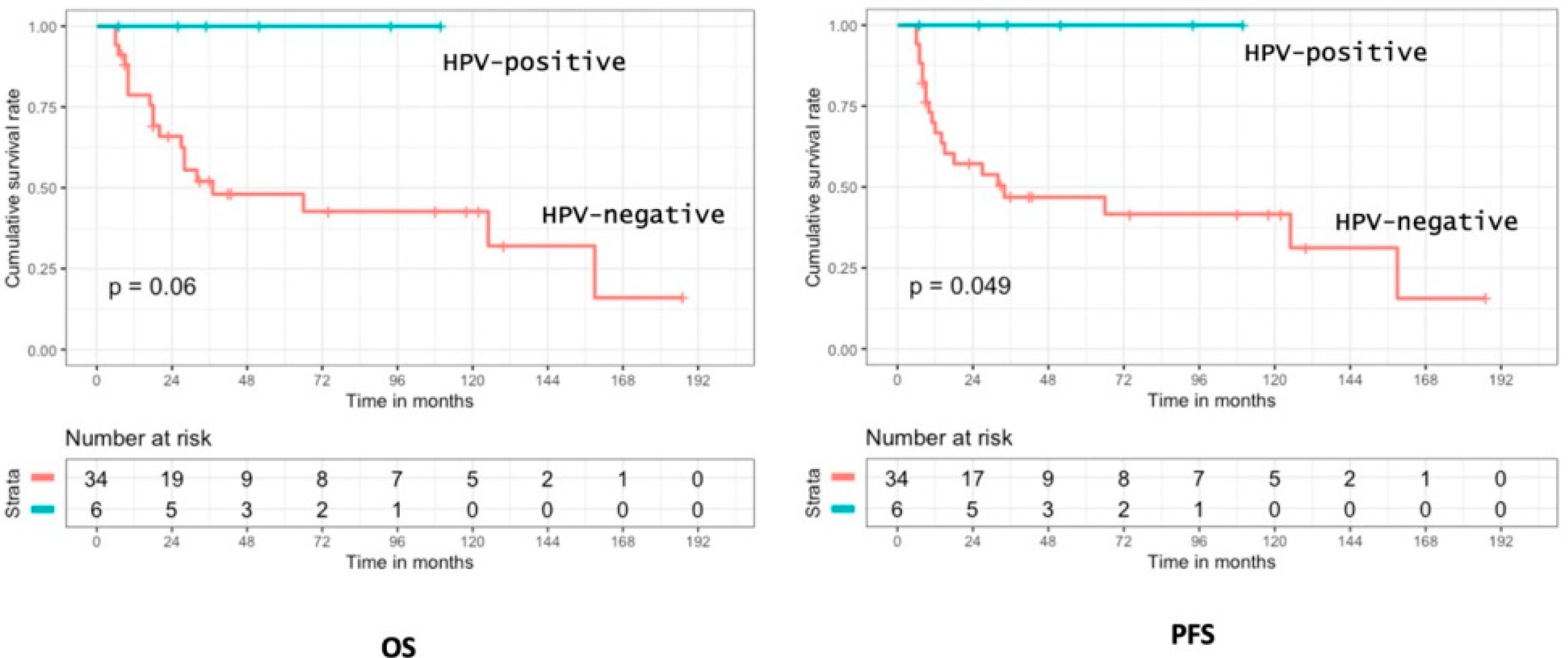
3.3. Oncologic Outcomes
3.4. HPV-Induced SNSCC Characterization
3.5. Prognostic Analysis of p16 Positivity and HPV Status
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sanghvi, S.; Khan, M.N.; Patel, N.R.; Yeldandi, S.; Baredes, S.; Eloy, J.A. Epidemiology of sinonasal squamous cell carcinoma: A comprehensive analysis of 4994 patients. Laryngoscope 2014, 124, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Tufano, R.P.; Mokadam, N.A.; Montone, K.T.; Weinstein, G.S.; Chalian, A.A.; Wolf, P.F.; Weber, R.S. Malignant tumors of the nose and paranasal sinuses: Hospital of the University of Pennsylvania experience 1990–1997. Am. J. Rhinol. 1999, 13, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Tang, A.L.; Takiar, V.; Wise-Draper, T.M. Langevin SM. Human Papillomavirus and Survival of Sinonasal Squamous Cell Carcinoma Patients: A Systematic Review and Meta-Analysis. Cancers 2021, 13, 3677. [Google Scholar] [CrossRef] [PubMed]
- Arnold, A.; Ziglinas, P.; Ochs, K.; Alter, N.; Geretschläger, A.; Lädrach, K.; Zbären, P.; Caversaccio, M. Therapy options and long-term results of sinonasal malignancies. Oral Oncol. 2012, 48, 1031–1037. [Google Scholar] [CrossRef]
- Khademi, B.; Moradi, A.; Hoseini, S.; Mohammadianpanah, M. Malignant neoplasms of the sinonasal tract: Report of 71 patients and literature review and analysis Oral Maxillofac Surg. J. Oral Maxillofac. Surg. 2009, 13, 191–199. [Google Scholar] [CrossRef]
- Porceddu, S.; Martin, J.; Shanker, G.; Weih, L.; Russell, C.; Rischin, D.; Corry, J.; Peters, L. Paranasal sinus tumors: Peter MacCallum Cancer Institute experience. Head Neck 2004, 26, 322–330. [Google Scholar] [CrossRef]
- Paré, A.; Blanchard, P.; Rosellini, S.; Aupérin, A.; Gorphe, P.; Casiraghi, O.; Temam, S.; Bidault, F.; Page, P.; Kolb, F.; et al. Outcomes of multimodal management for sinonasal squamous cell carcinoma. J. Craniomaxillofac. Surg. 2017, 45, 1124–1132. [Google Scholar] [CrossRef]
- Le, Q.T.; Fu, K.K.; Kaplan, M.; Terris, D.J.; Fee, W.E.; Goffinet, D.R. Treatment of maxillary sinus carcinoma: A comparison of the 1997 and 1977 American Joint Committee on cancer staging systems. Cancers 1999, 86, 1700–1711. [Google Scholar] [CrossRef]
- Recommandation REFCOR Pour la Pratique Clinique–Tumeurs Malignes Primitives des Fosses Nasales et des Sinus. 2022. Available online: Refcor.org (accessed on 15 January 2022).
- Sahovaler, A.; Kim, M.H.; Mendez, A.; Palma, D.; Fung, K.; Yoo, J.; Nichols, A.C.; MacNeil, S.D. Survival Outcomes in Human Papillomavirus–Associated Nonoropharyngeal Squamous Cell Carcinomas: A Systematic Review and Meta-analysis. JAMA Otolaryngol.-Head Neck Surg. 2020, 146, 1158–1166. [Google Scholar] [CrossRef]
- Lund, V.J.; Stammberger, H.; Nicolai, P.; Castelnuovo, P.; Beal, T.; Beham, A.; Bernal-Sprekelsen, M.; Braun, H.; Cappabianca, P.; Carrau, R.; et al. European position paper on endoscopic management of tumours of the nose, paranasal sinuses, and skull base. Rhino Support 2010, 22, 1–143. [Google Scholar]
- Kılıç, S.; Kılıç, S.S.; Baredes, S.; Park, R.C.W.; Mahmoud, O.; Suh, J.D.; Gray, S.T.; Eloy, J.A. Comparison of endoscopic and open resection of sinonasal squamous cell carcinoma: A propensity score-matched analysis of 652 patients. Int. Forum Allergy 2018, 8, 421–434. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.B.; Liu, I.Y.; Gornbein, J.A.; Nguyen, C.T. HPV-Positive Oropharyngeal Carcinoma: A Systematic Review of Treatment and Prognosis. Otolaryngol.–Head Neck Surg. 2015, 153, 758–769. [Google Scholar] [CrossRef] [PubMed]
- Mirghani, H.; Amen, F.; Tao, Y.; Deutsch, E.; Levy, A. Increased radiosensitivity of HPV-positive head and neck cancers: Molecular basis and therapeutic perspectives. Cancer Treat. Rev. 2015, 41, 844–852. [Google Scholar] [CrossRef]
- Fakhry, C.; Westra, W.H.; Li, S.; Cmelak, A.; Ridge, J.A.; Pinto, H.; Forastiere, A.; Gillison, M.L. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J. Natl. Cancer Inst. 2008, 100, 261–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gillison, M.L. Human papillomavirus-associated head and neck cancer is a distinct epidemiologic, clinical, and molecular entity. Semin. Oncol. 2004, 31, 744–754. [Google Scholar] [CrossRef]
- Weinberger, P.M.; Yu, Z.; Haffty, B.G.; Kowalski, D.; Harigopal, M.; Brandsma, M.; Sasaki, C.; Joe, J.; Camp, R.L.; Rimm, D.; et al. Molecular classification identifies a subset of human papillomavirus—associated oropharyngeal cancers with favorable prognosis. J. Clin. Oncol. 2006, 24, 736–747. [Google Scholar] [CrossRef]
- Kılıç, S.; Kılıç, S.S.; Kim, E.S.; Baredes, S.; Mahmoud, O.; Gray, S.T.; Eloy, J.A. Significance of human papillomavirus positivity in sinonasal squamous cell carcinoma. Int. Forum Allergy Rhinol. 2017, 7, 980–989. [Google Scholar] [CrossRef]
- Rassy, E.; Nicolai, P.; Pavlidis, N. Comprehensive management of HPV-related squamous cell carcinoma of the head and neck of unknown primary. Head Neck 2019, 41, 3700–3711. [Google Scholar] [CrossRef]
- Syrjänen, K.; Syrjänen, S. Detection of human papillomavirus in sinonasal carcinoma: Systematic review and meta-analysis. Hum. Pathol. 2013, 44, 983–991. [Google Scholar] [CrossRef]
- Augustin, J.; Lepine, C.; Morini, A.; Brunet, A.; Veyer, D.; Brochard, C.; Mirghani, H.; Péré, H.; Badoual, C. HPV Detection in Head and Neck Squamous Cell Carcinomas: What Is the Issue? Front. Oncol. 2020, 10, 1751. [Google Scholar] [CrossRef]
- Augustin, J.; Outh-Gauer, S.; Mandavit, M.; Gasne, C.; Grard, O.; Denize, T.; Nervo, M.; Mirghani, H.; Laccourreye, O.; Bonfils, P.; et al. Evaluation of the efficacy of the 4 tests (p16 immunochemistry, polymerase chain reaction, DNA, and RNA in situ hybridization) to evaluate a human papillomavirus infection in head and neck cancers: A cohort of 348 French squamous cell carcinomas. Hum Pathol. 2018, 78, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Veyer, D.; Wack, M.; Grard, O.; Bonfils, P.; Hans, S.; Bélec, L.; Badoual, C.; Péré, H. HPV detection and genotyping of head and neck cancer biopsies by molecular testing with regard to the new oropharyngeal squamous cell carcinoma classification based on HPV status. Pathology 2019, 51, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Sjöstedt, S.; von Buchwald, C.; Agander, T.K.; Aanaes, K. Impact of human papillomavirus in sinonasal cancer-a systematic review. Acta Oncol. 2021, 60, 1175–1191. [Google Scholar] [CrossRef] [PubMed]
- Jiromaru, R.; Yamamoto, H.; Yasumatsu, R.; Hongo, T.; Nozaki, Y.; Hashimoto, K.; Taguchi, K.; Masuda, M.; Nakagawa, T.; Oda, Y. HPV-related Sinonasal Carcinoma: Clinicopathologic Features, Diagnostic Utility of p16 and Rb Immunohistochemistry, and EGFR Copy Number Alteration. Am. J. Surg. Pathol. 2020, 44, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Torabi, S.J.; Yarbrough, W.G.; Mehra, S.; Osborn, H.A.; Judson, B. Association of Human Papillomavirus Status at Head and Neck Carcinoma Subsites With Overall Survival. JAMA Otolaryngol.-Head Neck Surg. 2018, 44, 519–525. [Google Scholar] [CrossRef] [Green Version]
- Boulikas, T.; Vougiouka, M. Cisplatin and platinum drugs at the molecular level. (Review). Oncol. Rep. 2003, 10, 1663–1682. [Google Scholar] [CrossRef] [PubMed]
- Hennequin, C.; Quero, L.; Favaudon, V. Réparation cellulaire et radiosensibilité tumorale: Focus sur le gène ATM [DNA repair and tumour radiosensitivity: Focus on ATM gene]. Bull Cancer 2011, 98, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Fukuoka, E.; Yamashita, K.; Tanaka, T.; Sawada, R.; Sugita, Y.; Arimoto, A.; Fujita, M.; Takaguchi, G.; Matsuda, T.; Oshikiri, T.; et al. Neoadjuvant Chemotherapy Increases PD-L1 Expression and CD8+ Tumor-infiltrating Lymphocytes in Esophageal Squamous Cell Carcinoma. Anticancer Res. 2019, 39, 4539–4548. [Google Scholar] [CrossRef]
- Miquelestorena-Standley, E.; Jourdan, M.L.; Collin, C.; Bouvier, C.; Larousserie, F.; Aubert, S.; Gomez-Brouchet, A.; Guinebretière, J.-M.; Tallegas, M.; Brulin, B.; et al. Effect of decalcification protocols on immunohistochemistry and molecular analyses of bone samples. Mod. Pathol. 2020, 33, 1505–1517. [Google Scholar] [CrossRef]
- Lewis, J.S., Jr.; Beadle, B.; Bishop, J.A.; Chernock, R.D.; Colasacco, C.; Lacchetti, C.; Moncur, J.T.; Rocco, J.W.; Schwartz, M.R.; Seethala, R.R.; et al. Human Papillomavirus Testing in Head and Neck Carcinomas: Guideline From the College of American Pathologists. Arch. Pathol. Lab. Med. 2018, 142, 559–597. [Google Scholar] [CrossRef] [Green Version]
- Alos, L.; Hakim, S.; Larque, A.B.; Oliva, J.D.L.; Rodriguez-Carunchio, L.; Caballero, M.; Nadal, A.; Marti, C.; Guimera, N.; Fernandez-Figueras, M.-T.; et al. p16 overexpression in high-grade neuroendocrine carcinomas of the head and neck: Potential diagnostic pitfall with HPV-related carcinomas. Virchows Arch. 2016, 469, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Lechner, M.; Chakravarthy, A.R.; Walter, V.; Masterson, L.; Feber, M.; Jay, A.; Weinberger, P.M.; McIndoe, R.A.; Forde, C.T.; Chester, K.; et al. Frequent HPV-independent p16/INK4A overexpression in head and neck cancer. Oral Oncol. 2018, 83, 32–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thavaraj, S. Human papillomavirus-associated neoplasms of the sinonasal tract and nasopharynx. Semin. Diagn. Pathol. 2016, 33, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Vermorken, J.B. Human Papillomavirus (HPV): A Criterion for Therapeutic Decision in Squamous Cell Carcinoma of the Head and Neck? Recent Results Cancer Res. 2017, 206, 137–147. [Google Scholar] [PubMed]
- Culié, D.; Garrel, R.; Viotti, J.; Schiappa, R.; Chamorey, E.; Fakhry, N.; Lallemant, B.; Vergez, S.; Dupret-Bories, A.; Dassonville, O.; et al. Impact of HPV-associated p16-expression and other clinical factors on therapeutic decision-making in patients with oropharyngeal cancer: A GETTEC multicentric study. Eur. J. Surg. Oncol. 2018, 44, 1908–1913. [Google Scholar] [CrossRef]
- Culié, D.; Lisan, Q.; Leroy, C.; Modesto, A.; Schiappa, R.; Chamorey, E.; Dassonville, O.; Poissonnet, G.; Guelfucci, B.; Bizeau, A.; et al. Oropharyngeal cancer: First relapse description and prognostic factor of salvage treatment according to p16 status, a GETTEC multicentric study. Eur. J. Cancer 2021, 143, 168–177. [Google Scholar] [CrossRef]
- Larque, A.B.; Hakim, S.; Ordi, J.; Nadal, A.; Diaz, A.; Pino, M.D.; Marimon, L.; Alobid, I.; Cardesa, A.; Also, L. High-risk human papillomavirus is transcriptionally active in a subset of sinonasal squamous cell carcinoma. Mod. Pathol. 2014, 27, 343–351. [Google Scholar] [CrossRef]
- Oliver, J.R.; Lieberman, S.M.; Tam, M.M.; Liu, C.Z.; Li, Z.; Hu, K.S.; Morris, L.G.T.; Givi, B. Human papillomavirus and survival of patients with sinonasal squamous cell carcinoma. Cancers 2020, 126, 1413–1423. [Google Scholar] [CrossRef]
- Ang, K.K.; Harris, J.; Wheeler, R.; Weber, R.; Rosenthal, D.I.; Nguyen-Tân, P.F.; Westra, W.H.; Chung, C.H.; Jordan, R.C.; Lu, C.; et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N. Engl. J. Med. 2010, 363, 24–35. [Google Scholar] [CrossRef] [Green Version]
- Cohen, E.; Coviello, C.; Menaker, S.; Martinez-Duarte, E.; Gomez, C.; Lo, K.; Kerr, D.; Franzmann, E.; Leibowitz, L.; Sargi, Z. P16 and human papillomavirus in sinonasal squamous cell carcinoma. Head Neck 2020, 42, 2021–2029. [Google Scholar] [CrossRef]
- Torabi, S.J.; Spock, T.; Cardoso, B.; Chao, J.; Morse, E.; Manes, R.P.; Judson, B.L. Margins in Sinonasal Squamous Cell Carcinoma: Predictors, Outcomes, and the Endoscopic Approach. Laryngoscope 2020, 130, E388–E396. [Google Scholar] [CrossRef] [PubMed]
- Gillison, M.L.; Trotti, A.M.; Harris, J.; Eisbruch, A.; Harari, P.M.; Adelstein, D.J.; Jordan, R.C.K.; Zhao, W.; Sturgis, E.M.; Burtness, B.; et al. Radiotherapy plus cetuximab or cisplatin in human papillomavirus-positive oro- pharyngeal cancer (NRG Oncology RTOG 1016): A randomised, multicentre, non- inferiority trial. Lancet 2019, 393, 40–50. [Google Scholar] [CrossRef]
- Moya-Plana, A.; Veyrat, M.; Honart, J.F.; de Fremicourt, K.; Alkhashnam, H.; Janot, F.; Leymarie, N.; Temam, S.; Kolb, F. Reconstruction of maxillectomy and midfacial defects using latissimus dorsi-scapular free flaps in a comprehensive cancer center. Oral Oncol. 2019, 99, 104468. [Google Scholar] [CrossRef]
- Abdelmeguid, A.S.; Teeramatwanich, W.; Roberts, D.B.; Amit, M.; Ferraroto, R.; Glisson, B.S.; Kupferman, M.E.; Su, S.Y.; Phan, J.; Garden, A.S.; et al. Neoadjuvant chemotherapy for locoregionally advanced squamous cell carcinoma of the paranasal sinuses. Cancers 2021, 127, 1788–1795. [Google Scholar] [CrossRef] [PubMed]
- Rooper, L.M.; Bishop, J.A.; Westra, W.H. Transcriptionally Active High-Risk Human Papillomavirus is Not a Common Etiologic Agent in the Malignant Transformation of Inverted Schneiderian Papillomas. Head Neck Pathol. 2017, 11, 346–353. [Google Scholar] [CrossRef] [Green Version]
- Sahnane, N.; Ottini, G.; Turri-Zanoni, M.; Furlan, D.; Battaglia, P.; Karligkiotis, A.; Albeni, C.; Cerutti, R.; Mura, E.; Chiaravalli, A.M.; et al. Comprehensive analysis of HPV infection, EGFR exon 20 mutations and LINE1 hypomethylation as risk factors for malignant transformation of sinonasal-inverted papilloma to squamous cell carcinoma. Int. J. Cancer. 2019, 144, 1313–1320. [Google Scholar] [CrossRef]
- Cabal, V.N.; Menendez, M.; Vivanco, B.; Potes-Ares, S.; Riobello, C.; Suarez-Fernandez, L.; Garcia-Marin, R.; Blanco-Lorenzo, V.; Lopez, F.; Alvarez-Marcos, C.; et al. EGFR mutation and HPV infection in sinonasal inverted papilloma and squamous cell carcinoma. Rhinology 2020, 58, 368–376. [Google Scholar] [CrossRef]
- Udager, A.M.; McHugh, J.B.; Goudsmit, C.M.; Weigelin, H.C.; Lim, M.S.; Elenitoba-Johnson, K.S.J. Human papillomavirus (HPV) and somatic EGFR mutations are essential, mutually exclusive oncogenic mechanisms for inverted sinonasal papillomas and associated sinonasal squamous cell carcinomas. Ann. Oncol. 2018, 29, 466–471. [Google Scholar] [CrossRef]


| Characteristics | Overall Cohort |
|---|---|
| Mean age (yrs) | 61 (+/−15.1) |
| Sex ratio (M/F) | 1.7 |
| Tobacco | |
| Smokers | 28 (48.2%) |
| Mean consumption (pack years) | 34.2 |
| Primary site | |
| Maxillary | 40 (69%) |
| Ethmoid | 5 (8.6%) |
| Sphenoid | 1 (1.7%) |
| Nasal cavity | 12 (20.7%) |
| TNM stage | |
| T1–T2; T3–T4 | 9 (15.5%); 49 (84.5%) |
| N+ | 16 (27.6%) |
| M+ | 1 (1.7%) |
| Treatment algorithm in curative intent (n = 55) | |
| ICT | 26 (44.8%) |
| Surgery | 43 (74.1%) |
| Adjuvant RT | 40 (70.7%) |
| CRT without surgery | 9 (15.5%) |
| Median follow-up (months) | 44.8 (+/−49.1) |
| Tumor recurrence | 20 |
| Local | 12 |
| Regional | 3 |
| Distant metastases | 1 |
| Multifocal (T+N+M) | 4 |
| Oncologic outcomes (patients treated curatively, n = 55) | |
| PFS at 2 and 5 years | 55.7%/45.5% |
| OS at 2 and 5 years | 70.2%/54.2% |
| Characteristics | HPV+ (n = 9) | HPV− (n = 50) | p |
|---|---|---|---|
| Mean age (yrs) | 48.8 | 61.1 | 0.029 |
| Sex ratio (M/F) | 1.25 | 1.78 | 0.71 |
| Tobacco | |||
| Smokers | 2 (22%) | 24 (50%) | 0.476 |
| Primary site | |||
| Maxillary | 1 (11%) | 40 (80%) | <10−4 |
| Ethmoid | None | 5 (10%) | |
| Sphenoid | None | 1 (2%) | |
| Nasal cavity | 8 (89%) | 4 (8%) | <10−4 |
| Pathologic findings | |||
| Poorly; Well-Differentiated | 3; 6 | 6/44 (12%) | 0.13 |
| Keratinizing; Non-Keratinizing | 3; 6 | 21/29 (42%) | 0.725 |
| TNM stage | |||
| T1–T2; T3–T4 | 3 (33%); 6(67%) | 6 (12%); 43 (88%) | 0.136 |
| N+ | 2 (22%) | 14 (29%) | 1 |
| M+ | None | 1 (2%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tendron, A.; Classe, M.; Casiraghi, O.; Pere, H.; Even, C.; Gorphe, P.; Moya-Plana, A. Prognostic Analysis of HPV Status in Sinonasal Squamous Cell Carcinoma. Cancers 2022, 14, 1874. https://doi.org/10.3390/cancers14081874
Tendron A, Classe M, Casiraghi O, Pere H, Even C, Gorphe P, Moya-Plana A. Prognostic Analysis of HPV Status in Sinonasal Squamous Cell Carcinoma. Cancers. 2022; 14(8):1874. https://doi.org/10.3390/cancers14081874
Chicago/Turabian StyleTendron, Alexandre, Marion Classe, Odile Casiraghi, Hélène Pere, Caroline Even, Philippe Gorphe, and Antoine Moya-Plana. 2022. "Prognostic Analysis of HPV Status in Sinonasal Squamous Cell Carcinoma" Cancers 14, no. 8: 1874. https://doi.org/10.3390/cancers14081874
APA StyleTendron, A., Classe, M., Casiraghi, O., Pere, H., Even, C., Gorphe, P., & Moya-Plana, A. (2022). Prognostic Analysis of HPV Status in Sinonasal Squamous Cell Carcinoma. Cancers, 14(8), 1874. https://doi.org/10.3390/cancers14081874






