Advanced or Metastatic Cutaneous Squamous Cell Carcinoma: The Current and Future Role of Radiation Therapy in the Era of Immunotherapy
Abstract
Simple Summary
Abstract
1. Introduction
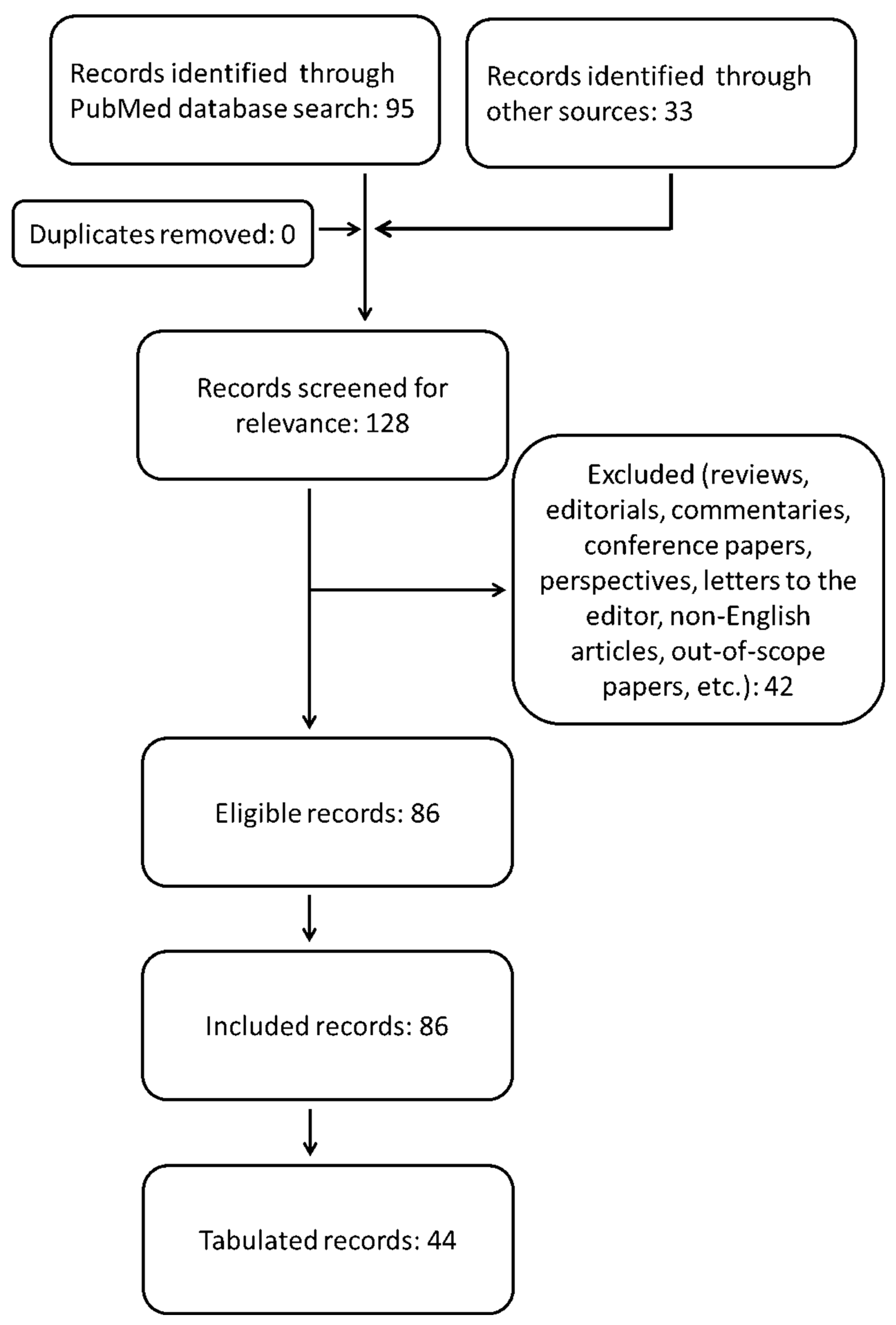
2. Methods
3. Operable Locally Advanced CSCC (LACSCC)
3.1. The Parotid and Neck Issues
3.1.1. The Patterns of Relapse despite Radiotherapy
3.1.2. Facial Nerve Involvement, Positive Surgical Margins and the Debate about the Classification of the Parotid Gland as a Cervical Lymph Node Level
3.1.3. The Problem of the Occult Disease and How to Face It
3.1.4. The Therapeutic Gain by Radiotherapy over Surgery Alone, Especially in the Presence of Adverse Prognostic Factors
3.1.5. Radiotherapy in the Management of a Limited Burden of Regional Disease
3.1.6. The Effect of Adding Systemic Therapy to Radiotherapy
3.2. Radiotherapy for Non-Head and Neck cSCCs
Comments
4. Inoperable LACSCC: Definitive Radiotherapy Combined or Not with Systemic Therapies
4.1. The Combined Treatments Borrowed from the HNSCC Management
4.2. Immunotherapy or Chemotherapy Drugs to Be Associated with Radiotherapy
4.3. The Research Efforts to Maximize the Efficacy of Radiotherapy by Using New Radiosensitizers or Fully Combining with Chemotherapy and Immunotherapy
4.4. The Feasibility of Hypofractionation of Radiation Dose
4.5. Brachytherapy
Comments
5. Distantly Metastatic CSCC (M1 CSCC): Does Radiotherapy Have a Role?
Comments
6. Limitations
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| cSCC | cutaneous squamous cell carcinoma |
| BCC | basal cell carcinoma |
| RT | radiation therapy |
| HNSCC | head and neck squamous cell carcinoma |
| IT | immunotherapy |
| DSS | disease-specific survival |
| LACSCC | locally advanced cutaneous squamous cell carcinoma |
| NCCN | National Comprehensive Cancer Network |
| DSS | disease-specific survival |
| DFS | disease-free survival |
| PFS | progression-free survival |
| OS | overall survival |
| CR | complete response |
| PR | partial response |
| SD | stable disease |
| 3D-CRT | 3-dimensional conformal radiotherapy |
| IMRT | intensity-modulated radiotherapy |
| SBRT | stereotactic body radiotherapy |
| END | elective neck dissection |
| ENI | elective neck irradiation |
| EGFR | epidermal growth factor |
| PD-1 | programmed cell death protein 1 |
| PD-L1 | programmed death-ligand 1 |
| ICI | immune checkpoint inhibitors |
| FDA | food and drug administration |
| BED | biological effective dose |
| Pt | platinum-based chemotherapy |
| Cx | cetuximab |
| CRT | chemoradiotherapy |
| PET | positron emission tomography |
| RECIST | response evaluation criteria in solid tumors |
| PERCIST | positron emission tomography (PET) response criteria in solid tumors |
References
- Que, S.K.T.; Zwald, F.O.; Schmults, C.D. Cutaneous squamous cell carcinoma: Incidence, risk factors, diagnosis, and staging. J. Am. Acad. Dermatol. 2018, 78, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Bordea, C.; Wojnarowska, F.; Millard, P.R.; Doll, H.; Welsh, K.; Morris, P.J. Skin cancers in renal-transplant recipients occur more frequently than previously recognized in a temperate climate. Transplantation 2004, 77, 574–579. [Google Scholar] [CrossRef] [PubMed]
- Schmults, C.D.; Karia, P.S.; Carter, J.B.; Han, J.; Qureshi, A.A. Factors Predictive of Recurrence and Death from Cutaneous Squamous Cell Carcinoma: A 10-year, single-institution cohort study. JAMA Dermatol. 2013, 149, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Okuyama, R.; Saida, T.; Uhara, H. Platinum and anthracycline therapy for advanced cutaneous squamous cell carcinoma. Int. J. Clin. Oncol. 2012, 18, 506–509. [Google Scholar] [CrossRef]
- AIRTUM Working Group; Busco, S.; Buzzoni, C.; Mallone, S.; Trama, A.; Castaing, M.; Bella, F.; Amodio, R.; Bizzoco, S.; Cassetti, T.; et al. I tumori in Italia—Rapporto 2015: I tumori rari in Italia. Epidemiol Prev 2016, 40, 1–120. [Google Scholar] [CrossRef]
- Migden, M.R.; Rischin, D.; Schmults, C.D.; Guminski, A.; Hauschild, A.; Lewis, K.D.; Chung, C.H.; Hernandez-Aya, L.F.; Lim, A.M.; Chang, A.L.S.; et al. PD-1 Blockade with Cemiplimab in Advanced Cutaneous Squamous-Cell Carcinoma. N. Engl. J. Med. 2018, 379, 341–351. [Google Scholar] [CrossRef]
- Harris, B.; Pipkorn, P.; Nguyen, K.N.B.; Jackson, R.; Rao, S.; Moore, M.G.; Farwell, D.G.; Bewley, A.F. Association of Adjuvant Radiation Therapy with Survival in Patients with Advanced Cutaneous Squamous Cell Carcinoma of the Head and Neck. JAMA Otolaryngol. Neck Surg. 2019, 145, 153. [Google Scholar] [CrossRef]
- Stratigos, A.J.; Garbe, C.; Dessinioti, C.; Lebbe, C.; Bataille, V.; Bastholt, L.; Dreno, B.; Fargnoli, M.C.; Forsea, A.M.; Frenard, C.; et al. European interdisciplinary guideline on invasive squamous cell carcinoma of the skin: Part Treatment. Eur. J. Cancer 2020, 128, 83–102. [Google Scholar] [CrossRef]
- Hillen, U.; Leiter, U.; Haase, S.; Kaufmann, R.; Becker, J.; Gutzmer, R.; Terheyden, P.; Krause-Bergmann, A.; Schulze, H.-J.; Hassel, J.; et al. Advanced cutaneous squamous cell carcinoma: A retrospective analysis of patient profiles and treatment patterns—Results of a non-interventional study of the De, COG. Eur. J. Cancer 2018, 96, 34–43. [Google Scholar] [CrossRef]
- Al-Mamgani, A.; Tans, L.; Van Rooij, P.H.E.; Noever, I.; de Jong, R.B.; Levendag, P.C. Hypofractionated radiotherapy denoted as the “Christie”: An effective means of palliating patients with head and neck cancers not suitable for curative treatment. Acta Oncol. 2009, 48, 562–570. [Google Scholar] [CrossRef]
- Bonomo, P.; Desideri, I.; Loi, M.; Russo, M.L.; Olmetto, E.; Maragna, V.; Francolini, G.; Paoli, C.D.; Grassi, R.; Pezzulla, D.; et al. Elderly patients affected by head and neck squamous cell carcinoma unfit for standard curative treatment: Is de-intensified, hypofractionated radiotherapy a feasible strategy? Oral Oncol. 2017, 74, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.K.; Kelley, B.F.; Prokop, L.J.; Murad, M.H.; Baum, C.L. Risk Factors for Cutaneous Squamous Cell Carcinoma Recurrence, Metastasis, and Disease-Specific Death: A Systematic Review and Meta-analysis. JAMA Dermatol. 2016, 152, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Ferini, G.; Molino, L.; Bottalico, L.; De Lucia, P.; Garofalo, F. A small case series about safety and effectiveness of a hypofractionated electron beam radiotherapy schedule in five fractions for facial non melanoma skin cancer among frail and elderly patients. Rep. Pr. Oncol. Radiother. 2021, 26, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Migden, M.R.; Khushalani, N.I.; Chang, A.L.S.; Lewis, K.D.; Schmults, C.D.; Hernandez-Aya, L.; Meier, F.; Schadendorf, D.; Guminski, A.; Hauschild, A.; et al. Cemiplimab in locally advanced cutaneous squamous cell carcinoma: Results from an open-label, phase 2, single-arm trial. Lancet Oncol. 2020, 21, 294–305. [Google Scholar] [CrossRef]
- Tsung, I.; Worden, F.P.; Fontana, R.J. A Pilot Study of Checkpoint Inhibitors in Solid Organ Transplant Recipients with Metastatic Cutaneous Squamous Cell Carcinoma. Oncol. 2020, 26, 133–138. [Google Scholar] [CrossRef]
- Bron, L.P.; Traynor, S.J.; Msc, E.B.M.; Ms, F.C.J.O. Primary and Metastatic Cancer of the Parotid: Comparison of Clinical Behavior in 232 Cases. Laryngoscope 2003, 113, 1070–1075. [Google Scholar] [CrossRef]
- Palme, C.E.; O’Brien, C.J.; Veness, M.J.; Mc Neil, E.B.; Bron, L.P.; Morgan, G.J. Extent of Parotid Disease Influences Outcome in Patients with Metastatic Cutaneous Squamous Cell Carcinoma. Arch. Otolaryngol. Head Neck Surg. 2003, 129, 750–753. [Google Scholar] [CrossRef]
- Dona, E.; Veness, M.J.; Cakir, B.; Morgan, G.J. Metastatic cutaneous squamous cell carcinoma to the parotid: The role of surgery and adjuvant radiotherapy to achieve best outcome. ANZ J. Surg. 2003, 73, 692–696. [Google Scholar] [CrossRef]
- Turner, S.J.; Morgan, G.J.; Palme, C.E.; Veness, M.J. Metastatic cutaneous squamous cell carcinoma of the external ear: A high-risk cutaneous subsite. J. Laryngol. Otol. 2010, 124, 26–31. [Google Scholar] [CrossRef]
- Audet, N.; Palme, C.E.; Gullane, P.J.; Gilbert, R.W.; Brown, D.H.; Irish, J.; Neligan, P. Cutaneous metastatic squamous cell carcinoma to the parotid gland: Analysis and outcome. Head Neck 2004, 26, 727–732. [Google Scholar] [CrossRef]
- Southwell, K.E.; Chaplin, J.M.; Eisenberg, R.L.; Mc Ivor, N.P.; Morton, R.P. Effect of immunocompromise on metastatic cutaneous squamous cell carcinoma in the parotid and neck. Head Neck 2006, 28, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Howle, J.R.; Morgan, G.J.; Kalnins, I.; Palme, C.E.; Veness, M.J. Metastatic cutaneous squamous cell carcinoma of the scalp. ANZ J. Surg. 2008, 78, 449–453. [Google Scholar] [CrossRef] [PubMed]
- Pramana, A.; Browne, L.; Graham, P.H. Metastatic cutaneous squamous cell carcinoma to parotid nodes: The role of bolus with adjuvant radiotherapy. J. Med Imaging Radiat. Oncol. 2012, 56, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Ch’Ng, S.; Maitra, A.; Allison, R.S.; Chaplin, J.M.; Gregor, R.T.; Lea, R.; Tan, S.T. Parotid and cervical nodal status predict prognosis for patients with head and neck metastatic cutaneous squamous cell carcinoma. J. Surg. Oncol. 2008, 98, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Iyer, N.G.; Clark, J.R.; Murali, R.; Gao, K.; O’Brien, C.J. Outcomes following parotidectomy for metastatic squamous cell carcinoma with microscopic residual disease: Implications for facial nerve preservation. Head Neck 2009, 31, 21–27. [Google Scholar] [CrossRef]
- Oddone, N.; Morgan, G.J.; Palme, C.E.; Perera, L.; Shannon, J.; Wong, E.; Gebski, V.; Veness, M.J. Metastatic cutaneous squamous cell carcinoma of the head and neck: The Immunosuppression, Treatment, Extranodal spread, and Margin status (ITEM) prognostic score to predict outcome and the need to improve survival. Cancer 2009, 115, 1883–1891. [Google Scholar] [CrossRef]
- Forest, V.-I.; Clark, J.J.; Veness, M.J.; Milross, C. N1S3: A revised staging system for head and neck cutaneous squamous cell carcinoma with lymph node metastases: Results of 2 Australian Centers. Cancer 2010, 116, 1298–1304. [Google Scholar] [CrossRef]
- Kirke, D.N.; Porceddu, S.; Wallwork, B.D.; Panizza, B.; Coman, W.B. Pathologic Occult Neck Disease in Patients with Metastatic Cutaneous Squamous Cell Carcinoma to the Parotid. Head Otolaryngol. Neck Surg. 2011, 144, 549–551. [Google Scholar] [CrossRef]
- Rotman, A.; Kerr, S.J.; Giddings, C.E.B. Elective neck dissection in metastatic cutaneous squamous cell carcinoma to the parotid gland: A systematic review and meta-analysis. Head Neck 2019, 41, 1131–1139. [Google Scholar] [CrossRef]
- Coombs, A.C.; Butler, A.; Allison, R. Metastatic cutaneous squamous cell carcinoma of the parotid gland: Prognostic factors. J. Laryngol. Otol. 2018, 132, 264–269. [Google Scholar] [CrossRef]
- Hirshoren, N.; Ruskin, O.; Mc Dowell, L.J.; Magarey, M.; Kleid, S.; Dixon, B.J. Management of Parotid Metastatic Cutaneous Squamous Cell Carcinoma: Regional Recurrence Rates and Survival. Otolaryngol. Neck Surg. 2018, 159, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.T.; Palme, C.E.; Morgan, G.J.; Gebski, V.; Wang, A.Y.; Veness, M.J. Predictors of outcome in patients with metastatic cuta-neous head and neck squamous cell carcinoma involving cervical lymph nodes: Improved survival with the addition of adjuvant radiotherapy. Head Neck 2012, 34, 1524–1528. [Google Scholar] [CrossRef] [PubMed]
- Sahovaler, A.; Krishnan, R.J.; Yeh, D.H.; Zhou, Q.; Palma, D.; Fung, K.; Yoo, J.; Nichols, A.; Mac Neil, S.D. Outcomes of Cutaneous Squamous Cell Carcinoma in the Head and Neck Region with Regional Lymph Node Metastasis: A Systematic Review and Meta-analysis. JAMA Otolaryngol. Neck Surg. 2019, 145, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.A.; Virk, S.; Palme, C.E.; Low, T.; Ch’Ng, S.; Gupta, R.; Gao, K.; Clark, J. Age is not a predictor of prognosis in metastatic cutaneous squamous cell carcinoma of the head and neck. ANZ J. Surg. 2016, 88, E273–E277. [Google Scholar] [CrossRef]
- Ferini, G.; Tripoli, A.; Umina, V.; Borzì, G.R.; Marchese, V.A.; Illari, S.I.; Cacciola, A.; Lillo, S.; Parisi, S.; Valenti, V. Radiation Proctitis: The Potential Role of Hyaluronic Acid in the Prevention and Restoration of Any Damage to the Rectal Mucosa among Prostate Cancer Patients Submitted to Curative External Beam Radiotherapy. Gastroenterol. Insights 2021, 12, 446–455. [Google Scholar] [CrossRef]
- Ferini, G.; Cacciola, A.; Parisi, S.; Lillo, S.; Molino, L.; Tamburella, C.; Davi, V.; Napoli, I.; Platania, A.; Settineri, N.; et al. Curative Radiotherapy in Elderly Patients with Muscle Invasive Bladder Cancer: The Prognostic Role of Sarcopenia. In Vivo 2021, 35, 571–578. [Google Scholar] [CrossRef]
- Sood, A.; Wykes, J.; Roshan, D.; Wang, L.Y.; Mc Guinness, J.; Forstner, D.; Fowler, A.; Lee, M.; Kernohan, M.; Ngo, Q.; et al. Number of nodal metastases and prognosis in metastatic cutaneous squamous cell carcinoma of the head and neck. ANZ J. Surg. 2019, 89, 863–867. [Google Scholar] [CrossRef]
- Wilkie, M.D.; Chudek, D.A.; Flynn, C.D.; Gaskell, P.; Loh, C.; Tandon, S.; Roland, N.J.; Jones, T.M.; Lancaster, J. Outcomes and prognosticators in regionally recurrent cutaneous squamous cell carcinoma of the head and neck. Eur. J. Surg. Oncol. (EJSO) 2020, 46, 2035–2041. [Google Scholar] [CrossRef]
- Wong, W.K.; Morton, R.P. Elective management of cervical and parotid lymph nodes in stage N0 cutaneous squamous cell carcinoma of the head and neck: A decision analysis. European Archives of Oto-Rhino-Laryngology 2014, 271, 3011–3019. [Google Scholar] [CrossRef]
- Kadakia, S.; Ducic, Y.; Marra, D.; Saman, M. The role of elective superficial parotidectomy in the treatment of temporal region squamous cell carcinoma. Oral Maxillofac. Surg. 2015, 20, 143–147. [Google Scholar] [CrossRef]
- Kampel, L.; Dorman, A.; Horovitz, G.; Warshavsky, A.; Gutfeld, O.; Muhanna, N. The role of parotidectomy for advanced cutaneous squamous cell carcinoma of the head and neck. Eur. Arch. Oto-Rhino-Laryngol. 2021, 278, 3955–3963. [Google Scholar] [CrossRef] [PubMed]
- Kampel, L.; Dorman, A.; Horowitz, G.; Fliss, D.M.; Gutfeld, O.; Muhanna, N. Surgically Treated Advanced Cutaneous Squamous Cell Carcinoma of the Head and Neck: Outcome Predictors and the Role of Adjuvant Radiation Therapy. Ann. Otol. Rhinol. Laryngol. 2021, 130, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Hazim, A.Z.; Reed, C.T.; Price, K.A.; Foote, R.L.; Ma, D.J.; Neben-Wittich, M.; De Lone, D.R.; Ms, S.M.J.; Bs, C.Y.S.; Mbbs, A.V.C. Survival outcomes in locally advanced cutaneous squamous cell carcinoma presenting with clinical perineural invasion alone. Head Neck 2021, 43, 1995–2001. [Google Scholar] [CrossRef]
- Trosman, S.J.; Zhu, A.; Nicolli, E.A.; Leibowitz, J.M.; Sargi, Z.B. High-Risk Cutaneous Squamous Cell Cancer of the Head and Neck: Risk Factors for Recurrence and Impact of Adjuvant Treatment. Laryngoscope 2021, 131. [Google Scholar] [CrossRef]
- Porceddu, S.V.; Bressel, M.; Poulsen, M.G.; Stoneley, A.; Veness, M.J.; Kenny, L.M.; Wratten, C.; Corry, J.; Cooper, S.; Fogarty, G.B.; et al. Postoperative Concurrent Chemoradiotherapy Versus Postoperative Radiotherapy in High-Risk Cutaneous Squamous Cell Carcinoma of the Head and Neck: The Randomized Phase III TROG 05.01 Trial. J. Clin. Oncol. 2018, 36, 1275–1283. [Google Scholar] [CrossRef] [PubMed]
- Sweeny, L.; Do, N.R.D.; Magnuson, J.S.; Carroll, W.R.; Ms, E.E.H.; Bs, S.O.H.; Desmond, R.L.; Rosenthal, E.L. EGFR expression in advanced head and neck cutaneous squamous cell carcinoma. Head Neck 2012, 34, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Heath, C.H.; Deep, N.L.; Nabell, L.; Carroll, W.R.; Desmond, R.; Clemons, L.; Spencer, S.; Magnuson, J.S.; Rosenthal, E.L. Phase 1 Study of Erlotinib Plus Radiation Therapy in Patients with Advanced Cutaneous Squamous Cell Carcinoma. Int. J. Radiat. Oncol. 2013, 85, 1275–1281. [Google Scholar] [CrossRef] [PubMed]
- Goh, A.; Howle, J.; Hughes, M.; Veness, M.J. Managing patients with cutaneous squamous cell carcinoma metastatic to the axilla or groin lymph nodes. Australas. J. Dermatol. 2009, 51, 113–117. [Google Scholar] [CrossRef]
- Fogarty, G.; Cassumbhoy, R.; Martin, J.; Fay, M.; Ainslie, J.; Martin, J. Technique for axillary radiotherapy using computer-assisted planning for high-risk skin cancer. Australas. Radiol. 2007, 51, 267–275. [Google Scholar] [CrossRef]
- Yang, P.F.; Veness, M.J.; Cooper, E.A.; Fox, R.; Smee, R.I.; Lehane, C.; Crowe, P.J.; Howle, J.R.; Thompson, S.R. Outcomes of patients with metastatic cutaneous squamous cell carcinoma to the axilla: A multicentre cohort study. ANZ J. Surg. 2021, 91, 878–884. [Google Scholar] [CrossRef]
- Veness, M.J.; Morgan, G.J.; Palme, C.E.; Gebski, V. Surgery and Adjuvant Radiotherapy in Patients with Cutaneous Head and Neck Squamous Cell Carcinoma Metastatic to Lymph Nodes: Combined Treatment Should be Considered Best Practice. Laryngoscope 2005, 115, 870–875. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network. Available online: https://www.nccn.org/professionals/physician_gls/pdf/squamous.pdf (accessed on 10 March 2022).
- O’Brien, C.J.; Mc Neil, E.B.; Mc Mahon, J.D.; Pathak, I.; Lauer, C.S.; Jackson, M.A.B.; Mc Mahon, J.D.; Pathak, I.; Lauer, C.S.; Jackson, M.A. Significance of clinical stage, extent of surgery, and pathologic findings in metastatic cutaneous squamous carcinoma of the parotid gland. Head Neck 2002, 24, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Kacew, A.; Harris, E.J.; Lorch, J.H.; Haddad, R.I.; Chau, N.G.; Rabinowits, G.; Le Boeuf, N.R.; Schmults, C.D.; Thakuria, M.; Mac Conaill, L.E.; et al. Chromosome 3q arm gain linked to immunotherapy response in advanced cutaneous squamous cell carcinoma. Eur. J. Cancer 2019, 113, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Amoils, M.; Kim, J.; Lee, C.; Sunwoo, J.B.; Colevas, A.D.; Aasi, S.Z.; Hollmig, S.T.; Ma, Y.; Divi, V.; Divi, A.V. PD-L1 Expression and Tumor-Infiltrating Lymphocytes in High-Risk and Metastatic Cutaneous Squamous Cell Carcinoma. Otolaryngol. Neck Surg. 2018, 160, 93–99. [Google Scholar] [CrossRef]
- Kraft, S.; Gadkaree, S.K.; Deschler, D.G.; Lin, D.T.; Hoang, M.P.; Emerick, K.S. Programmed cell death ligand-1 and cytotoxic T cell infiltrates in metastatic cutaneous squamous cell carcinoma of the head and neck. Head Neck 2020, 42, 3226–3234. [Google Scholar] [CrossRef]
- Tanvetyanon, T.; Padhya, T.; Mc Caffrey, J.; Kish, J.A.; Deconti, R.C.; Trotti, A.; Rao, N.G. Postoperative concurrent chemotherapy and radiotherapy for high-risk cutaneous squamous cell carcinoma of the head and neck. Head Neck 2015, 37, 840–845. [Google Scholar] [CrossRef]
- Lu, S.M.; Lien, W.W. Concurrent Radiotherapy with Cetuximab or Platinum-based Chemotherapy for Locally Advanced Cutaneous Squamous Cell Carcinoma of the Head and Neck. Am. J. Clin. Oncol. 2018, 41, 95–99. [Google Scholar] [CrossRef]
- Samstein, R.; Ho, A.L.; Lee, N.Y.; Barker, C.A. Locally Advanced and Unresectable Cutaneous Squamous Cell Carcinoma: Outcomes of Concurrent Cetuximab and Radiotherapy. J. Ski. Cancer 2014, 2014, 1–7. [Google Scholar] [CrossRef]
- Joseph, K.; Alkaabi, K.; Warkentin, H.; Ghosh, S.; Jha, N.; Smylie, M.; Walker, J. Cetuximab-radiotherapy combination in the management of locally advanced cutaneous squamous cell carcinoma. J. Med Imaging Radiat. Oncol. 2019, 63, 257–263. [Google Scholar] [CrossRef]
- Nottage, M.K.; Lin, C.; Hughes, B.G.M.; Kenny, L.; Smith, D.D.; Houston, K.; Francesconi, A. Prospective study of definitive chemoradiation in locally or regionally advanced squamous cell carcinoma of the skin. Head Neck 2017, 39, 679–683. [Google Scholar] [CrossRef]
- Amaral, T.; Osewold, M.; Presser, D.; Meiwes, A.; Garbe, C.; Leiter, U. Advanced cutaneous squamous cell carcinoma: Real world data of patient profiles and treatment patterns. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Cowey, C.L.; Robert, N.J.; Espirito, J.L.; Davies, K.; Frytak, J.; Lowy, I.; Fury, M.G. Clinical outcomes among unresectable, locally advanced, and metastatic cutaneous squamous cell carcinoma patients treated with systemic therapy. Cancer Med. 2020, 9, 7381–7387. [Google Scholar] [CrossRef] [PubMed]
- Ogata, D.; Namikawa, K.; Otsuka, M.; Asai, J.; Kato, H.; Yasuda, M.; Maekawa, T.; Fujimura, T.; Kato, J.; Takenouchi, T.; et al. Systemic treatment of patients with advanced cutaneous squamous cell carcinoma: Response rates and outcomes of the regimes used. Eur. J. Cancer 2020, 127, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Perry, J.R.; Minaei, E.; Engels, E.; Ashford, B.G.; Mc Alary, L.; Clark, J.R.; Gupta, R.; Tehei, M.; Corde, S.; Carolan, M.; et al. Thulium oxide nanoparticles as radioenhancers for the treatment of metastatic cutaneous squamous cell carcinoma. Phys. Med. Biol. 2020, 65, 215018. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Ballah, T.; Nottage, M.; Hay, K.; Chua, B.; Kenny, L.; Thomas, P.; Teng, M.; Keller, J.; Le, T.; et al. A prospective study investigating the efficacy and toxicity of definitive Chemo, Radiation and Immun, Otherapy (CRIO) in locally and/or regionally advanced unresectable cutaneous squamous cell carcinoma. Radiat. Oncol. 2021, 16, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Lavaud, J.; Blom, A.; Longvert, C.; Fort, M.; Funck-Brentano, E.; Saiag, P. Pembrolizumab and concurrent hypo-fractionated radiotherapy for advanced non-resectable cutaneous squamous cell carcinoma. Eur. J. Dermatol. 2019, 29, 636–640. [Google Scholar] [CrossRef] [PubMed]
- De Felice, F.; Musio, D.; De Falco, D.; Grapulin, L.; Magnante, A.L.; Caiazzo, R.; Bulzonetti, N.; Tombolini, V. Definitive weekly hypofractionated radiotherapy in cutaneous squamous cell carcinoma: Response rates and outcomes in elderly patients unfit for surgery. Int. J. Dermatol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Toya, R.; Saito, T.; Yamaguchi, K.; Matsuyama, T.; Watakabe, T.; Matsumoto, T.; Yoshida, R.; Hirosue, A.; Murakami, D.; Orita, Y.; et al. Hypofractionated palliative volumetric modulated arc radiotherapy with the Radiation Oncology Study Group 8502 “QUAD shot” regimen for incurable head and neck cancer. Radiat. Oncol. 2020, 15, 1–8. [Google Scholar] [CrossRef]
- Fan, D.; Kang, J.J.; Fan, M.; Wang, H.; Lee, A.; Yu, Y.; Chen, L.; Tsai, C.J.; Mc Bride, S.M.; Riaz, N.; et al. Last-line local treatment with the Quad Shot regimen for previously irradiated head and neck cancers. Oral Oncol. 2020, 104, 104641. [Google Scholar] [CrossRef]
- Voruganti, I.S.; Poon, I.; Husain, Z.A.; Bayley, A.; Barnes, E.A.; Zhang, L.; Chin, L.; Erler, D.; Higgins, K.; Enepekides, D.; et al. Stereotactic body radiotherapy for head and neck skin cancer. Radiother. Oncol. 2021, 165, 1–7. [Google Scholar] [CrossRef]
- Palmisciano, P.; Ferini, G.; Ogasawara, C.; Wahood, W.; Bin Alamer, O.; Gupta, A.D.; Scalia, G.; Larsen, A.M.G.; Yu, K.; Umana, G.E.; et al. Orbital Metastases: A Systematic Review of Clinical Characteristics, Management Strategies, and Treatment Outcomes. Cancers 2021, 14, 94. [Google Scholar] [CrossRef] [PubMed]
- Whitley, M.J.; Cardones, A.R.; Craciunescu, O.I.; Kirsch, D.G. Externally applied high-dose-rate brachytherapy for deeply invasive cutaneous squamous cell carcinoma in an older patient. Pract. Radiat. Oncol. 2015, 6, e141–e144. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tagliaferri, L.; Fionda, B.; Bussu, F.; Parrilla, C.; Lancellotta, V.; Deodato, F.; Cammelli, S.; Boldrini, L.; Gambacorta, M.A.; Morganti, A.G.; et al. Interventional radiotherapy (brachytherapy) for squamous cell carcinoma of the nasal vestibule: A multidisciplinary systematic review. Eur. J. Dermatol. 2019, 29, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.M.; Dasgeb, B.; Liem, S.; Ali, A.; Harrison, A.; Finkelstein, M.; Cha, J.; Anne, R.; Greenbaum, S.; Sherwin, W.; et al. High-Dose-Rate Brachytherapy for the Treatment of Basal and Squamous Cell Carcinomas on Sensitive Areas of the Face: A Report of Clinical Outcomes and Acute and Subacute Toxicities. Adv. Radiat. Oncol. 2021, 6, 100616. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.W.; Buzurovic, I.M.; Mahal, B.V.; Hwang, W.; Oladeru, O.; O’farrell, D.A.; Harris, T.C.; Margalit, D.N.; Lam, M.; Devlin, P.M. Combined interstitial and surface high-dose-rate brachytherapy treatment of squamous cell carcinoma of the hand. J. Contemp. Brachyther. 2020, 12, 48–52. [Google Scholar] [CrossRef]
- Bellia, S.R.; Feliciani, G.; Del Duca, M.; Monti, M.; Turri, V.; Sarnelli, A.; Romeo, A.; Kelson, I.; Keisari, Y.; Popovtzer, A.; et al. Clinical evidence of abscopal effect in cutaneous squamous cell carcinoma treated with diffusing alpha emitters radiation therapy: A case report. J. Contemp. Brachyther. 2019, 11, 449–457. [Google Scholar] [CrossRef]
- Notz, G.; Cognetti, D.; Murchison, A.P.; Bilyk, J.R. Perineural Invasion of Cutaneous Squamous Cell Carcinoma Along the Zygomaticotemporal Nerve. Ophthalmic Plast. Reconstr. Surg. 2014, 30, e49–e52. [Google Scholar] [CrossRef]
- Panizza, B.J.; Redmond, M.J. Intracranial Management of Perineural Spread in the Trigeminal Nerve. J. Neurol. Surg. Part B Skull Base 2016, 77, 150–160. [Google Scholar] [CrossRef]
- Suk, S.; Shin, H.W.; Yoon, K.C.; Kim, J. Aggressive cutaneous squamous cell carcinoma of the scalp. Arch. Craniofacial Surg. 2020, 21, 363–367. [Google Scholar] [CrossRef]
- Kadakia, S.; Ducic, Y.; Marra, D.; Chan, D.; Saman, M.; Sawhney, R.; Mourad, M. Cutaneous squamous cell carcinoma of the scalp in the immunocompromised patient: Review of 53 cases. Oral Maxillofac. Surg. 2016, 20, 171–175. [Google Scholar] [CrossRef]
- Van De Vijfeijken, S.E.; Slot, M.; Strackee, S.; Becking, A.G.; De Lange, J.; Smeele, L.E.; Schreuder, W.H. Is Three-Dimensional Virtual Planning in Cranial Reconstruction for Advanced Cutaneous Squamous Cell Carcinoma of the Skull a Feasible Option? J. Craniofacial Surg. 2019, 30, 2362–2367. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Fischbein, N.J.; Murphy, J.D.; Chu, K.P.; Bavan, B.; Dieterich, S.; Hara, W.; Kaplan, M.J.; Colevas, A.D.; Le, Q.-T. Stereotactic Radiosurgery for Retreatment of Gross Perineural Invasion in Recurrent Cutaneous Squamous Cell Carcinoma of the Head and Neck. Am. J. Clin. Oncol. 2013, 36, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Pontoriero, A.; Iatì, G.; Conti, A.; Minutoli, F.; Bottari, A.; Pergolizzi, S.; De Renzis, C. Treatment of periocular basal cell carcinoma using an advanced stereotactic device. Anticancer Res. 2014, 34, 873–875. [Google Scholar] [PubMed]
- Ferini, G.; Valenti, V.; Puliafito, I.; Illari, S.I.; Marchese, V.A.; Borzì, G.R. Volumetric Modulated Arc Therapy Capabilities for Treating Lower-Extremity Skin Affected by Several Merkel Cell Carcinoma Nodules: When Technological Advances Effectively Achieve the Palliative Therapeutic Goal while Minimising the Risk of Potential Toxicities. Medicina 2021, 57, 1379. [Google Scholar] [CrossRef] [PubMed]
- Tagliaferri, L.; Kovács, G.; Aristei, C.; De Sanctis, V.; Barbera, F.; Morganti, A.G.; Casà, C.; Pieters, B.R.; Russi, E.; Livi, L.; et al. Current state of interventional radiotherapy (brachytherapy) education in Italy: Results of the INTERACTS survey. J. Contemp. Brachyther. 2019, 11, 48–53. [Google Scholar] [CrossRef]
- Argenziano, G.; Fargnoli, M.C.; Fantini, F.; Gattoni, M.; Gualdi, G.; Pastore, F.; Pellacani, G.; Quaglino, P.; Queirolo, P.; Troiani, T. Identifying candidates for immunotherapy with cemiplimab to treat advanced cutaneous squamous cell carcinoma: An expert opinion. Ther. Adv. Med Oncol. 2022, 14, 1–13. [Google Scholar] [CrossRef]
- David, J.M.; Ho, A.S.; Luu, M.; Yoshida, E.J.; Kim, S.; Mita, A.C.; Scher, K.S.; Shiao, S.L.; Tighiouart, M.; Zumsteg, Z.S. Treatment at high-volume facilities and academic centers is independently associated with improved survival in patients with locally advanced head and neck cancer. Cancer 2017, 123, 3933–3942. [Google Scholar] [CrossRef]
- Keeping, S.; Xu, Y.; Chen, C.-I.; Cope, S.; Mojebi, A.; Kuznik, A.; Konidaris, G.; Ayers, D.; Sasane, M.; Allen, R.; et al. Comparative efficacy of cemiplimab versus other systemic treatments for advanced cutaneous squamous cell carcinoma. Futur. Oncol. 2021, 17, 611–627. [Google Scholar] [CrossRef]
- Hughes, B.; Munoz-Couselo, E.; Mortier, L.; Bratland, Å.; Gutzmer, R.; Roshdy, O.; Mendoza, R.G.; Schachter, J.; Arance, A.; Grange, F.; et al. Pembrolizumab for locally advanced and recurrent/metastatic cutaneous squamous cell carcinoma (KEYNOTE-629 study): An open-label, nonrandomized, multicenter, phase II trial. Ann. Oncol. 2021, 32, 1276–1285. [Google Scholar] [CrossRef]
- Foote, M.C.; Mc Grath, M.; Guminski, A.; Hughes, B.; Meakin, J.; Thomson, D.; Zarate, D.; Simpson, F.; Porceddu, S.V. Phase II study of single-agent panitumumab in patients with incurable cutaneous squamous cell carcinoma. Ann. Oncol. 2014, 25, 2047–2052. [Google Scholar] [CrossRef]
- Gold, K.A.; Kies, M.S.; William, W.N.; Johnson, F.M.; Lee, J.J.; Glisson, B.S. Erlotinib in the treatment of recurrent or metastatic cutaneous squamous cell carcinoma: A single-arm phase 2 clinical trial. Cancer 2018, 124, 2169–2173. [Google Scholar] [CrossRef] [PubMed]
- Hanna, G.J.; Ruiz, E.S.; Le Boeuf, N.R.; Thakuria, M.; Schmults, C.D.; Decaprio, J.A.; Silk, A.W. Real-world outcomes treating patients with advanced cutaneous squamous cell carcinoma with immune checkpoint inhibitors (CPI). Br. J. Cancer 2020, 123, 1535–1542. [Google Scholar] [CrossRef] [PubMed]
- Salzmann, M.; Leiter, U.; Loquai, C.; Zimmer, L.; Ugurel, S.; Gutzmer, R.; Thoms, K.-M.; Enk, A.H.; Hassel, J.C. Programmed cell death protein 1 inhibitors in advanced cutaneous squamous cell carcinoma: Real-world data of a retrospective, multicenter study. Eur. J. Cancer 2020, 138, 125–132. [Google Scholar] [CrossRef] [PubMed]
- In, G.K.; Vaidya, P.; Filkins, A.; Hermel, D.J.; King, K.G.; Ragab, O.; Tseng, W.W.; Swanson, M.; Kokot, N.; Lang, J.E.; et al. PD-1 inhibition therapy for advanced cutaneous squamous cell carcinoma: A retrospective analysis from the University of Southern California. J. Cancer Res. Clin. Oncol. 2021, 147, 1803–1811. [Google Scholar] [CrossRef] [PubMed]
- Rischin, D.; Migden, M.R.; Lim, A.M.; Schmults, C.D.; Khushalani, N.I.; Hughes, B.G.M.; Schadendorf, D.; Dunn, L.A.; Hernandez-Aya, L.; Chang, A.L.S.; et al. Phase 2 study of cemiplimab in patients with metastatic cutaneous squamous cell carcinoma: Primary analysis of fixed-dosing, long-term outcome of weight-based dosing. J. Immunother. Cancer 2020, 8, e000775. [Google Scholar] [CrossRef]
- Tam, S.; Gajera, M.; Luo, X.; Glisson, B.S.; Ferrarotto, R.; Johnson, F.M.; Mott, F.E.; Gillison, M.L.; Lu, C.; Le, X.; et al. Cytotoxic and targeted systemic therapy in patients with advanced cutaneous squamous cell carcinoma in the head and neck. Head Neck 2021, 43, 1592–1603. [Google Scholar] [CrossRef]
- Lipson, E.J.; Naqvi, F.F.; Loss, M.J.; Schollenberger, M.D.; Pardoll, D.M.; Moore, J.; Brennan, D.C. Kidney retransplantation after anti–programmed cell death-1 (PD-1)–related allograft rejection. Am. J. Transplant. 2020, 20, 2264–2268. [Google Scholar] [CrossRef]
- Trager, M.H.; Coley, S.M.; Dube, G.; Khan, S.; Ingham, M.; Samie, F.H.; Geskin, L.J.; Mc Donnell, D.; Brouder, D.; Saenger, Y.; et al. Combination checkpoint blockade for metastatic cutaneous malignancies in kidney transplant recipients. J. Immunother. Cancer 2020, 8, e000908. [Google Scholar] [CrossRef]
- Matsushita, S.; Kawai, K.; Tada, K.; Mera, K.; Kubo, H.; Ibusuki, A.; Yoshii, N.; Kanekura, T. Metastatic cutaneous squamous cell carcinoma treated successfully with surgery, radiotherapy and S-1/cisplatin chemotherapy. J. Dermatol. 2010, 37, 666–670. [Google Scholar] [CrossRef]
- Mazzola, R.; Jereczek-Fossa, B.A.; Antognoni, P.; Di Muzio, N.; Nicosia, L.; Lancia, A.; Fazio, I.; Chiesa, S.; Osti, M.F.; Pergolizzi, S.; et al. OLIGO-AIRO: A national survey on the role of radiation oncologist in the management of OLIGO-metastatic patients on the behalf of AIRO. Med. Oncol. 2021, 38, 1–5. [Google Scholar] [CrossRef]
- Santarpia, M.; Altavilla, G.; Borsellino, N.; Girlando, A.; Mancuso, G.; Pergolizzi, S.; Piazza, D.; Pontoriero, A.; Valerio, M.R.; Gebbia, V. High-dose Radiotherapy for Oligo-progressive NSCLC Receiving EGFR Tyrosine Kinase Inhibitors: Real World Data. In Vivo 2020, 34, 2009–2014. [Google Scholar] [CrossRef] [PubMed]
- Cacciola, A.; Parisi, S.; Tamburella, C.; Lillo, S.; Ferini, G.; Molino, L.; Iatì, G.; Pontoriero, A.; Bottari, A.; Mazziotti, S.; et al. Stereotactic body radiation therapy and radiofrequency ablation for the treatment of liver metastases: How and when? Rep. Pract. Oncol. Radiother. 2020, 25, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Weissmann, T.; Höfler, D.; Hecht, M.; Semrau, S.; Haderlein, M.; Filimonova, I.; Frey, B.; Bert, C.; Lettmaier, S.; Mantsopoulos, K.; et al. Oligometastatic head and neck cancer: Which patients benefit from radical local treatment of all tumour sites? Radiat. Oncol. 2021, 16, 1–10. [Google Scholar] [CrossRef]
- Lazim, N.M.; Elliott, M.; Wykes, J.; Clark, J. Oligometastases in head and neck carcinoma and their impact on management. ANZ J. Surg. 2021, 91, 2617–2623. [Google Scholar] [CrossRef]
- Franzese, C.; Badalamenti, M.; Teriaca, A.; De Virgilio, A.; Mercante, G.; Cavina, R.; Ferrari, D.; Santoro, A.; Spriano, G.; Scorsetti, M. Metastasis-directed stereotactic body radiation therapy in the management of oligometastatic head and neck cancer. J. Cancer Res. Clin. Oncol. 2021, 147, 1307–1313. [Google Scholar] [CrossRef] [PubMed]
- Ferini, G.; Viola, A.; Valenti, V.; Tripoli, A.; Molino, L.; Marchese, V.A.; Illari, S.I.; Borzì, G.R.; Prestifilippo, A.; Umana, G.E.; et al. WHOle Brain Irradiation or STEreotactic Radiosurgery for five or more brain metastases (WHOBI-STER): A prospective comparative study of neurocognitive outcomes, level of autonomy in daily activities and quality of life. Clin. Transl. Radiat. Oncol. 2021. [Google Scholar] [CrossRef]
- Bauman, G.S.; Corkum, M.T.; Fakir, H.; Nguyen, T.K.; Palma, D.A. Ablative radiation therapy to restrain everything safely treatable (ARREST): Study protocol for a phase I trial treating polymetastatic cancer with stereotactic radiotherapy. BMC Cancer 2021, 21, 405. [Google Scholar] [CrossRef]
- La Motte, G.; Caillot, A.; Di Palma, C.; Lechapt-Zalcman, E.; Bénateau, H.; Cogez, J. Intramedullary metastasis of a cutaneous squamous cell carcinoma. Rev. Neurol. 2014, 170, 230–232. [Google Scholar] [CrossRef]
- Ferini, G.; Valenti, V.; Tripoli, A.; Illari, S.; Molino, L.; Parisi, S.; Cacciola, A.; Lillo, S.; Giuffrida, D.; Pergolizzi, S. Lattice or Oxygen-Guided Radiotherapy: What If They Converge? Possible Future Directions in the Era of Immunotherapy. Cancers 2021, 13, 3290. [Google Scholar] [CrossRef]
- Ferini, G.; Castorina, P.; Valenti, V.; Illari, I.S.; Sachpazidis, I.; Castorina Pergolizzi, S. A Novel Radiotherapeutic Approach to Treat Bulky Metastases even from Cutaneous Squamous Cell Carcinoma: Its Rationale and a Look at the Re-liability of the Linear Quadratic Model to Explain its Radiobiological Effects. Front. Oncol. 2022, 12, 809279. [Google Scholar] [CrossRef]
- Parisi, S.; Napoli, I.; Lillo, S.; Cacciola, A.; Ferini, G.; Iatì, G.; Pontoriero, A.; Tamburella, C.; Davì, V.; Pergolizzi, S. Spine eburnation in a metastatic lung cancer patient treated with immunotherapy and radiotherapy. The first case report of bystander effect on bone. J. Oncol. Pharm. Pr. 2021, 28, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, L.J.C.; Gongora, A.B.L.; Barbosa, F.G.; Dos Anjos, C.H.; Munhoz, R.R. Atypical response with bone pseudoprogression in a patient receiving nivolumab for advanced cutaneous squamous cell carcinoma. J. Immunother. Cancer 2018, 6, 130. [Google Scholar] [CrossRef] [PubMed]
- Mc Lean, L.S.; Cavanagh, K.; Hicks, R.J.; Callahan, J.; Xie, J.; Cardin, A.; Lim, A.M.; Rischin, D. FDG-PET/CT imaging for evaluating durable responses to immune check point inhibitors in patients with advanced cutaneous squamous cell carcinoma. Cancer Imaging 2021, 21, 1–6. [Google Scholar] [CrossRef]
- Duong, T.; Wong, D.; Barrett, A.; Price, H. Successful use of immunotherapy to treat advanced cutaneous squamous cell carcinoma in recessive dystrophic epidermolysis bullosa. BMJ Case Rep. 2021, 14, e238966. [Google Scholar] [CrossRef]
- Meattini, I.; Franco, P.; Belgioia, L.; Boldrini, L.; Botticella, A.; De Santis, M.C.; Marvaso, G.; Montesi, G.; Parisi, S.; Triggiani, L.; et al. Radiation therapy during the coronavirus disease 2019 (COVID-19) pandemic in Italy: A view of the nation’s young oncologists. ESMO Open. 2020, 5, e000779. [Google Scholar] [CrossRef]
- Cangkrama, M.; Wietecha, M.; Mathis, N.; Okumura, R.; Ferrarese, L.; Al-Nuaimi, D.; Antsiferova, M.; Dummer, R.; Innocenti, M.; Werner, S. A paracrine activin A–m, Dia2 axis promotes squamous carcinogenesis via fibroblast reprogramming. EMBO Mol. Med. 2020, 12, e11466. [Google Scholar] [CrossRef]
- Bordignon, P.; Bottoni, G.; Xu, X.; Popescu, A.S.; Truan, Z.; Guenova, E.; Kofler, L.; Jafari, P.; Ostano, P.; Röcken, M.; et al. Dualism of FGF and TGF-β Signaling in Heterogeneous Cancer-Associated Fibroblast Activation with ETV1 as a Critical Determinant. Cell Rep. 2019, 28, 2358–2372. [Google Scholar] [CrossRef]
| Authors Year | Study Size | Surgery Type No. Patients (Percentage) | Radiation Protocol No. Patients (Percentage) | Systemic Therapy No. Patients (Percentage) | Outcomes | Adverse Events (Grade ≥ 3) No. Patients (Percentage) | |
|---|---|---|---|---|---|---|---|
| 1 | Bron et al.—2003 [16] | 101 | Parotidectomy 101 (100%) Neck dissection 75 (74.3%) | EBRT 101 (100%) | N/A | LC 5-year 94% DSS 5-year 65% | N/A |
| 2 | Dona et al.—2003 [18] | 74 | Parotidectomy 74 (100%) Neck dissection 52 (70.3%) | EBRT Parotid 74 (100%), Neck 56 (75.7%) | N/A | LC 2-year 76%; 5-year 73% | 0 (0%) |
| 3 | Palme et al.—2003 [17] | 126 | Parotidectomy 88 (69.8%) Neck dissection 87 (69%) | EBRT 126 (100%) | N/A | LC 5-year 80% DSS 5-year 68% | N/A |
| 4 | Audet et al.—2004 [20] | 56 | Parotidectomy 44 (78.6%) Neck dissection 28 (50%) | EBRT 56 (100%) | N/A | DSS 3-year 72% Recurrence 29% | N/A |
| 5 | Southwell et al.—2006 [21] | 49 | Parotidectomy 46 (93.9%) Neck dissection 43 (87.8%) | EBRT 49 (100%) | N/A | Recurrence 56% OS 1-year 88%; 2-year 80% | N/A |
| 6 | Ch’ng et al.—2008 [24] | 170 | Parotidectomy 135 (79.4%) Neck dissection 150 (88.2%) | EBRT 170 (100%) 50–70 Gy | N/A | DFS 5-year 59% DSS 5-year 69% OS 5-year 48% Recurrence 36% | N/A |
| 7 | Howle et al.—2008 [22] | 27 | Parotidectomy 16 (59.3%) Neck dissection 29 (96.3%) | EBRT 27 (100%) 60 Gy in 30 fractions | N/A | Recurrence 48% PFS 6 months (2–29) OS 9 months (1–73) | N/A |
| 8 | Iyer et al.—2009 [25] | 176 | Parotidectomy 176 (100%) Neck dissection 136 (77.3%) | EBRT 176 (100%) 54 Gy (45–66) in 27 fr | N/A | LC 5-year 80% OS 5-year 60% | N/A |
| 9 | Oddone et al.—2009 [26] | 250 | Parotidectomy 152 (61%) Neck dissection 223 (89.2%) | EBRT 250 (100%) 60 Gy (50–74) in 30 fr | N/A | Recurrence 28% PFS 8 months (2–34) | N/A |
| 10 | Forest et al.—2010 [27] | 215 | Parotidectomy 198 (92.1%) Neck dissection 166 (77.2%) | EBRT 215 (100%) 54 Gy parotid, 50 Gy neck | N/A | OS 2-year 82%: 5-year 69% DSS 2-year 87%; 5-year 77% LC 2-year 81%; 5-year 73% | N/A |
| 11 | Goh et al.—2010 [48] | 26 | N/A | EBRT 26 (100%) 50 Gy (45–66) | Chemotherapy 2 (7.7%) | Recurrence 27% PFS 2.2 months (0.5–14.1) OS 18.5 months (0.5–74.5) | N/A |
| 12 | Turner et al.—2010 [19] | 43 | Parotidectomy 36 (83.7%) Neck dissection 35 (81.4%) | EBRT 43 (100%) 60 Gy (36–74) in 30 fr | N/A | Recurrence 35% PFS 5 months (4–20) OS 13 months (4–89) | N/A |
| 13 | Kirke et al.—2011 [28] | 51 | Parotidectomy 51 (100%) Neck dissection 34 (66.7%) | EBRT 51 (100%) 60 Gy in 30 fr | N/A | Recurrence 17.6% | N/A |
| 14 | Pramana et al.—2012 [23] | 75 | Parotidectomy 28 (37%) Neck dissection 47 (63%) | EBRT 75 (100%) 60 Gy (42–70) in 28 fr BED 72 Gy (50–84) | N/A | LC 5-year 67% DSS 5-year 66% OS 5-year 52% | Dermatitis 41 (55%) ORN 4 (5%) |
| 15 | Sweeny et al.—2012 [46] | 56 | N/A | EBRT 56 (100%) | N/A | OS 2-year 64%; 5-year 56% | N/A |
| 16 | Wang et al.—2012 [32] | 122 | Neck dissection 122 (100%) | EBRT 122 (100%) 60 Gy in 30 fr | N/A | Recurrence 28% DFS 5-year 56% | N/A |
| 17 | Heath et al.—2013 [47] | 15 | Neck dissection 15 (100%) | EBRT 15 (100%) 60–66 Gy | Erlotinib 15 (100%) | OS 1-year 83%; 2-year 65% DFS 1-year 73%; 2-year 60% Recurrence 26.7% | Dermatitis 10 (67%) |
| 18 | Smith et al.—2016 [34] | 442 | N/A | EBRT 442 (100%) | N/A | Recurrence 17% | N/A |
| 19 | Hirshoren et al.—2018 [31] | 78 | Parotidectomy 78 (100%) Neck dissection 25 (32.1%) | EBRT 78 (100%) | N/A | LC 5-year 76% OS 5-year 46% | N/A |
| 20 | Porceddu et al.—2018 [45] | 310 | Neck dissection 310 (100%) | EBRT 310 (100%) 60 Gy in 30 fr | Carboplatin 153 (49.4%) | DFS 2-year 83%; 5-year 73% OS 2-year 88%; 5-year 79% | Hearing loss 17 (5.5%) ORN 10 (3.2%) Tinnitus 6 (1.9%) Neuropathy 4 (1.3%) Cataract 1 (0.3%) |
| 21 | Sood et al.—2019 [37] | 101 | Parotidectomy 78 (77.2%) Neck dissection 90 (89.1%) | EBRT 101 (100%) | N/A | Recurrence 24.8% | N/A |
| 22 | Trosman et al.—2020 [44] | 104 | N/A | EBRT 104 (100%) | Carboplatin 38 (37%) | OS 2-year 91%; 5-year 82% DFS 2-year 64%; 5-year 64% | N/A |
| 23 | Wilkie et al.—2020 [38] | 91 | Parotidectomy 71 (78%) Neck dissection 20 (22%) | EBRT 91 (100%) | N/A | Recurrence 36.3% PFS 9 months (3–38) OS 42 months (12–104) OS 5-year 43.8% DFS 5-year (36.2%) DSS 5-year 63.8% | N/A |
| 24 | Hazim et al.—2021 [43] | 21 | N/A | EBRT 21 (100%) Photon 11 (52%) 70 Gy Proton 10 (48%) 70 GyRBE | Cisplatin 10 (48%) Cetuximab 2 (10%) Cemiplimab 1 (5%) Paclitaxel 1 (5%) | Recurrence 40.8% PFS 2-year 44.5% OS 2-year 84.8% | Dermatitis 4 (19%) Thrombocytopenia 8 (38%) Mucositis 2 (9.5%) |
| 25 | Kampel et al.—2021 [42] | 74 | Parotidectomy 48 (65%) Neck dissection 63 (85%) | EBRT 74 (100%) | Chemotherapy 7 (9.5%) | OS 5-year 54.1% DFS 5-year 77% | N/A |
| 26 | Yang et al.—2021 [50] | 74 | N/A | EBRT 74 (100%) 50 Gy in 25 fr | N/A | DFS 2-year 49%; 5-year 49% OS 2-year 68%; 5-year 51% | N/A |
| Authors Year | Study Size | Radiation Protocol No. Patients (Percentage) | Systemic Therapy No. Patients (Percentage) | Outcomes | Adverse Events (Grade ≥ 3) No. Patients (Percentage) | |
|---|---|---|---|---|---|---|
| 1 | Samstein et al.—2014 [59] | 12 | EBRT 12 (100%) 60 Gy (12–80) in 30 fr | Cetuximab 12 (100%) | RR 64%; DC 91% DSS 2-year 51% OS 2-year 40% | Dermatitis 2 (16.7%) Thrombocytopenia 2 (16.7%) Mucositis 1 (8.3%) |
| 2 | Lu et al.—2015 [58] | 23 | EBRT 23 (100%) 60 Gy in 30 fr | N/A | Recurrence 12 (52%) PFS 8 months (1-31) | N/A |
| 3 | Tanvetyanon et al.—2015 [57] | 61 | EBRT 61 (100%) 60–66 Gy in 30 fr | Carboplatin or Cisplatin 61 (100%) | Recurrence 50% PS 23.5 months (7.4–39.5) | Leukopenia 3 (4.9%) Mucositis 3 (4.9%) Neurological 3 (4.9%) |
| 4 | Nottage et al.—2017 [61] | 21 | EBRT 21 (100%) 70 Gy in 35 fr | Cisplatin 21 (100%) | LC 1-year 61.9% OS 1-year 80.2% DFS 1-year 100% | Thrombocytopenia 6 (28.6%) Anemia/Fibrosis 5 (23.8%) Hearing loss 4 (19%) Leukopenia/ORN 2 (9.5%) |
| 5 | Joseph et al.—2018 [60] | 8 | EBRT 8 (100%) 55–66 Gy in 22–30 fr | Cetuximab 8 (100%) | DFS 2-year 87.5% PFS 2-year 83.3% OS 2-year 87.5% | Dermatitis 4 (50%) ACS/fatigue/mucositis 1 (12.5%) |
| 6 | Cowey et al.—2019 [63] | 82 | EBRT 82 (100%) | Carboplatin and Paclitaxel 22 (26.8%) Cetuximab 20 (24.4%) Cisplatin and 5-FU 6 (7.3%) Cisplatin 5 (6.1%) CarboP, PacliT and Cetux 5 (6.1%) CisP, Cetux and 5-FU 3 (2.7%) Other 21 (25.6%) | OS 1-year 56.1%; 2-year 30.2%; 3-year 15.6% | N/A |
| 7 | Lavaud et al.—2019 [67] | 4 | Hypofractionated EBRT 4 (100%) 26 Gy in 4 fr | Pembrolizumab 4 (100%) | PFS 14.4 months OS 15.6 months | 0 (0%) |
| 8 | Fan et al.—2020 [70] | 166 | Hypofractionated EBRT 166 (100%) Photon 92 (55%) Proton 74 (45%) 45 Gy in 12 fr | Cetuximab 32 (39%) Chemotherapy 30 (36%) Immunotherapy 11 (13%) Combination 10 (12%) | RR 66% OS 1-year 25.3% PFS 1-year 17.7% | Dysphagia 11 (6.6%) Trismus 5 (3%) Dermatitis 3 (1.8%) Mucositis/ORN/OSM 1 (0.9%) |
| 9 | Ogata et al.—2020 [64] | 130 | EBRT 62 (48%) | Carbo/Cisplatin 74 (57%) Cetuximab 5 (3.8%) Other 51 (39.2%) | PFS 5-year platinum 14%, no 22% OS 5-year platinum 29%, no 26% PFS 5-year non-RT 8%, RT 29% OS 5-year non-RT 15%, RT 42% PFS 5-year RT-plat 20%, RT-no 41% OS 5-year RT-plat 25%, RT-no 48% | Skin ulcer 3 (2.3%) Anemia/Hyponatriemia 2 (1.5%) Duodenal ulcer/Heart failure/Febrile neutropenia/Erythema multiforme 1 (0.8%) |
| 10 | De Felice et al.—2021 [68] | 18 | Ultra-hypofractionated EBRT 18 (100%) 56-64 Gy in 7–8 fr | N/A | OS 1-year 66%; 2-year 26.4% PFS 1-year 58.7%; 2-year 23.5% | 0 (0%) |
| 11 | Voruganti et al.—2021 [71] | 77 (out of 106 various skin cancers) | SBRT 106 (100%) | N/A | OS 1-year 44%; 2-year 26% PFS 1-year 60%; 2-year 44% | Dermatitis 31 (29.2%) Mucositis 1 (1%) Skin ulceration 1 (1%) Fibrosis 7 (6.6%) ORN 1 (1%) |
| Authors Year | Study Size | Radiation Protocol No. Patients (Percentage) | Systemic Therapy No. Patients (Percentage) | Outcomes | Adverse Events (Grade ≥ 3) No. Patients (Percentage) | |
|---|---|---|---|---|---|---|
| 1 | Foote et al.—2014 [91] | 16 | Previous EBRT 14 (87.5%) | Panitumumab 16 (100%) | PFS 8 months OS 11 months OS 2-year 37.5% | Dermatitis 4 (25%) Fatigue 1 (6%) |
| 2 | Gold et al.—2018 [92] | 39 | Previous EBRT 32 (82%) | Erlotinib 39 (100%) | DC 72% PFS 4.7 months (3.5–6.2) OS 13 months 88.4–20.5) OS 1-year 53%; 3-year 19% | Fatigue 4 (10%) Dermatitis 3 (8%) |
| 3 | Hanna et al.—2020 [93] | 61 | Previous EBRT 36 (59%) | Cemiplimab/Nivolumab/Pembrolizumab 61 (100%) | PFS 6-month 50.3% OS 1-year 46.1% | Gastrointestinal 5 (8.2%) Rheumatologic 4 (6.6%) Skin 2 (3.3%) Muscular 1 (1.6%) Neurologic 1 (1.6%) |
| 4 | In et al.—2020 [95] | 26 | Previous EBRT 10 (38.5%) | Cemiplimab 13 (50%) Pembrolizumab 7 (26.9%) Nivolumab 6 (23.1%) | PFS 5.4 months RR 42.3% DR 7.6 months (2.8–28.8) | DKA 2 (7.7%) Cardiomyopathy 1 (3.8%) Coagulopathy 1 (3.8%) Pneumonitis 1 (3.8%) |
| 5 | Rischin et al.—2020 [96] | 115 | Previous EBRT 88 (76.5%) | Cemiplimab 26 (100%) | DC 67.8% DR 1-year 90%) OS 1-year 80.7% | Anemia 7 (6.1%) Fatigue 4 (3.5%) Pneumonitis 3 (2.6%) Dyspnea 2 (1.7%) Rash 1 (0.9%) |
| 6 | Salzmann et al.—2020 [94] | 46 | - | Pembrolizumab 28 (61%) Nivolumab 19 (22%) Cemiplimab 8 (17%) | RR 58.7% DC 80.4% PFS 1-year 58.8%; 2-year 52.3% OS 1-year 79.3%; 2-year 67.1% | Myositis 2 (4.3%) Pneumonitis 2 (4.3%) Arthritis 1 (2.2%) Dermatitis 1 (2.2%) Thyreoiditis 1 (2.2%) |
| 7 | Hughes et al.—2021 [90] | 105 | Previous CT-RT 17 (16.2%) | Pembrolizumab 105 (100%) | RR 35.2% DC 52.4% DR 1-year 77.8% PFS 1-year 36.4% OS 1-year 48,4% | Hepatitis 2 (1.3%) Dermatitis 1 (0.6%) Fatigue 1 (0.6%) Nephritis 1 (0.6%) Pneumonitis 1 (0.6%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferini, G.; Palmisciano, P.; Forte, S.; Viola, A.; Martorana, E.; Parisi, S.; Valenti, V.; Fichera, C.; Umana, G.E.; Pergolizzi, S. Advanced or Metastatic Cutaneous Squamous Cell Carcinoma: The Current and Future Role of Radiation Therapy in the Era of Immunotherapy. Cancers 2022, 14, 1871. https://doi.org/10.3390/cancers14081871
Ferini G, Palmisciano P, Forte S, Viola A, Martorana E, Parisi S, Valenti V, Fichera C, Umana GE, Pergolizzi S. Advanced or Metastatic Cutaneous Squamous Cell Carcinoma: The Current and Future Role of Radiation Therapy in the Era of Immunotherapy. Cancers. 2022; 14(8):1871. https://doi.org/10.3390/cancers14081871
Chicago/Turabian StyleFerini, Gianluca, Paolo Palmisciano, Stefano Forte, Anna Viola, Emanuele Martorana, Silvana Parisi, Vito Valenti, Corrado Fichera, Giuseppe Emmanuele Umana, and Stefano Pergolizzi. 2022. "Advanced or Metastatic Cutaneous Squamous Cell Carcinoma: The Current and Future Role of Radiation Therapy in the Era of Immunotherapy" Cancers 14, no. 8: 1871. https://doi.org/10.3390/cancers14081871
APA StyleFerini, G., Palmisciano, P., Forte, S., Viola, A., Martorana, E., Parisi, S., Valenti, V., Fichera, C., Umana, G. E., & Pergolizzi, S. (2022). Advanced or Metastatic Cutaneous Squamous Cell Carcinoma: The Current and Future Role of Radiation Therapy in the Era of Immunotherapy. Cancers, 14(8), 1871. https://doi.org/10.3390/cancers14081871









