The Extent of Necrosis in Brain Metastases May Predict Subtypes of Primary Cancer and Overall Survival in Patients Receiving Craniotomy
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. MRI
2.3. Image Postprocessing and Analysis
2.4. Statistical Analyses
3. Results
3.1. Patient Characteristics
3.2. Most Influential Variable Predicting BM with Abundant Necrosis
3.3. Relationship between NTR and Lung Cancer Subtypes
3.4. Effect of BM Necrosis on Overall Patient Survival
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Tabouret, E.; Chinot, O.; Metellus, P.; Tallet, A.; Viens, P.; Goncalves, A. Recent trends in epidemiology of brain metastases: An overview. Anticancer Res. 2012, 32, 4655–4662. [Google Scholar] [PubMed]
- Park, K.; Bae, G.H.; Kim, W.K.; Yoo, C.J.; Park, C.W.; Kim, S.K.; Cha, J.; Kim, J.W.; Jung, J. Radiotherapy for brain metastasis and long-term survival. Sci. Rep. 2021, 11, 8046. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Song, Q.; Qasem, S.; O′Neill, S.; Lee, J.; Furdui, C.M.; Pasche, B.; Metheny-Barlow, L.; Masters, A.H.; Lo, H.W.; et al. Multi-omics analysis of brain metastasis outcomes following craniotomy. Front. Oncol. 2020, 10, 615472. [Google Scholar] [CrossRef]
- Berghoff, A.S.; Preusser, M. New developments in brain metastases. Ther. Adv. Neurol. Disord. 2018, 11, 1756286418785502. [Google Scholar] [CrossRef]
- Majigsuren, M.; Abe, T.; Kageji, T.; Matsuzaki, K.; Takeuchi, M.; Iwamoto, S.; Otomi, Y.; Uyama, N.; Nagahiro, S.; Harada, M. Comparison of brain tumor contrast-enhancement on t1-cube and 3d-spgr images. Magn. Reson. Med. Sci. 2016, 15, 34–40. [Google Scholar] [CrossRef]
- Ahn, S.J.; Chung, T.S.; Chang, J.H.; Lee, S.K. The added value of double dose gadolinium enhanced 3d t2 fluid-attenuated inversion recovery for evaluating small brain metastases. Yonsei Med. J. 2014, 55, 1231–1237. [Google Scholar] [CrossRef]
- Caivano, R.; Lotumolo, A.; Rabasco, P.; Zandolino, A.; D′Antuono, F.; Villonio, A.; Lancellotti, M.I.; Macarini, L.; Cammarota, A. 3 tesla magnetic resonance spectroscopy: Cerebral gliomas vs. Metastatic brain tumors. Our experience and review of the literature. Int. J. Neurosci. 2013, 123, 537–543. [Google Scholar] [CrossRef]
- Server, A.; Orheim, T.E.; Graff, B.A.; Josefsen, R.; Kumar, T.; Nakstad, P.H. Diagnostic examination performance by using microvascular leakage, cerebral blood volume, and blood flow derived from 3-t dynamic susceptibility-weighted contrast-enhanced perfusion mr imaging in the differentiation of glioblastoma multiforme and brain metastasis. Neuroradiology 2011, 53, 319–330. [Google Scholar]
- Atanasov, G.; Schierle, K.; Hau, H.M.; Dietel, C.; Krenzien, F.; Brandl, A.; Wiltberger, G.; Englisch, J.P.; Robson, S.C.; Reutzel-Selke, A.; et al. Prognostic significance of tumor necrosis in hilar cholangiocarcinoma. Ann. Surg. Oncol. 2017, 24, 518–525. [Google Scholar] [CrossRef]
- Warburg, O. On the origin of cancer cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Ciminera, A.K.; Jandial, R.; Termini, J. Metabolic advantages and vulnerabilities in brain metastases. Clin. Exp. Metastasis 2017, 34, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Sambade, M.J.; Prince, G.; Deal, A.M.; Trembath, D.; McKee, M.; Garrett, A.; Keith, K.; Ramirez, J.; Midkiff, B.; Blackwell, K.; et al. Examination and prognostic implications of the unique microenvironment of breast cancer brain metastases. Breast Cancer Res. Treat. 2019, 176, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Bahna, M.; Heimann, M.; Bode, C.; Borger, V.; Eichhorn, L.; Guresir, E.; Hamed, M.; Herrlinger, U.; Ko, Y.D.; Lehmann, F.; et al. Tumor-associated epilepsy in patients with brain metastases: Necrosis-to-tumor ratio forecasts postoperative seizure freedom. Neurosurg. Rev. 2021, 45, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Suh, J.H. Stereotactic radiosurgery for the management of brain metastases. N. Engl. J. Med. 2010, 362, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- Yushkevich, P.A.; Yang, G.; Gerig, G. Itk-snap: An interactive tool for semi-automatic segmentation of multi-modality biomedical images. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2016, 2016, 3342–3345. [Google Scholar] [PubMed]
- Martens, K.; Meyners, T.; Rades, D.; Tronnier, V.; Bonsanto, M.M.; Petersen, D.; Dunst, J.; Dellas, K. The prognostic value of tumor necrosis in patients undergoing stereotactic radiosurgery of brain metastases. Radiat. Oncol. 2013, 8, 162. [Google Scholar] [CrossRef]
- Fink, K.R.; Fink, J.R. Imaging of brain metastases. Surg. Neurol. Int. 2013, 4, S209–S219. [Google Scholar] [CrossRef]
- Natekin, A.; Knoll, A. Gradient boosting machines, a tutorial. Front. Neurorobot. 2013, 7, 21. [Google Scholar] [CrossRef]
- Yee, P.P.; Li, W. Tumor necrosis: A synergistic consequence of metabolic stress and inflammation. Bioessays 2021, 43, e2100029. [Google Scholar] [CrossRef]
- Hammoud, M.A.; Sawaya, R.; Shi, W.; Thall, P.F.; Leeds, N.E. Prognostic significance of preoperative mri scans in glioblastoma multiforme. J. Neurooncol. 1996, 27, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, M.; Abi-Said, D.; Fourney, D.R.; Gokaslan, Z.L.; Shi, W.; DeMonte, F.; Lang, F.F.; McCutcheon, I.E.; Hassenbusch, S.J.; Holland, E.; et al. A multivariate analysis of 416 patients with glioblastoma multiforme: Prognosis, extent of resection, and survival. J. Neurosurg. 2001, 95, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Graeber, T.G.; Osmanian, C.; Jacks, T.; Housman, D.E.; Koch, C.J.; Lowe, S.W.; Giaccia, A.J. Hypoxia-mediated selection of cells with diminished apoptotic potential in solid tumours. Nature 1996, 379, 88–91. [Google Scholar] [CrossRef] [PubMed]
- Milross, C.G.; Tucker, S.L.; Mason, K.A.; Hunter, N.R.; Peters, L.J.; Milas, L. The effect of tumor size on necrosis and polarographically measured po2. Acta Oncol. 1997, 36, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Ju, M.K.; Jeon, H.M.; Jeong, E.K.; Lee, Y.J.; Kim, C.H.; Park, H.G.; Han, S.I.; Kang, H.S. Regulation of tumor progression by programmed necrosis. Oxid. Med. Cell. Longev. 2018, 2018, 3537471. [Google Scholar] [CrossRef] [PubMed]
- Caruso, R.; Parisi, A.; Bonanno, A.; Paparo, D.; Quattrocchi, E.; Branca, G.; Scardigno, M.; Fedele, F. Histologic coagulative tumour necrosis as a prognostic indicator of aggressiveness in renal, lung, thyroid and colorectal carcinomas: A brief review. Oncol. Lett. 2012, 3, 16–18. [Google Scholar] [CrossRef] [PubMed]
- Fasano, M.; Della Corte, C.M.; Papaccio, F.; Ciardiello, F.; Morgillo, F. Pulmonary large-cell neuroendocrine carcinoma: From epidemiology to therapy. J. Thorac. Oncol. 2015, 10, 1133–1141. [Google Scholar] [CrossRef]
- Travis, W.D. Update on small cell carcinoma and its differentiation from squamous cell carcinoma and other non-small cell carcinomas. Mod. Pathol. 2012, 25 (Suppl. 1), S18–S30. [Google Scholar] [CrossRef]
- Kienast, Y.; von Baumgarten, L.; Fuhrmann, M.; Klinkert, W.E.; Goldbrunner, R.; Herms, J.; Winkler, F. Real-time imaging reveals the single steps of brain metastasis formation. Nat. Med. 2010, 16, 116–122. [Google Scholar] [CrossRef]
- Berk, B.A.; Nagel, S.; Hering, K.; Paschke, S.; Hoffmann, K.T.; Kortmann, R.D.; Gaudino, C.; Seidel, C. White matter lesions reduce number of brain metastases in different cancers: A high-resolution mri study. J. Neurooncol. 2016, 130, 203–209. [Google Scholar] [CrossRef]
- Schneider, T.; Kemmling, A.; Schroeder, J.; Pantel, K.; Glatzel, M.; Schoen, G.; Mohme, M.; Fiehler, J.; Gellissen, S. Inverse perfusion requirements of supra- and infratentorial brain metastases formation. Front. Neurol. 2018, 9, 391. [Google Scholar] [CrossRef] [PubMed]
- Da Cunha Santos, G.; Shepherd, F.A.; Tsao, M.S. Egfr mutations and lung cancer. Annu. Rev. Pathol. Mech. Dis. 2011, 6, 49–69. [Google Scholar] [CrossRef] [PubMed]
- Eichler, A.F.; Kahle, K.T.; Wang, D.L.; Joshi, V.A.; Willers, H.; Engelman, J.A.; Lynch, T.J.; Sequist, L.V. Egfr mutation status and survival after diagnosis of brain metastasis in nonsmall cell lung cancer. Neuro-Oncology 2010, 12, 1193–1199. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.J.; Kwon, H.; Yang, J.J.; Park, M.; Cha, Y.J.; Suh, S.H.; Lee, J.M. Contrast-enhanced t1-weighted image radiomics of brain metastases may predict egfr mutation status in primary lung cancer. Sci. Rep. 2020, 10, 8905. [Google Scholar] [CrossRef]
- Sperduto, P.W.; Berkey, B.; Gaspar, L.E.; Mehta, M.; Curran, W. A new prognostic index and comparison to three other indices for patients with brain metastases: An analysis of 1,960 patients in the rtog database. Int. J. Radiat. Oncol. 2008, 70, 510–514. [Google Scholar] [CrossRef]
- Sperduto, P.W.; Chao, S.T.; Sneed, P.K.; Luo, X.; Suh, J.; Roberge, D.; Bhatt, A.; Jensen, A.W.; Brown, P.D.; Shih, H.; et al. Diagnosis-specific prognostic factors, indexes, and treatment outcomes for patients with newly diagnosed brain metastases: A multi-institutional analysis of 4,259 patients. Int. J. Radiat. Oncol. Biol. Phys. 2010, 77, 655–661. [Google Scholar] [CrossRef]
- Pu, R.T.; Schott, A.F.; Sturtz, D.E.; Griffith, K.A.; Kleer, C.G. Pathologic features of breast cancer associated with complete response to neoadjuvant chemotherapy: Importance of tumor necrosis. Am. J. Surg. Pathol. 2005, 29, 354–358. [Google Scholar] [CrossRef]
- Lin, P.P.; Jaffe, N.; Herzog, C.E.; Costelloe, C.M.; Deavers, M.T.; Kelly, J.S.; Patel, S.R.; Madewell, J.E.; Lewis, V.O.; Cannon, C.P. Chemotherapy response is an important predictor of local recurrence in ewing sarcoma. Cancer Interdiscip. Int. J. Am. Cancer Soc. 2007, 109, 603–611. [Google Scholar] [CrossRef]
- Shim, B.; Jung, J.H.; Cho, H.M.; Kim, H.J.; Hong, J.H.; Kim, S.W. Neoadjuvant treatment response as a tumor necrosis grade for patients with rectal cancer. Am. Soc. Clin. Oncol. 2013, 31, e14563. [Google Scholar] [CrossRef]
- Boire, A.; Brastianos, P.K.; Garzia, L.; Valiente, M. Brain metastasis. Nat. Rev. Cancer 2020, 20, 4–11. [Google Scholar] [CrossRef]
- Zakaria, R.; Das, K.; Radon, M.; Bhojak, M.; Rudland, P.R.; Sluming, V.; Jenkinson, M.D. Diffusion-weighted mri characteristics of the cerebral metastasis to brain boundary predicts patient outcomes. BMC Med. Imaging 2014, 14, 26. [Google Scholar] [CrossRef] [PubMed]
- Jung, W.S.; Park, C.H.; Hong, C.K.; Suh, S.H.; Ahn, S.J. Diffusion-weighted imaging of brain metastasis from lung cancer: Correlation of mri parameters with the histologic type and gene mutation status. AJNR Am. J. Neuroradiol. 2018, 39, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Cheok, S.; Chung, L.K.; Ung, N.; Thill, K.; Voth, B.; Kwon, D.H.; Kim, J.H.; Kim, C.J.; Tenn, S.; et al. Characteristics and treatments of large cystic brain metastasis: Radiosurgery and stereotactic aspiration. Brain Tumor Res. Treat. 2015, 3, 1–7. [Google Scholar] [CrossRef] [PubMed]
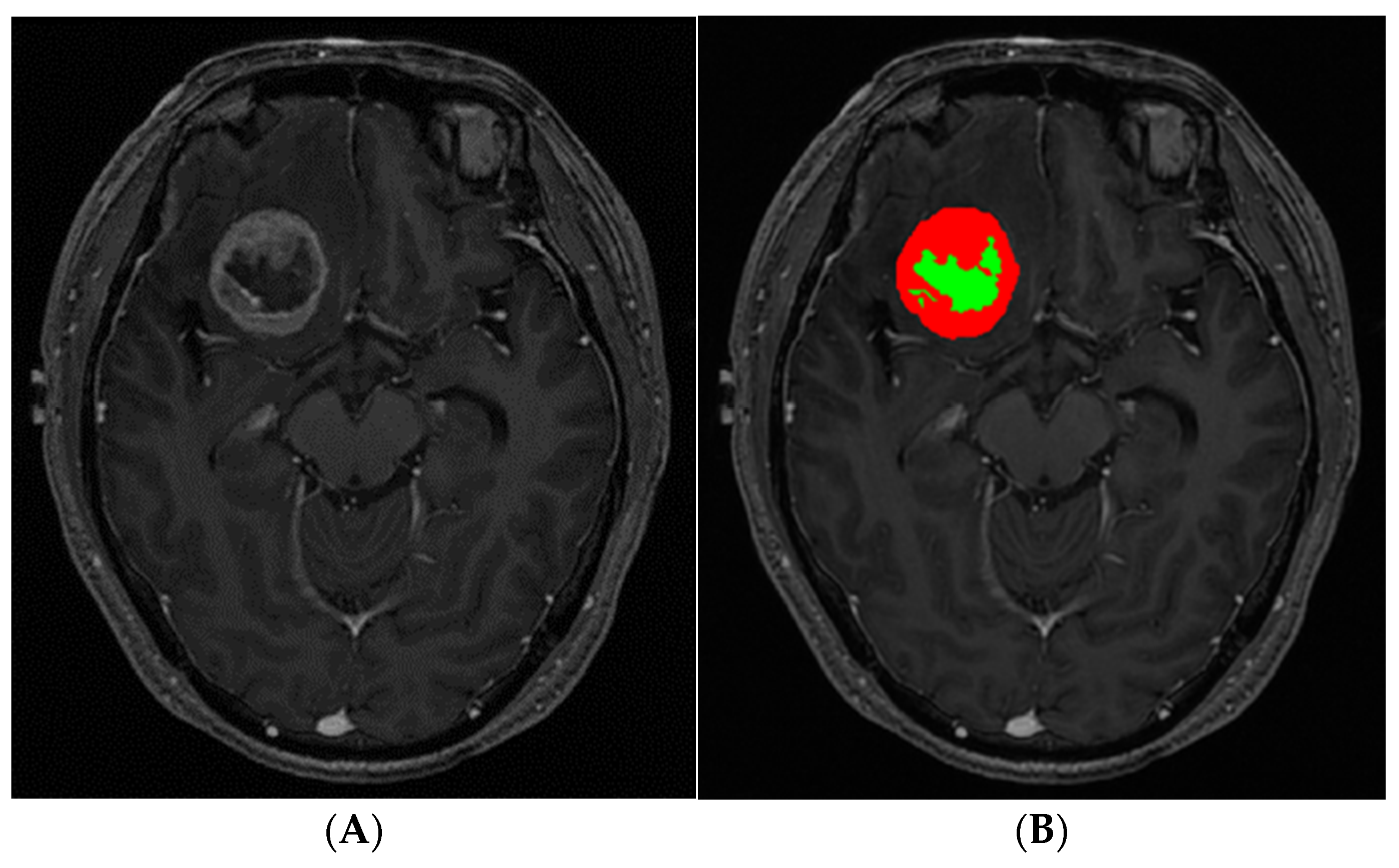
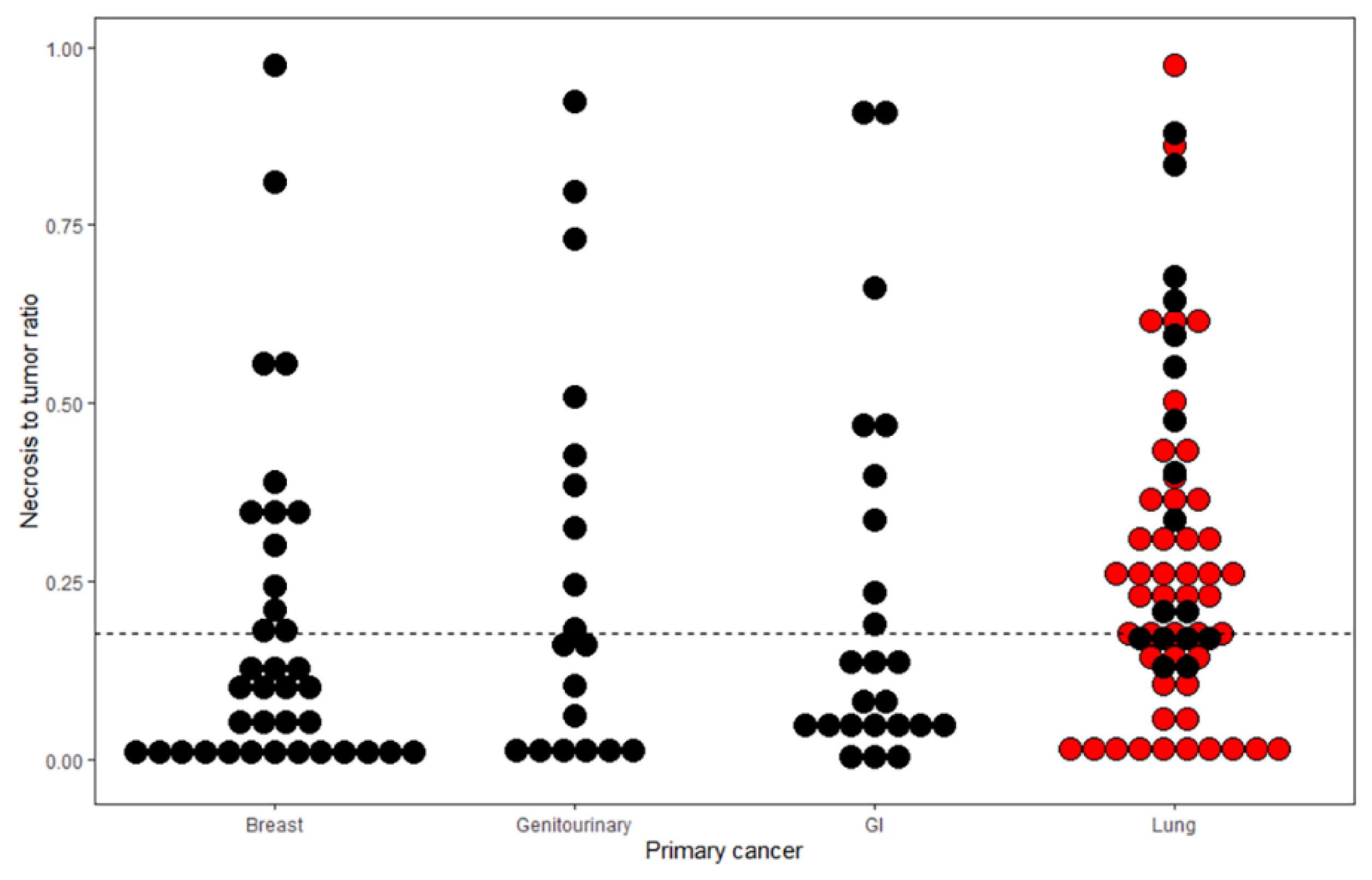
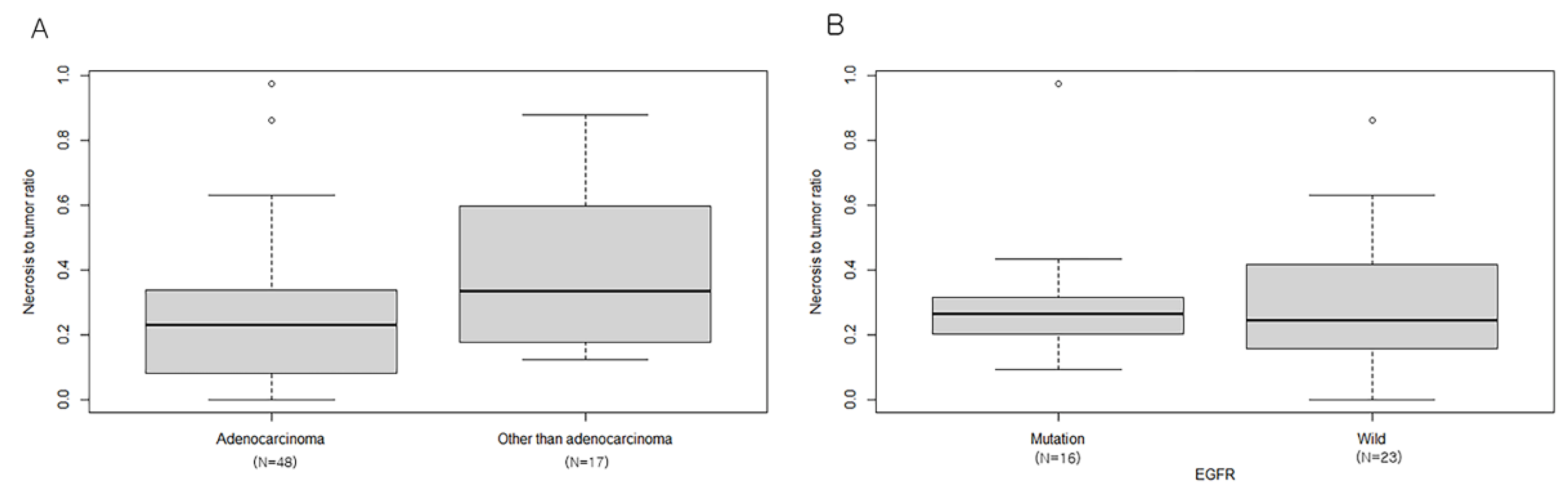
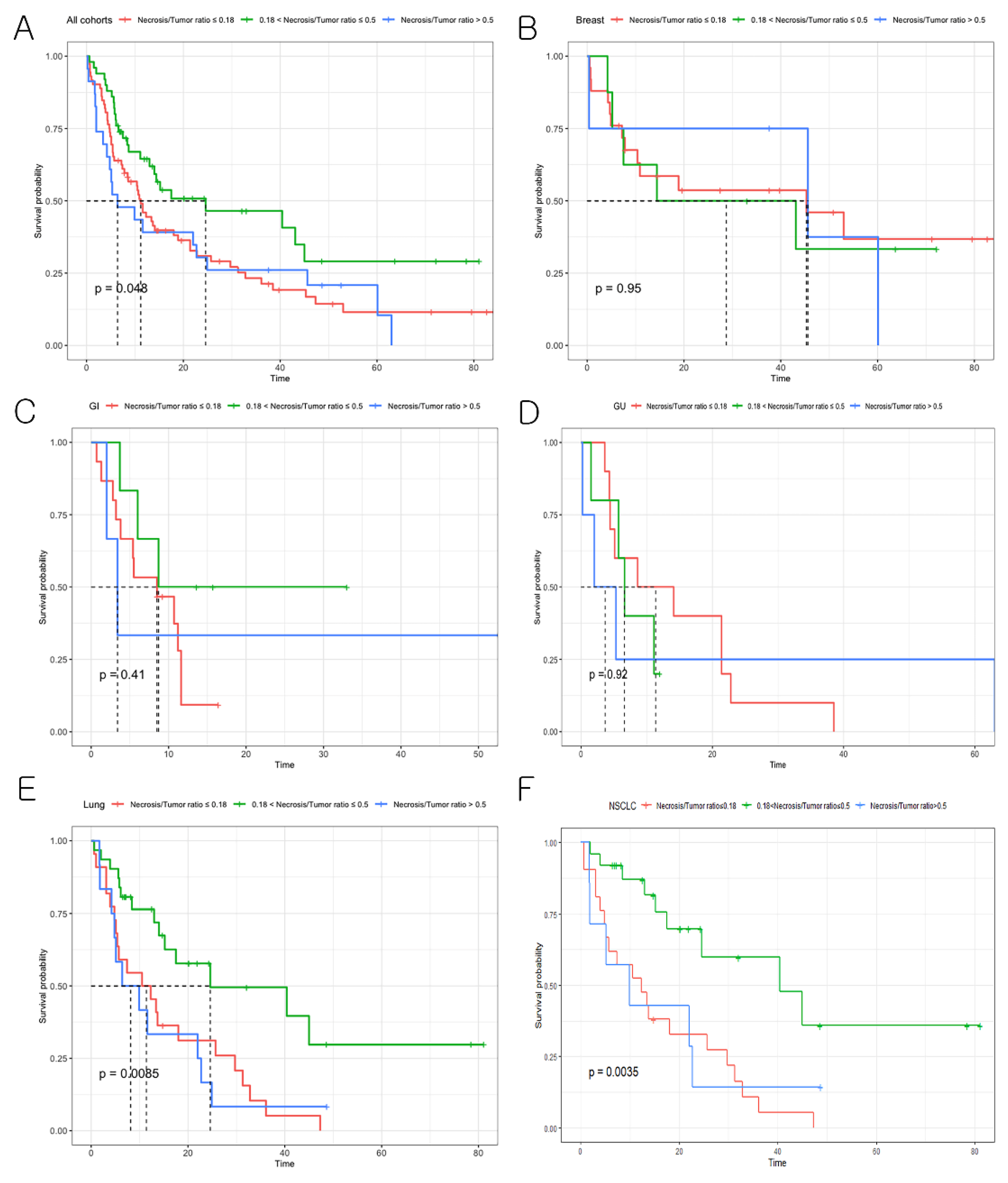
| BMs with Sparse Necrosis (n = 73) | BM with Abundant Necrosis (n = 72) | Total (n = 145) | p | |
|---|---|---|---|---|
| Primary cancer | <0.01 * | |||
| Breast | 25 (34.25%) | 12 (16.67%) | 37 (25.52%) | |
| Genitourinary | 10 (13.70%) | 9 (12.50%) | 19 (13.10%) | |
| Gastrointestinal | 15 (20.55%) | 9 (12.50%) | 24 (16.55%) | |
| Lung | 23 (31.51%) | 42 (58.33%) | 65 (44.83%) | |
| Lung vs. others | <0.01 * | |||
| Lung | 23 (31.51%) | 42 (58.33%) | 65 (44.83%) | |
| Other tumors | 50 (68.49%) | 30 (41.67%) | 80 (55.17%) | |
| Sex | 0.08 | |||
| F | 44 (60.27%) | 32 (44.44%) | 76 (52.41%) | |
| M | 29 (39.73%) | 40 (55.56%) | 69 (47.59%) | |
| Age | 57.62 ± 11.51 | 59.68 ± 12.04 | 58.64 ± 11.78 | 0.29 |
| Time interval to BM resection (months) | 33.65 ± 50.60 | 23.50 ± 26.16 | 28.61 ± 40.54 | 0.13 |
| Chemotherapy | 0.12 | |||
| No | 20 (27.78%) | 29 (41.43%) | 49 (34.51%) | |
| Yes | 52 (72.22%) | 41 (58.57%) | 93 (65.49%) | |
| BM volume (cm3) | 12.07 ± 15.77 | 25.74 ± 28.09 | 18.86 ± 23.68 | <0.01 * |
| BM location | 0.02 * | |||
| Cerebellum | 27 (36.99%) | 15 (20.83%) | 42 (28.97%) | |
| Frontal | 20 (27.40%) | 22 (30.56%) | 42 (28.97%) | |
| Occipital | 2 (2.74%) | 6 (8.33%) | 8 (5.52%) | |
| Parietal | 15 (20.55%) | 26 (36.11%) | 41 (28.28%) | |
| Temporal | 5 (6.85%) | 3 (4.17%) | 8 (5.52%) | |
| Subcortex | 4 (5.48%) | 0 (0.0%) | 4 (2.76%) | |
| Preoperative KPS | 0.46 | |||
| <70 | 10 (13.70%) | 10 (13.89%) | 20 (13.79%) | |
| 70–80 | 55 (75.34%) | 49 (68.06%) | 104 (71.72%) | |
| 90–100 | 8 (10.96%) | 13 (18.06%) | 21 (14.48%) | |
| Postoperative complication | ||||
| No | 66 (90.41%) | 67 (93.06%) | 133 (91.72%) | 0.78 |
| Yes | 7 (9.59%) | 5 (6.94%) | 12 (8.28%) |
| Odds Ratio | p-Value | |
|---|---|---|
| Primary cancer | <0.01 | |
| Breast Genitourinary Gastrointestinal cancer | 1.0 | |
| Lung cancer | 3.33 (1.49–7.69) | |
| Sex | 0.81 | |
| Female | 1.0 | |
| Male | 1.11 (0.47–2.54) | |
| Age | 1 (0.97–1.03) | 0.92 |
| Tumor volume (cm3) | 1.04 (1.02–1.07) | <0.01 |
| BM location | 0.13 | |
| Infratentorial BM | 1.0 | |
| Supratentorial BM | 1.83 (0.83–4.13) |
| Random Forest | Gradient Boosting | |
|---|---|---|
| Mean Decrease Accuracy | Relative Influence | |
| Tumor volume (cm3) | 15.81 | 65.50 |
| Lung vs. others | 13.47 | 23.51 |
| Sex | 3.12 | 1.41 |
| Chemotherapy | 3.26 | 0.96 |
| BM location | 1.36 | 3.68 |
| Time interval to BM resection (months) | −1.98 | 1.95 |
| Age | 0.31 | 2.95 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoo, J.; Cha, Y.J.; Park, H.H.; Park, M.; Joo, B.; Suh, S.H.; Ahn, S.J. The Extent of Necrosis in Brain Metastases May Predict Subtypes of Primary Cancer and Overall Survival in Patients Receiving Craniotomy. Cancers 2022, 14, 1694. https://doi.org/10.3390/cancers14071694
Yoo J, Cha YJ, Park HH, Park M, Joo B, Suh SH, Ahn SJ. The Extent of Necrosis in Brain Metastases May Predict Subtypes of Primary Cancer and Overall Survival in Patients Receiving Craniotomy. Cancers. 2022; 14(7):1694. https://doi.org/10.3390/cancers14071694
Chicago/Turabian StyleYoo, Jihwan, Yoon Jin Cha, Hun Ho Park, Mina Park, Bio Joo, Sang Hyun Suh, and Sung Jun Ahn. 2022. "The Extent of Necrosis in Brain Metastases May Predict Subtypes of Primary Cancer and Overall Survival in Patients Receiving Craniotomy" Cancers 14, no. 7: 1694. https://doi.org/10.3390/cancers14071694
APA StyleYoo, J., Cha, Y. J., Park, H. H., Park, M., Joo, B., Suh, S. H., & Ahn, S. J. (2022). The Extent of Necrosis in Brain Metastases May Predict Subtypes of Primary Cancer and Overall Survival in Patients Receiving Craniotomy. Cancers, 14(7), 1694. https://doi.org/10.3390/cancers14071694







