Metastatic Renal Medullary and Collecting Duct Carcinoma in the Era of Antiangiogenic and Immune Checkpoint Inhibitors: A Multicentric Retrospective Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
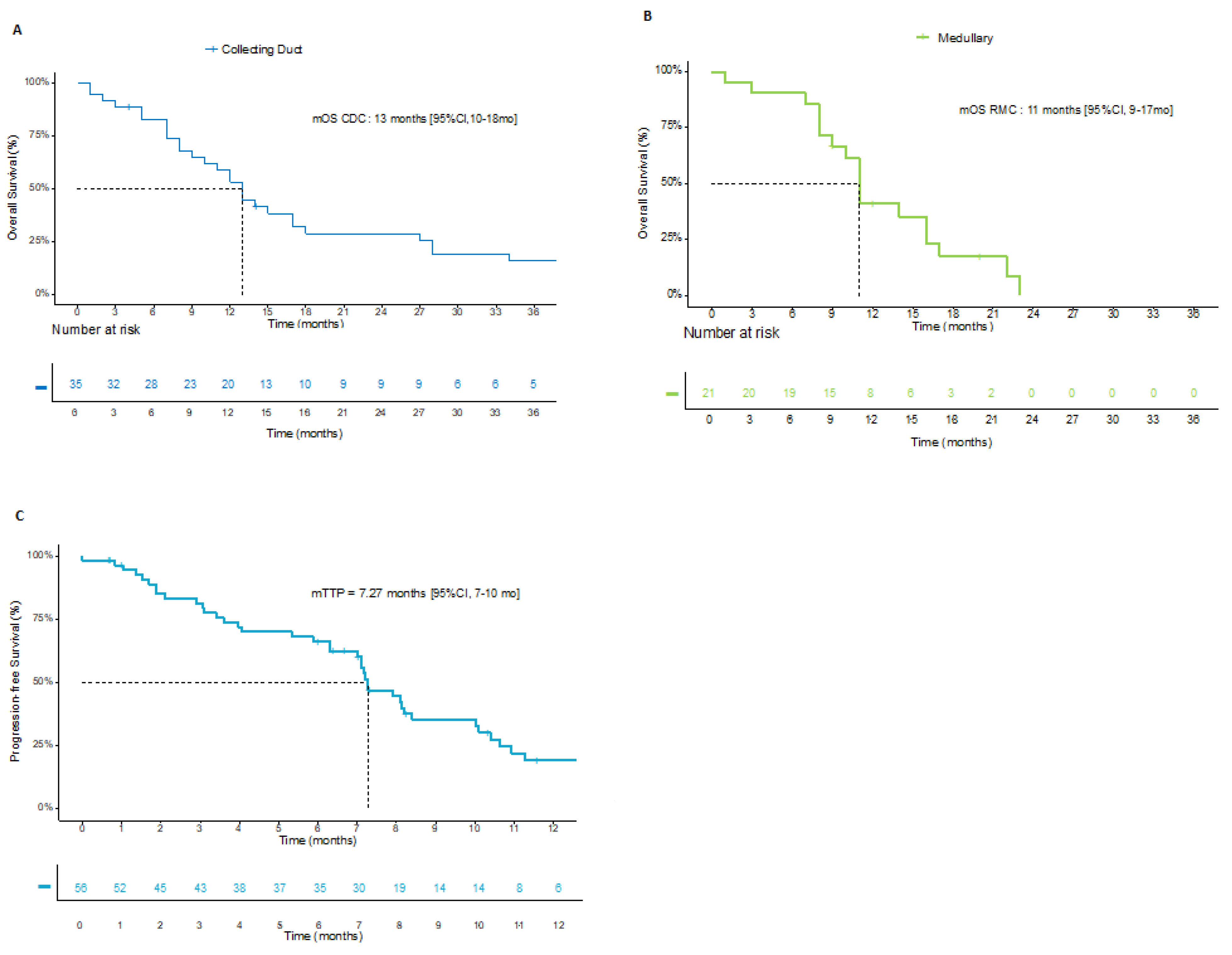
3.1. Population
3.2. First-Line Treatment
3.3. Subsequent Treatments
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Teghom, C.; Gachet, J.; Scotté, F.; ElAidi, R.; Oudard, S. La tumeur de Bellini. Bull. du Cancer 2011, 98, 1230–1232. [Google Scholar] [CrossRef]
- Abern, M.R.; Tsivian, M.; Polascik, T.J.; Coogan, C.L. Characteristics and Outcomes of Tumors Arising from the Distal Nephron. Urology 2012, 80, 140–146. [Google Scholar] [CrossRef]
- Dason, S.; Allard, C.; Sheridan–Jonah, A.; Gill, J.; Jamshaid, H.; Aziz, T.; Kajal, B.; Kapoor, A. Management of Renal Collecting Duct Carcinoma: A Systematic Review and the McMaster Experience. Curr. Oncol. 2013, 20, 223–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oudard, S.; Banu, E.; Vieillefond, A.; Fournier, L.; Priou, F.; Medioni, J.; Banu, A.; Duclos, B.; Rolland, F.; Escudier, B.; et al. Prospective Multicenter Phase II Study of Gemcitabine Plus Platinum Salt for Metastatic Collecting Duct Carcinoma: Results of a GETUG (Groupe d’Etudes des Tumeurs Uro-Génitales) Study. J. Urol. 2007, 177, 1698–1702. [Google Scholar] [CrossRef] [PubMed]
- Procopio, G.; Sepe, P.; Buti, S.; Claps, M.; Colecchia, M.; De Cecco, L.; Andrea, D.; Dugo, M.; Gargiuli, C.; Giannatempo, P.; et al. A phase 2 prospective trial of cabozantinib as first-line treatment for metastatic collecting ducts renal cell carcinoma: The BONSAI trial (Meeturo 2) clinical trial information—NCT03354884. J. Clin. Oncol. 2021, 39, 4571. [Google Scholar] [CrossRef]
- Sui, W.; Matulay, J.T.; Robins, D.J.; James, M.B.; Onyeji, I.C.; Roychoudhury, A.; Wenske, S.; DeCastro, G.J. Collecting duct carcinoma of the kidney: Disease characteristics and treatment outcomes from the National Cancer Database. Urol. Oncol. Semin. Orig. Investig. 2017, 35, 540.e13–540.e18. [Google Scholar] [CrossRef] [PubMed]
- Pepek, J.M.; Johnstone, P.A.; Jani, A.B. Influence of Demographic Factors on Outcome of Collecting Duct Carcinoma: A Surveillance, Epidemiology, and End Results (SEER) Database Analysis. Clin. Genitourin. Cancer 2009, 7, E24–E27. [Google Scholar] [CrossRef]
- Kwon, K.A.; Oh, S.Y.; Kim, H.Y.; Kim, H.S.; Lee, H.Y.; Kim, T.M.; Lim, H.Y.; Lee, N.-R.; Lee, H.J.; Hong, S.H.; et al. Clinical Features and Treatment of Collecting Duct Carcinoma of the Kidney from the Korean Cancer Study Group Genitourinary and Gynecology Cancer Committee. Cancer Res. Treat. 2014, 46, 141–147. [Google Scholar] [CrossRef]
- Mego, M.; Sycova-Mila, Z.; Rychly, B.; Obertova, J.; Rajec, J.; Hes, O.; Mardiak, J.; Rejlekova, K. Sunitinib in the treatment of tubulocystic carcinoma of the kidney. A case report. Ann. Oncol. 2008, 19, 1655–1656. [Google Scholar] [CrossRef]
- Staehler, M.; Schöppler, G.; Haseke, N.; Städler, T.; Karl, A.; Siebels, M.; Ihrler, S.; Stief, C.G. Carcinoma of the Collecting Ducts of Bellini of the Kidney: Adjuvant Chemotherapy Followed by Multikinase Inhibition with Sunitinib. Clin. Genitourin. Cancer 2009, 7, 58–61. [Google Scholar] [CrossRef]
- Mennitto, A.; Verzoni, E.; Peverelli, G.; Alessi, A.; Procopio, G. Management of Metastatic Collecting Duct Carcinoma: An Encouraging Result in a Patient Treated with Cabozantinib. Clin. Genitourin. Cancer 2018, 16, e521–e523. [Google Scholar] [CrossRef]
- Mizutani, K.; Horie, K.; Nagai, S.; Tsuchiya, T.; Saigo, C.; Kobayashi, K.; Miyazaki, T.; Deguchi, T. Response to nivolumab in metastatic collecting duct carcinoma expressing PD-L1: A case report. Mol. Clin. Oncol. 2017, 7, 988–990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yasuoka, S.; Hamasaki, T.; Kuribayashi, E.; Nagasawa, M.; Kawaguchi, T.; Nagashima, Y.; Kondo, Y. Nivolumab therapy for metastatic collecting duct carcinoma after nephrectomy: A case report. Medicine 2018, 97, e13173. [Google Scholar] [CrossRef] [PubMed]
- Rimar, K.J.; Meeks, J.J.; Kuzel, T.M. Anti-programmed Death Receptor 1 Blockade Induces Clinical Response in a Patient With Metastatic Collecting Duct Carcinoma. Clin. Genitourin. Cancer 2016, 14, e431–e434. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Billis, A.; Shah, R.B.; Moch, H.; Osunkoya, A.O.; Jochum, W.; Hes, O.; Bacchi, C.E.; De Castro, M.G.; Hansel, D.E.; et al. Carcinoma of the Collecting Ducts of Bellini and Renal Medullary Carcinoma. Am. J. Surg. Pathol. 2012, 36, 1265–1278. [Google Scholar] [CrossRef]
- Ohe, C.; Smith, S.C.; Sirohi, D.; Divatia, M.; de Peralta-Venturina, M.; Paner, G.P.; Agaimy, A.; Amin, M.B.; Argani, P.; Chen, Y.-B.; et al. Reappraisal of Morphologic Differences Between Renal Medullary Carcinoma, Collecting Duct Carcinoma, and Fumarate Hydratase–deficient Renal Cell Carcinoma. Am. J. Surg. Pathol. 2018, 42, 279–292. [Google Scholar] [CrossRef]
- Pal, S.K.; Choueiri, T.K.; Wang, K.; Khaira, D.; Karam, J.A.; Van Allen, E.; Palma, N.A.; Stein, M.N.; Johnson, A.; Squillace, R.; et al. Characterization of Clinical Cases of Collecting Duct Carcinoma of the Kidney Assessed by Comprehensive Genomic Profiling. Eur. Urol. 2016, 70, 516–521. [Google Scholar] [CrossRef]
- Wang, J.; Papanicolau-Sengos, A.; Chintala, S.; Wei, L.; Liu, B.; Hu, Q.; Miles, K.M.; Conroy, J.M.; Glenn, S.T.; Costantini, M.; et al. Collecting duct carcinoma of the kidney is associated with CDKN2A deletion and SLC family gene up-regulation. Oncotarget 2016, 7, 29901–29915. [Google Scholar] [CrossRef] [Green Version]
- Malouf, G.G.; Compérat, E.; Yao, H.; Mouawad, R.; Lindner, V.; Rioux-Leclercq, N.; Verkarre, V.; Leroy, X.; Dainese, L.; Classe, M.; et al. Unique Transcriptomic Profile of Collecting Duct Carcinomas Relative to Upper Tract Urothelial Carcinomas and other Kidney Carcinomas. Sci. Rep. 2016, 6, 30988. [Google Scholar] [CrossRef] [Green Version]
- Bronchud, M.H.; Castillo, S.; De Romaní, S.E.; Mourelo, S.; Fernández, A.; Baena, C.; Murillo, J.; Julia, J.C.; Esquius, J.; Romero, R.; et al. HER2 Blockade in Metastatic Collecting Duct Carcinoma (CDC) of the Kidney: A Case Report. Onkologie 2012, 35, 776–779. [Google Scholar] [CrossRef]
- Walsh, A.; Kelly, D.; Vaid, Y.N.; Hilliard, L.M.; Friedman, G.K. Complete response to carboplatin, gemcitabine, and paclitaxel in a patient with advanced metastatic renal medullary carcinoma. Pediatr. Blood Cancer 2010, 55, 1217–1220. [Google Scholar] [CrossRef] [PubMed]
- Gangireddy, V.G.R.; Liles, G.B.; Sostre, G.D.; Coleman, T. Response of Metastatic Renal Medullary Carcinoma to Carboplatinum and Paclitaxel Chemotherapy. Clin. Genitourin. Cancer 2012, 10, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Diao, B.; Paule, B.; Esquivel, S.; Abbou, C.; Salomon, L.; De La Taille, A. Carcinome médullaire du rein: Rapport d’une rémission sous gemcitabine-cisplatine et revue des perspectives thérapeutiques. Progrès Urol. 2010, 20, 538–541. [Google Scholar] [CrossRef] [PubMed]
- Wetmore, C.; Boyett, J.; Li, S.; Lin, T.; Bendel, A.; Gajjar, A.; Orr, B.A. Alisertib is active as single agent in recurrent atypical teratoid rhabdoid tumors in 4 children. Neuro-Oncology 2015, 17, 882–888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carugo, A.; Minelli, R.; Sapio, L.; Soeung, M.; Carbone, F.; Robinson, F.S.; Tepper, J.; Chen, Z.; Lovisa, S.; Svelto, M.; et al. p53 Is a Master Regulator of Proteostasis in SMARCB1-Deficient Malignant Rhabdoid Tumors. Cancer Cell 2019, 35, 204–220.e9. [Google Scholar] [CrossRef] [Green Version]
| Characteristic | CDC n = 35 | RMC n= 22 | Total Population n = 57 |
|---|---|---|---|
| Patient characteristics | |||
| Median age (IQR), years | 61 (52–66) | 33 (24–38) | 53 (34–63) |
| Male, n (%) | 23 (66) | 17 (77) | 40 (70) |
| Disease characteristics | |||
| Histology n (%) | |||
| CDC | 35 (100) | 0 | |
| RMC | 0 | 22 (100) | 35 (61) |
| Sickle cell traits, n (%) | 1 (3) | 15 (68) | 22 (39) |
| Missing data | 14 | 3 | 16 (28) |
| INI1 mutation, n (%) | |||
| Loss | 3 (9) | 15 (68) | 18 (32) |
| Missing data | 26 (74) | 6 (27.2) | 32 (56) |
| Metastases at diagnosis, n (%) | 21 (60) | 13 (59) | 34 (60) |
| Metastatic sites, n (%) | |||
| Lymph nodes | 28 (80) | 16 (73) | 44 (77) |
| Bones | 14 (40) | 11 (50) | 25 (44) |
| Liver | 10 (29) | 10 (45) | 20 (35) |
| Lung | 18 (51) | 14 (64) | 32 (56) |
| Other | 15 (43) | 9 (41) | 24 (42) |
| Pathologic stage at diagnosis n (%) | |||
| pT1–T2 | 5 (14) | 1 (5) | 6 (11) |
| pT3–T4 | 26 (74) | 16 (73) | 42 (74) |
| Missing data | 4 | 5 | 9 |
| Lymph node invasion, n (%) | 19 (54) | 15 (68) | 34 (60) |
| Missing data | 13 | 7 | 21 |
| Radical nephrectomy, n (%) | 27 (77) | 13 (59) | 40 (70) |
| Median number of treatment lines (IQR) | 2 (1–5) | 2.5 (1–4) | 2 (1–5) |
| 1 line (%) | 46 | 23 | 37 |
| 2 lines (%) | 23 | 27 | 24 |
| ≥3 lines (%) | 31 | 50 | 39 |
| Type of Treatment | All Platinum-Based Chemotherapies n = 57 | CG Regimen and Bevacizumab n = 29 | CG Regimen n = 19 | MVAC dd n = 9 |
|---|---|---|---|---|
| ORR, n (%) Complete response Partial response Stable disease Progressive disease Missing data | 22 (39) 1 (2) 21 (37) 16 (28) 8 (14) 8 (14) | 12 (41) 0 12 (41) 11 (38) 2 (7) 2 (7) | 5 (26) 0 5 (26) 5 (26) 4 (21) 4 (21) | 5 (56) 1 (11) 4 (44) 0 2 (22) 2 (22) |
| Type of Treatment | First-Line Therapy | Subsequent-Line Therapy | |||
|---|---|---|---|---|---|
| Platinum-Based Regimen ± Bevacizumab n = 57 | TKI a n = 12 | ICI b n = 20 | CT c n = 34 | Other Treatment d n = 6 | |
| ORR, n (%) DCR, n (%) -Complete response -Partial response -Stable disease -Progressive disease -Missing data | 22 (39) 38 (67) 1 (2) 21 (37) 16 (28) 8 (14) 8 (14) | 1 (8) 6 (50) 0 1 (8) 5 (42) 3 (25) 3 (25) | 2 (10) 6 (30) 0 2 (10) 4 (20) 14 (70) 0 | 4 (12) 8 (24) 0 4 (12) 4 (12) 20 (59) 6 (18) | 2 (33) 2 (33) 0 2 (33) 0 4 (67) 0 |
| Median duration of treatment, months | 4 | 3 | 2 | 2 | 1 |
| No. | First-Line Therapy | Best Response at First Line | Reason for First-Line Discontinuation | Second-Line Therapy | Best Response at Second Line | Reason for Second-Line Discontinuation | Third-Line Therapy | Best Response at Third Line | Reason for Third-Line Discontinuation | Overall Survival, Msonths |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Cisplatin + gemcitabine + bevacizumab | SD | End of treatment | N/A | N/A | N/A | N/A | N/A | N/A | 51 |
| 2 1 | MVAC dd | PD | Progression | Cisplatin + gemcitabine + bevacizumab | PR | End of treatment | Carboplatin + gemcitabine + bevacizumab | PR | Progression | 30 |
| 3 | Carboplatin + gemcitabine + bevacizumab | PR | End of treatment | Carboplatin + gemcitabine | PD | Progression | Nivolumab | PR | Still on treatment | 43 |
| 4 | MVAC dd | SD | End of treatment | Carboplatin + gemcitabine + bevacizumab | PR | Toxicity | N/A | N/A | N/A | 69 |
| 5 | Carboplatin + gemcitabine + bevacizumab | SD | Toxicity | Nivolumab | PD | Progression | Cabozantinib | PR | Toxicity | 104 |
| 6 2 | Cisplatin + gemcitabine + bevacizumab | Dissociated | End of treatment | Trametinib | PR | Toxicity | Trametinib + crizotinib | PR | Progression | 28 |
| 7 3 | Carboplatin + gemcitabine + bevacizumab | PR | Toxicity | Carboplatin | PD | Progression | Paclitaxel | SD | Progression | 27 |
| 8 | Carboplatin + gemcitabine + bevacizumab | SD | Toxicity | Nivolumab + ipilimumab | PD | Toxicity | Cabozantinib | SD | Progression | 34 |
| 9 | Carboplatin + gemcitabine + bevacizumab | PR | Toxicity | Nivolumab + ipilimumab | SD | Toxicity | N/A | N/A | N/A | 48 |
| 10 4 | Cisplatin + gemcitabine + bevacizumab | PR | End of treatment | Nivolumab | PR | Progression | Cabozantinib | SD | Progression | 22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guillaume, Z.; Colomba, E.; Thouvenin, J.; Saldana, C.; Campedel, L.; Dumont, C.; Laguerre, B.; Maillet, D.; Vicier, C.; Rolland, F.; et al. Metastatic Renal Medullary and Collecting Duct Carcinoma in the Era of Antiangiogenic and Immune Checkpoint Inhibitors: A Multicentric Retrospective Study. Cancers 2022, 14, 1678. https://doi.org/10.3390/cancers14071678
Guillaume Z, Colomba E, Thouvenin J, Saldana C, Campedel L, Dumont C, Laguerre B, Maillet D, Vicier C, Rolland F, et al. Metastatic Renal Medullary and Collecting Duct Carcinoma in the Era of Antiangiogenic and Immune Checkpoint Inhibitors: A Multicentric Retrospective Study. Cancers. 2022; 14(7):1678. https://doi.org/10.3390/cancers14071678
Chicago/Turabian StyleGuillaume, Zoé, Emeline Colomba, Jonathan Thouvenin, Carolina Saldana, Luca Campedel, Clément Dumont, Brigitte Laguerre, Denis Maillet, Cécile Vicier, Frédéric Rolland, and et al. 2022. "Metastatic Renal Medullary and Collecting Duct Carcinoma in the Era of Antiangiogenic and Immune Checkpoint Inhibitors: A Multicentric Retrospective Study" Cancers 14, no. 7: 1678. https://doi.org/10.3390/cancers14071678
APA StyleGuillaume, Z., Colomba, E., Thouvenin, J., Saldana, C., Campedel, L., Dumont, C., Laguerre, B., Maillet, D., Vicier, C., Rolland, F., Borchiellini, D., Barthelemy, P., Albiges, L., Auclin, E., Roulleaux Dugage, M., Oudard, S., & Thibault, C. (2022). Metastatic Renal Medullary and Collecting Duct Carcinoma in the Era of Antiangiogenic and Immune Checkpoint Inhibitors: A Multicentric Retrospective Study. Cancers, 14(7), 1678. https://doi.org/10.3390/cancers14071678







