Outcomes of Patients with Metastatic Melanoma—A Single-Institution Retrospective Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
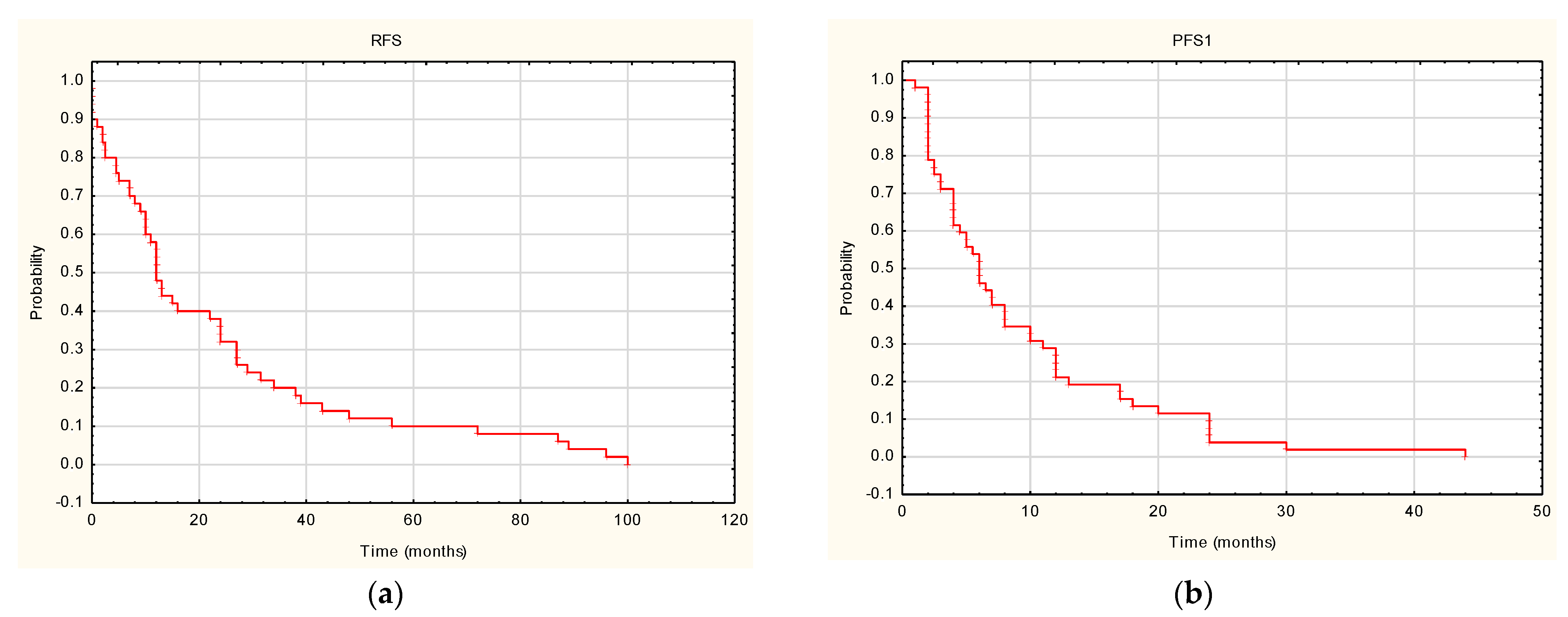
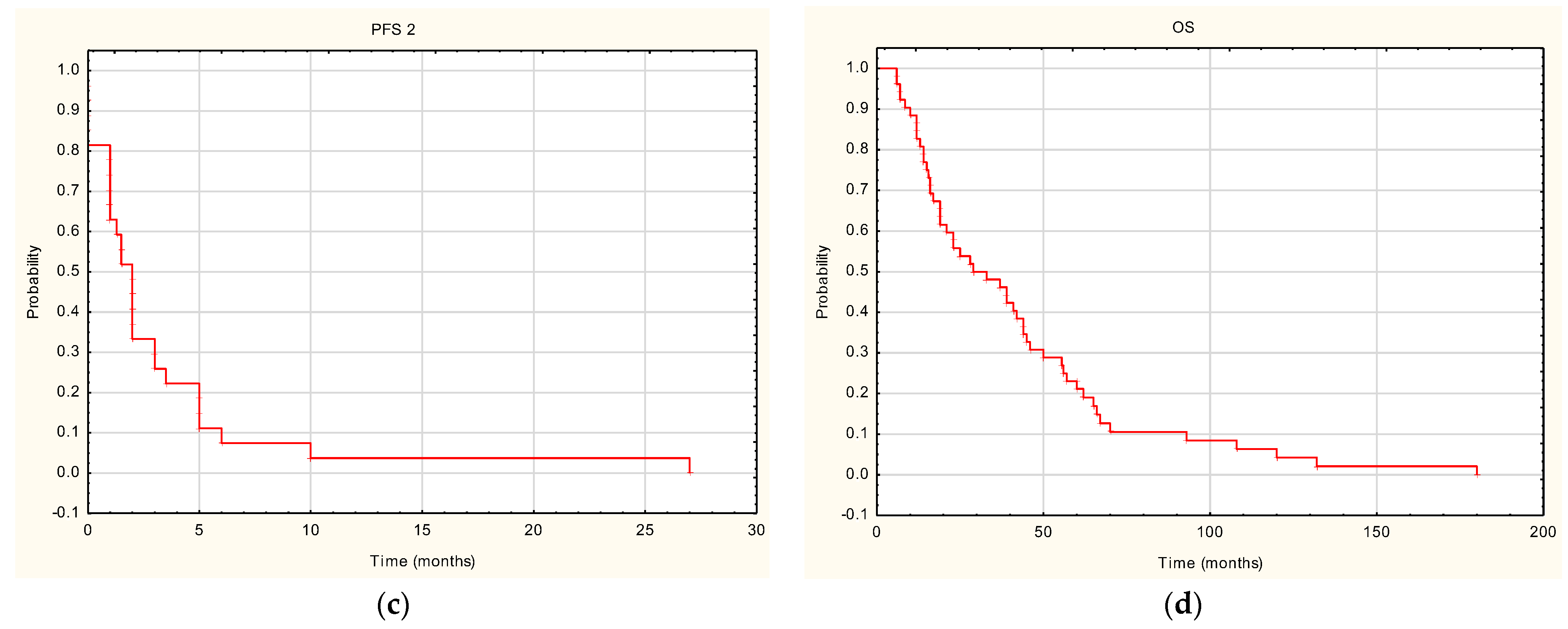
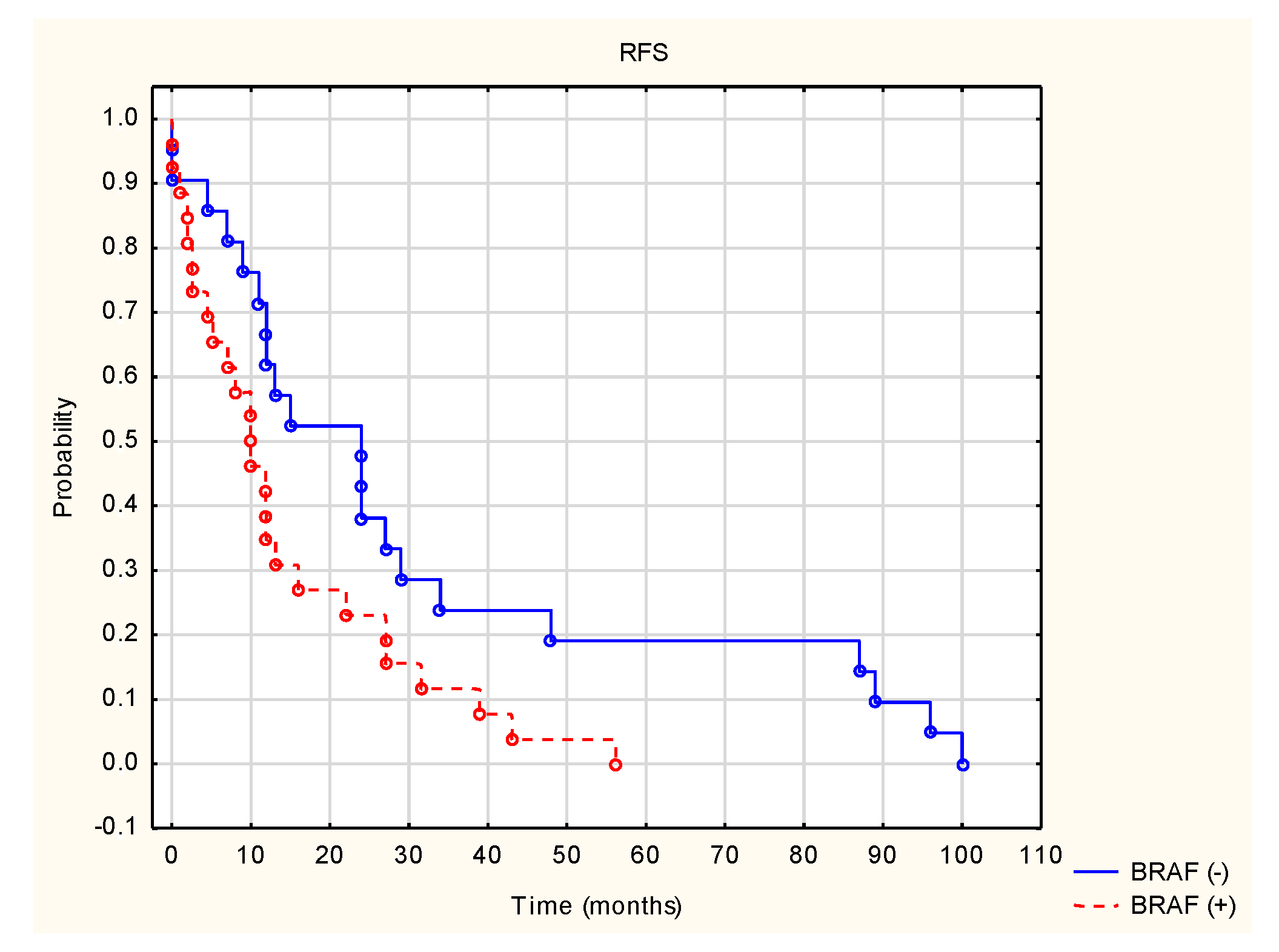
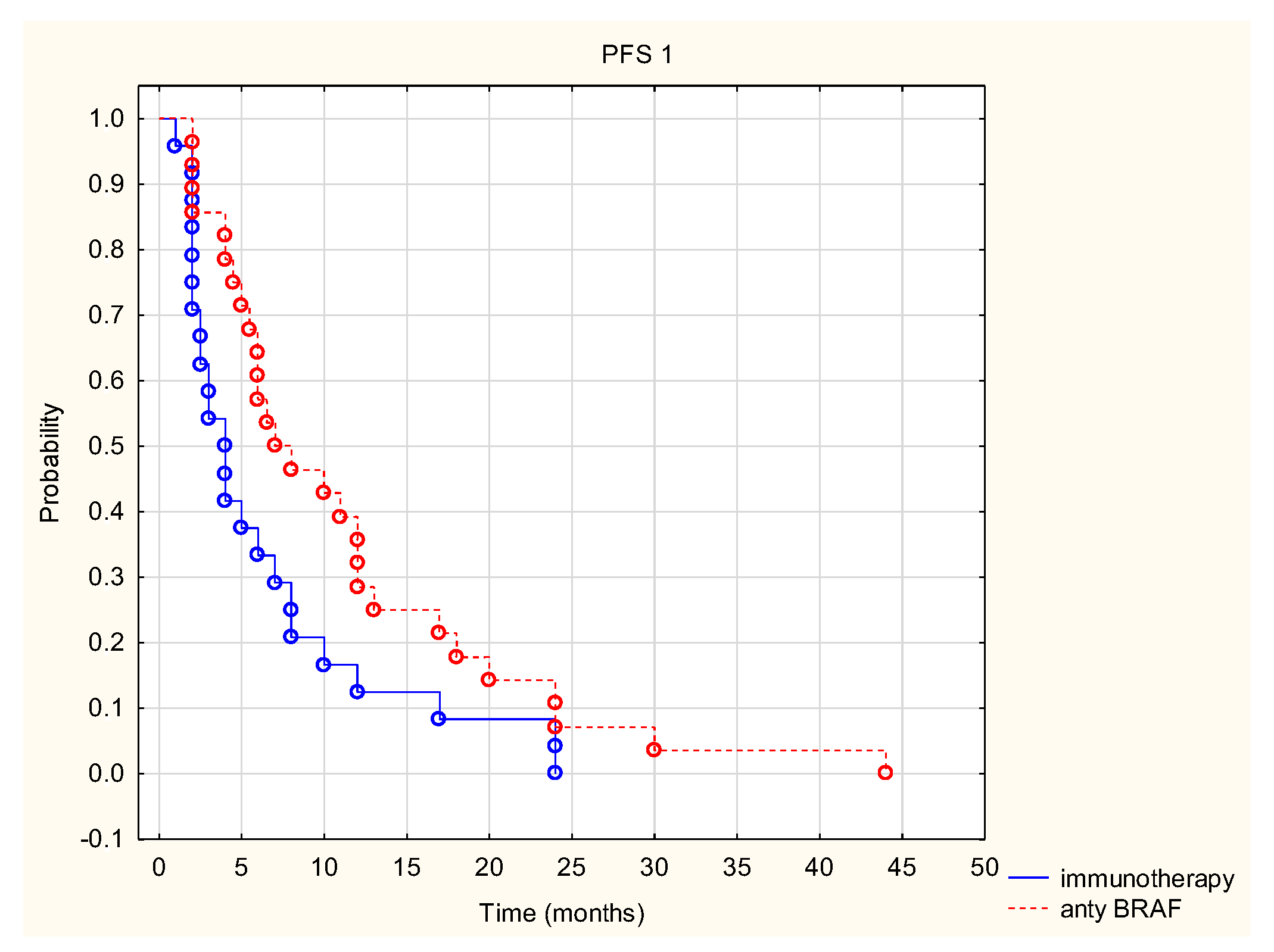
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Didkowska, J.; Wojciechowska, U.; Olasek, P. Cancers in Poland in 2015. Available online: http://onkologia.org.pl/wp-content/uploads/Nowotwory_2015.pdf (accessed on 20 December 2021).
- Rutkowski, P.; Wysocki, P.J.; Nasierowska-Guttmejer, A.; Jeziorski, A.; Wysocki, W.M.; Kalinka-Warzocha, E.; Świtaj, T.; Kozak, K.; Kamińska-Winciorek, G.; Wixniewski, P.; et al. Cutaneous melanomas. Oncol. Clin. Pract. 2020, 16, 163–182. [Google Scholar] [CrossRef]
- Didkowska, J.U.; Wojciechowska, U.; Zatoński, W. Cancer in Poland in 2009. Available online: http://onkologia.org.pl/wp-content/uploads/Nowotwory_2009.pdf (accessed on 20 December 2021).
- United States Cancer Statistics. Available online: https://gis.cdc.gov/Cancer/USCS/#/AtAGlance/ (accessed on 23 December 2021).
- Ward, W.H.; Farma, J.M. Cutaneous Melanoma: Etiology and Therapy, 1st ed.; Codon Publications: Brisbane, Australia, 2017; pp. 3–23. [Google Scholar] [CrossRef]
- Rutkowski, P.; Wysocki, P.J.; Nasierowska-Guttmejer, A.; Jeziorski, A.; Wysocki, W.M.; Kalinka-Warzocha, E.; Świtaj, T.; Kozak, K.; Kamińska-Winciorek, G.; Czarnecka, A.M.; et al. Cutaneous melanomas. Oncol. Clin. Pract. 2019, 15, 1–19. [Google Scholar] [CrossRef]
- Eggermont, A.M.M.; Chiarion-Sileni, V.; Grob, J.-J.; Dummer, R.; Wolchok, J.D.; Schmidt, H.; Hamid, O.; Robert, C.; A Ascierto, P.; Richards, J.M.; et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): A randomised, double-blind, phase 3 trial. Lancet Oncol. 2015, 16, 522–530. [Google Scholar] [CrossRef]
- Kirkwood, J.M.; Strawderman, M.H.; Ernstoff, M.S.; Smith, T.J.; Borden, E.C.; Blum, R.H. Interferon alfa-2b adjuvant therapy of high-risk resected cutaneous melanoma: The Eastern Cooperative Oncology Group Trial EST 1684. J. Clin. Oncol. 1996, 14, 7–17. [Google Scholar] [CrossRef]
- Stachyra-Strawa, P.; Ciesielka, M.; Janiszewski, M.; Grzybowska-Szatkowska, L. The role of immunotherapy and molecular-targeted therapy in the treatment of melanoma (Review). Oncol. Rep. 2021, 46, 158. [Google Scholar] [CrossRef]
- Herndon, T.M.; Demko, S.G.; Jiang, X.; He, K.; Gootenberg, J.E.; Cohen, M.H.; Keegan, P.; Pazdur, R.U.S. Food and Drug Administration Approval: Peginterferon-alfa-2b for the Adjuvant Treatment of Patients with Melanoma. Oncologist 2012, 17, 1323–1328. [Google Scholar] [CrossRef]
- Atkins, M.B.; Lotze, M.T.; Dutcher, J.P.; Fisher, R.I.; Weiss, G.; Margolin, K.; Abrams, J.; Sznol, M.; Parkinson, D.; Hawkins, M.; et al. High-Dose Recombinant Interleukin 2 Therapy for Patients With Metastatic Melanoma: Analysis of 270 Patients Treated Between 1985 and 1993. J. Clin. Oncol. 1999, 17, 2105–2116. [Google Scholar] [CrossRef]
- Sileni, V.C.; Nortilli, R.; Aversa, S.M.L.; Paccagnella, A.; Medici, M.; Corti, L.; Favaretto, A.; Cetto, G.L.; Monfardini, S. Phase II randomized study of dacarbazine, carmustine, cisplatin and tamoxifen versus dacarbazine alone in advanced melanoma patients. Melanoma Res. 2001, 11, 189–196. [Google Scholar] [CrossRef]
- Su, P.J.; Chen, J.S.; Liaw, C.C.; Chang, H.K.; Wang, H.M.; Yang, T.S.; Lin, Y.C.; Liau, C.T.; Yang, H.Y.; Yeh, K.Y.; et al. Biochemotherapy with carmustine, cisplatin, dacarbazine, tamoxifen and low dose interleukin-2 for patients with metastatic malignant melanoma. Chang Gung Med. J. 2011, 34, 478–486. [Google Scholar]
- Velho, T.R. Metastatic melanoma—A review of current and future drugs. Drugs Context 2012, 2012, 212242. [Google Scholar] [CrossRef]
- Gołąb, J.; Jakóbisiak, M.; Lasek, W.; Stokłosa, T. Immunologia, 6th ed.; PWN: Warszawa, Poland, 2014; pp. 163–165. [Google Scholar]
- Malesa, A.; Nowak, J.; Skórka, K.; Karp, M.; Giannopoulos, K. Immunoterapia z użyciem przeciwciał monoklonalnych ukierunkowanych na szlak PD-1/PD-L1 w chorobach nowotworowych. Acta Haematol. Pol. 2018, 49, 207–227. [Google Scholar] [CrossRef]
- Wilczyński, J.R.; Nowak, M.; Wilczyński, M. Targeted therapy with monoclonal antibodies: Do we observe progress in the management of female genital tract cancers? Postepy Higieny I Medycyny Doswiadczalnej 2018, 72, 192–204. [Google Scholar] [CrossRef]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef]
- Muenst, S.; Soysal, S.; Gao, F.; Obermann, E.C.; Oertli, D.; E Gillanders, W. The presence of programmed death 1 (PD-1)-positive tumor-infiltrating lymphocytes is associated with poor prognosis in human breast cancer. Breast Cancer Res. Treat. 2013, 139, 667–676. [Google Scholar] [CrossRef]
- Wan, P.T.; Garnett, M.J.; Roe, S.M.; Lee, S.; Niculescu-Duvaz, D.; Good, V.M.; Cancer Genome Project; Jones, C.M.; Marshall, C.J.; Springer, C.J.; et al. Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell 2004, 116, 855–867. [Google Scholar] [CrossRef]
- Zaleśna, I.; Hartman, M.L.; Czyż, M. BRAF mutation in progression and therapy of melanoma, papillary thyroid carcinoma and colorectal adenocarcinoma. Postepy Hig. Med. Dosw. 2016, 70, 471–488. [Google Scholar] [CrossRef]
- Viros, A.; Fridlyand, J.; Bauer, J.; Lasithiotakis, K.; Garbe, C.; Pinkel, D.; Bastian, B.C. Improving Melanoma Classification by Integrating Genetic and Morphologic Features. PLoS Med. 2008, 5, e120. [Google Scholar] [CrossRef]
- Dummer, R.; Hauschild, A.; Santinami, M.; Atkinson, V.; Mandalà, M.; Kirkwood, J.M.; Sileni, V.C.; Larkin, J.; Nyakas, M.; Dutriaux, C.; et al. Five-Year Analysis of Adjuvant Dabrafenib plus Trametinib in Stage III Melanoma. N. Engl. J. Med. 2020, 383, 1139–1148. [Google Scholar] [CrossRef]
- Robert, C.; Thomas, L.; Bondarenko, I.; O’Day, S.; Weber, J.; Garbe, C.; Lebbe, C.; Baurain, J.-F.; Testori, A.; Grob, J.-J.; et al. Ipilimumab plus Dacarbazine for Previously Untreated Metastatic Melanoma. N. Engl. J. Med. 2011, 364, 2517–2526. [Google Scholar] [CrossRef]
- Geering, B.; Fussenegger, M. Synthetic immunology: Modulating the human immune system. Trends Biotechnol. 2015, 33, 65–79. [Google Scholar] [CrossRef]
- Shuptrine, C.W.; Surana, R.; Weiner, L.M. Monoclonal antibodies for the treatment of cancer. Semin. Cancer Biol. 2012, 22, 3–13. [Google Scholar] [CrossRef]
- Fellner, C. Ipilimumab (yervoy) prolongs survival in advanced melanoma: Serious side effects and a hefty price tag may limit its use. Pharm. Ther. 2012, 37, 503–530. [Google Scholar]
- Weber, J.; Mandalà, M.; Del Vecchio, M.; Gogas, H.J.; Arance, A.M.; Cowey, C.L.; Dalle, S.; Schenker, M.; Chiarion-Sileni, V.; Marquez-Rodas, I.; et al. Adjuvant Nivolumab versus Ipilimumab in Resected Stage III or IV Melanoma. N. Engl. J. Med. 2017, 377, 1824–1835. [Google Scholar] [CrossRef]
- Wolchok, J.D.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.-J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. CheckMate 067: 6.5-year outcomes in patients (pts) with advanced melanoma. J. Clin. Oncol. 2021, 39, 9506. [Google Scholar] [CrossRef]
- Robert, C.; Ribas, A.; Schachter, J.; Arance, A.; Grob, J.-J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.M.; Lotem, M.; et al. Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE-006): Post-hoc 5-year results from an open-label, multicentre, randomised, controlled, phase 3 study. Lancet Oncol. 2019, 20, 1239–1251. [Google Scholar] [CrossRef]
- Hamid, O.; Robert, C.; Daud, A.; Hodi, F.S.; Hwu, W.J.; Kefford, R.; Wolchok, J.D.; Hersey, P.; Joseph, R.; Weber, J.S.; et al. Five-year survival outcomes for patients with advanced melanoma treated with pembrolizumab in KEYNOTE-001. Ann. Oncol. 2019, 30, 582–588. [Google Scholar] [CrossRef]
- Proietti, I.; Skroza, N.; Michelini, S.; Mambrin, A.; Balduzzi, V.; Bernardini, N.; Marchesiello, A.; Tolino, E.; Volpe, S.; Maddalena, P.; et al. BRAF Inhibitors: Molecular Targeting and Immunomodulatory Actions. Cancers 2020, 12, 1823. [Google Scholar] [CrossRef]
- Unsworth, A.; Bye, A.P.; Kriek, N.; Sage, T.; Osborne, A.; Donaghy, D.; Gibbins, J.M. Cobimetinib and trametinib inhibit platelet MEK but do not cause platelet dysfunction. Platelets 2018, 30, 762–772. [Google Scholar] [CrossRef]
- Hauschild, A.; Grobb, J.; Demidov, L.; Jouary, T.; Gutzmer, R.; Millward, M.; Rutkowski, P.; Blank, C.; Miller, W.; Martin-Algarra, S.; et al. An Update on Overall Survival (Os) and Follow-On Therapies in Break-3, a Phase Iii, Randomized Trial: Dabrafenib (D) Vs. Dacarbazine (Dtic) in Patients (Pts) with Braf V600E Mutation-Positive Metastatic Melanoma (Mm). Ann. Oncol. 2014, 25, iv378. [Google Scholar] [CrossRef]
- Chapman, P.B.; Hauschild, A.; Robert, C.; Haanen, J.B.; Ascierto, P.; Larkin, J.; Dummer, R.; Garbe, C.; Testori, A.; Maio, M.; et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N. Engl. J. Med. 2011, 364, 2507–2516. [Google Scholar] [CrossRef]
- Robert, C.; Grob, J.J.; Stroyakovskiy, D.; Karaszewska, B.; Hauschild, A.; Levchenko, E.; Chiarion Sileni, V.; Schachter, J.; Garbe, C.; Bondarenko, I.; et al. Five-Year Outcomes with Dabrafenib plus Trametinib in Metastatic Melanoma. N. Engl. J. Med. 2019, 381, 626–636. [Google Scholar] [CrossRef]
- Larkin, J.; Ascierto, P.A.; Dréno, B.; Atkinson, V.; Liszkay, G.; Maio, M.; Mandalà, M.; Demidov, L.; Stroyakovskiy, D.; Thomas, L.; et al. Combined Vemurafenib and Cobimetinib in BRAF-Mutated Melanoma. N. Engl. J. Med. 2014, 371, 1867–1876. [Google Scholar] [CrossRef]
- Gutzmer, R.; Stroyakovskiy, D.; Gogas, H.; Robert, C.; Lewis, K.; Protsenko, S.; Pereira, R.P.; Eigentler, T.; Rutkowski, P.; Demidov, L.; et al. Atezolizumab, vemurafenib, and cobimetinib as first-line treatment for unresectable advanced BRAFV600 mutation-positive melanoma (IMspire150): Primary analysis of the randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2020, 395, 1835–1844. [Google Scholar] [CrossRef]
- Picard, M.; Dang, N.P.; D’Incan, M.; Mansard, S.; Déchelotte, P.; Pereira, B.; Mondié, J.; Barthélémy, I. Is BRAF a prognostic factor in stage III skin melanoma? A retrospective study of 72 patients after positive sentinel lymph node dissection. Br. J. Dermatol. 2014, 171, 108–114. [Google Scholar] [CrossRef]
- Mann, G.; Pupo, G.M.; Campain, A.E.; Carter, C.D.; Schramm, S.-J.; Pianova, S.; Gerega, S.K.; De Silva, C.; Lai, K.; Wilmott, J.; et al. BRAF Mutation, NRAS Mutation, and the Absence of an Immune-Related Expressed Gene Profile Predict Poor Outcome in Patients with Stage III Melanoma. J. Investig. Dermatol. 2013, 133, 509–517. [Google Scholar] [CrossRef]
- Ellerhorst, J.A.; Greene, V.R.; Ekmekcioglu, S.; Warneke, C.L.; Johnson, M.M.; Cooke, C.P.; Wang, L.-E.; Prieto, V.G.; Gershenwald, J.E.; Wei, Q.; et al. Clinical Correlates ofNRASandBRAFMutations in Primary Human Melanoma. Clin. Cancer Res. 2010, 17, 229–235. [Google Scholar] [CrossRef]
- Meckbach, D.; Bauer, J.; Pflugfelder, A.; Meier, F.; Busch, C.; Eigentler, T.; Capper, D.; von Deimling, A.; Mittelbronn, M.; Perner, S.; et al. Survival According to BRAF-V600 Tumor Mutations—An Analysis of 437 Patients with Primary Melanoma. PLoS ONE 2014, 9, e86194. [Google Scholar] [CrossRef][Green Version]
- Ardekani, G.S.; Jafarnejad, S.M.; Tan, L.; Saeedi, A.; Li, G. The Prognostic Value of BRAF Mutation in Colorectal Cancer and Melanoma: A Systematic Review and Meta-Analysis. PLoS ONE 2012, 7, e47054. [Google Scholar] [CrossRef]
- Rutkowski, P.; Gos, A.; Jurkowska, M.; Świtaj, T.; Dziewirski, W.; Zdzienicki, M.; Ptaszyński, K.; Michej, W.; Tysarowski, A.; Siedlecki, J. Molecular alterations in clinical stage III cutaneous melanoma: Correlation with clinicopathological features and patient outcome. Oncol. Lett. 2014, 8, 47–54. [Google Scholar] [CrossRef][Green Version]
- Long, G.V.; Menzies, A.M.; Nagrial, A.M.; Haydu, L.E.; Hamilton, A.L.; Mann, G.J.; Hughes, T.; Thompson, J.F.; Scolyer, R.A.; Kefford, R.F. Prognostic and Clinicopathologic Associations of Oncogenic BRAF in Metastatic Melanoma. J. Clin. Oncol. 2011, 29, 1239–1246. [Google Scholar] [CrossRef]
| Patient Parameters | Patient Data |
|---|---|
| Number of patients | 52 |
| Sex | |
| Women | 15 |
| Men | 37 |
| Median age (range) | 59.9 (29–65) |
| Type of recurrence | |
| Distant | 33 |
| Local | 2 |
| Distant and local | 16 |
| Location of primary lesion | |
| Peripheral (extremities) | 25 |
| Central (head and torso) | 8 |
| Unknown primary | 19 |
| BRAF status | |
| BRAF V600 | 28 |
| BRAF-wildtype | 21 |
| Unknown | 3 |
| Blood parameters | |
| HGB ≥12.5 g/dL | 35 |
| HGB <12.5 g/dL | 17 |
| MCV 80–95 fl | 39 |
| MCV >95 fl | 5 |
| MCV <80 fl | 8 |
| MCH 27–32 pg | 41 |
| MCH >32 pg | 3 |
| MCH <27 pg | 8 |
| MCHC 27–32 g/dL | 48 |
| MCHC >32 g/dL | 3 |
| MCHC <27 g/dL | 0 |
| PLT 150.000–450.000 | 48 |
| PLT >450.000 | 4 |
| PLT <150.000 | 0 |
| LDH ≤240 U/L | 27 |
| LDH 241–480 U/L | 16 |
| LDH >481 U/L | 3 |
| Unknown LDH | 6 |
| ALT ≤40 U/L | 49 |
| ALT 41–200 U/L | 3 |
| AST ≤40 U/L | 48 |
| AST 41–200 U/L | 4 |
| Level of invasion (Clark classification) | |
| I | 1 |
| II | 1 |
| III | 4 |
| IV | 24 |
| V | 14 |
| Metastases | |
| Lymph nodes | 38 |
| Lung | 30 |
| Brain | 18 |
| Skin and subcutaneous tissue | 18 |
| Liver | 16 |
| Bones | 10 |
| Other | 15 |
| Primary surgical treatment | 47 |
| First-line treatment | 52 |
| Anti-BRAF/MEK | 28 |
| Vemurafenib | 24 |
| Dabrafenib | 3 |
| Vemurafenib + cobimetinib | 1 |
| Anti-PD-1/anti-CTLA4 | 24 |
| Pembrolizumab | 10 |
| Ipilimumab | 10 |
| Nivolumab | 4 |
| Second-line treatment | 27 |
| Ipilimumab | 16 |
| Nivolumab | 6 |
| Pembrolizumab | 5 |
| Other treatment | |
| Palliative radiotherapy | 23 |
| Surgical treatment of metastases | 14 |
| Surgical treatment of local recurrence | 11 |
| Analyzed Parameter | RFS | PFS 1 | PFS 2 | OS | ||||
|---|---|---|---|---|---|---|---|---|
| Test Value | p | Test Value | p | Test Value | p | Test Value | p | |
| Sex | 1.058 | 0.289 | 1.815 | 0.069 | 1.726 | 0.084 | 0.720 | 0.471 |
| Age | 0.076 | 0.782 | 0.187 | 0.664 | 0.506 | 0.476 | 0.708 | 0.400 |
| Type of recurrence | 0.837 | 0.402 | −0.409 | 0.682 | 0.055 | 0.955 | 1.740 | 0.081 |
| Location of primary lesion | −0.208 | 0.835 | 0.274 | 0.7831 | −0.382 | 0.701 | 0.134 | 0.892 |
| BRAF status | −2.211 | 0.027 | 1.266 | 0.205 | 0.546 | 0.584 | −0.530 | 0.595 |
| HGB (<12.5 vs. ≥12.5 g/dL) | −0.265 | 0.790 | −1.312 | 0.189 | −1.553 | 0.120 | −1.415 | 0.157 |
| MCV (norm vs. beyond the norm) | 1.224 | 0.542 | 0.624 | 0.731 | 1.280 | 0.527 | 0.534 | 0.765 |
| MCH (norm vs. beyond the norm) | 0.245 | 0.884 | 1.566 | 0.457 | 5.065 | 0.079 | 0.587 | 0.745 |
| MCHC (norm vs. beyond the norm) | −0.456 | 0.648 | −1.132 | 0.257 | −0.701 | 0.482 | −0.804 | 0.421 |
| PLT (norm vs. beyond the norm) | 0.771 | 0.440 | −0.833 | 0.404 | −0.354 | 0.722 | −0.283 | 0.776 |
| LDH (continuous value) | 0.128 | 0.719 | 1.582 | 0.208 | 2.051 | 0.152 | 1.946 | 0.162 |
| Primary surgical treatment | −1.630 | 0.103 | 0.182 | 0.856 | −0.741 | 0.458 | −0.748 | 0.454 |
| Level of invasion (Clark classification) | 4.977 | 0.418 | 2.447 | 0.784 | NA | NA | 3.322 | 0.650 |
| Type of treatment (anti-BRAF vs. immunotherapy) | NA * | NA | 1.998 | 0.046 | NA | NA | 0.238 | 0.812 |
| Lung metastases | NA | NA | NA | NA | NA | NA | 0.521 | 0.602 |
| Liver metastases | NA | NA | NA | NA | NA | NA | 0.176 | 0.860 |
| Brain metastases | NA | NA | NA | NA | NA | NA | 0.344 | 0.730 |
| Lymph node metastases | NA | NA | NA | NA | NA | NA | −0.389 | 0.697 |
| Skin and subcutaneous tissue metastases | NA | NA | NA | NA | NA | NA | −0.003 | 0.997 |
| Bone metastases | NA | NA | NA | NA | NA | NA | 0.224 | 0.823 |
| Other | NA | NA | NA | NA | NA | NA | −0.024 | 0.980 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szatkowska, L.; Sieczek, J.; Tekiela, K.; Ziętek, M.; Stachyra-Strawa, P.; Cisek, P.; Matkowski, R. Outcomes of Patients with Metastatic Melanoma—A Single-Institution Retrospective Analysis. Cancers 2022, 14, 1672. https://doi.org/10.3390/cancers14071672
Szatkowska L, Sieczek J, Tekiela K, Ziętek M, Stachyra-Strawa P, Cisek P, Matkowski R. Outcomes of Patients with Metastatic Melanoma—A Single-Institution Retrospective Analysis. Cancers. 2022; 14(7):1672. https://doi.org/10.3390/cancers14071672
Chicago/Turabian StyleSzatkowska, Lidia, Jan Sieczek, Katarzyna Tekiela, Marcin Ziętek, Paulina Stachyra-Strawa, Paweł Cisek, and Rafał Matkowski. 2022. "Outcomes of Patients with Metastatic Melanoma—A Single-Institution Retrospective Analysis" Cancers 14, no. 7: 1672. https://doi.org/10.3390/cancers14071672
APA StyleSzatkowska, L., Sieczek, J., Tekiela, K., Ziętek, M., Stachyra-Strawa, P., Cisek, P., & Matkowski, R. (2022). Outcomes of Patients with Metastatic Melanoma—A Single-Institution Retrospective Analysis. Cancers, 14(7), 1672. https://doi.org/10.3390/cancers14071672







